Preliminary Assessment of Polysaccharide-Based Emulgels Containing Delta-Aminolevulinic Acid for Oral Lichen planus Treatment
Abstract
:1. Introduction
2. Results and Discussion
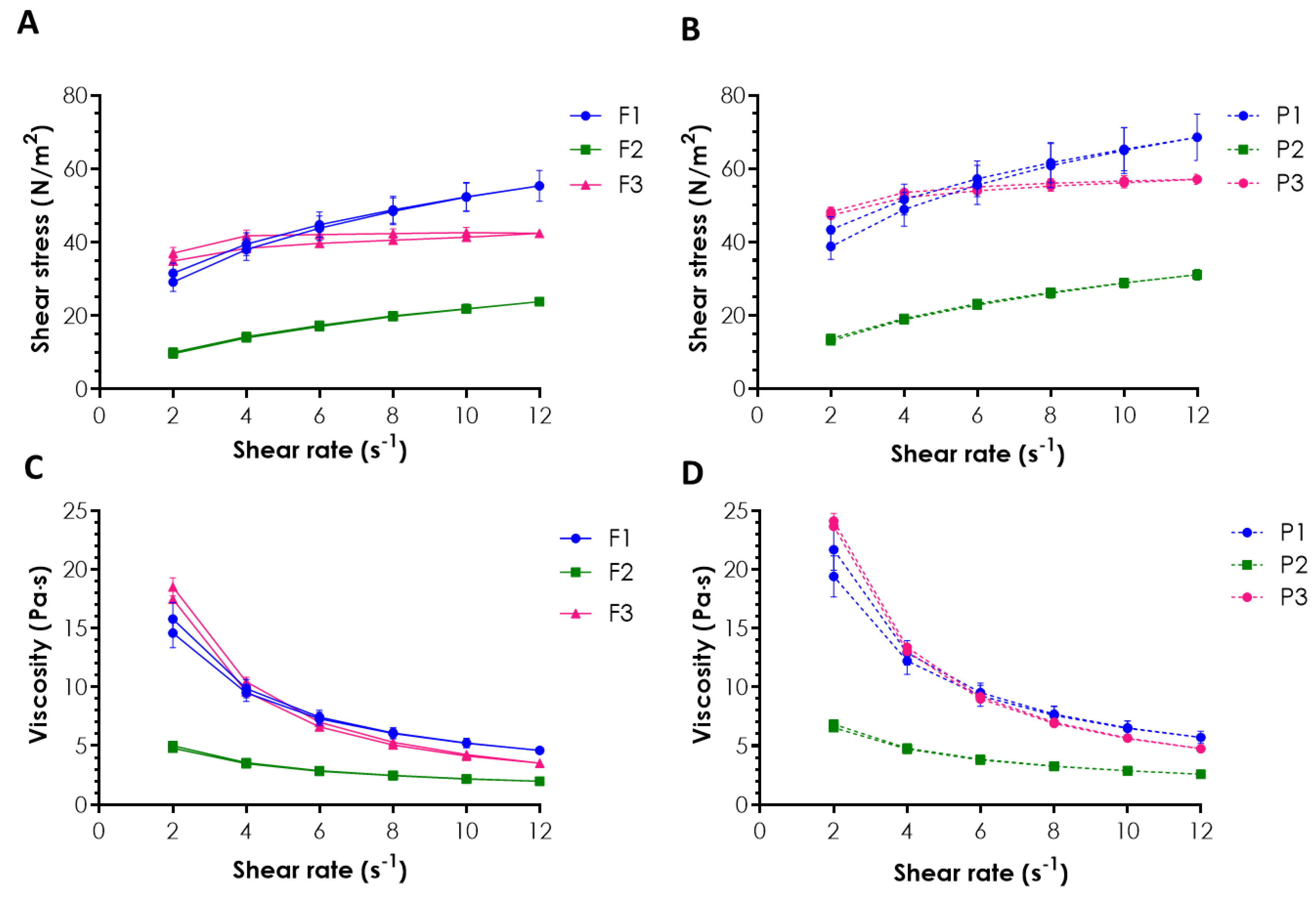
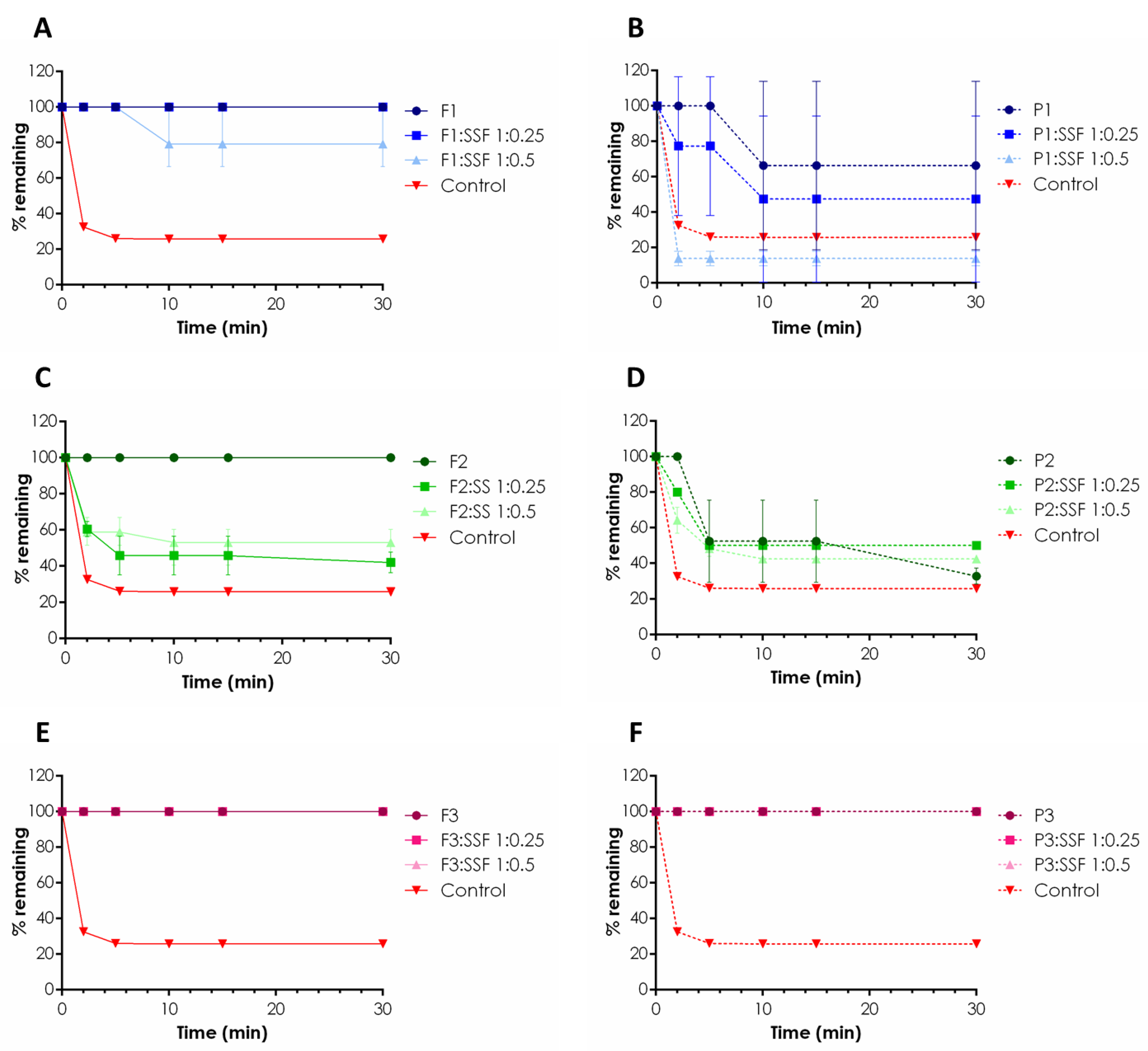
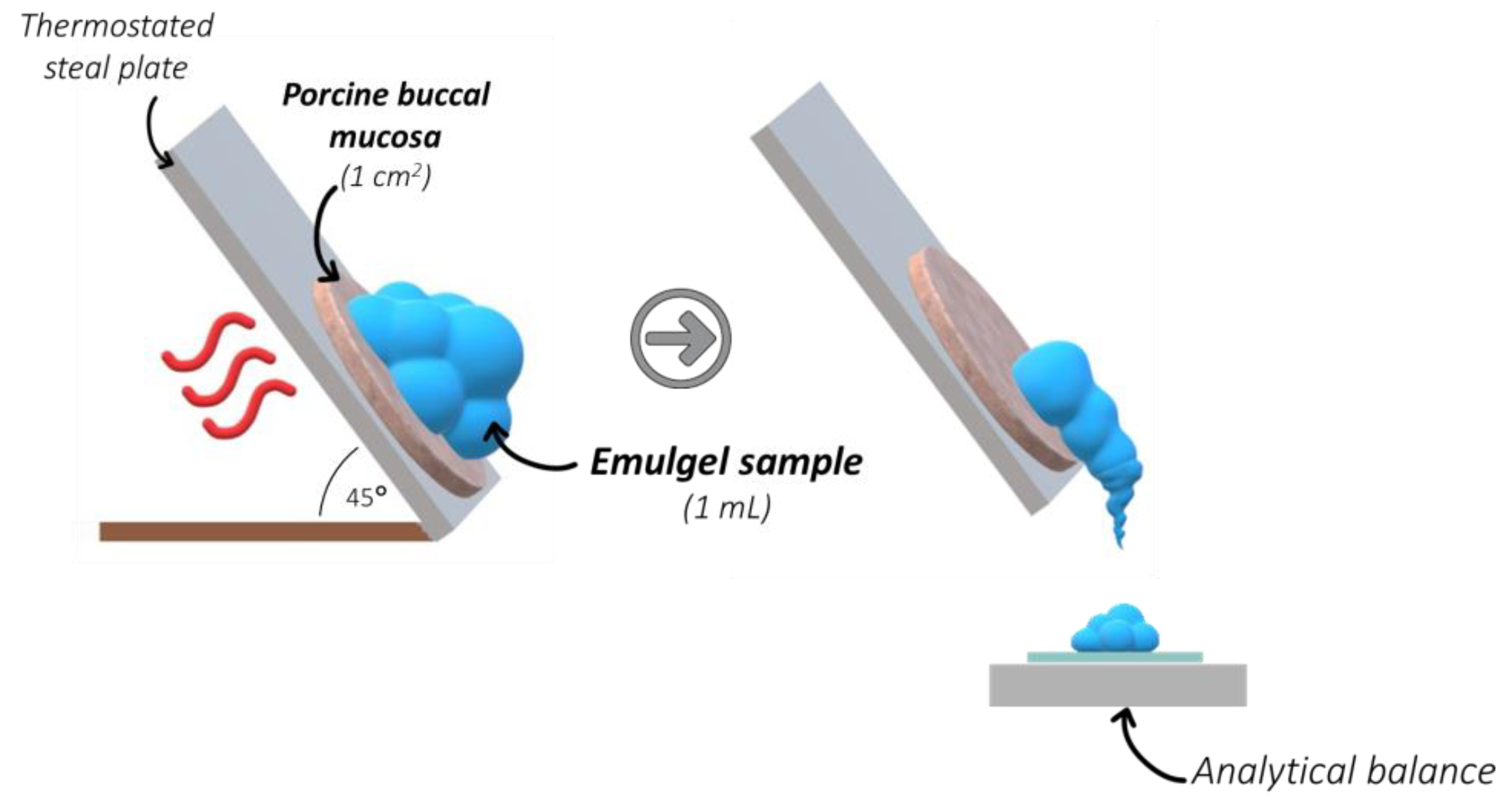
2.1. Rheological Behavior and Mucoretention Characteristics
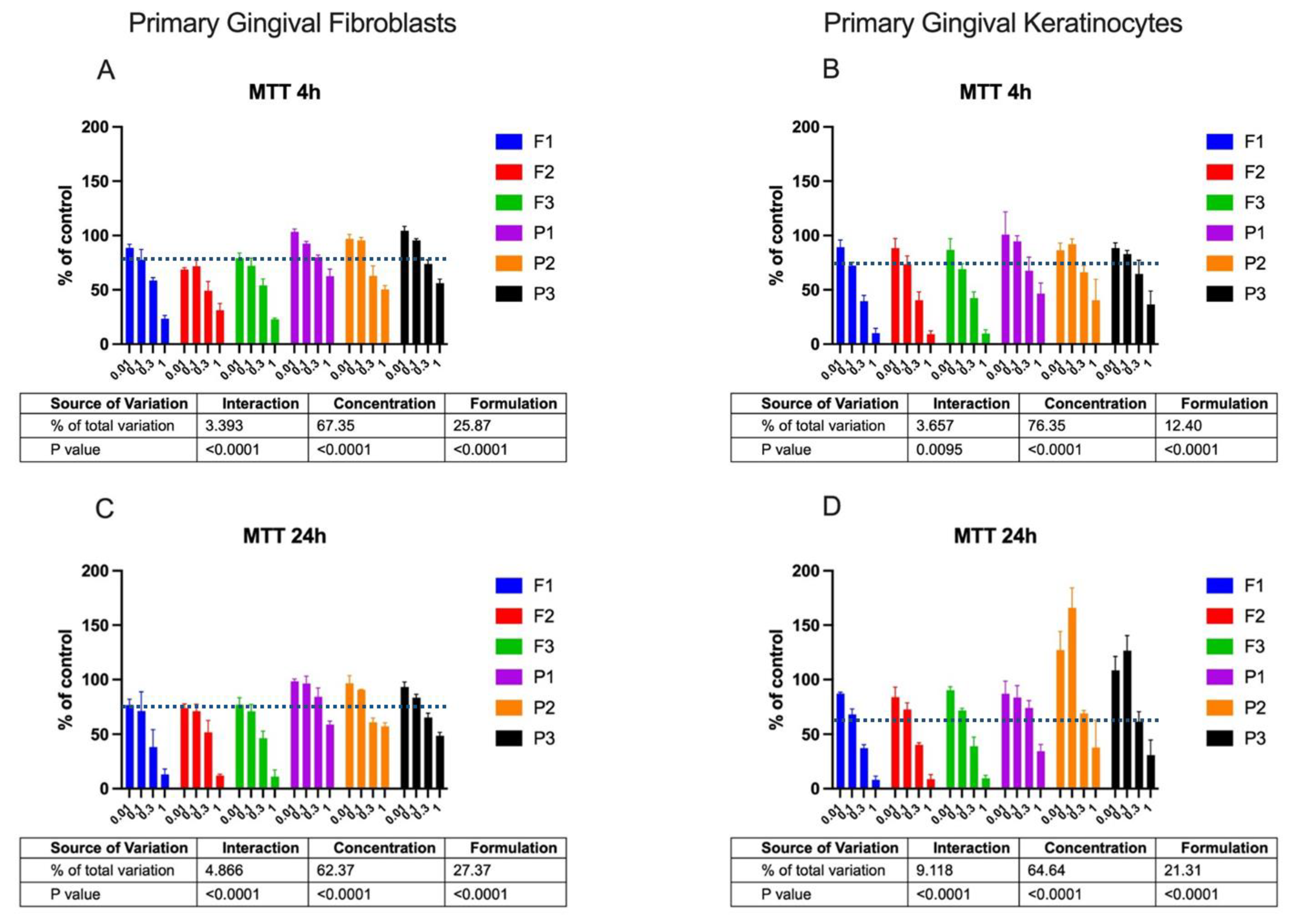
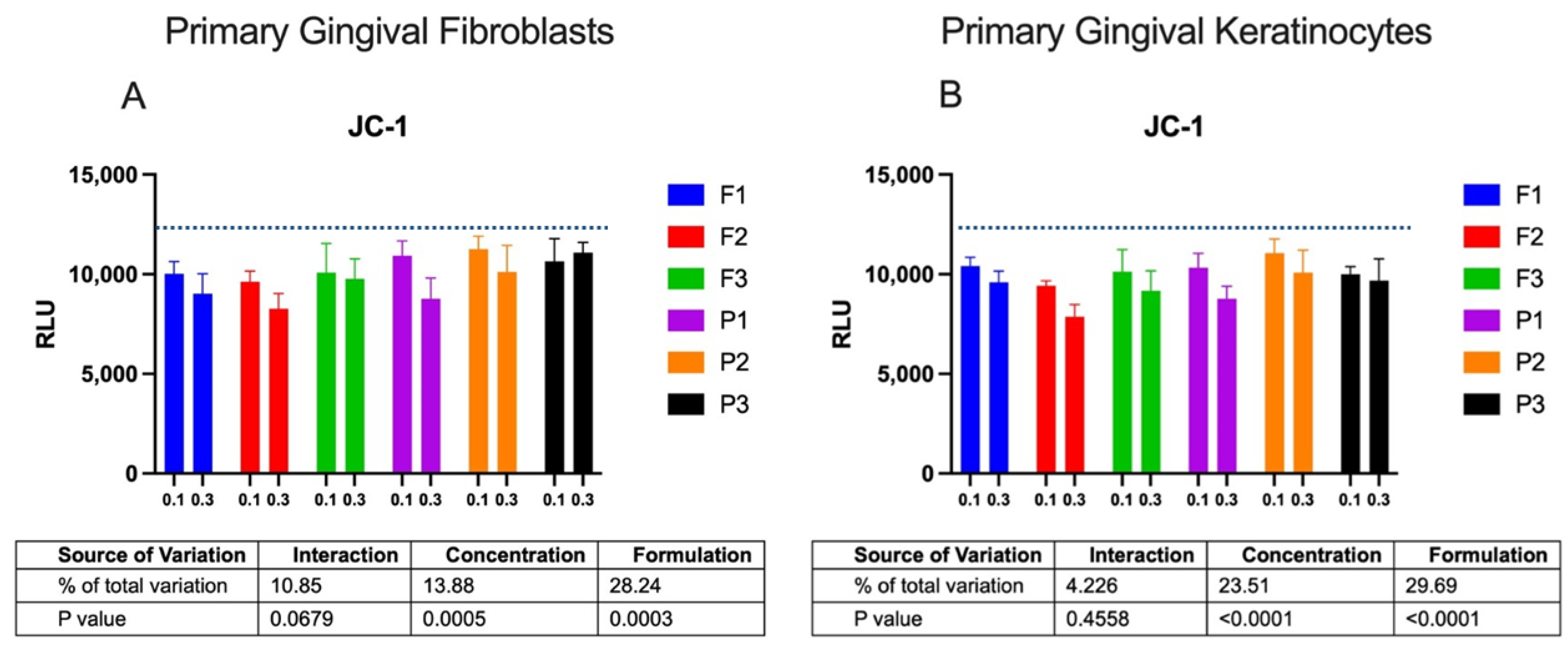
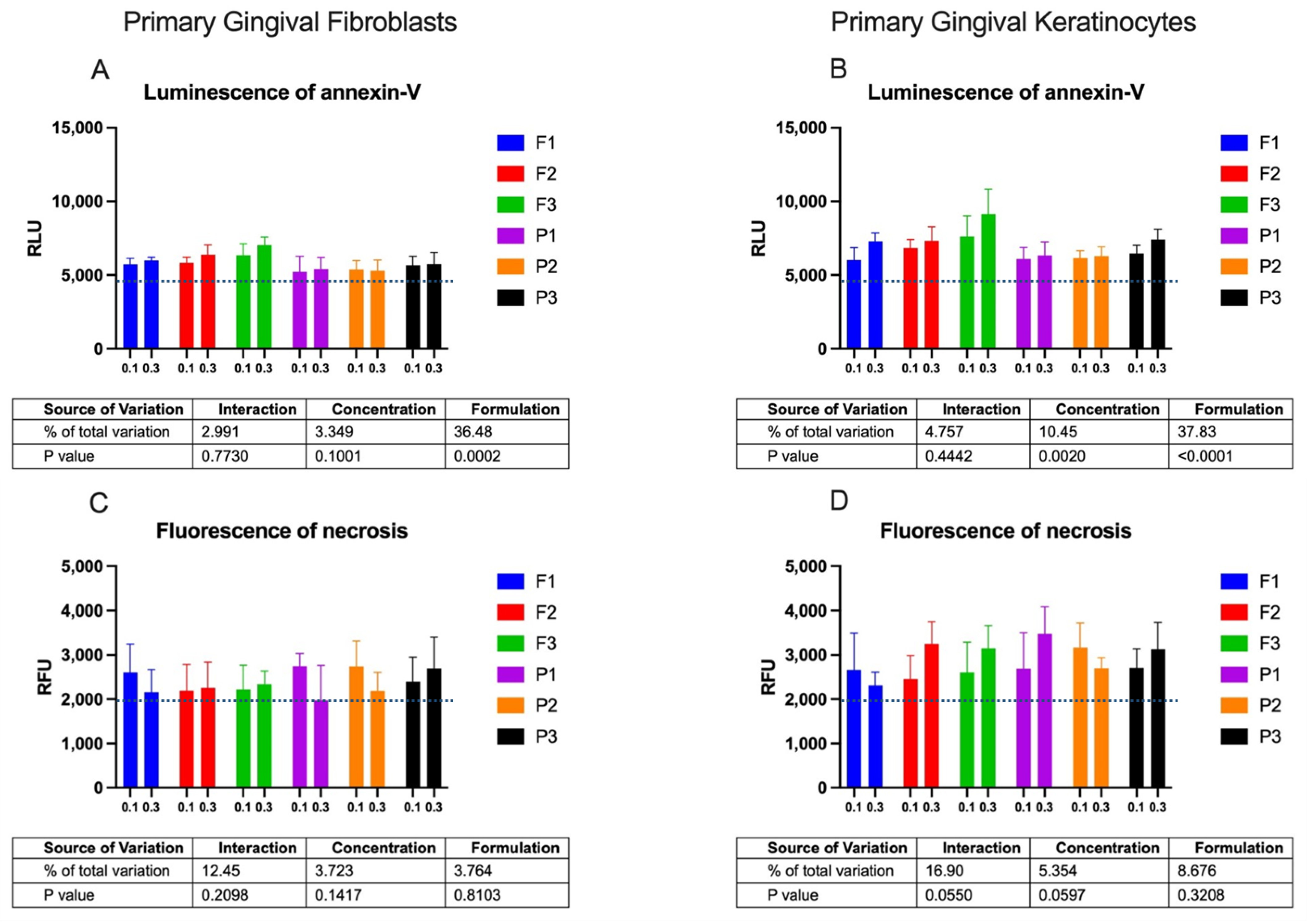
2.2. Cytotoxicity Profile
3. Materials and Methods
3.1. Materials
3.2. Emulgel Preparation and Characterization
3.3. Rheological Measurements
3.4. Mucoretention Measurements
- W0—initial sample weight applied on the tissue,
- Wt—weight of the sample detached from the tissue at predetermined time point.
3.5. Cytotoxicity Profile
3.5.1. Cells
3.5.2. Sample Dilutions for Cytotoxicity Studies
3.5.3. MTT Assay
3.5.4. RealTime-GloTM Annexin V Assay
3.5.5. JC-1 Assay
3.6. Statistical Analysis
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lavanya, N.; Jayanthi, P.; Rao, U.K.; Ranganathan, K. Oral Lichen Planus: An Update on Pathogenesis and Treatment. J. Oral Maxillofac. Pathol. 2011, 15, 127–132. [Google Scholar] [CrossRef]
- Ismail, S.B.; Kumar, S.K.S.; Zain, R.B. Oral Lichen Planus and Lichenoid Reactions: Etiopathogenesis, Diagnosis, Management and Malignant Transformation. J. Oral Sci. 2007, 49, 89–106. [Google Scholar] [CrossRef] [PubMed]
- Rotaru, D.; Chisnoiu, R.; Picos, A.M.; Picos, A.; Chisnoiu, A. Treatment Trends in Oral Lichen Planus and Oral Lichenoid Lesions (Review). Exp. Ther. Med. 2020, 20, 198. [Google Scholar] [CrossRef]
- Romano, A.; Di Stasio, D.; Gentile, E.; Petruzzi, M.; Serpico, R.; Lucchese, A. The Potential Role of Photodynamic Therapy in Oral Premalignant and Malignant Lesions: A Systematic Review. J. Oral Pathol. Med. 2021, 50, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Sulewska, M.; Duraj, E.; Sobaniec, S.; Graczyk, A.; Milewski, R.; Wróblewska, M.; Pietruski, J.; Pietruska, M. A Clinical Evaluation of the Efficacy of Photodynamic Therapy in the Treatment of Erosive Oral Lichen Planus: A Case Series. Photodiagn. Photodyn. Ther. 2017, 18, 12–19. [Google Scholar] [CrossRef]
- Choudhary, R.; Reddy, S.S.; Nagi, R.; Nagaraju, R.; Kunjumon, S.P.; Sen, R. The Effect of Photodynamic Therapy on Oral-Premalignant Lesions: A Systematic Review. J. Clin. Exp. Dent. 2022, 14, e285–e292. [Google Scholar] [CrossRef] [PubMed]
- Casas, A. Clinical Uses of 5-Aminolaevulinic Acid in Photodynamic Treatment and Photodetection of Cancer: A Review. Cancer Lett. 2020, 490, 165–173. [Google Scholar] [CrossRef]
- Rkein, A.M.; Ozog, D.M. Photodynamic Therapy. Dermatol. Clin. 2014, 32, 415–425. [Google Scholar] [CrossRef]
- Morton, C.A.; Szeimies, R.-M.; Sidoroff, A.; Braathen, L.R. European Guidelines for Topical Photodynamic Therapy Part 1: Treatment Delivery and Current Indications—Actinic Keratoses, Bowen’s Disease, Basal Cell Carcinoma. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Tewari, K.M.; Eggleston, I.M. Chemical Approaches for the Enhancement of 5-Aminolevulinic Acid-Based Photodynamic Therapy and Photodiagnosis. Photochem. Photobiol. Sci. 2018, 17, 1553–1572. [Google Scholar] [CrossRef] [PubMed]
- Gilhotra, R.M.; Ikram, M.; Srivastava, S.; Gilhotra, N. A Clinical Perspective on Mucoadhesive Buccal Drug Delivery Systems. J. Biomed. Res. 2014, 28, 81–97. [Google Scholar] [CrossRef]
- Sulewska, M.E.; Tomaszuk, J.; Sajewicz, E.; Pietruski, J.; Starzyńska, A.; Pietruska, M. Treatment of Reticular Oral Lichen Planus with Photodynamic Therapy: A Case Series. J. Clin. Med. 2023, 12, 875. [Google Scholar] [CrossRef] [PubMed]
- Potaś, J.; Szymańska, E.; Basa, A.; Hafner, A.; Winnicka, K. Tragacanth Gum/Chitosan Polyelectrolyte Complexes-Based Hydrogels Enriched with Xanthan Gum as Promising Materials for Buccal Application. Materials 2020, 14, 86. [Google Scholar] [CrossRef]
- Villar-Padilla, A.; Del Prado-Audelo, M.L.; González-Torres, M.; Giraldo-Gomez, D.M.; Caballero-Florán, I.H.; González-Del Carmen, M.; Sharifi-Rad, J.; Figueroa-González, G.; Reyes-Hernández, O.D.; Cortés, H.; et al. Development of a Xanthan Gum Film for the Possible Treatment of Vaginal Infections. Cell Mol. Biol. 2021, 67, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Jelkmann, M.; Leichner, C.; Zaichik, S.; Laffleur, F.; Bernkop-Schnürch, A. A Gellan Gum Derivative as In-Situ Gelling Cationic Polymer for Nasal Drug Delivery. Int. J. Biol. Macromol. 2020, 158, 1037–1046. [Google Scholar] [CrossRef]
- Arslan, D.; Akbal Dağıstan, Ö.; Sagirli, O.; Mulazimoglu, L.; Cevher, E.; Yildiz-Pekoz, A. Development and Evaluation of Combined Effect Buccal Films for Treatment of Oral Candidiasis. AAPS Pharm. Sci. Tech. 2022, 24, 23. [Google Scholar] [CrossRef]
- Zhang, R.; Gao, T.; Wang, D. Photodynamic Therapy (PDT) for Oral Leukoplakia: A Systematic Review and Meta-Analysis of Single-Arm Studies Examining Efficacy and Subgroup Analyses. BMC Oral Health 2023, 23, 568. [Google Scholar] [CrossRef]
- The European Pharmacopoeia 10.0; Council of Europe: Strasbourg, France, 2020; Volume 1.
- Singh, M.; Kanoujia, J.; Singh, P.; Parashar, P.; Arya, M.; Tripathi, C.B.; Sinha, V.R.; Saraf, S.A. Development of an α-Linolenic Acid Containing a Soft Nanocarrier for Oral Delivery-Part II: Buccoadhesive Gel. RSC Adv. 2016, 6, 101602–101612. [Google Scholar] [CrossRef]
- Lee, C.H.; Moturi, V.; Lee, Y. Thixotropic Property in Pharmaceutical Formulations. J. Control. Release 2009, 136, 88–98. [Google Scholar] [CrossRef]
- Sandri, G.; Rossi, S.; Bonferoni, M.C.; Ferrari, F.; Mori, M.; Caramella, C. The Role of Chitosan as a Mucoadhesive Agent in Mucosal Drug Delivery. J. Drug Deliv. Sci. Technol. 2012, 22, 275–284. [Google Scholar] [CrossRef]
- Bandi, S.P.; Bhatnagar, S.; Venuganti, V.V.K. Advanced Materials for Drug Delivery across Mucosal Barriers. Acta Biomater. 2021, 119, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-Y.; Lee, C.-J.; Lin, Y.-Y. Drug-Polymer Interaction Affecting the Mechanical Properties, Adhesion Strength and Release Kinetics of Piroxicam-Loaded Eudragit E Films Plasticized with Different Plasticizers. J. Control. Release 1995, 33, 375–381. [Google Scholar] [CrossRef]
- Boddupalli, B.M.; Mohammed, Z.N.K.; Nath, R.A.; Banji, D. Mucoadhesive Drug Delivery System: An Overview. J. Adv. Pharm. Technol. Res. 2010, 1, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Buri, P.A. Surface, Interfacial and Molecular Aspects of Polymer Bioadhesion on Soft Tissues. J. Control. Release 1985, 2, 257–275. [Google Scholar] [CrossRef]
- Gu, J.M.; Robinson, J.R.; Leung, S.H. Binding of Acrylic Polymers to Mucin/Epithelial Surfaces: Structure-Property Relationships. Crit. Rev. Ther. Drug Carr. Syst. 1988, 5, 21–67. [Google Scholar]
- Marques, M.R.C.; Loebenberg, R.; Almukainzi, M. Simulated Biological Fluids with Possible Application in Dissolution Testing. Dissolution Technol. 2011, 18, 15–28. [Google Scholar] [CrossRef]
- Namjoshi, S.; Caccetta, R.; Edwards, J.; Benson, H.A.E. Liquid Chromatography Assay for 5-Aminolevulinic Acid: Application to in Vitro Assessment of Skin Penetration via Dermaportation. J. Chromatogr. B 2007, 852, 49–55. [Google Scholar] [CrossRef]
- Rathbone, M.J.; Şenel, S.; Pather, I. Systemic oral mucosal drug delivery systems and delivery systems. In Oral Mucosal Drug Delivery; Gilles, P., Ghazali, F.A., Eds.; Springer: New York, NY, USA, 2015. [Google Scholar]
- Präbst, K.; Engelhardt, H.; Ringgeler, S.; Hübner, H. Basic Colorimetric Proliferation Assays: MTT, WST, and Resazurin BT. In Cell Viability Assays: Methods and Protocols; Gilbert, D.F., Friedrich, O., Eds.; Springer: New York, NY, USA, 2017; pp. 1–17. [Google Scholar] [CrossRef]
| F1 | F2 | F3 | |
|---|---|---|---|
| Compound | Concentration (%, w/w) | ||
| 5-aminolevulinic acid | 5.0 | 5.0 | 5.0 |
| Tragacanth gum | 5.0 | 5.0 | - |
| Xanthan gum | 1.0 | - | 2.0 |
| Gellan gum | - | - | 0.7 |
| Castor oil | 2.0 | 2.0 | 2.0 |
| Lecithin | 0.5 | 0.5 | 0.5 |
| Propylene glycol | 5.0 | 5.0 | 5.0 |
| Disodium dihydrogen ethylenediaminetetraacetate | 0.1 | 0.1 | 0.1 |
| Sodium benzoate | 0.1 | 0.1 | 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymańska, E.; Potaś, J.; Maciejczyk, M.; Sulewska, M.E.; Pietruska, M.; Zalewska, A.; Pietruska, A.; Winnicka, K. Preliminary Assessment of Polysaccharide-Based Emulgels Containing Delta-Aminolevulinic Acid for Oral Lichen planus Treatment. Pharmaceuticals 2023, 16, 1534. https://doi.org/10.3390/ph16111534
Szymańska E, Potaś J, Maciejczyk M, Sulewska ME, Pietruska M, Zalewska A, Pietruska A, Winnicka K. Preliminary Assessment of Polysaccharide-Based Emulgels Containing Delta-Aminolevulinic Acid for Oral Lichen planus Treatment. Pharmaceuticals. 2023; 16(11):1534. https://doi.org/10.3390/ph16111534
Chicago/Turabian StyleSzymańska, Emilia, Joanna Potaś, Mateusz Maciejczyk, Magdalena Ewa Sulewska, Małgorzata Pietruska, Anna Zalewska, Aleksandra Pietruska, and Katarzyna Winnicka. 2023. "Preliminary Assessment of Polysaccharide-Based Emulgels Containing Delta-Aminolevulinic Acid for Oral Lichen planus Treatment" Pharmaceuticals 16, no. 11: 1534. https://doi.org/10.3390/ph16111534







