Experimental Evaluation of the Hypersensitivity Reactions of a New Glycopeptide Antibiotic Flavancin in Animal Models
Abstract
:1. Introduction
2. Results
2.1. Anaphylactogenic Properties of Flavancin
2.2. Histamine-Releasing Effect of Flavancin and Vancomycin
2.3. Pseudoallergic Reaction
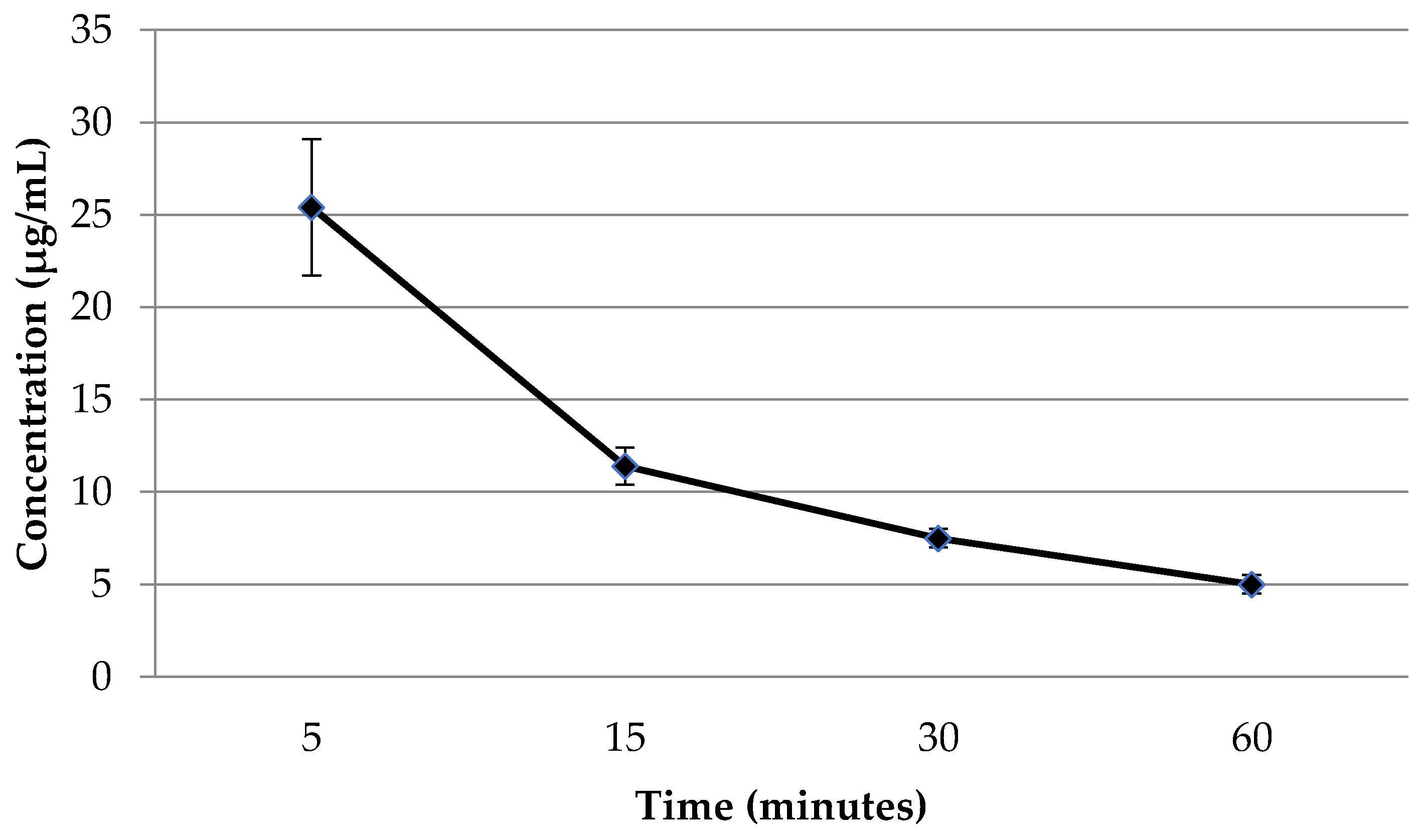
2.4. The Concentration of Flavancin in the Blood Plasma of Rats after Intravenous Administration
3. Discussion
4. Materials and Methods
4.1. Anaphylactogenic Properties of Flavancin
4.1.1. Generalized Anaphylaxis (Anaphylactic Shock)
4.1.2. Active Skin Anaphylaxis
4.2. Determination of Histamine in Blood Plasma
4.3. Evaluation of Pseudoallergic Reaction
4.4. Determination of Flavancin Concentrations in Rat Plasma by a High-Performance Liquid Chromatography
4.4.1. Chromatography Conditions for Flavancin
4.4.2. Sample Preparation
4.4.3. Preparation of Standard Samples
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Griffith, R.S. Vancomycin use: An historical review. J. Antimicrob. Chemother. 1984, 14 (Suppl. D), 1–5. [Google Scholar] [CrossRef]
- De Lucaa, J.F.; Holmesb, N.E.; Trubiano, J.A. Adverse reactions to vancomycin and crossreactivity with other antibiotics. Curr. Opin. Allergy Clin. Immunol. 2020, 20, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.P. Comparative safety of teicoplanin and vancomycin. Int. J. Antimicrob. Agents 1998, 10, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Sivagnanam, S.; Deleu, D. Red man syndrome. Crit. Care 2003, 7, 119–120. [Google Scholar] [CrossRef] [PubMed]
- Polk, R.E.; Healy, D.P.; Schwartz, L.B.; Rock, D.T.; Garson, M.L.; Roller, K. Vancomycin and the red-man syndrome: Pharmacodynamics of histamine release. J. Infect. Dis. 1988, 157, 502–507. [Google Scholar] [CrossRef]
- Horinouchi, Y.; Abe, K.; Kubo, K.; Oka, M. Mechanisms of vancomycin-induced histamine release from rat peritoneal mast cells. Agents Actions 1993, 40, 28–36. [Google Scholar] [CrossRef]
- Williams, P.D.; Laska, D.A.; Shetler, T.J.; McGrath, J.P.; White, S.L.; Hoover, D.M. Vancomycin-induced release of histamine from rat peritoneal mast cells and a rat basophil cell line (RBL-1). Agents Actions 1991, 32, 217–223. [Google Scholar] [CrossRef]
- Blumenthal, K.G.; Peter, J.G.; Trubiano, J.A.; Phillips, E.J. Antibiotic allergy. Lancet 2019, 12, 183–198. [Google Scholar] [CrossRef]
- Azimi, E.; Reddy, V.B.; Lerner, E.A. Brief communication: MRGPRX2, atopic dermatitis and red man syndrome. Itch 2017, 2, e5. [Google Scholar] [CrossRef]
- McNeil, B.D.; Pundir, P.; Meeker, S.; Han, L.; Undem, B.J.; Kulka, M.; Dong, X. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature 2014, 519, 237–241. [Google Scholar] [CrossRef]
- Gaudenzio, N.; Sibilano, R.; Marichal, T.; Starkl, P.; Reber, L.L.; Cenac, N.; McNeil, B.D.; Dong, X.; Hernandez, J.D.; Sagi-Eisenberg, R.; et al. Different activation signals induce distinct mast cell degranulation strategies. J. Clin. Investig. 2016, 126, 3981–3998. [Google Scholar] [CrossRef] [PubMed]
- Sahai, J.V.; Fuller, S.H.; Polk, R.E. Vancomycin-Induced Histamine Release and “Red Man Syndrome”: Comparison of 1- and 2-Hour Infusions. Antimicrob. Agents Chemother. 1990, 34, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Elbarbry, F. Vancomycin Dosing and Monitoring: Critical Evaluation of the Current Practice. Eur. J. Drug Metab. Pharmacokinet. 2018, 43, 259–268. [Google Scholar] [CrossRef]
- Mulubwa, M.; Griesel, H.A.; Mugabo, P.; Dippenaar, R.; van Wyk, L. Assessment of Vancomycin Pharmacokinetics and Dose Regimen Optimisation in Preterm Neonates. Drugs 2020, 20, 105–113. [Google Scholar] [CrossRef]
- Drennan, P.G.; Begg, E.J.; Gardiner, S.J.; Kirkpatrick, C.M.; Chambers, S.T. The dosing and monitoring of vancomycin: What is the best way forward? Int. J. Antimicrob. Agents 2019, 53, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Nix, D.E.; Davis, L.E.; Matthias, K.R. The relationship of vancomycin 24-hour AUC and trough concentration. Am. J. Health Syst. Pharm. 2022, 79, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Moiseenko, E.I.; Erdei, R.; Grammatikova, N.E.; Mirchink, E.P.; Isakova, E.B.; Pereverzeva, E.R.; Batta, G.; Shchekotikhin, A.E. Aminoalkylamides of Eremomycin Exhibit an Improved Antibacterial Activity. Pharmaceuticals 2021, 14, 379. [Google Scholar] [CrossRef] [PubMed]
- Treshchalin, M.I.; Polozkova, V.A.; Moiseenko, E.I.; Treshalina, H.M.; Shchekotikhin, A.E.; Pereverzeva, E.R. Evaluation of Toxic Properties of New Glycopeptide Flavancin on Rats. Pharmaceuticals 2022, 15, 661. [Google Scholar] [CrossRef]
- Solensky, R.; Khan, D.A. Drug allergy: An updated practice parameter. Ann. Allergy Asthma Immunol. 2010, 105, 259–273. [Google Scholar] [CrossRef]
- Minhas, J.S.; Wickner, P.G.; Long, A.A.; Banerji, A.; Blumenthal, K.G. Immune-mediated reactions to vancomycin. Ann. Allergy Asthma Immunol. 2016, 116, 544–553. [Google Scholar] [CrossRef]
- Hall, V.; Wong, M.; Munsif, M.; Stevenson, B.R.; Elliott, K.; Lucas, M.; Baird, A.J.; Athan, E.; Young, M.; Pickles, R.; et al. Antimicrobial anaphylaxis: The changing face of severe antimicrobial allergy. J. Antimicrob. Chemother. 2020, 75, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Ruggero, M.A.; Abdelghany, O.; Topal, J.E. Vancomycin-induced thrombocytopenia without isolation of a drug-dependent antibody. Pharmacotherapy 2012, 32, e321-5. [Google Scholar] [CrossRef] [PubMed]
- Ebo, D.G.; Clarke, R.C.; Mertes, P.-M.; Platt, P.R.; Sabato, V.; Sadleir, P.H. Molecular mechanisms and pathophysiology of perioperative hypersensitivity and anaphylaxis: A narrative review. Br. J. Anaesth. 2019, 123, e38–e49. [Google Scholar] [CrossRef] [PubMed]
- Renz, C.L.; Laroche, D.; Thurn, J.D.; Finn, H.A.; Lynch, J.P.; Thisted, R.; Moss, J. Tryptase levels are not increased during vancomycin-induced anaphylactoid reactions. Anesthesiology 1998, 89, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Council of Europe European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes. Available online: https://rm.coe.int/168007a67b (accessed on 28 August 2018).
- GOST P 53434-2009; Principles of Good Laboratory Practice. Order of the Federal Agency for Technical Regulation and Metrology: Moscow, Russia, 2 March 2010.
- Verdier, F.; Chazal, I.; Descotes, J. Anaphylaxis models in the guinea-pig. Toxicology 1994, 93, 55–61. [Google Scholar] [CrossRef]
- Freireich, E.J.; Gehan, E.A.; Rall, D.P.; Schmidt, L.H.; Skipper, H.E. Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey, and man. Cancer Chemother. Rep. 1966, 50, 219–244. [Google Scholar]
- Best, J.; Nijhout, H.F.; Samaranayake, S.; Hashemi, P.; Reed, M. A mathematical model for histamine synthesis, release, and control in varicosities. Theor. Biol. Med. Model. 2017, 14, 24. [Google Scholar] [CrossRef]
- Supotnitskiy, M.V.; Gorbunova, E.V.; Korsun, L.V.; Lebedinskaya, E.V. Aspects of preclinical studies at the stage of developing non-viral vector constructions designed for gene therapy. SCEEMP 2014, 3, 30–38. Available online: https://cyberleninka.ru/article/n/osobennosti-doklinicheskih-issledovaniy-na-etape-razrabotki-nevirusnyh-vektornyh-konstruktsiy-prednaznachennyh-dlya-tseley-gennoy/viewer (accessed on 28 August 2018). (In Russian).
| Sensitizing Doses, mg/kg | Resolving Doses, mg/kg | Effect | ||
|---|---|---|---|---|
| Subcutaneous | Intramuscularly | Intramuscularly | Intracardially | |
| flavancin | flavancin | flavancin | flavancin | no |
| 0.5 | 0.5 | 0.5 | 3.0 | |
| flavancin | flavancin | flavancin | flavancin | no |
| 1.5 | 1.5 | 1.5 | 3.0 | |
| saline solution | saline solution | saline solution | flavancin | no |
| 1.0 mL | 1.0 mL | 1.0 mL | 3.0 | |
| cattle serum | cattle serum | cattle serum | cattle serum | Severe anaphylactic reaction. After two hours, 8 pigs out of 10 died. A day later, the remaining two pigs died. |
| 0.1 mL | 0.1 mL | 0.1 mL | 0.5 mL | |
| Sensitizing Doses, mg/kg | Resolving Doses | Blue Spot Diameter, cm | ||
|---|---|---|---|---|
| Subcutaneous | Intramuscularly | Intramuscularly | Intracardially | |
| flavancin | flavancin | flavancin | flavancin | 2.3 ± 0.6 |
| 0.5 | 0.5 | 0.5 | 0.05 mL per pig | |
| flavancin | flavancin | flavancin | flavancin | 2.4 ± 0.5 |
| 1.5 | 1.5 | 1.5 | 0.05 mL per pig | |
| saline solution | saline solution | saline solution | flavancin | 2.5 ± 0.5 |
| 1.0 mL | 1.0 mL | 1.0 mL | 0.05 mL per pig | |
| Agent | Dose, mg/kg | Time, Minutes | Histamine Level, µg/L |
|---|---|---|---|
| Vancomycin | 300 | 0.5 | 120.3 ± 3.1 |
| 1.5 | 145.0 ± 10.1 | ||
| 3 | 170.6 ± 20.9 | ||
| Flavancin | 100 | 0.5 | 549.9 ± 92.6 |
| 1.5 | 796.2 ± 56.3 | ||
| 3 | 297.7 ± 54.9 | ||
| Control | <0.4 |
| Agent | Dose, mg/kg | Time, Minutes | Histamine Level, µg/L |
|---|---|---|---|
| Vancomycin | 7.5 | 0.5 | 112.6 ± 27.8 |
| 1 | 85.2 ± 14.7 | ||
| 1.5 | 13.4 ± 9.3 | ||
| Flavancin | 2.5 | 0.5 | 33.3 ± 7.0 |
| 1 | 16.8 ± 7.7 | ||
| 1.5 | <1.1 | ||
| Control | <1.1 |
| Drug | The Mass of the Hind Leg Foot (mg) | Relative Increase Me Over Mc, % | RI, % | |
|---|---|---|---|---|
| Con A Me Mean ± SD | Saline Mc Mean ± SD | |||
| Vancomycin, MTD | 149.4 ± 3.0 | 131.8 ± 3.6 | 13.4 | 63 |
| Vancomycin, TD | 158.1 ± 3.8 | 140.4 ± 2.3 | 12.6 | 54 |
| Flavancin, MTD | 151.5 ± 3.0 | 139.3 ± 2.9 | 8.8 | 7.3 |
| Flavancin, TD | 154.3 ± 2.1 | 142.1 ± 2.0 | 8.5 | 3.7 |
| Control | 148.2 ± 2.4 | 137.0 ± 2.3 | 8.2 | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Treshchalin, M.I.; Polozkova, V.A.; Moiseenko, E.I.; Shchekotikhin, A.E.; Dovzhenko, S.A.; Kobrin, M.B.; Pereverzeva, E.R. Experimental Evaluation of the Hypersensitivity Reactions of a New Glycopeptide Antibiotic Flavancin in Animal Models. Pharmaceuticals 2023, 16, 1569. https://doi.org/10.3390/ph16111569
Treshchalin MI, Polozkova VA, Moiseenko EI, Shchekotikhin AE, Dovzhenko SA, Kobrin MB, Pereverzeva ER. Experimental Evaluation of the Hypersensitivity Reactions of a New Glycopeptide Antibiotic Flavancin in Animal Models. Pharmaceuticals. 2023; 16(11):1569. https://doi.org/10.3390/ph16111569
Chicago/Turabian StyleTreshchalin, Michael I., Vasilisa A. Polozkova, Elena I. Moiseenko, Andrey E. Shchekotikhin, Svetlana A. Dovzhenko, Mikhail B. Kobrin, and Eleonora R. Pereverzeva. 2023. "Experimental Evaluation of the Hypersensitivity Reactions of a New Glycopeptide Antibiotic Flavancin in Animal Models" Pharmaceuticals 16, no. 11: 1569. https://doi.org/10.3390/ph16111569
APA StyleTreshchalin, M. I., Polozkova, V. A., Moiseenko, E. I., Shchekotikhin, A. E., Dovzhenko, S. A., Kobrin, M. B., & Pereverzeva, E. R. (2023). Experimental Evaluation of the Hypersensitivity Reactions of a New Glycopeptide Antibiotic Flavancin in Animal Models. Pharmaceuticals, 16(11), 1569. https://doi.org/10.3390/ph16111569






