Enhancing Tamoxifen Therapy with α-Mangostin: Synergistic Antiproliferative Effects on Breast Cancer Cells and Potential Reduced Endometrial Impact
Abstract
:1. Introduction
2. Results
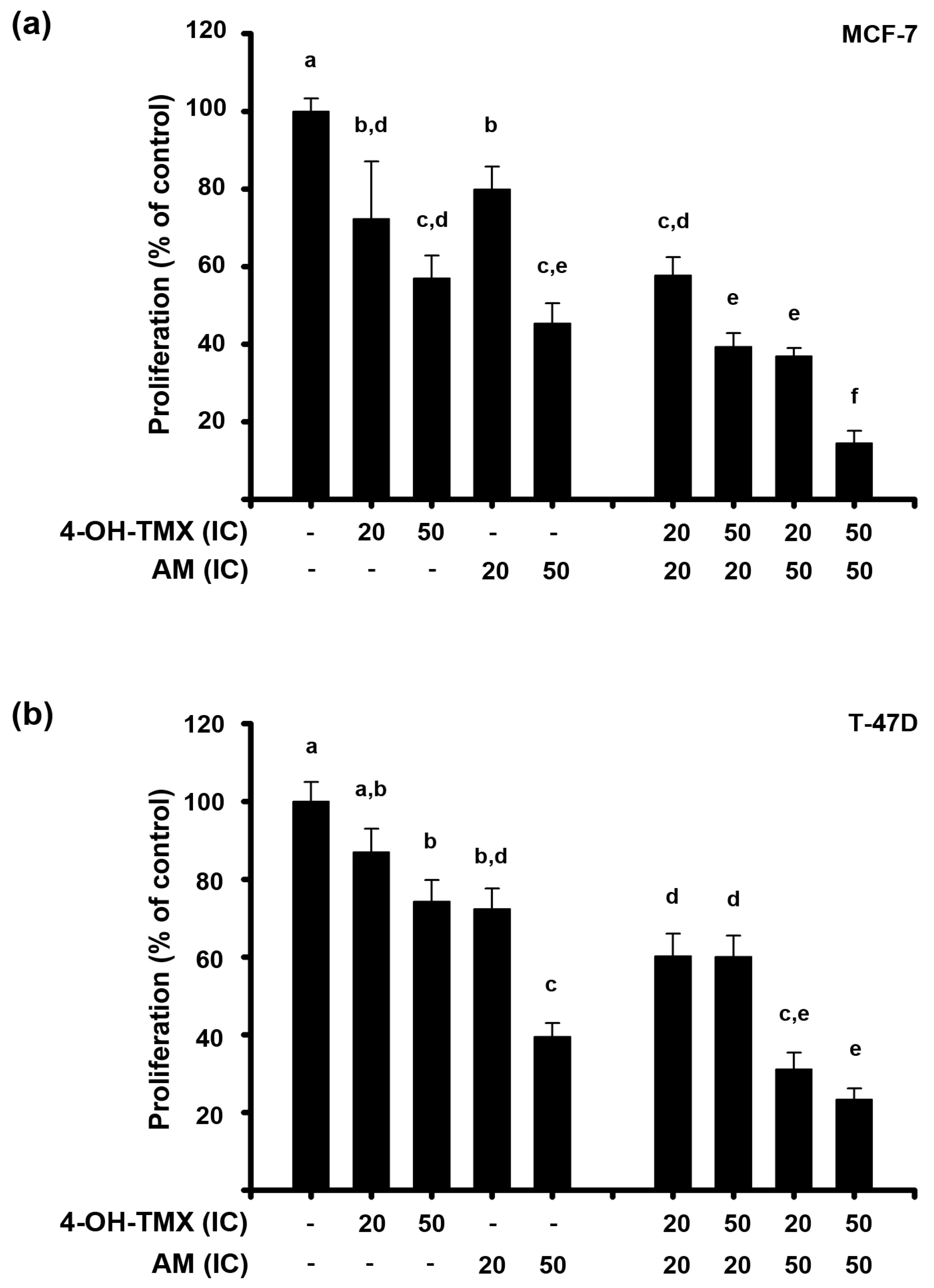
2.1. AM and 4-OH-TMX Inhibited ER+ Breast Cancer Cells Proliferation in a Concentration-Dependent Manner
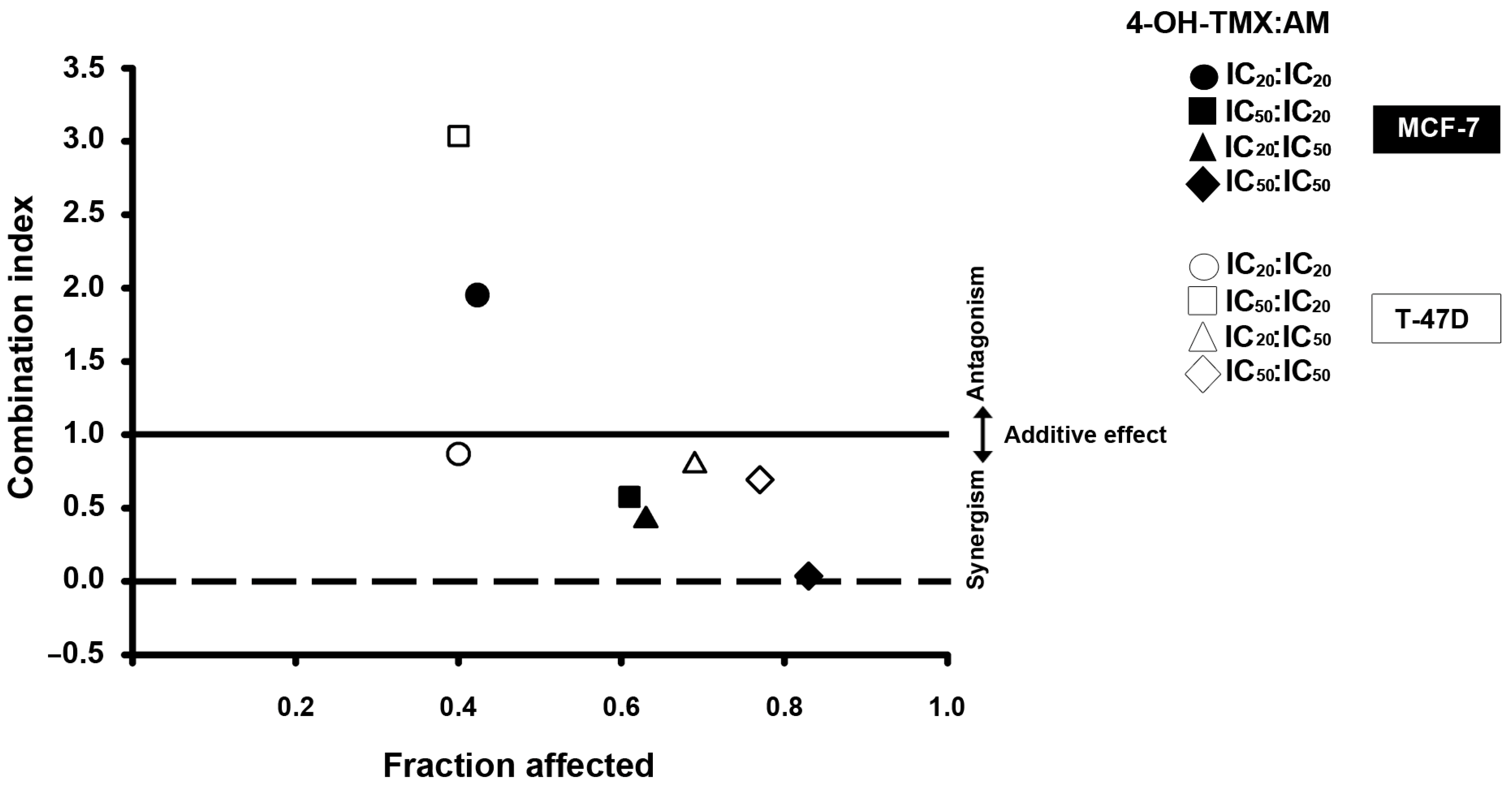
2.2. The Combination of AM with 4-OH-TMX Acted Synergistically to Inhibit Cell Growth, Allowing for a Significant Dose Reduction While Maintaining Their Efficacy
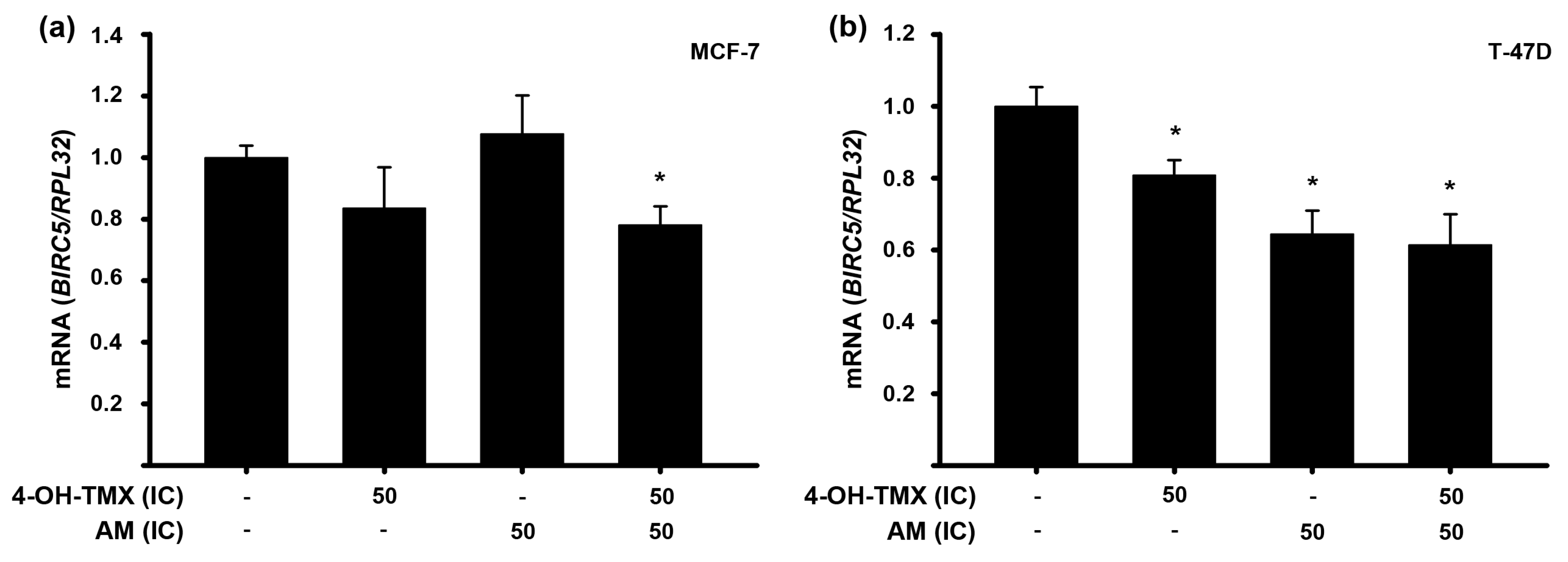
2.3. The Combination of AM with 4-OH-TMX Enhanced Its Inhibitory Effects upon mRNA Expression of Some Genes Involved in Oncogenesis, Cell Cycle Progression, and Apoptosis in Breast Cancer Cells
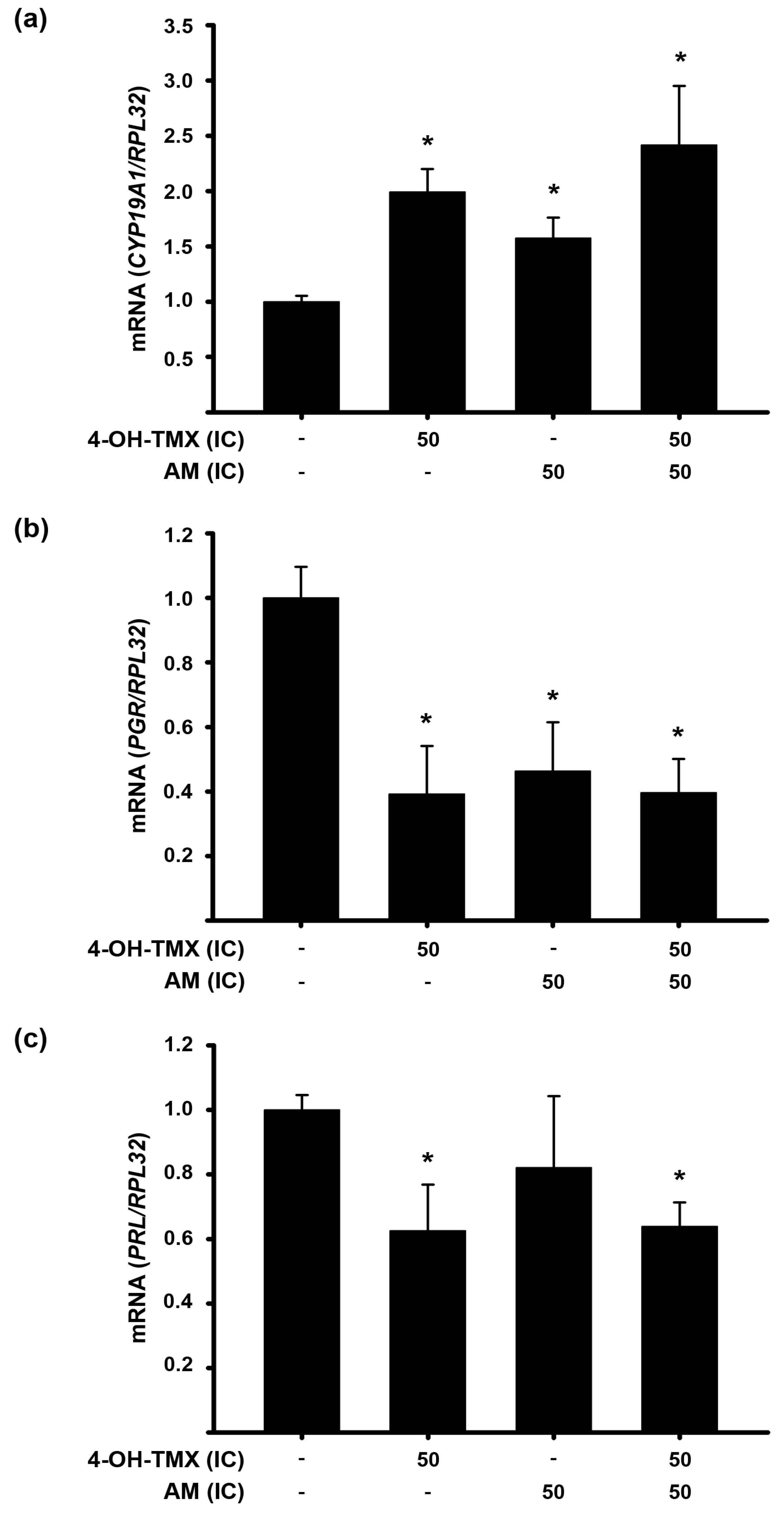
2.4. The Treatment with 4-OH-TMX or AM Differentially Modified mRNA Expression of Genes Involved in ER+ Signaling in Breast Cancer Cells
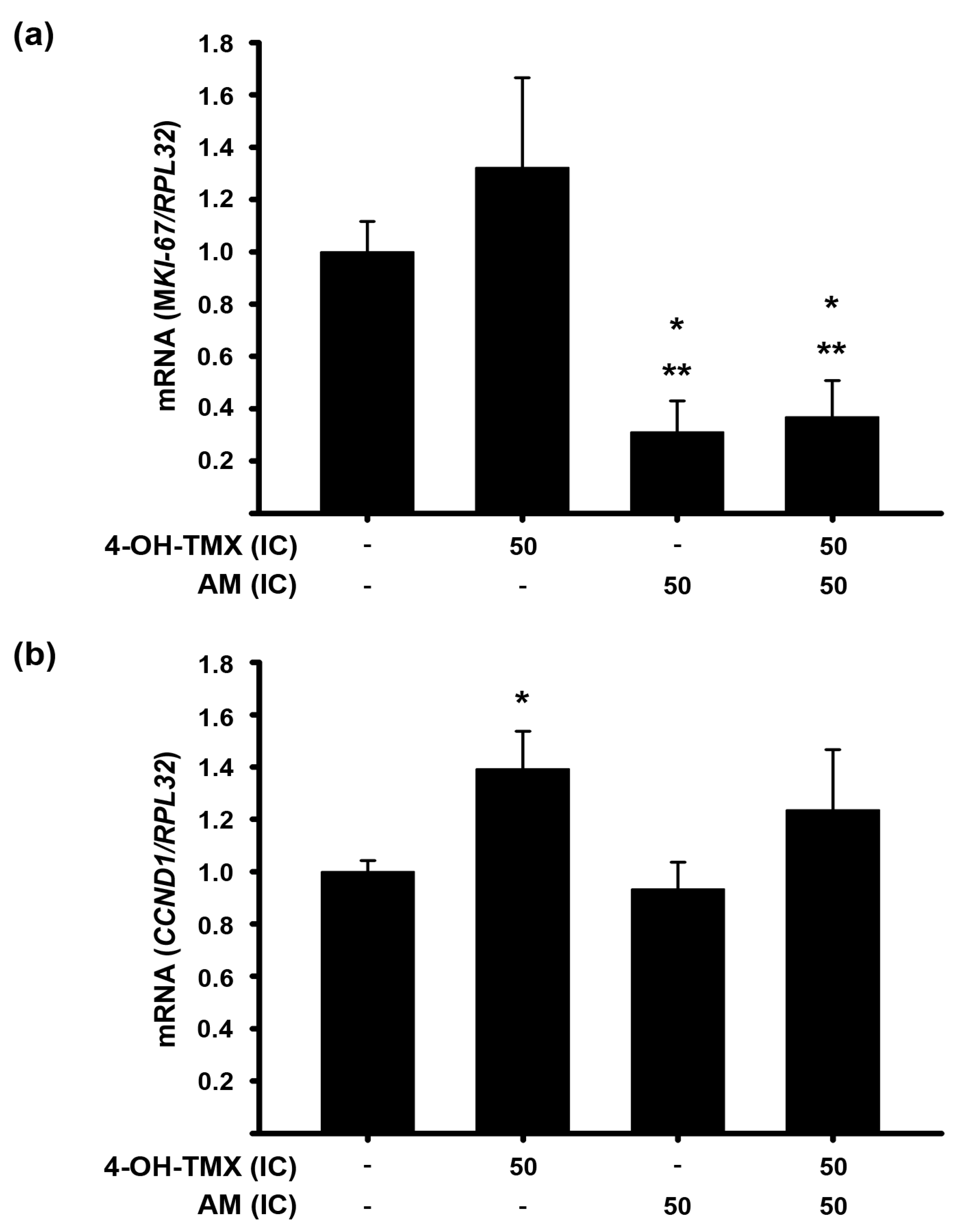
2.5. AM Decreases the 4-OH-TMX-Dependent Expression Upregulation of Genes Involved in Cell Proliferation in Endometrium Cells
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Lines
4.3. Proliferation Studies
4.4. Combination Index and Dose Reduction Index Determination
4.5. PCR Amplification
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Sotiriou, C.; Wirapati, P.; Loi, S.; Harris, A.; Fox, S.; Smeds, J.; Nordgren, H.; Farmer, P.; Praz, V.; Haibe-Kains, B.; et al. Gene expression profiling in breast cancer: Understanding the molecular basis of histologic grade to improve prognosis. J. Natl. Cancer Inst. 2006, 98, 262–272. [Google Scholar] [CrossRef]
- Zattarin, E.; Leporati, R.; Ligorio, F.; Lobefaro, R.; Vingiani, A.; Pruneri, G.; Vernieri, C. Hormone Receptor Loss in Breast Cancer: Molecular Mechanisms, Clinical Settings, and Therapeutic Implications. Cells 2020, 9, 2644. [Google Scholar] [CrossRef]
- Grimm, S.L.; Hartig, S.M.; Edwards, D.P. Progesterone Receptor Signaling Mechanisms. J. Mol. Biol. 2016, 428, 3831–3849. [Google Scholar] [CrossRef]
- Hughes-Davies, L.; Caldas, C.; Wishart, G.C. Tamoxifen: The drug that came in from the cold. Br. J. Cancer 2009, 101, 875–878. [Google Scholar] [CrossRef] [PubMed]
- Howell, A.; Howell, S.J. Tamoxifen evolution. Br. J. Cancer 2023, 128, 421–425. [Google Scholar] [CrossRef]
- Nasu, K.; Takai, N.; Nishida, M.; Narahara, H. Tumorigenic effects of tamoxifen on the female genital tract. Clin. Med. Pathol. 2008, 1, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am. J. Clin. Nutr. 2003, 78, 517S–520S. [Google Scholar] [CrossRef]
- Ovalle-Magallanes, B.; Eugenio-Perez, D.; Pedraza-Chaverri, J. Medicinal properties of mangosteen (Garcinia mangostana L.): A comprehensive update. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2017, 109 Pt 1, 102–122. [Google Scholar] [CrossRef]
- Zhang, K.J.; Gu, Q.L.; Yang, K.; Ming, X.J.; Wang, J.X. Anticarcinogenic Effects of alpha-Mangostin: A Review. Planta Med. 2017, 83, 188–202. [Google Scholar]
- Ibrahim, M.Y.; Hashim, N.M.; Mariod, A.A.; Mohan, S.; Abdulla, M.A.; Abdelwahab, S.I.; Arbab, I.A. α-Mangostin from Garcinia mangostana Linn: An updated review of its pharmacological properties. Arab. J. Chem. 2016, 9, 317–329. [Google Scholar] [CrossRef]
- Bissoli, I.; Muscari, C. Doxorubicin and alpha-Mangostin oppositely affect luminal breast cancer cell stemness evaluated by a new retinaldehyde-dependent ALDH assay in MCF-7 tumor spheroids. Biomed. Pharmacother. Biomed. Pharmacother. 2020, 124, 109927. [Google Scholar]
- Lara-Sotelo, G.D.N.; García-Becerra, R.; Avila, E.; Prado-Garcia, H.; Morales-Guadarrama, G.; Ibarra-Sánchez, M.J.; Esparza-López, J.; Larrea, F.; García-Quiroz, J. A-Mangostin Synergizes the Antineoplastic Effects of 5-Fluorouracil Allowing a Significant Dose Reduction in Breast Cancer Cells. Processes 2021, 9, 12. [Google Scholar] [CrossRef]
- Klein, D.J.; Thorn, C.F.; Desta, Z.; Flockhart, D.A.; Altman, R.B.; Klein, T.E. PharmGKB summary: Tamoxifen pathway, pharmacokinetics. Pharm. Genom. 2013, 23, 643–647. [Google Scholar] [CrossRef]
- Pawlik, A.; Slominska-Wojewodzka, M.; Herman-Antosiewicz, A. Sensitization of estrogen receptor-positive breast cancer cell lines to 4-hydroxytamoxifen by isothiocyanates present in cruciferous plants. Eur. J. Nutr. 2016, 55, 1165–1180. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Li, J.; Ning, H.; Yuan, Z.; Zhong, Y.; Wu, S.; Zeng, J.Z. Alpha-Mangostin Induces Apoptosis and Inhibits Metastasis of Breast Cancer Cells via Regulating RXRalpha-AKT Signaling Pathway. Front. Pharm. 2021, 12, 739658. [Google Scholar] [CrossRef]
- Chou, T.C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef]
- Chou, T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharm. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef] [PubMed]
- Hemmerlein, B.; Weseloh, R.M.; Mello de Queiroz, F.; Knotgen, H.; Sanchez, A.; Rubio, M.E.; Martin, S.; Schliephacke, T.; Jenke, M.; Heinz Joachim, R.; et al. Overexpression of Eag1 potassium channels in clinical tumours. Mol. Cancer 2006, 5, 41. [Google Scholar] [CrossRef]
- Diaz, L.; Bernadez-Vallejo, S.V.; Vargas-Castro, R.; Avila, E.; Gomez-Ceja, K.A.; Garcia-Becerra, R.; Segovia-Mendoza, M.; Prado-Garcia, H.; Lara-Sotelo, G.; Camacho, J.; et al. The Phytochemical alpha-Mangostin Inhibits Cervical Cancer Cell Proliferation and Tumor Growth by Downregulating E6/E7-HPV Oncogenes and KCNH1 Gene Expression. Int. J. Mol. Sci. 2023, 24, 3055. [Google Scholar] [CrossRef]
- Jeffreys, S.A.; Becker, T.M.; Khan, S.; Soon, P.; Neubauer, H.; de Souza, P.; Powter, B. Prognostic and Predictive Value of CCND1/Cyclin D1 Amplification in Breast Cancer With a Focus on Postmenopausal Patients: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2022, 13, 895729. [Google Scholar] [CrossRef]
- Oparina, N.; Erlandsson, M.C.; Faldt Beding, A.; Parris, T.; Helou, K.; Karlsson, P.; Einbeigi, Z.; Bokarewa, M.I. Prognostic Significance of BIRC5/Survivin in Breast Cancer: Results from Three Independent Cohorts. Cancers 2021, 13, 2209. [Google Scholar] [CrossRef] [PubMed]
- Garg, H.; Suri, P.; Gupta, J.C.; Talwar, G.P.; Dubey, S. Survivin: A unique target for tumor therapy. Cancer Cell Int. 2016, 16, 49. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.G.; Yu, F.; Yao, Q.; Chen, J.H.; Wang, L. The role of survivin in diagnosis, prognosis and treatment of breast cancer. J. Thorac. Dis. 2010, 2, 100–110. [Google Scholar]
- Davies, C.; Pan, H.; Godwin, J.; Gray, R.; Arriagada, R.; Raina, V.; Abraham, M.; Medeiros Alencar, V.H.; Badran, A.; Bonfill, X.; et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 2013, 381, 805–816. [Google Scholar] [CrossRef]
- Ghanavati, M.; Khorshidi, Y.; Shadnoush, M.; Akbari, M.E.; Ardehali, S.H.; Chavarri-Guerra, Y.; Akbari, A.; Barragan-Carrillo, R.; Amin Amlashi, M.; Javid, Z.; et al. Tamoxifen use and risk of endometrial cancer in breast cancer patients: A systematic review and dose-response meta-analysis. Cancer Rep. 2023, 6, e1806. [Google Scholar] [CrossRef]
- Fisher, B.; Costantino, J.P.; Wickerham, D.L.; Cecchini, R.S.; Cronin, W.M.; Robidoux, A.; Bevers, T.B.; Kavanah, M.T.; Atkins, J.N.; Margolese, R.G.; et al. Tamoxifen for the prevention of breast cancer: Current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J. Natl. Cancer Inst. 2005, 97, 1652–1662. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Tian, W.; Ma, X. Alpha-mangostin inhibits intracellular fatty acid synthase and induces apoptosis in breast cancer cells. Mol. Cancer 2014, 13, 138. [Google Scholar] [CrossRef]
- Kurose, H.; Shibata, M.A.; Iinuma, M.; Otsuki, Y. Alterations in cell cycle and induction of apoptotic cell death in breast cancer cells treated with alpha-mangostin extracted from mangosteen pericarp. J. Biomed. Biotechnol. 2012, 2012, 672428. [Google Scholar] [CrossRef]
- Xu, Q.; Ma, J.; Lei, J.; Duan, W.; Sheng, L.; Chen, X.; Hu, A.; Wang, Z.; Wu, Z.; Wu, E.; et al. alpha-Mangostin suppresses the viability and epithelial-mesenchymal transition of pancreatic cancer cells by downregulating the PI3K/Akt pathway. BioMed Res. Int. 2014, 2014, 546353. [Google Scholar] [CrossRef]
- Neuman, E.; Ladha, M.H.; Lin, N.; Upton, T.M.; Miller, S.J.; DiRenzo, J.; Pestell, R.G.; Hinds, P.W.; Dowdy, S.F.; Brown, M.; et al. Cyclin D1 stimulation of estrogen receptor transcriptional activity independent of cdk4. Mol. Cell. Biol. 1997, 17, 5338–5347. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Rasool, M.; Chaoudhry, H.; Pushparaj, N.P.; Jha, P.; Hafiz, A.; Mahfooz, M.; Abdus Sami, G.; Azhar Kamal, M.; Bashir, S.; et al. Molecular mechanisms and mode of tamoxifen resistance in breast cancer. Bioinformation 2016, 12, 135–139. [Google Scholar] [CrossRef]
- Gao, A.; Sun, T.; Ma, G.; Cao, J.; Hu, Q.; Chen, L.; Wang, Y.; Wang, Q.; Sun, J.; Wu, R.; et al. LEM4 confers tamoxifen resistance to breast cancer cells by activating cyclin D-CDK4/6-Rb and ERalpha pathway. Nat. Commun. 2018, 9, 4180. [Google Scholar] [CrossRef] [PubMed]
- Jirstrom, K.; Stendahl, M.; Ryden, L.; Kronblad, A.; Bendahl, P.O.; Stal, O.; Landberg, G. Adverse effect of adjuvant tamoxifen in premenopausal breast cancer with cyclin D1 gene amplification. Cancer Res. 2005, 65, 8009–8016. [Google Scholar] [CrossRef] [PubMed]
- Lundgren, K.; Brown, M.; Pineda, S.; Cuzick, J.; Salter, J.; Zabaglo, L.; Howell, A.; Dowsett, M.; Landberg, G.; Trans, A.I. Effects of cyclin D1 gene amplification and protein expression on time to recurrence in postmenopausal breast cancer patients treated with anastrozole or tamoxifen: A Trans ATAC study. Breast Cancer Res. 2012, 14, R57. [Google Scholar] [CrossRef]
- Stendahl, M.; Kronblad, A.; Ryden, L.; Emdin, S.; Bengtsson, N.O.; Landberg, G. Cyclin D1 overexpression is a negative predictive factor for tamoxifen response in postmenopausal breast cancer patients. Br. J. Cancer 2004, 90, 1942–1948. [Google Scholar] [CrossRef]
- Ouadid-Ahidouch, H.; Le Bourhis, X.; Roudbaraki, M.; Toillon, R.A.; Delcourt, P.; Prevarskaya, N. Changes in the K+ current-density of MCF-7 cells during progression through the cell cycle: Possible involvement of a h-ether. a-gogo K+ channel. Recept. Channels 2001, 7, 345–356. [Google Scholar]
- Ouadid-Ahidouch, H.; Roudbaraki, M.; Delcourt, P.; Ahidouch, A.; Joury, N.; Prevarskaya, N. Functional and molecular identification of intermediate-conductance Ca2+-activated K+ channels in breast cancer cells: Association with cell cycle progression. Am. J. Physiol. Cell Physiol. 2004, 287, C125–C134. [Google Scholar] [CrossRef]
- Borowiec, A.S.; Hague, F.; Gouilleux-Gruart, V.; Lassoued, K.; Ouadid-Ahidouch, H. Regulation of IGF-1-dependent cyclin D1 and E expression by hEag1 channels in MCF-7 cells: The critical role of hEag1 channels in G1 phase progression. Biochim. Biophys. Acta 2011, 1813, 723–730. [Google Scholar] [CrossRef]
- Song, J.; Su, H.; Zhou, Y.Y.; Guo, L.L. Prognostic value of survivin expression in breast cancer patients: A meta-analysis. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 2013, 34, 2053–2062. [Google Scholar] [CrossRef]
- Moriai, R.; Tsuji, N.; Moriai, M.; Kobayashi, D.; Watanabe, N. Survivin plays as a resistant factor against tamoxifen-induced apoptosis in human breast cancer cells. Breast Cancer Res. Treat. 2009, 117, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Won, Y.S.; Lee, J.H.; Kwon, S.J.; Kim, J.Y.; Park, K.H.; Lee, M.K.; Seo, K.I. alpha-Mangostin-induced apoptosis is mediated by estrogen receptor alpha in human breast cancer cells. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2014, 66, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Balunas, M.J.; Su, B.; Brueggemeier, R.W.; Kinghorn, A.D. Xanthones from the botanical dietary supplement mangosteen (Garcinia mangostana) with aromatase inhibitory activity. J. Nat. Prod. 2008, 71, 1161–1166. [Google Scholar] [CrossRef] [PubMed]
- Mardianingrum, R.; Yusuf, M.; Hariono, M.; Mohd Gazzali, A.; Muchtaridi, M. alpha-Mangostin and its derivatives against estrogen receptor alpha. J. Biomol. Struct. Dyn. 2022, 40, 2621–2634. [Google Scholar] [CrossRef]
- Shevra, C.R.; Ghosh, A.; Kumar, M. Cyclin D1 and Ki-67 expression in normal, hyperplastic and neoplastic endometrium. J. Postgrad. Med. 2015, 61, 15–20. [Google Scholar]
- Kosmas, K.; Stamoulas, M.; Marouga, A.; Kavantzas, N.; Patsouris, E.; Athanassiadou, P. Expression of Ki-67 as proliferation biomarker in imprint smears of endometrial carcinoma. Diagn. Cytopathol. 2013, 41, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Mu, K.; Wang, Y.; Zhou, Z.; Zhang, J.; Sheng, Y.; Zhang, T. CyclinD1, a prominent prognostic marker for endometrial diseases. Diagn. Pathol. 2013, 8, 138. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Jia, M.; Hu, J.; Huang, Z.; Deng, Y.; Lai, L.; Ding, S.; Hu, Z. Prognostic Value of Ki67 in Patients with Stage 1-2 Endometrial Cancer: Validation of the Cut-off Value of Ki67 as a Predictive Factor. OncoTargets Ther. 2020, 13, 10841–10850. [Google Scholar] [CrossRef]
- Tamm-Rosenstein, K.; Simm, J.; Suhorutshenko, M.; Salumets, A.; Metsis, M. Changes in the transcriptome of the human endometrial Ishikawa cancer cell line induced by estrogen, progesterone, tamoxifen, and mifepristone (RU486) as detected by RNA-sequencing. PLoS ONE 2013, 8, e68907. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Lan, L.; Liu, R.; Wu, Y.; Qu, Q.; Wen, K. Tamoxifen has a proliferative effect in endometrial carcinoma mediated via the GPER/EGFR/ERK/cyclin D1 pathway: A retrospective study and an in vitro study. Mol. Cell. Endocrinol. 2016, 437, 51–61. [Google Scholar] [CrossRef]
- Thomas, D.; Gut, B.; Karsai, S.; Wimmer, A.B.; Wu, K.; Wendt-Nordahl, G.; Zhang, W.; Kathofer, S.; Schoels, W.; Katus, H.A.; et al. Inhibition of cloned HERG potassium channels by the antiestrogen tamoxifen. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2003, 368, 41–48. [Google Scholar] [CrossRef]
- Hussaarts, K.; Berger, F.A.; Binkhorst, L.; Oomen-de Hoop, E.; van Leeuwen, R.W.F.; van Alphen, R.J.; Mathijssen-van Stein, D.; de Groot, N.M.S.; Mathijssen, R.H.J.; van Gelder, T. The Risk of QTc-Interval Prolongation in Breast Cancer Patients Treated with Tamoxifen in Combination with Serotonin Reuptake Inhibitors. Pharm. Res. 2019, 37, 7. [Google Scholar] [CrossRef]
- Xie, M.; Zhu, S.; Liu, G.; Wu, Y.; Zhou, W.; Yu, D.; Wan, J.; Xing, S.; Wang, S.; Gan, L.; et al. A Novel Quantitative Electrocardiography Strategy Reveals the Electroinhibitory Effect of Tamoxifen on the Mouse Heart. J. Cardiovasc. Transl. Res. 2023, 16, 1232–1248. [Google Scholar] [CrossRef]
- Cubeddu, L.X. Drug-induced Inhibition and Trafficking Disruption of ion Channels: Pathogenesis of QT Abnormalities and Drug-induced Fatal Arrhythmias. Curr. Cardiol. Rev. 2016, 12, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Eisvand, F.; Imenshahidi, M.; Ghasemzadeh Rahbardar, M.; Tabatabaei Yazdi, S.A.; Rameshrad, M.; Razavi, B.M.; Hosseinzadeh, H. Cardioprotective effects of alpha-mangostin on doxorubicin-induced cardiotoxicity in rats. Phytother. Res. PTR 2022, 36, 506–524. [Google Scholar] [CrossRef]
- Yu, J.; Berga, S.L.; Johnston-MacAnanny, E.B.; Sidell, N.; Bagchi, I.C.; Bagchi, M.K.; Taylor, R.N. Endometrial Stromal Decidualization Responds Reversibly to Hormone Stimulation and Withdrawal. Endocrinology 2016, 157, 2432–2446. [Google Scholar] [CrossRef] [PubMed]
- Vichai, V.; Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- Sebaugh, J.L. Guidelines for accurate EC50/IC50 estimation. Pharm. Stat. 2011, 10, 128–134. [Google Scholar] [CrossRef] [PubMed]


| Cell Line | AM (µM) | 4-OH-TMX (µM) | ||
|---|---|---|---|---|
| IC20 | IC50 | IC20 | IC50 | |
| MCF-7 | 2.35 ± 0.28 | 3.53 ± 0.23 | 0.73 ± 0.58 | 2.44 ± 2.35 |
| T-47D | 4.60 ± 0.22 | 7.15 ± 0.16 | 0.0094 ± 0.0072 | 0.1584 ± 0.082 |
| Cell Line | Combination Schemes | DRI (Folds) | |
|---|---|---|---|
| 4-OH-TMX:AM | 4-OH-TMX | AM | |
| MCF-7 | IC20:IC20 | 1.38 | 0.82 |
| IC50:IC20 | 3.44 | 3.52 | |
| IC20:IC50 | 15.28 | 2.86 | |
| IC50:IC50 | 156.18 | 32.63 | |
| T-47D | IC20:IC20 | 7.55 | 1.36 |
| IC50:IC20 | 0.43 | 1.37 | |
| IC20:IC50 | 85.16 | 1.29 | |
| IC50:IC50 | 113.67 | 1.46 | |
| Gen | Accession Number | Upper Primer | Lower Primer | Probe Number * |
|---|---|---|---|---|
| KCNH1 | AF078741.1 | cctggaggtgatccaagatg | ccaaacacgtctccttttcc | 49 |
| MKI67 | X65550.1 | ggtgtgcagaaaatccaaga | actgtccctatgacttcttctggttg | 63 |
| CCND1 | NM_053056.2 | gaagatcgtcgccacctg | gacctcctcctcgcacttct | 67 |
| BIRC5 | NM_001012271.2 | gcccagtgtttcttctgctt | aaccggacgaatgcttttta | 11 |
| ESR1 | X03635.1 | ccttcttcaagagaagtattcaagg | gtttttatcaatggtgcactgg | 83 |
| CYP19A1 | NM_00103.2 | gaattcatgcgagtctggatct | tcattatgtggaacatacttgagga | 55 |
| PGR | NM_001271162 | tcaagcttcaagttagccaaga | gacttcgtagcccttccaaa | 6 |
| PRL | NM_000948.2 | aaaggatcgccatggaaag | gcacaggagcaggtttgac | 18 |
| RPL32 | NM_000994.3 | gaagttcctggtccacaacg | gagcgatctcggcacagta | 17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargas-Castro, R.; García-Becerra, R.; Díaz, L.; Avila, E.; Ordaz-Rosado, D.; Bernadez-Vallejo, S.V.; Cano-Colín, S.; Camacho, J.; Larrea, F.; García-Quiroz, J. Enhancing Tamoxifen Therapy with α-Mangostin: Synergistic Antiproliferative Effects on Breast Cancer Cells and Potential Reduced Endometrial Impact. Pharmaceuticals 2023, 16, 1576. https://doi.org/10.3390/ph16111576
Vargas-Castro R, García-Becerra R, Díaz L, Avila E, Ordaz-Rosado D, Bernadez-Vallejo SV, Cano-Colín S, Camacho J, Larrea F, García-Quiroz J. Enhancing Tamoxifen Therapy with α-Mangostin: Synergistic Antiproliferative Effects on Breast Cancer Cells and Potential Reduced Endometrial Impact. Pharmaceuticals. 2023; 16(11):1576. https://doi.org/10.3390/ph16111576
Chicago/Turabian StyleVargas-Castro, Rafael, Rocío García-Becerra, Lorenza Díaz, Euclides Avila, David Ordaz-Rosado, Samantha V. Bernadez-Vallejo, Saúl Cano-Colín, Javier Camacho, Fernando Larrea, and Janice García-Quiroz. 2023. "Enhancing Tamoxifen Therapy with α-Mangostin: Synergistic Antiproliferative Effects on Breast Cancer Cells and Potential Reduced Endometrial Impact" Pharmaceuticals 16, no. 11: 1576. https://doi.org/10.3390/ph16111576








