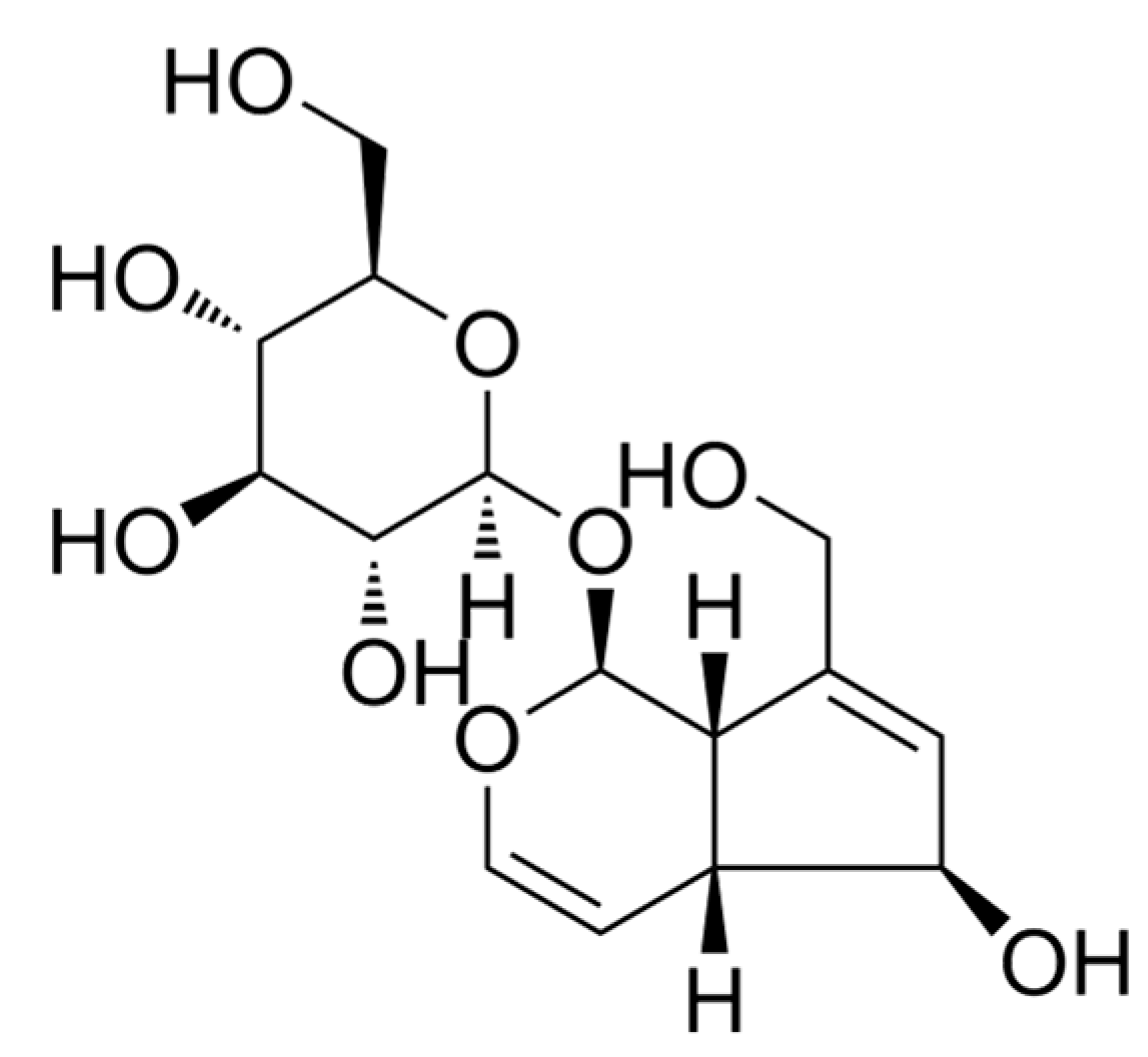
The Analgesia Effect of Aucubin on CFA-Induced Inflammatory Pain by Inhibiting Glial Cells Activation-Mediated Inflammatory Response via Activating Mitophagy
Abstract
:1. Introduction
2. Results
2.1. Effect of Aucubin on CFA-Induced Inflammatory Pain in Mice
2.2. Effects of Aucubin on Inflammatory Responses in Mice Induced by CFA
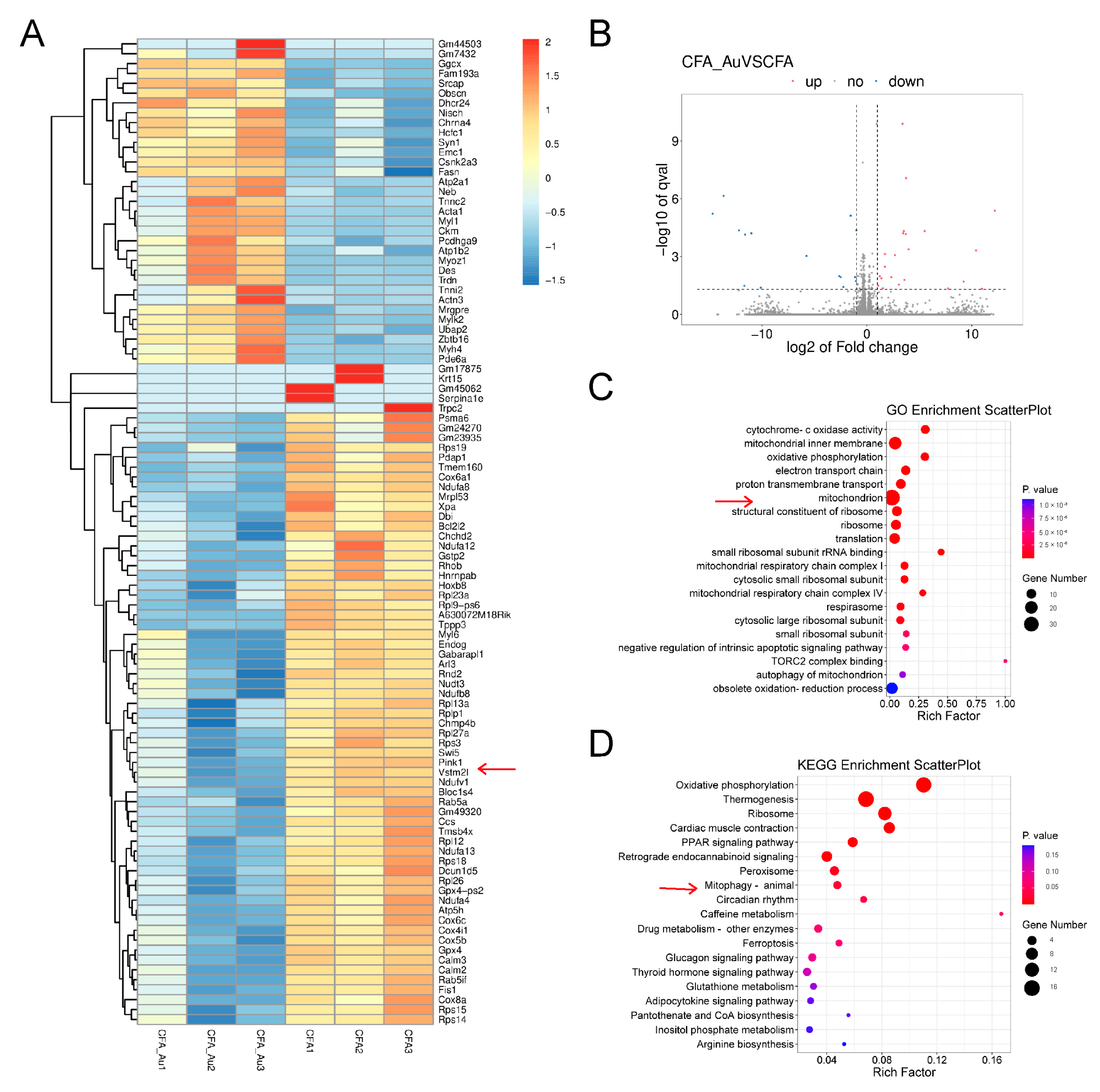
2.3. RNA-Seq Analysis in the Spinal Cord of CFA-Injected Mice Treated with Aucubin
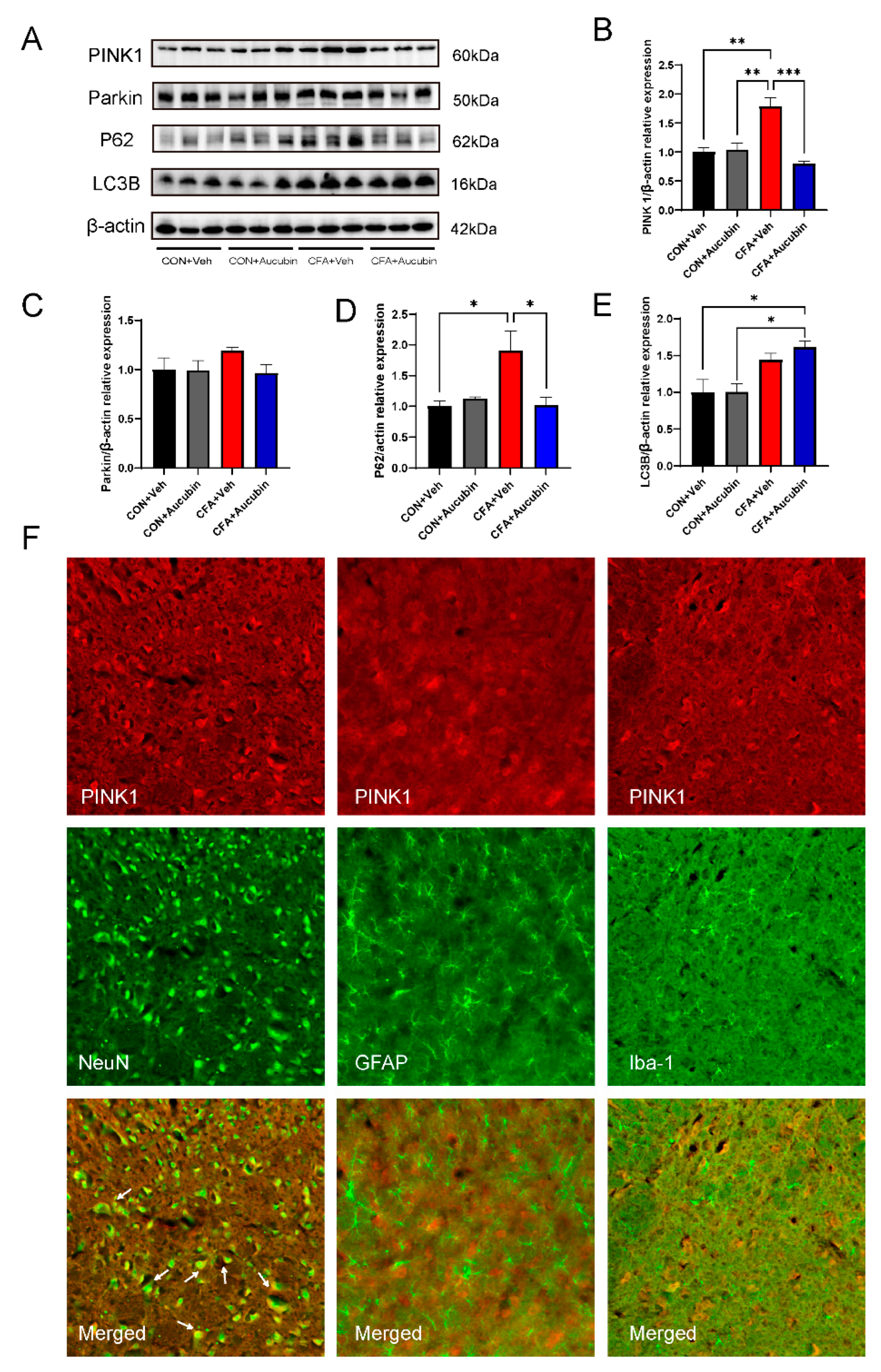
2.4. Effects of Aucubin on Mitophagy in CFA-Injected Mice
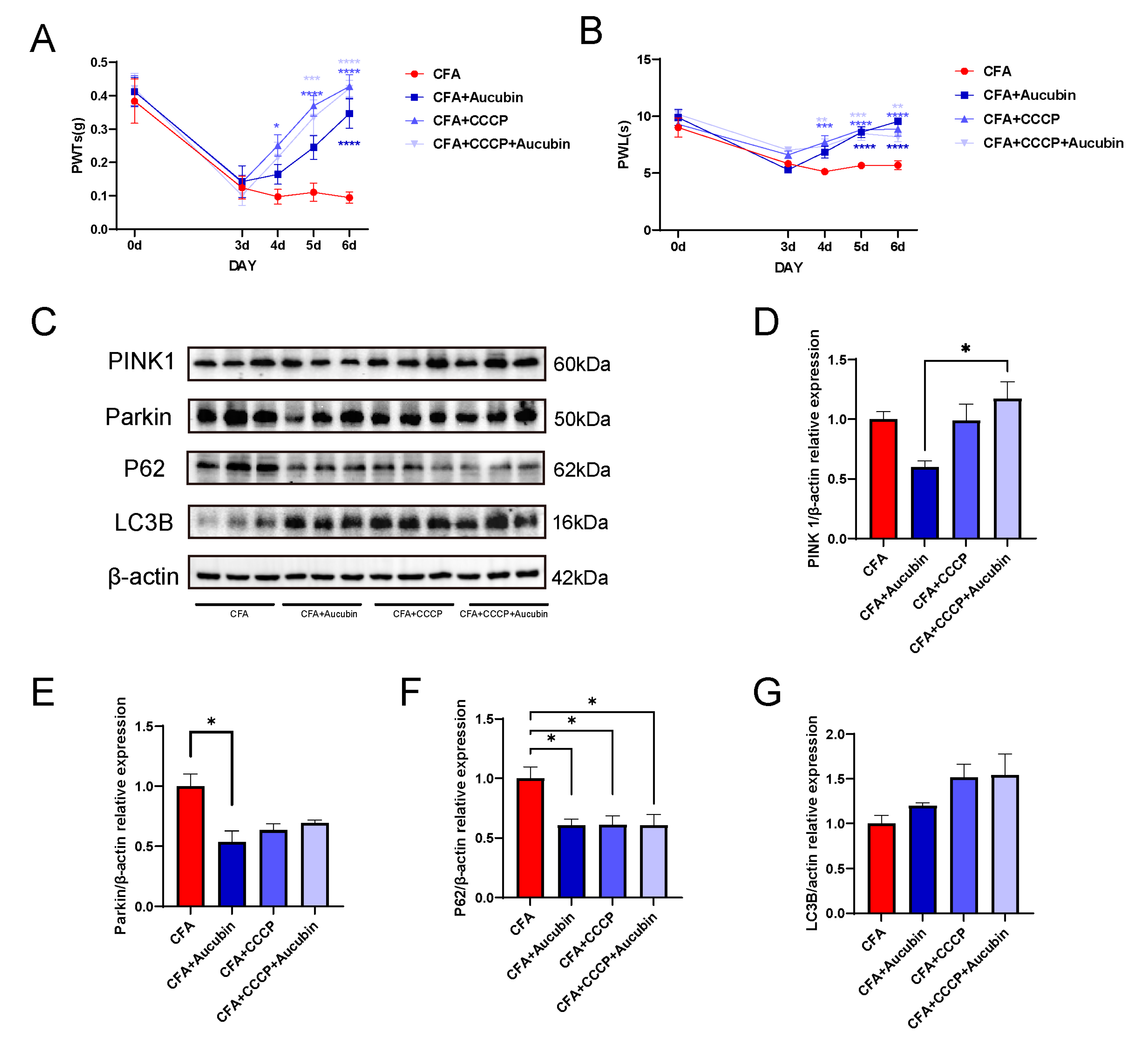
2.5. CCCP Significantly Alleviated CFA-Induced Inflammatory Pain in Mice by Activating Mitophagy
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Animals
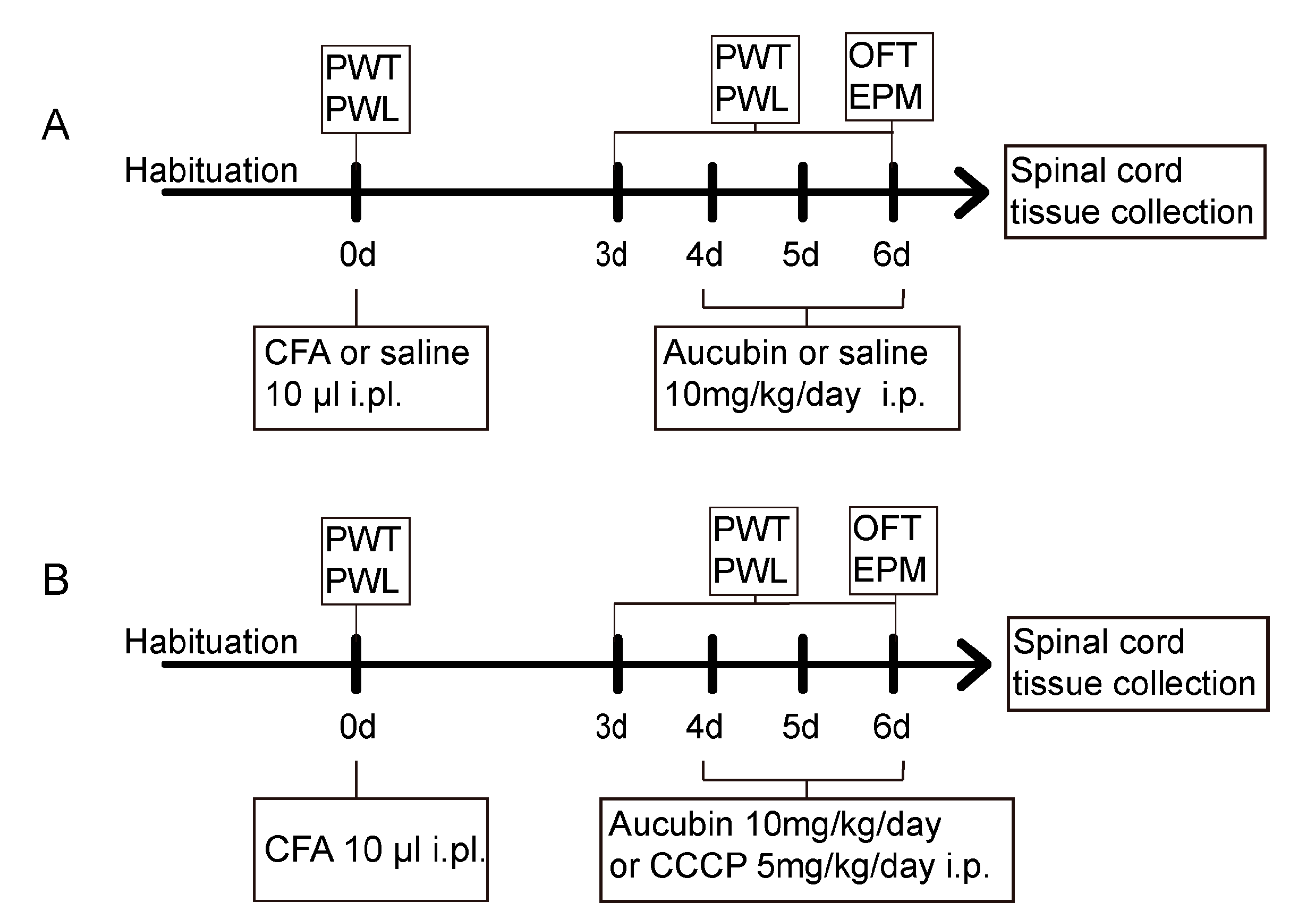
4.3. Experimental Protocol and Treatment Schedule
4.4. Mechanical Allodynia
4.5. Thermal Hyperalgesia
4.6. Elevated Plus Maze Test
4.7. Open Field Test
4.8. Immunofluorescence Staining
4.9. Western Blot Analysis
4.10. RNA Sequencing
4.11. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Djouhri, L.; Al Otaibi, M.; Kahlat, K.; Smith, T.; Sathish, J.; Weng, X. Persistent hindlimb inflammation induces changes in activation properties of hyperpolarization-activated current (Ih) in rat C-fiber nociceptors in vivo. Neuroscience 2015, 301, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Mills, S.E.E.; Nicolson, K.P.; Smith, B.H. Chronic pain: A review of its epidemiology and associated factors in population-based studies. Br. J. Anaesth. 2019, 123, e273–e283. [Google Scholar] [CrossRef] [PubMed]
- Yekkirala, A.S.; Roberson, D.P.; Bean, B.P.; Woolf, C.J. Breaking barriers to novel analgesic drug development. Nat. Rev. Drug Discov. 2017, 16, 545–564. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, C.R.; Andriessen, A.S.; Chen, G.; Wang, K.; Jiang, C.; Maixner, W.; Ji, R.-R. Central Nervous System Targets: Glial Cell Mechanisms in Chronic Pain. Neurotherapeutics 2020, 17, 846–860. [Google Scholar] [CrossRef]
- Grace, P.M.; Hutchinson, M.R.; Maier, S.F.; Watkins, L.R. Pathological pain and the neuroimmune interface. Nat. Rev. Immunol. 2014, 14, 217–231. [Google Scholar] [CrossRef]
- Ji, R.-R.; Nackley, A.; Huh, Y.; Terrando, N.; Maixner, W. Neuroinflammation and Central Sensitization in Chronic and Widespread Pain. Anesthesiology 2018, 129, 343–366. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Abdelmohsen, K.; Abe, A.; Abedin, M.J.; Abeliovich, H.; Arozena, A.A.; Adachi, H.; Adams, C.M.; Adams, P.D.; Adeli, K.; et al. Guidelines for the use and interpretation of assays for monitoring autophagy. In Autophagy, 3rd ed.; Taylor & Francis: New York, NY, USA, 2016; Volume 12, pp. 1–222. [Google Scholar] [CrossRef]
- Menzies, F.M.; Fleming, A.; Rubinsztein, D.C. Compromised autophagy and neurodegenerative diseases. Nat. Rev. Neurosci. 2015, 16, 345–357. [Google Scholar] [CrossRef]
- Martinez-Vicente, M. Neuronal Mitophagy in Neurodegenerative Diseases. Front. Mol. Neurosci. 2017, 10, 64. [Google Scholar] [CrossRef]
- McWilliams, T.G.; Muqit, M.M. PINK1 and Parkin: Emerging themes in mitochondrial homeostasis. Curr. Opin. Cell Biol. 2017, 45, 83–91. [Google Scholar] [CrossRef]
- Eiyama, A.; Okamoto, K. PINK1/Parkin-mediated mitophagy in mammalian cells. Curr. Opin. Cell Biol. 2015, 33, 95–101. [Google Scholar] [CrossRef]
- Eldeeb, M.A.; Thomas, R.A.; Ragheb, M.A.; Fallahi, A.; Fon, E.A. Mitochondrial quality control in health and in Parkinson’s disease. Physiol. Rev. 2022, 102, 1721–1755. [Google Scholar] [CrossRef] [PubMed]
- Bjørkøy, G.; Lamark, T.; Brech, A.; Outzen, H.; Perander, M.; Øvervatn, A.; Stenmark, H.; Johansen, T. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J. Cell Biol. 2005, 171, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Huang, M.; Yao, Y.-M. Autophagy and proinflammatory cytokines: Interactions and clinical implications. Cytokine Growth Factor Rev. 2018, 43, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, P.S.S.; Willemen, H.L.D.M.; Eijkelkamp, N. Mitochondria and sensory processing in inflammatory and neuropathic pain. Front. Pain Res. 2022, 3, 1013577. [Google Scholar] [CrossRef]
- Yang, P.; Zhang, Q.; Shen, H.; Bai, X.; Liu, P.; Zhang, T. Research progress on the protective effects of aucubin in neurological diseases. Pharm. Biol. 2022, 60, 1088–1094. [Google Scholar] [CrossRef]
- Chen, S.; Zeng, X.; Zong, W.; Wang, X.; Chen, L.; Zhou, L.; Li, C.; Huang, Q.; Huang, X.; Zeng, G.; et al. Aucubin Alleviates Seizures Activity in Li-Pilocarpine-Induced Epileptic Mice: Involvement of Inhibition of Neuroinflammation and Regulation of Neurotransmission. Neurochem. Res. 2019, 44, 472–484. [Google Scholar] [CrossRef]
- Zhu, Y.-L.; Sun, M.-F.; Jia, X.-B.; Zhang, P.-H.; Xu, Y.-D.; Zhou, Z.-L.; Xu, Z.-H.; Cui, C.; Chen, X.; Yang, X.-S.; et al. Aucubin alleviates glial cell activation and preserves dopaminergic neurons in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonian mice. NeuroReport 2018, 29, 1075–1083. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, L.-D.; Wang, J.-Y.; Wang, H.; Chen, X.-Y.; Zhang, L.; Yuan, Y. Anti-inflammatory effects of aucubin in cellular and animal models of rheumatoid arthritis. Chin. J. Nat. Med. 2022, 20, 458–472. [Google Scholar] [CrossRef]
- Xiao, S.; Zhong, N.; Yang, Q.; Li, A.; Tong, W.; Zhang, Y.; Yao, G.; Wang, S.; Liu, J.; Liu, Z. Aucubin promoted neuron functional recovery by suppressing inflammation and neuronal apoptosis in a spinal cord injury model. Int. Immunopharmacol. 2022, 111, 109163. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Huang, W.-H.; Zeng, X.-C.; Li, X.-H.; Li, J.; Zhou, J.; Xiao, J.; Xiao, B.; Ouyang, D.-S.; et al. The Protective Effect of Aucubin from Eucommia ulmoides Against Status Epilepticus by Inducing Autophagy and Inhibiting Necroptosis. Am. J. Chin. Med. 2017, 45, 557–573. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.; Jin, H.; Zhang, X.; Li, W.; Wang, D.; Tong, P.; Liu, Y.; Tan, Z. Aucubin prevents steroid-induced osteoblast apoptosis by enhancing autophagy via AMPK activation. J. Cell. Mol. Med. 2021, 25, 10175–10184. [Google Scholar] [CrossRef] [PubMed]
- Rong, Z.; Yang, L.; Chen, Y.; Qin, Y.; Cheng, C.-Y.; Zhao, J.; Li, L.-F.; Ma, X.; Wu, Y.-M.; Liu, S.-B.; et al. Sophoridine alleviates hyperalgesia and anxiety-like behavior in an inflammatory pain mouse model induced by complete freund’s adjuvant. Mol. Pain 2023, 19. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra, V.; Tanga, F.Y.; DeLeo, J.A. Complete Freunds adjuvant-induced peripheral inflammation evokes glial activation and proinflammatory cytokine expression in the CNS. Eur. J. Neurosci. 2004, 20, 467–473. [Google Scholar] [CrossRef]
- Rüb, C.; Wilkening, A.; Voos, W. Mitochondrial quality control by the Pink1/Parkin system. Cell Tissue Res. 2017, 367, 111–123. [Google Scholar] [CrossRef]
- Tang, Q.; Zheng, G.; Feng, Z.; Chen, Y.; Lou, Y.; Wang, C.; Zhang, X.; Zhang, Y.; Xu, H.; Shang, P.; et al. Trehalose ameliorates oxidative stress-mediated mitochondrial dysfunction and ER stress via selective autophagy stimulation and autophagic flux restoration in osteoarthritis development. Cell Death Dis. 2017, 8, e3081. [Google Scholar] [CrossRef]
- Onishi, M.; Yamano, K.; Sato, M.; Matsuda, N.; Okamoto, K. Molecular mechanisms and physiological functions of mitophagy. EMBO J. 2021, 40, e104705. [Google Scholar] [CrossRef]
- Li, C.; Wang, X.-G.; Yang, S.; Lyu, Y.-H.; Gao, X.-J.; Cao, J.; Zang, W.-D. Treadmill exercise alleviates neuropathic pain by regulating mitophagy of the anterior cingulate cortex in rats. Sheng Li Xue Bao 2023, 75, 160–170. [Google Scholar]
- Kuner, R. Central mechanisms of pathological pain. Nat. Med. 2010, 16, 1258–1266. [Google Scholar] [CrossRef]
- Ren, K.; Dubner, R. Neuron-glia crosstalk gets serious: Role in pain hypersensitivity. Curr. Opin. Anaesthesiol. 2008, 21, 570–579. [Google Scholar] [CrossRef]
- Fang, E.F.; Hou, Y.; Palikaras, K.; Adriaanse, B.A.; Kerr, J.S.; Yang, B.; Lautrup, S.; Hasan-Olive, M.M.; Caponio, D.; Dan, X.; et al. Mitophagy inhibits amyloid-β and tau pathology and reverses cognitive deficits in models of Alzheimer’s disease. Nat. Neurosci. 2019, 22, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Bar-Yosef, T.; Damri, O.; Agam, G. Dual Role of Autophagy in Diseases of the Central Nervous System. Front. Cell. Neurosci. 2019, 13, 196. [Google Scholar] [CrossRef] [PubMed]
- Durcan, T.M.; Fon, E.A. The three ’P’s of mitophagy: PARKIN, PINK1, and post-translational modifications. Genes Dev. 2015, 29, 989–999. [Google Scholar] [PubMed]
- Shin, H.J.; Park, H.; Shin, N.; Kwon, H.H.; Yin, Y.; Hwang, J.-A.; Song, H.-J.; Kim, J.; Kim, D.W.; Beom, J. Pink1-Mediated Chondrocytic Mitophagy Contributes to Cartilage Degeneration in Osteoarthritis. J. Clin. Med. 2019, 8, 1849. [Google Scholar] [CrossRef] [PubMed]
- Piao, Y.; Gwon, D.H.; Kang, D.-W.; Hwang, T.W.; Shin, N.; Kwon, H.H.; Shin, H.J.; Yin, Y.; Kim, J.-J.; Hong, J.; et al. TLR4-mediated autophagic impairment contributes to neuropathic pain in chronic constriction injury mice. Mol. Brain 2018, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.-H.; Shin, J.; Shin, N.; Yin, Y.; Lee, S.Y.; Kim, C.-S.; Kim, S.R.; Zhang, E.; Kim, D.W. PINK1 mediates spinal cord mitophagy in neuropathic pain. J. Pain Res. 2019, 12, 1685–1699. [Google Scholar]
- Mizushima, N.; Yoshimori, T.; Levine, B. Methods in Mammalian Autophagy Research. Cell 2010, 140, 313–326. [Google Scholar] [CrossRef]
- Komatsu, M.; Waguri, S.; Koike, M.; Sou, Y.S.; Ueno, T.; Hara, T.; Mizushima, N.; Iwata, J.I.; Ezaki, J.; Murata, S.; et al. Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell 2007, 131, 1149–1163. [Google Scholar]
- Liu, C.; Zheng, X.; Liu, L.; Hu, Y.; Zhu, Q.; Zhang, J.; Wang, H.; Gu, E.W.; Yang, Z.; Xu, G. Caloric Restriction Alleviates CFA-Induced Inflammatory Pain via Eleva ting β-Hydroxybutyric Acid Expression and Restoring Autophagic Flux in the Spinal Cord. Front. Neurosci. 2022, 16, 828278. [Google Scholar]
- Wang, Y.; Shi, Y.; Huang, Y.; Liu, W.; Cai, G.; Huang, S.; Zeng, Y.; Ren, S.; Zhan, H.; Wu, W. Resveratrol mediates mechanical allodynia through modulating inflammatory response via the TREM2-autophagy axis in SNI rat model. J. Neuroinflamm. 2020, 17, 311. [Google Scholar] [CrossRef]
- Chen, H.; Hu, Y.; Xie, K.; Chen, Y.; Wang, H.; Bian, Y.; Wang, Y.; Dong, A.; Yu, Y. Effect of autophagy on allodynia, hyperalgesia and astrocyte activatio n in a rat model of neuropathic pain. Int. J. Mol. Med. 2018, 42, 2009–2019. [Google Scholar]
- Zhang, E.; Yi, M.H.; Ko, Y.; Kim, H.W.; Seo, J.H.; Lee, Y.H.; Lee, W.; Kim, D.W. Expression of LC3 and Beclin 1 in the spinal dorsal horn following spi nal nerve ligation-induced neuropathic pain. Brain Res. 2013, 1519, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.-S.; Jing, P.B.; Wang, J.A.; Zhang, R.; Jiang, B.C.; Gao, Y.J.; Zhang, Z.J. Increased autophagic activity in dorsal root ganglion attenuates neuro pathic pain following peripheral nerve injury. Neurosci. Lett. 2015, 599, 158–163. [Google Scholar] [CrossRef]
- Chaplan, S.R.; Bach, F.W.; Pogrel, J.W.; Chung, J.M.; Yaksh, T.L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 1994, 53, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, K.; Dubner, R.; Brown, F.; Flores, C.; Joris, J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988, 32, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Walf, A.A.; Frye, C.A. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc. 2007, 2, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Kyi-Tha-Thu, C.; Htway, S.-M.; Suzuki, T.; Nohara, K.; Win-Shwe, T.-T. Gestational arsenic exposure induces anxiety-like behaviors in F1 female mice by dysregulation of neurological and immunological markers. Environ. Health Prev. Med. 2023, 28, 43. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, D.; Wang, Y.; Chen, Y.; Chen, G. The Analgesia Effect of Aucubin on CFA-Induced Inflammatory Pain by Inhibiting Glial Cells Activation-Mediated Inflammatory Response via Activating Mitophagy. Pharmaceuticals 2023, 16, 1545. https://doi.org/10.3390/ph16111545
Yao D, Wang Y, Chen Y, Chen G. The Analgesia Effect of Aucubin on CFA-Induced Inflammatory Pain by Inhibiting Glial Cells Activation-Mediated Inflammatory Response via Activating Mitophagy. Pharmaceuticals. 2023; 16(11):1545. https://doi.org/10.3390/ph16111545
Chicago/Turabian StyleYao, Dandan, Yongjie Wang, Yeru Chen, and Gang Chen. 2023. "The Analgesia Effect of Aucubin on CFA-Induced Inflammatory Pain by Inhibiting Glial Cells Activation-Mediated Inflammatory Response via Activating Mitophagy" Pharmaceuticals 16, no. 11: 1545. https://doi.org/10.3390/ph16111545
APA StyleYao, D., Wang, Y., Chen, Y., & Chen, G. (2023). The Analgesia Effect of Aucubin on CFA-Induced Inflammatory Pain by Inhibiting Glial Cells Activation-Mediated Inflammatory Response via Activating Mitophagy. Pharmaceuticals, 16(11), 1545. https://doi.org/10.3390/ph16111545





