Cancer-Cachexia-Induced Human Skeletal Muscle Myotube Degeneration Is Prevented via Cannabinoid Receptor 2 Agonism In Vitro
Abstract
:1. Introduction
2. Results
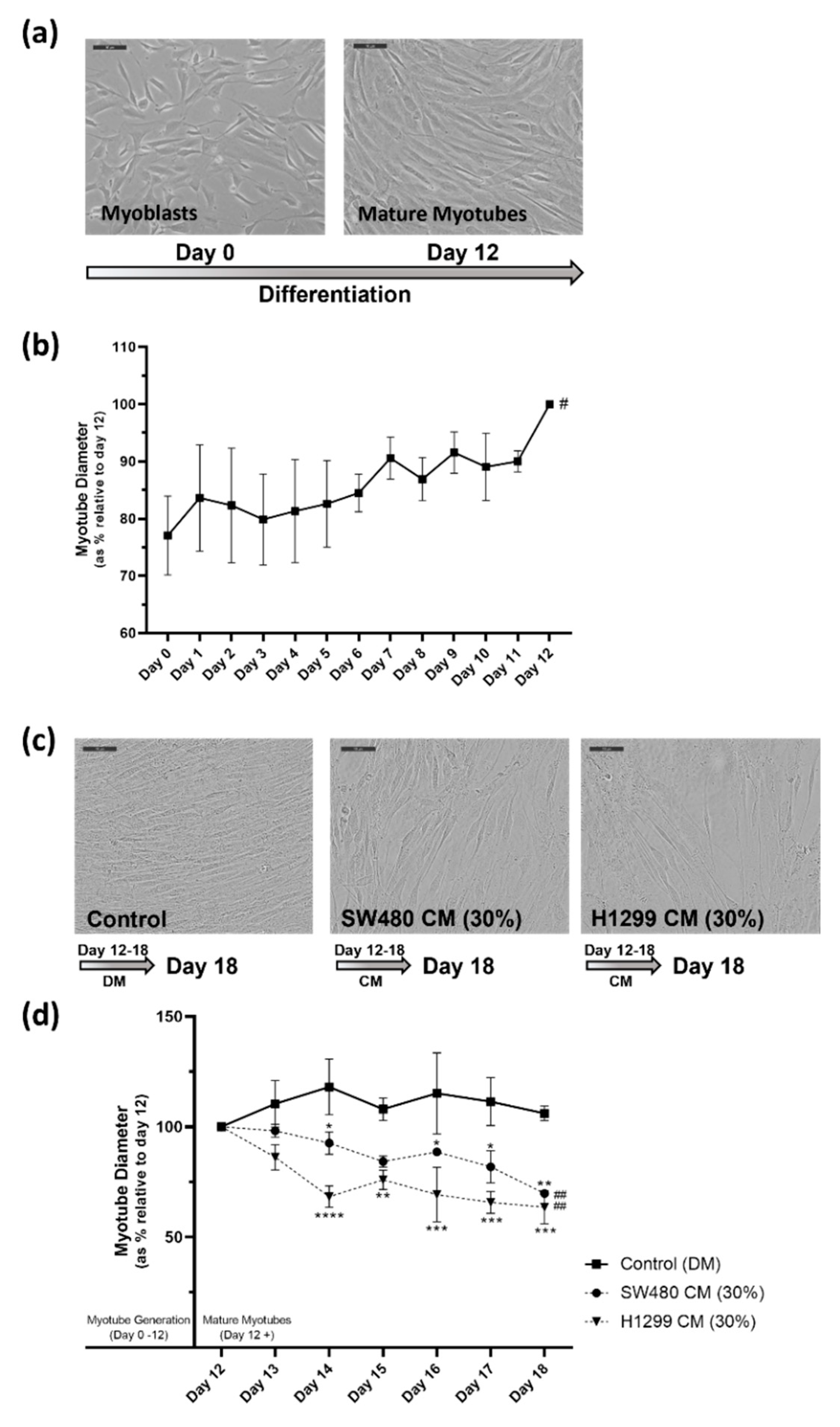
2.1. In Vitro Human Skeletal Muscle Cancer Cachexia Cell Model Is Established Using Cancer Conditioned Media Which Induces Significant Degeneration of Mature Myotubes
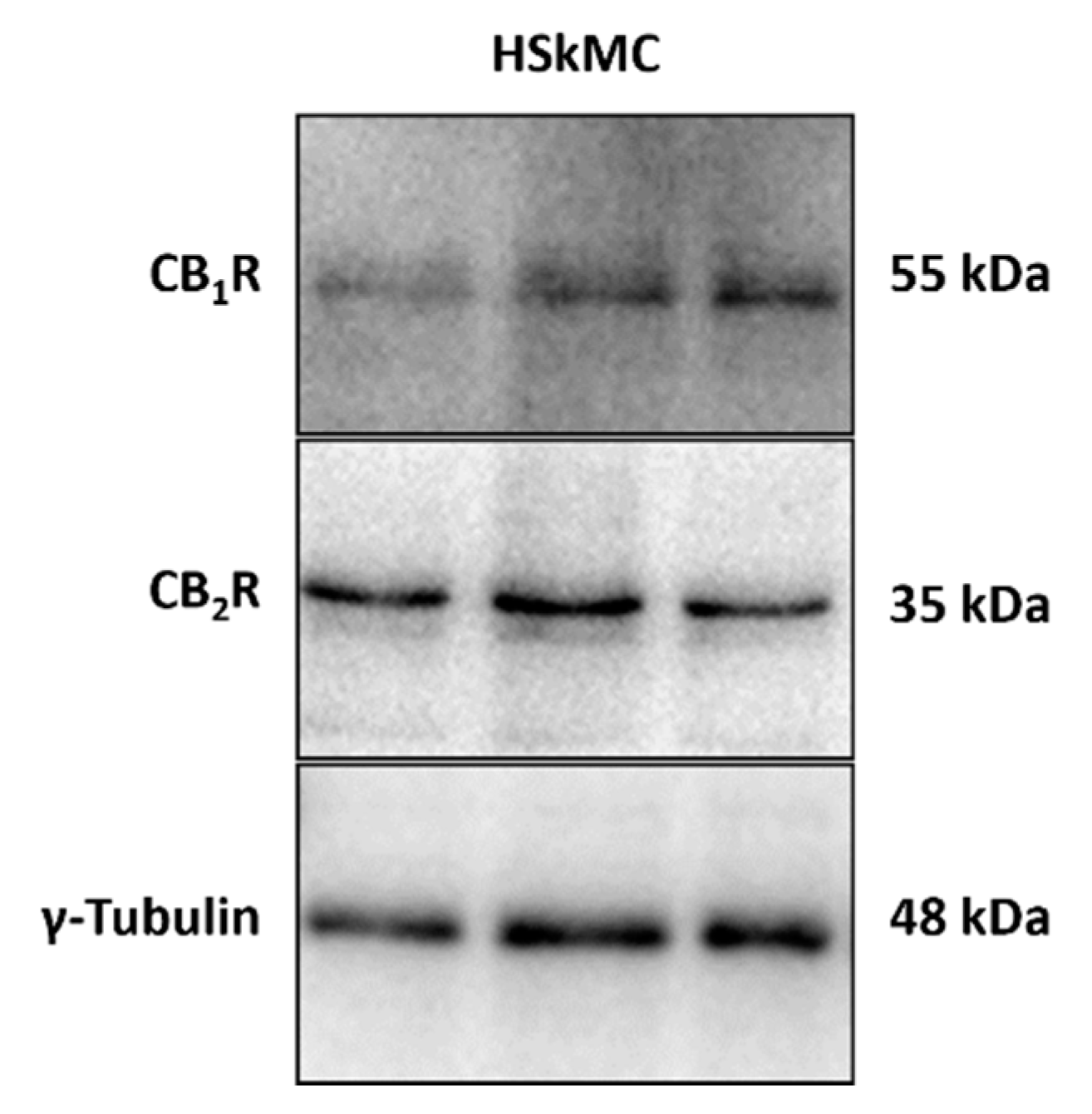
2.2. Cannabinoid 1 and 2 Receptors Are Expressed in the HSkMC Used in this Study
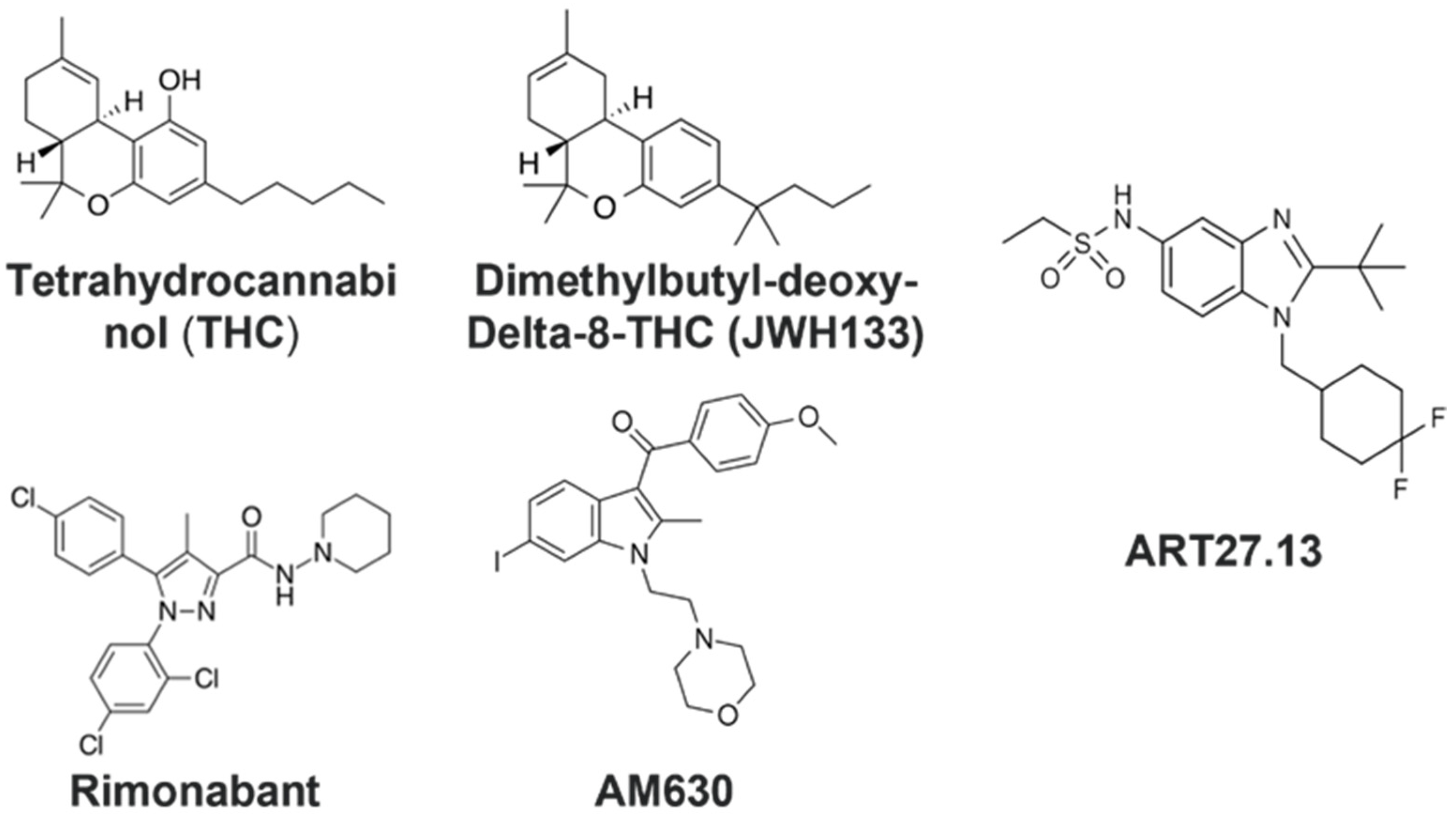
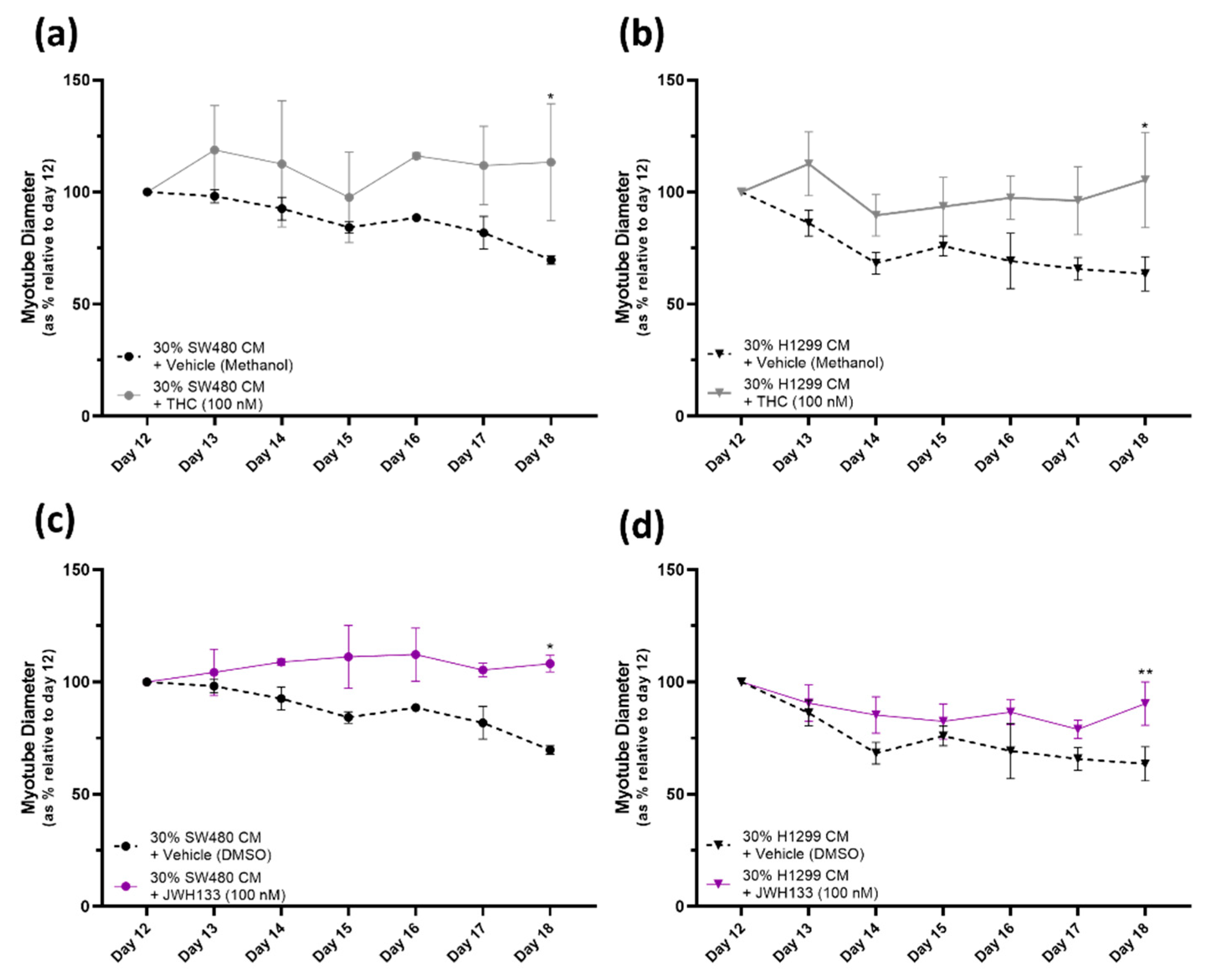
2.3. Cannabinoid Receptor Agonists, THC and JWH133, Provide a Protective Effect against Myotube Degeneration in the Cancer Cachexia Model, with SW480 and H1299 Cancers
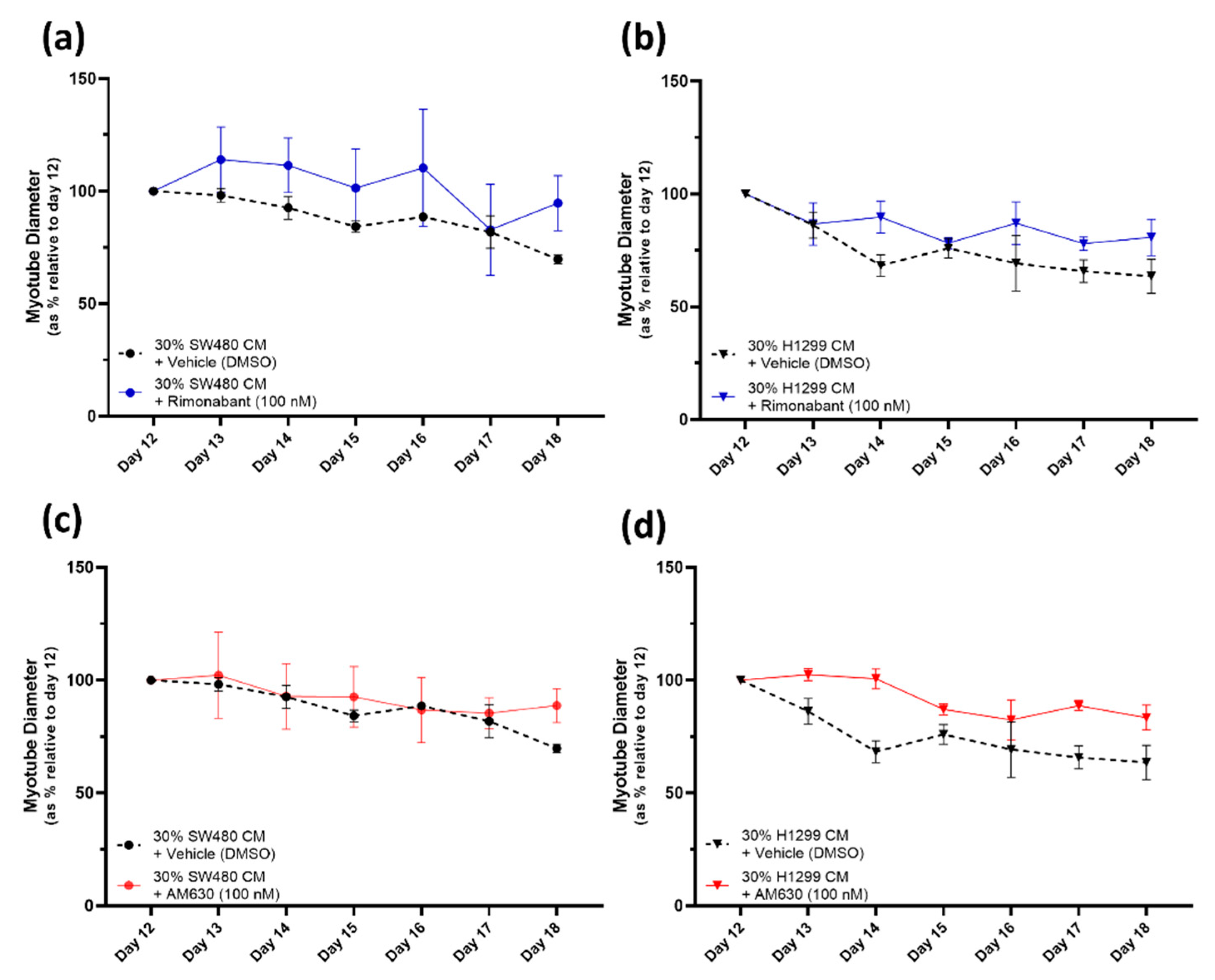
2.4. CB1R Antagonist Rimonabant or CB2R Antagonist AM630 Do Not Affect the Myotube Degeneration Induced by Either the SW480 or H1299 Cancer Cachexia Model
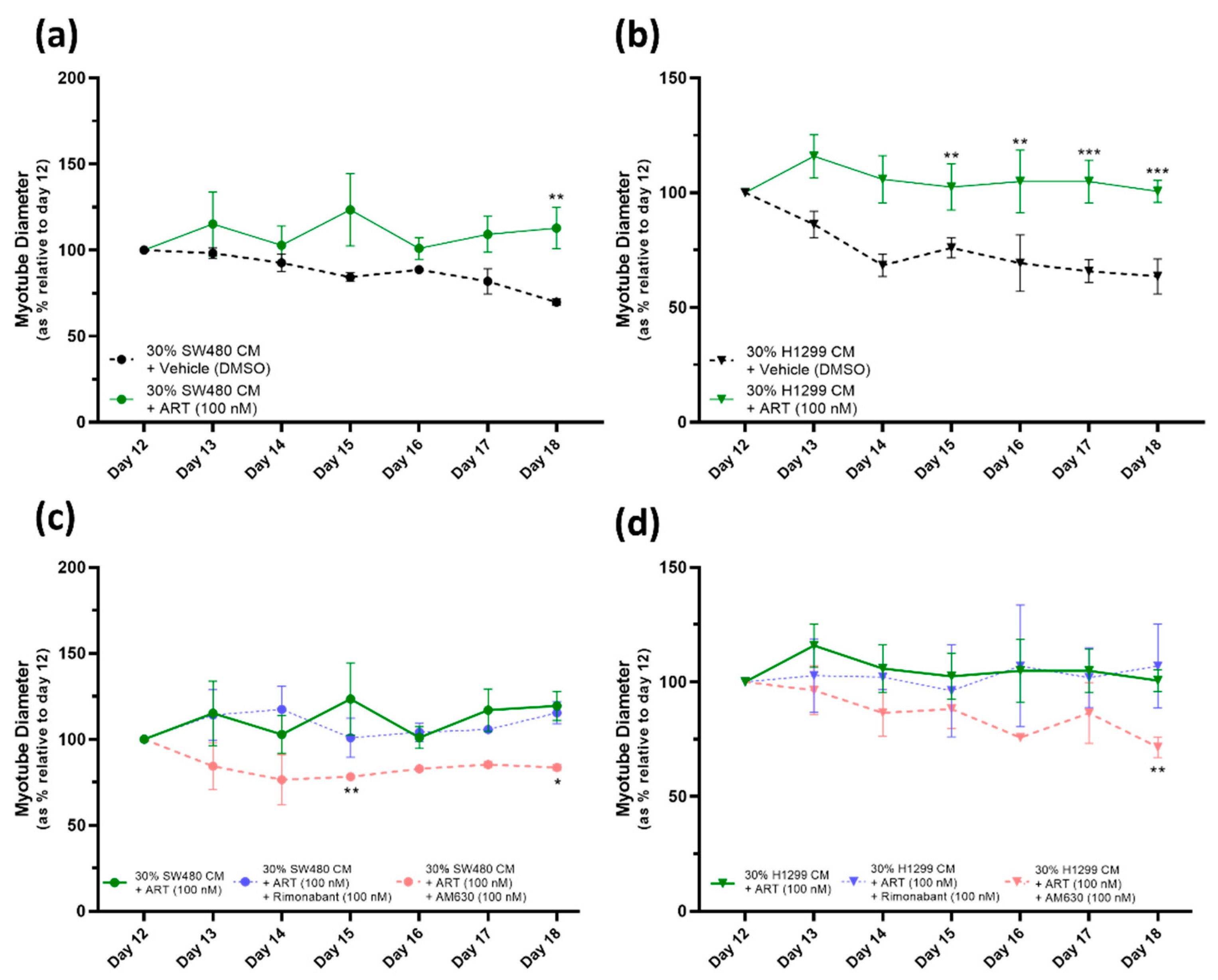
2.5. ART27.13 Protects against Cancer-Induced Skeletal Muscle Cachexia via Agonism of the Cannabinoid Receptor Type 2
3. Discussion
4. Materials and Methods
4.1. Primary Human Skeletal Muscle Cell (HSkMC) Culture and Differentiation
4.1.1. Human Skeletal Muscle Cell Culture
4.1.2. Differentiation of Human Skeletal Muscle Myoblasts to Mature Myotubes
4.2. Measurement of Myotube Diameter
4.3. In Vitro Cancer Cachexia Model
4.3.1. Conditioned Media from Cancer Cell Lines
4.3.2. Culture of Mature Myotubes with Cancer Conditioned Media
4.4. Treatment of Myotubes with Cannabinoid Receptor Agonists/Antagonists
4.5. Immunoblotting (Cannabinoid Receptor 1 and 2 Expression)
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aversa, Z.; Costelli, P.; Muscaritoli, M. Cancer-induced muscle wasting: Latest findings in prevention and treatment. Ther. Adv. Med. Oncol. 2017, 9, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Porporato, P. Understanding cachexia as a cancer metabolism syndrome. Oncogenesis 2016, 5, e200. [Google Scholar] [CrossRef]
- Jackman, R.W.; Floro, J.; Yoshimine, R.; Zitin, B.; Eiampikul, M.; El-Jack, K.; Seto, D.N.; Kandarian, S.C. Continuous release of tumor-derived factors improves the modeling of cachexia in muscle cell culture. Front. Physiol. 2017, 8, 738. [Google Scholar] [CrossRef]
- Seto, D.N.; Kandarian, S.C.; Jackman, R.W. A key role for leukemia inhibitory factor in C26 cancer cachexia. J. Biol. Chem. 2015, 290, 19976–19986. [Google Scholar] [CrossRef]
- Wu, C.; Zhu, M.; Lu, Z.; Zhang, Y.; Li, L.; Li, N.; Yin, L.; Wang, H.; Song, W.; Xu, H. L-carnitine ameliorates the muscle wasting of cancer cachexia through the AKT/FOXO3a/MaFbx axis. Nutr. Metab. 2021, 18, 98. [Google Scholar] [CrossRef]
- Hain, B.A.; Xu, H.; VanCleave, A.M.; Gordon, B.S.; Kimball, S.R.; Waning, D.L. REDD1 deletion attenuates cancer cachexia in mice. J. Appl. Physiol. 2021, 131, 1718–1730. [Google Scholar] [CrossRef]
- Simon, L.; Baldwin, C.; Kalea, A.Z.; Slee, A. Cannabinoid interventions for improving cachexia outcomes in cancer: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2022, 13, 23–41. [Google Scholar] [CrossRef] [PubMed]
- Mücke, M.; Weier, M.; Carter, C.; Copeland, J.; Degenhardt, L.; Cuhls, H.; Radbruch, L.; Häuser, W.; Conrad, R. Systematic review and meta-analysis of cannabinoids in palliative medicine. J. Cachexia Sarcopenia Muscle 2018, 9, 220–234. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.; Birdsell, L.; MacDonald, N.; Reiman, T.; Clandinin, M.T.; McCargar, L.J.; Murphy, R.; Ghosh, S.; Sawyer, M.B.; Baracos, V.E. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J. Clin. Oncol. 2013, 31, 1539–1547. [Google Scholar] [CrossRef]
- Baracos, V.E.; Martin, L.; Korc, M.; Guttridge, D.C.; Fearon, K.C. Cancer-associated cachexia. Nat. Rev. Dis. Primers 2018, 4, 17105. [Google Scholar] [CrossRef]
- Singlár, Z.; Ganbat, N.; Szentesi, P.; Osgonsandag, N.; Szabó, L.; Telek, A.; Fodor, J.; Dienes, B.; Gönczi, M.; Csernoch, L. Genetic Manipulation of CB1 Cannabinoid Receptors Reveals a Role in Maintaining Proper Skeletal Muscle Morphology and Function in Mice. Int. J. Mol. Sci. 2022, 23, 15653. [Google Scholar] [CrossRef] [PubMed]
- Eckardt, K.; Sell, H.; Taube, A.; Koenen, M.; Platzbecker, B.; Cramer, A.; Horrighs, A.; Lehtonen, M.; Tennagels, N.; Eckel, J. Cannabinoid type 1 receptors in human skeletal muscle cells participate in the negative crosstalk between fat and muscle. Diabetologia 2009, 52, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Iannotti, F.A.; Silvestri, C.; Mazzarella, E.; Martella, A.; Calvigioni, D.; Piscitelli, F.; Ambrosino, P.; Petrosino, S.; Czifra, G.; Bíró, T. The endocannabinoid 2-AG controls skeletal muscle cell differentiation via CB1 receptor-dependent inhibition of Kv7 channels. Proc. Natl. Acad. Sci. USA 2014, 111, E2472–E2481. [Google Scholar] [CrossRef] [PubMed]
- Langer, H.T.; Avey, A.; Baar, K. Cannabidiol does not impact acute anabolic or inflammatory signaling in skeletal muscle in vitro. Cannabis Cannabinoid Res. 2022, 7, 628–636. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, S.E.; Yates, A.S.; Porter, R.K. The peripheral cannabinoid receptor type 1 (CB1) as a molecular target for modulating body weight in man. Molecules 2021, 26, 6178. [Google Scholar] [CrossRef]
- Esposito, I.; Proto, M.C.; Gazzerro, P.; Laezza, C.; Miele, C.; Alberobello, A.T.; D’Esposito, V.; Beguinot, F.; Formisano, P.; Bifulco, M. The cannabinoid CB1 receptor antagonist Rimonabant stimulates 2-deoxyglucose uptake in skeletal muscle cells by regulating the expression of phosphatidylinositol-3-kinase. Mol. Pharmacol. 2008, 74, 1678–1686. [Google Scholar] [CrossRef]
- Oláh, T.; Bodnár, D.; Tóth, A.; Vincze, J.; Fodor, J.; Reischl, B.; Kovács, A.; Ruzsnavszky, O.; Dienes, B.; Szentesi, P. Cannabinoid signalling inhibits sarcoplasmic Ca2+ release and regulates excitation–contraction coupling in mammalian skeletal muscle. J. Physiol. 2016, 594, 7381–7398. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, M.; Wang, L.; Yu, T.; Jiang, S.; Jiang, P.; Sun, Y.; Pi, J.; Zhao, R.; Guan, D. Activation of cannabinoid type 2 receptor protects skeletal muscle from ischemia-reperfusion injury partly via Nrf2 signaling. Life Sci. 2019, 230, 55–67. [Google Scholar] [CrossRef]
- Jiang, P.; Wang, L.; Zhang, M.; Zhang, M.; Wang, C.; Zhao, R.; Guan, D. Cannabinoid type 2 receptor manipulates skeletal muscle regeneration partly by regulating macrophage M1/M2 polarization in IR injury in mice. Life Sci. 2020, 256, 117989. [Google Scholar] [CrossRef]
- Strasser, F.; Luftner, D.; Possinger, K.; Ernst, G.; Ruhstaller, T.; Meissner, W.; Ko, Y.-D.; Schnelle, M.; Reif, M.; Cerny, T. Comparison of orally administered cannabis extract and delta-9-tetrahydrocannabinol in treating patients with cancer-related anorexia-cachexia syndrome: A multicenter, phase III, randomized, double-blind, placebo-controlled clinical trial from the Cannabis-In-Cachexia-Study-Group. J. Clin. Oncol. 2006, 24, 3394–3400. [Google Scholar]
- Turcott, J.G.; del Rocío Guillen Núñez, M.; Flores-Estrada, D.; Oñate-Ocaña, L.F.; Zatarain-Barrón, Z.L.; Barrón, F.; Arrieta, O. The effect of nabilone on appetite, nutritional status, and quality of life in lung cancer patients: A randomized, double-blind clinical trial. Support. Care Cancer 2018, 26, 3029–3038. [Google Scholar] [CrossRef]
- Chasen, M. Safety and Efficacy of Inhaled Synthetic THC/CBD for Improving Physical Functioning and for Modulating Cachexia Progression in Patients with Advanced Cancer and Associated Cachexia. 2020. Available online: https://clinicaltrials.gov/study/NCT04001010 (accessed on 29 October 2023).
- Costiniuk, C.T.; Mills, E.; Cooper, C.L. Evaluation of oral cannabinoid-containing medications for the management of interferon and ribavirin-induced anorexia, nausea and weight loss in patients treated for chronic hepatitis C virus. Can. J. Gastroenterol. Hepatol. 2008, 22, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Andries, A.; Frystyk, J.; Flyvbjerg, A.; Støving, R.K. Dronabinol in severe, enduring anorexia nervosa: A randomized controlled trial. Int. J. Eat. Disord. 2014, 47, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G. Cannabinoid pharmacology: The first 66 years. Br. J. Pharmacol. 2006, 147, S163–S171. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G. Pharmacological Actions of Cannabinoids; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Kalliomäki, J.; Annas, P.; Huizar, K.; Clarke, C.; Zettergren, A.; Karlsten, R.; Segerdahl, M. Evaluation of the analgesic efficacy and psychoactive effects of AZD 1940, a novel peripherally acting cannabinoid agonist, in human capsaicin-induced pain and hyperalgesia. Clin. Exp. Pharmacol. Physiol. 2013, 40, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Kalliomäki, J.; Segerdahl, M.; Webster, L.; Reimfelt, A.; Huizar, K.; Annas, P.; Karlsten, R.; Quiding, H. Evaluation of the analgesic efficacy of AZD1940, a novel cannabinoid agonist, on post-operative pain after lower third molar surgical removal. Scand. J. Pain 2013, 4, 17–22. [Google Scholar] [CrossRef]
- Schou, M.; Varnäs, K.; Jucaite, A.; Gulyás, B.; Halldin, C.; Farde, L. Radiolabeling of the cannabinoid receptor agonist AZD1940 with carbon-11 and PET microdosing in non-human primate. Nucl. Med. Biol. 2013, 40, 410–414. [Google Scholar] [CrossRef]
- Laird, B.J.A. ISRCTN15607817. A Trial of the Synthetic Cannabinoid ART27.13 to Stimulate Appetite in Patients with Cancer Anorexia and Weight Loss. 2020. Available online: https://www.isrctn.com/ISRCTN15607817 (accessed on 12 October 2023). [CrossRef]
- Noone, J.; Rochfort, K.D.; O’Sullivan, F.; O’Gorman, D.J. SIRT4 is a regulator of human skeletal muscle fatty acid metabolism influencing inner and outer mitochondrial membrane-mediated fusion. Cell. Signal. 2023, 17, 112. [Google Scholar] [CrossRef]
- Levitt, D.E.; Adler, K.A.; Simon, L. HEMA 3 staining: A simple alternative for the assessment of myoblast differentiation. Curr. Protoc. Stem Cell Biol. 2019, 51, e101. [Google Scholar] [CrossRef]
- Crespillo, A.; Suárez, J.; Bermúdez-Silva, F.J.; Rivera, P.; Vida, M.; Alonso, M.; Palomino, A.; Lucena, M.A.; Serrano, A.; Pérez-Martín, M. Expression of the cannabinoid system in muscle: Effects of a high-fat diet and CB1 receptor blockade. Biochem. J. 2011, 433, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Haspula, D.; Clark, M.A. Cannabinoid receptors: An update on cell signaling, pathophysiological roles and therapeutic opportunities in neurological, cardiovascular, and inflammatory diseases. Int. J. Mol. Sci. 2020, 21, 7693. [Google Scholar] [CrossRef]
- Dalle, S.; Koppo, K. Cannabinoid receptor 1 expression is higher in muscle of old vs. young males, and increases upon resistance exercise in older adults. Sci. Rep. 2021, 11, 18349. [Google Scholar] [CrossRef] [PubMed]
- Iannotti, F.A.; Pagano, E.; Guardiola, O.; Adinolfi, S.; Saccone, V.; Consalvi, S.; Piscitelli, F.; Gazzerro, E.; Busetto, G.; Carrella, D. Genetic and pharmacological regulation of the endocannabinoid CB1 receptor in Duchenne muscular dystrophy. Nat. Commun. 2018, 9, 3950. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Schcolnik-Cabrera, A.; Chávez-Blanco, A.; Domínguez-Gómez, G.; Dueñas-González, A. Understanding tumor anabolism and patient catabolism in cancer-associated cachexia. Am. J. Cancer Res. 2017, 7, 1107. [Google Scholar]
- Ferrer, M.; Anthony, T.G.; Ayres, J.S.; Biffi, G.; Brown, J.C.; Caan, B.J.; Feliciano, E.M.C.; Coll, A.P.; Dunne, R.F.; Goncalves, M.D. Cachexia: A systemic consequence of progressive, unresolved disease. Cell 2023, 186, 1824–1845. [Google Scholar] [CrossRef]
- Cavuoto, P.; McAinch, A.J.; Hatzinikolas, G.; Janovská, A.; Game, P.; Wittert, G.A. The expression of receptors for endocannabinoids in human and rodent skeletal muscle. Biochem. Biophys. Res. Commun. 2007, 364, 105–110. [Google Scholar] [CrossRef]
- Nagappan, A.; Shin, J.; Jung, M.H. Role of cannabinoid receptor type 1 in insulin resistance and its biological implications. Int. J. Mol. Sci. 2019, 20, 2109. [Google Scholar] [CrossRef]
- Haddad, M. The impact of CB1 receptor on nuclear receptors in skeletal muscle cells. Pathophysiology 2021, 28, 457–470. [Google Scholar] [CrossRef]
- Dalle, S.; Schouten, M.; Meeus, G.; Slagmolen, L.; Koppo, K. Molecular networks underlying cannabinoid signaling in skeletal muscle plasticity. J. Cell. Physiol. 2022, 237, 3517–3540. [Google Scholar] [CrossRef] [PubMed]
- Hermanson, D.J.; Marnett, L.J. Cannabinoids, endocannabinoids, and cancer. Cancer Metastasis Rev. 2011, 30, 599–612. [Google Scholar] [CrossRef] [PubMed]
- Young, L.; Sung, J.; Stacey, G.; Masters, J.R. Detection of Mycoplasma in cell cultures. Nat. Protoc. 2010, 5, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.E.; Krohn, R.I.; Hermanson, G.; Mallia, A.; Gartner, F.; Provenzano, M.; Fujimoto, E.; Goeke, N.; Olson, B.; Klenk, D. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noone, J.; Rooney, M.F.; Karavyraki, M.; Yates, A.; O’Sullivan, S.E.; Porter, R.K. Cancer-Cachexia-Induced Human Skeletal Muscle Myotube Degeneration Is Prevented via Cannabinoid Receptor 2 Agonism In Vitro. Pharmaceuticals 2023, 16, 1580. https://doi.org/10.3390/ph16111580
Noone J, Rooney MF, Karavyraki M, Yates A, O’Sullivan SE, Porter RK. Cancer-Cachexia-Induced Human Skeletal Muscle Myotube Degeneration Is Prevented via Cannabinoid Receptor 2 Agonism In Vitro. Pharmaceuticals. 2023; 16(11):1580. https://doi.org/10.3390/ph16111580
Chicago/Turabian StyleNoone, John, Mary F. Rooney, Marilena Karavyraki, Andrew Yates, Saoirse E. O’Sullivan, and Richard K. Porter. 2023. "Cancer-Cachexia-Induced Human Skeletal Muscle Myotube Degeneration Is Prevented via Cannabinoid Receptor 2 Agonism In Vitro" Pharmaceuticals 16, no. 11: 1580. https://doi.org/10.3390/ph16111580
APA StyleNoone, J., Rooney, M. F., Karavyraki, M., Yates, A., O’Sullivan, S. E., & Porter, R. K. (2023). Cancer-Cachexia-Induced Human Skeletal Muscle Myotube Degeneration Is Prevented via Cannabinoid Receptor 2 Agonism In Vitro. Pharmaceuticals, 16(11), 1580. https://doi.org/10.3390/ph16111580





