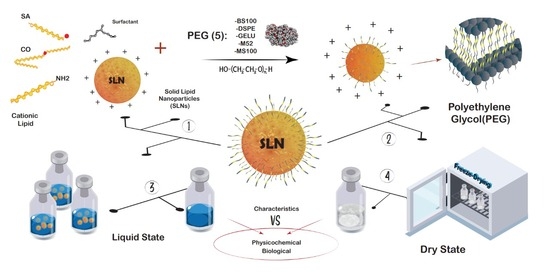
Comparative Analysis of the Physicochemical and Biological Characteristics of Freeze-Dried PEGylated Cationic Solid Lipid Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Properties of the Formulations
2.1.1. Factorial Study
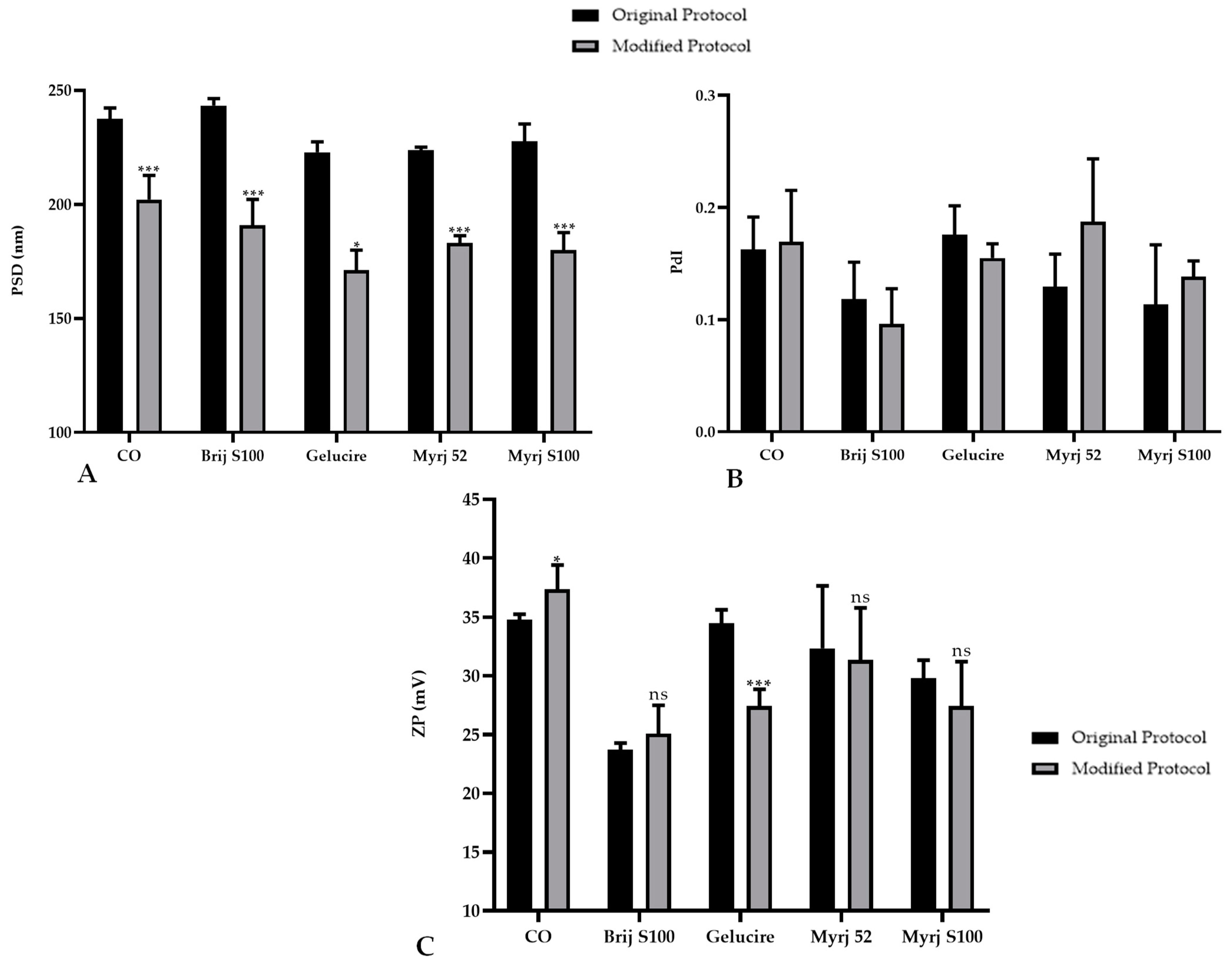
2.1.2. Particle Size, Polydispersity Index and Zeta Potential
2.2. Impact of PEG Incorporation in the Formulation
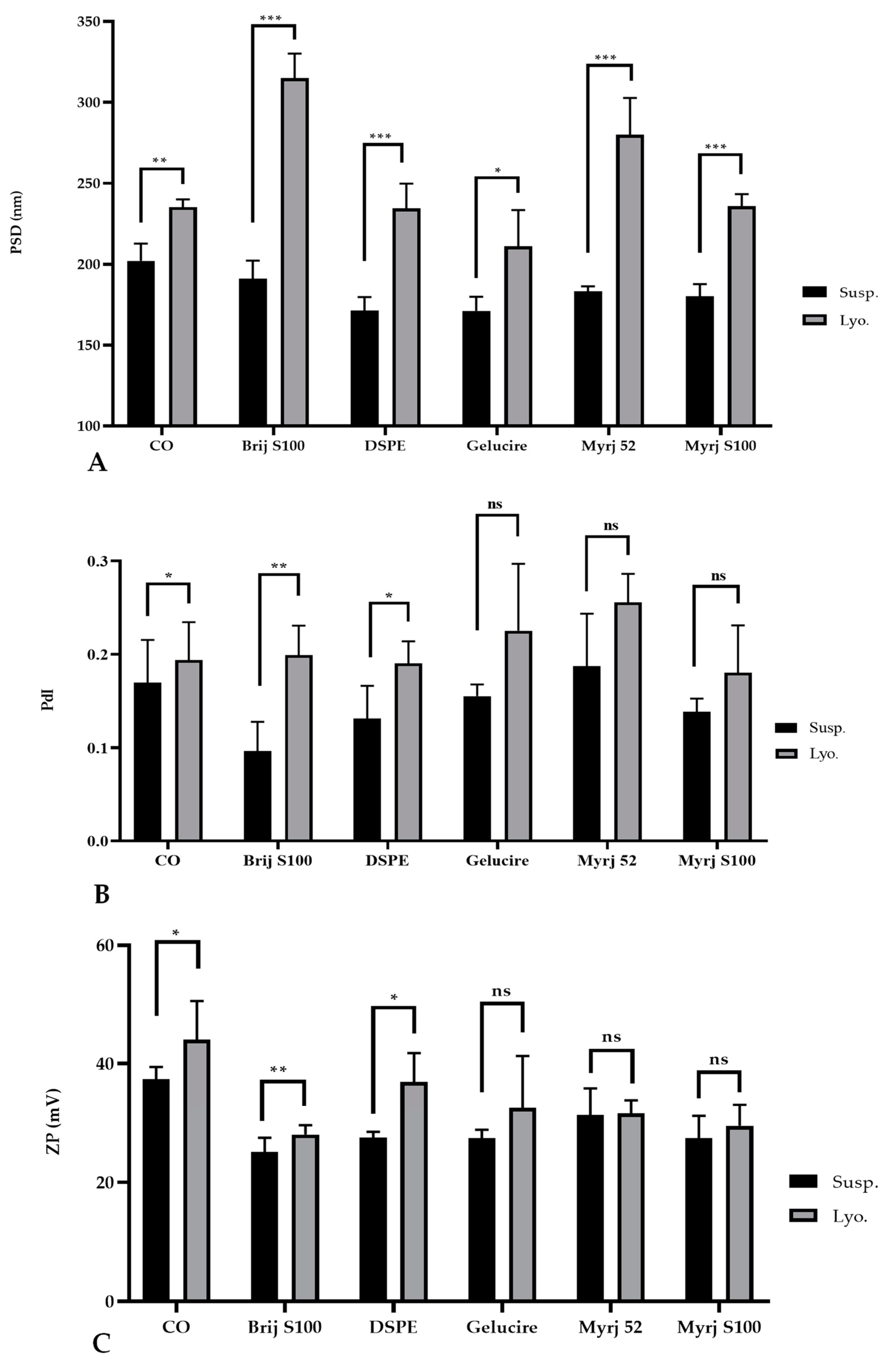
2.3. Freeze-Drying of PEG-cSLNs
2.3.1. Glass Transition Temperature (Tg)
2.3.2. Lyophilization of the PEG-cSLNs
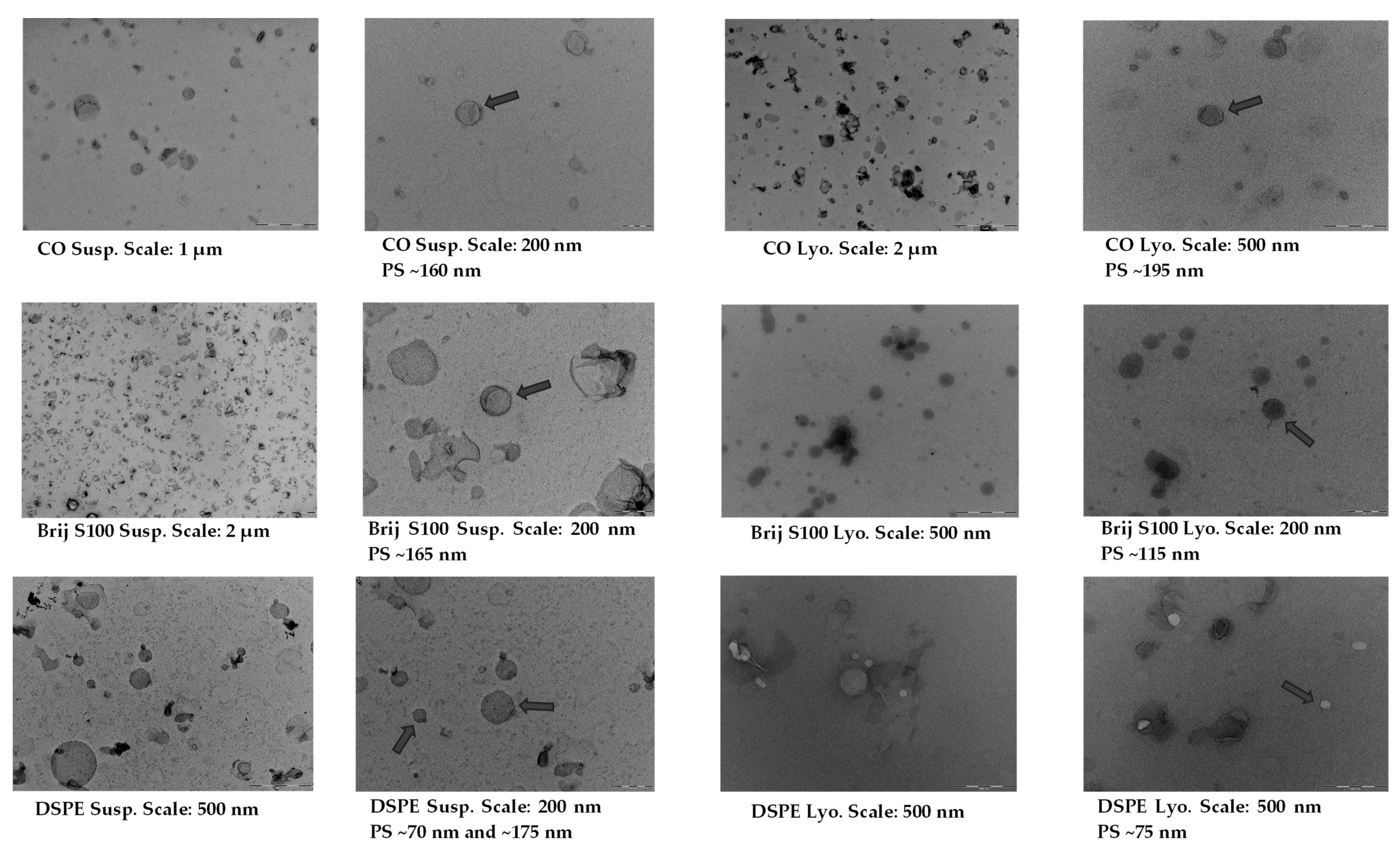
2.4. Morphology of Suspended and Freeze-Dried PEG-cSLNs
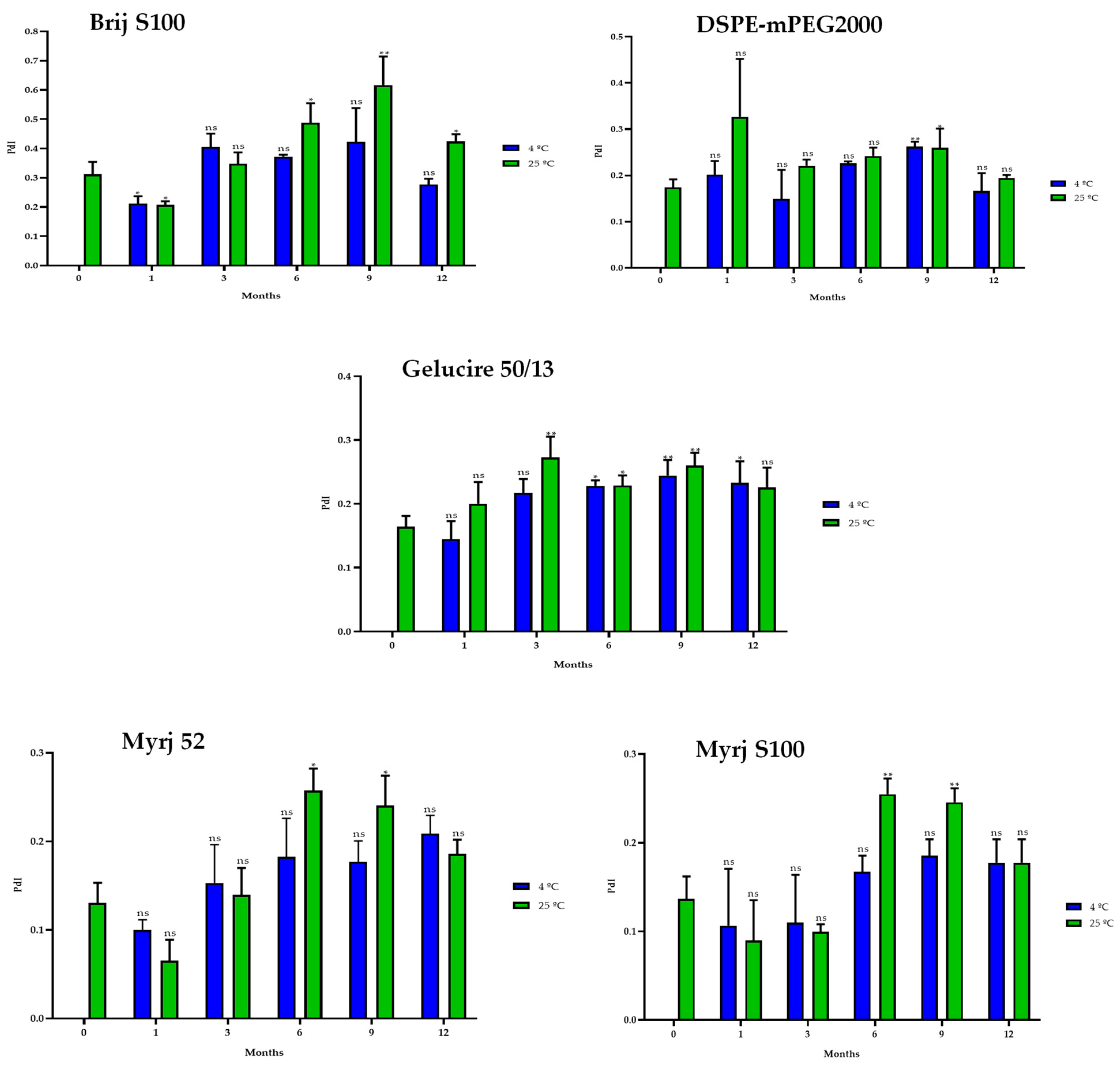
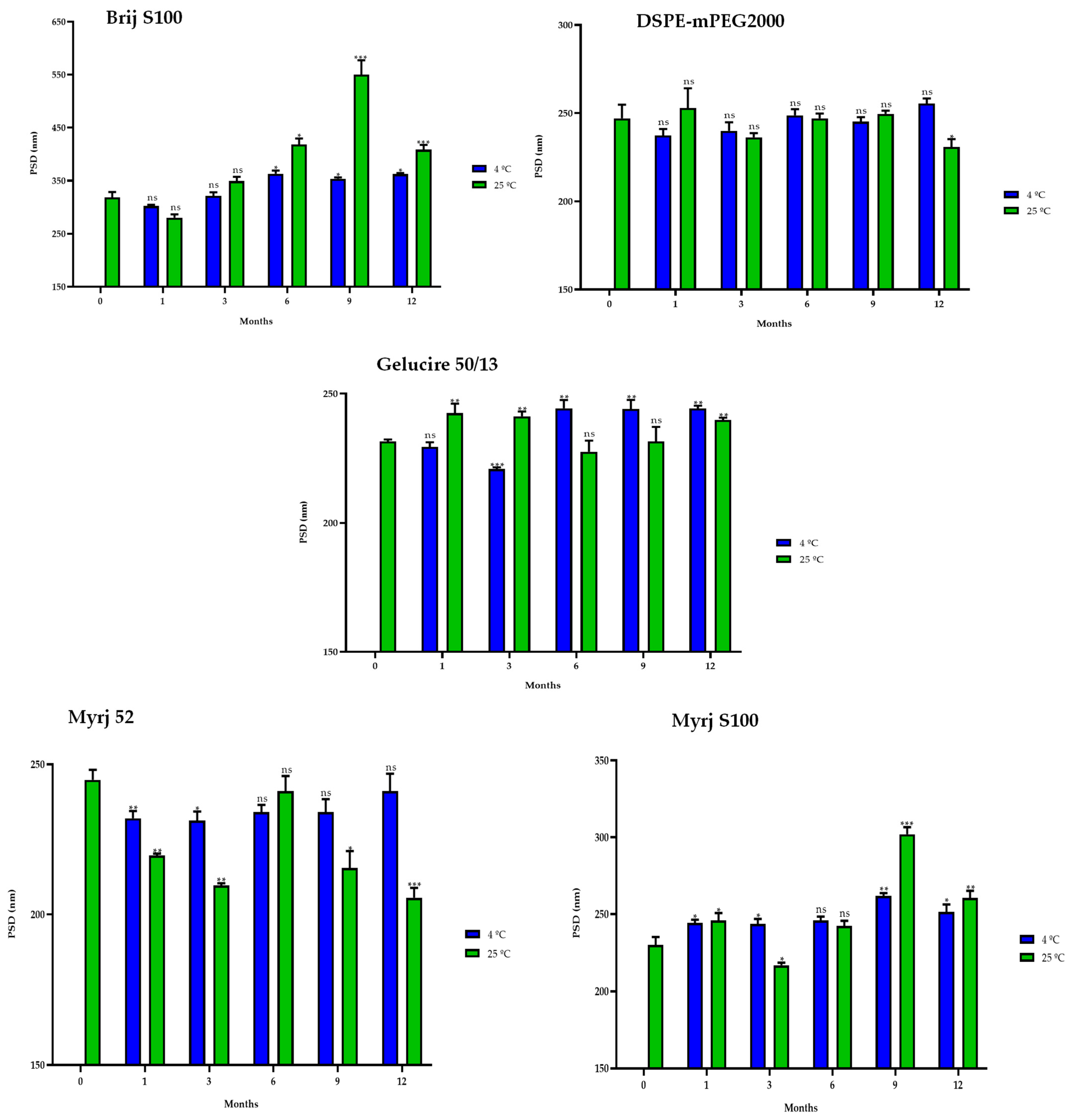
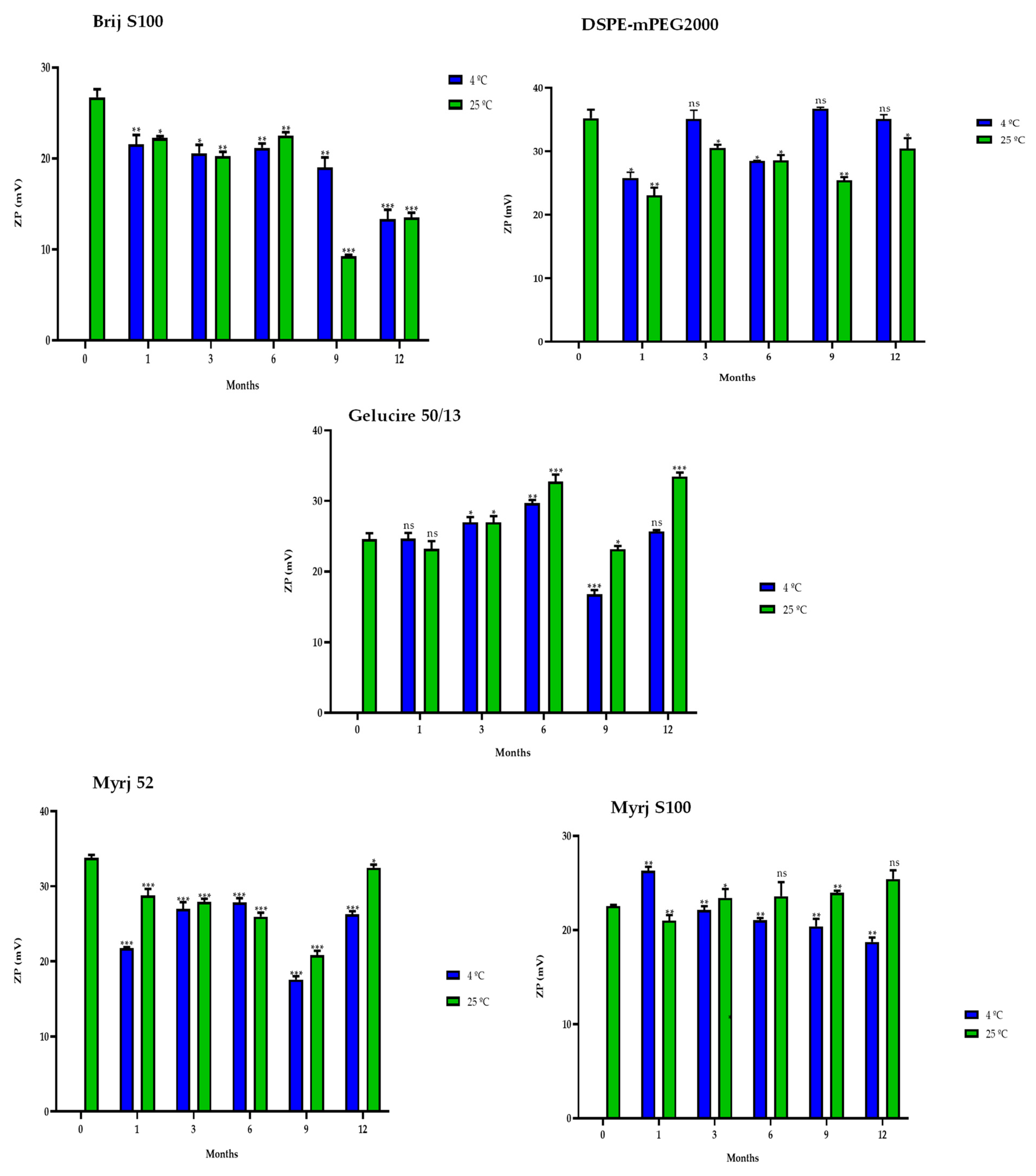
2.5. Stability Study of the PEG-cSLNs
2.5.1. One-Month Stability
2.5.2. One-Year Stability
2.6. Biological Assays
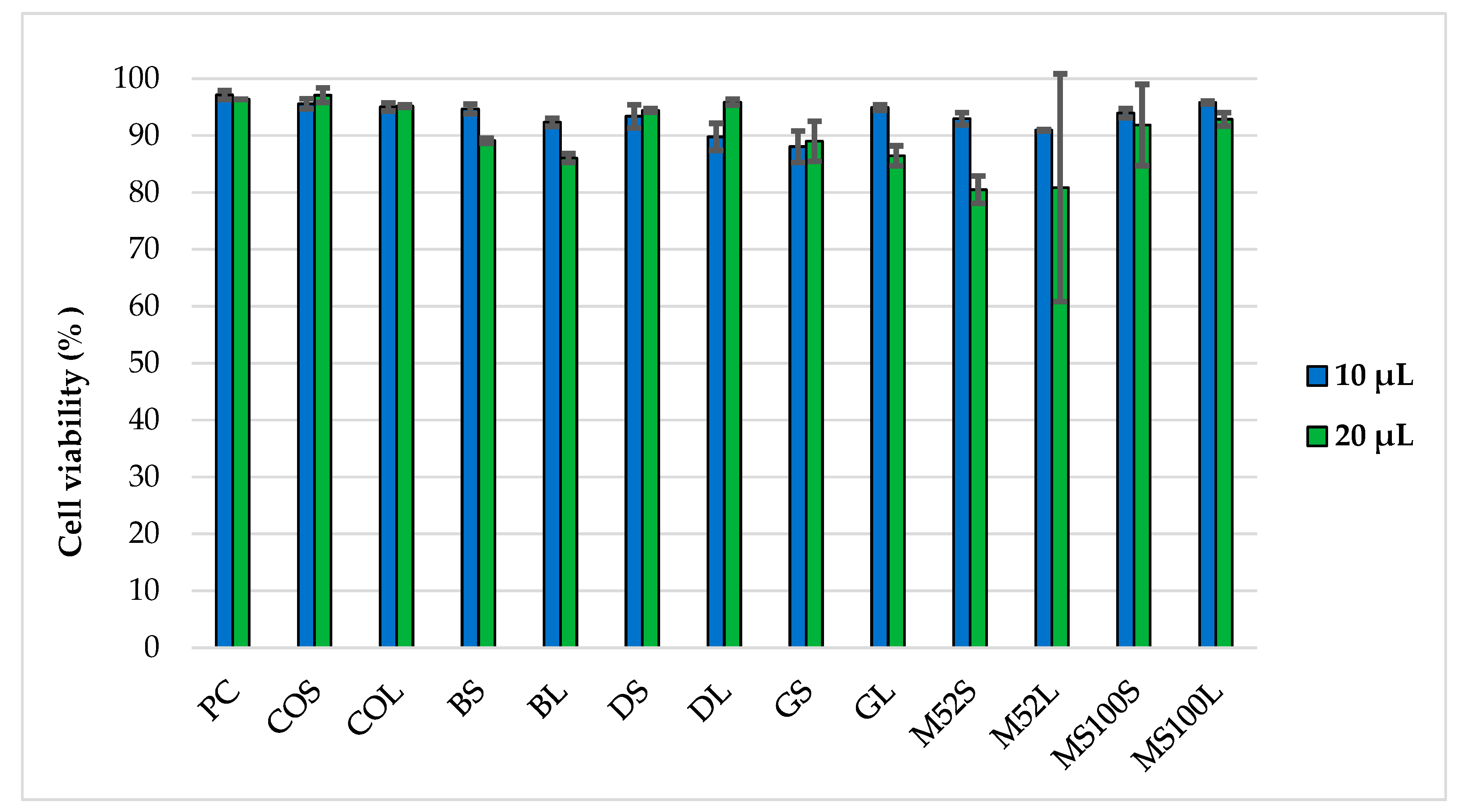
2.6.1. Cytotoxicity of PEG-cSLNs
2.6.2. SLNplex Formation with PEG-cSLNs
3. Materials and Methods
3.1. Materials
3.2. Method of Preparation of PEG-cSLNs
3.3. Physicochemical Characterization of the PEG-cSLNs
3.3.1. Size Characterization
3.3.2. Zeta Potential Analysis
3.4. Effect of PEGylation on the Physicochemical Characteristics of CO-cSLNs
3.5. Freeze-Drying of Nanoparticles
3.5.1. Glass Transition Temperature (Tg)
3.5.2. Freeze-Drying
3.5.3. Comparison between the Different Conditions after Freeze-Drying
3.6. Morphological Analysis of the PEG-cSLNs
3.7. Physical Stability Study
3.8. Biological Characterization of PEG-cSLNs
3.8.1. Cytotoxicity Assay
3.8.2. SLNplexes Formation
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geszke-Moritz, M.; Moritz, M. Solid lipid nanoparticles as attractive drug vehicles: Composition, properties and therapeutic strategies. Mater. Sci. Eng. C 2016, 68, 982–994. [Google Scholar] [CrossRef] [PubMed]
- Paunovska, K.; Loughrey, D.; Dahlman, J.E. Drug delivery systems for RNA therapeutics. Nat. Rev. Genet. 2022, 23, 265–280. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Q.; Liu, X.; Yang, T.; Cui, K.; Kong, L.; Yang, C.; Zhang, Z. Nanomedicine for acute respiratory distress syndrome: The latest application, targeting strategy, and rational design. Acta Pharm. Sin. B 2021, 11, 3060–3091. [Google Scholar] [CrossRef]
- De Jesus, M.B.; Zuhorn, I.S. Solid lipid nanoparticles as nucleic acid delivery system: Properties and molecular mechanisms. J. Control Release 2015, 201, 1–13. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Coura, R.; Nardi, N.B. A role for adeno-associated viral vectors in gene therapy. Genet. Mol. Biol. 2008, 31, 1–11. [Google Scholar] [CrossRef]
- Hanlon, K.S.; Kleinstiver, B.P.; Garcia, S.P.; Zaborowski, M.P.; Volak, A.; Spirig, S.E.; Muller, A.; Sousa, A.A.; Tsai, S.Q.; Bengtsson, N.E.; et al. High levels of AAV vector integration into CRISPR-induced DNA breaks. Nat. Commun. 2019, 10, 4439. [Google Scholar] [CrossRef] [PubMed]
- Takada, R.K.; Maheshwari, R.; Tekade, M.; Chougule, M.B. Solid lipid nanoparticles for targeting and delivery of drugs and genes. In Nanotechnology-Based Approaches for Targeting and Delivery of Drugs and Genes; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 256–286. ISBN 9780128097182. [Google Scholar]
- Suñé-Pou, M.; Prieto-Sánchez, S.; El Yousfi, Y.; Boyero-Corral, S.; Nardi-Ricart, A.; Nofrerias-Roig, I.; Pérez-Lozano, P.; García-Montoya, E.; Miñarro-Carmona, M.; Ticó, J.R.; et al. Cholesteryl oleate-loaded cationic solid lipid nanoparticles as carriers for efficient gene-silencing therapy. Int. J. Nanomed. 2018, 13, 3223–3233. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, J.A.; Cullis, P.R.; Van Der Meel, R. Lipid nanoparticles enabling gene therapies: From concepts to clinical utility. Nucleic Acid Ther. 2018, 28, 146–157. [Google Scholar] [CrossRef]
- Rotshild, V.; Hirsh-Raccah, B.; Miskin, I.; Muszkat, M.; Matok, I. Comparing the clinical efficacy of COVID-19 vaccines: A systematic review and network meta-analysis. Sci. Rep. 2021, 11, 22777. [Google Scholar] [CrossRef]
- Kalaycioglu, G.D.; Aydogan, N. Preparation and investigation of solid lipid nanoparticles for drug delivery. Colloids Surf. A Physicochem. Eng. Asp. 2016, 510, 77–86. [Google Scholar] [CrossRef]
- Suñé-Pou, M.; Limeres, M.J.; Moreno-Castro, C.; Hernández-Munain, C.; Suñé-Negre, J.M.; Cuestas, M.L.; Suñé, C. Innovative therapeutic and delivery approaches using nanotechnology to correct splicing defects underlying disease. Front. Genet. 2020, 11, 731. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, P.; Narayanasamy, D. Lipid nanoparticles: Different preparation techniques, characterization, hurdles, and strategies for the production of solid lipid nanoparticles and nanostructured lipid carriers for oral drug delivery. Sustain. Chem. Pharm. 2017, 6, 37–56. [Google Scholar] [CrossRef]
- Severino, P.; Szymanski, M.; Favaro, M.; Azzoni, A.R.; Chaud, M.V.; Santana, M.H.A.; Silva, A.M.; Souto, E.B. Development and characterization of a cationic lipid nanocarrier as non-viral vector for gene therapy. Eur. J. Pharm. Sci. 2015, 66, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Rodenak-Kladniew, B.; Islan, G.A.; de Bravo, M.G.; Durán, N.; Castro, G.R. Design, characterization and in vitro evaluation of linalool-loaded solid lipid nanoparticles as potent tool in cancer therapy. Colloids Surf. B Biointerfaces 2017, 154, 123–132. [Google Scholar] [CrossRef]
- Rompicharla, S.V.K.; Bhatt, H.; Shah, A.; Komanduri, N.; Vijayasarathy, D.; Ghosh, B.; Biswas, S. Formulation optimization, characterization, and evaluation of in vitro cytotoxic potential of curcumin loaded solid lipid nanoparticles for improved anticancer activity. Chem. Phys. Lipids 2017, 208, 10–18. [Google Scholar] [CrossRef]
- Alnylam Pharmaceuticals RNAi Therapeutics|Our Approved Products|Alnylam® Pharmaceuticals. Available online: https://www.alnylam.com/our-products (accessed on 7 July 2023).
- Ban, C.; Jo, M.; Lim, S.; Choi, Y.J. Control of the gastrointestinal digestion of solid lipid nanoparticles using PEGylated emulsifiers. Food Chem. 2018, 239, 442–452. [Google Scholar] [CrossRef]
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U.S. Poly(ethylene glycol) in drug delivery: Pros and cons as well as potential alternatives. Angew. Chemie-Int. Ed. 2010, 49, 6288–6308. [Google Scholar] [CrossRef]
- Schoenmaker, L.; Witzigmann, D.; Kulkarni, J.A.; Verbeke, R.; Kersten, G.; Jiskoot, W.; Crommelin, D.J.A. mRNA-lipid nanoparticle COVID-19 vaccines: Structure and stability. Int. J. Pharm. 2021, 601, 120586. [Google Scholar] [CrossRef]
- Ngamcherdtrakul, W.; Sangvanich, T.; Reda, M.; Gu, S.; Bejan, D.; Yantasee, W. Lyophilization and stability of antibody-conjugated mesoporous silica nanoparticle with cationic polymer and PEG for siRNA delivery. Int. J. Nanomed. 2018, 13, 4015. [Google Scholar] [CrossRef]
- Yoo, J.-W.; Chambers, E.; Mitragotri, S. Factors that control the circulation time of nanoparticles in blood: Challenges, solutions and future prospects. Curr. Pharm. Des. 2010, 16, 2298–2307. [Google Scholar] [CrossRef]
- Evers, M.J.W.; Kulkarni, J.A.; van der Meel, R.; Cullis, P.R.; Vader, P.; Schiffelers, R.M. State-of-the-art design and rapid-mixing production techniques of lipid nanoparticles for nucleic acid delivery. Small Methods 2018, 2, 1700375. [Google Scholar] [CrossRef]
- Suñé-Pou, M.; Limeres, M.J.; Nofrerias, I.; Nardi-Ricart, A.; Prieto-Sánchez, S.; El-Yousfi, Y.; Pérez-Lozano, P.; García-Montoya, E.; Miñarro-Carmona, M.; Ticó, J.R.; et al. Improved synthesis and characterization of cholesteryl oleate-loaded cationic solid lipid nanoparticles with high transfection efficiency for gene therapy applications. Colloids Surf. B Biointerfaces 2019, 180, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Limeres, M.; Suñé-Pou, M.; Prieto-Sánchez, S.; Moreno-Castro, C.; Nusblat, A.; Casrto, G.; Su, C. Development and characterization of an improved formulation of cholesteryl oleate-loaded cationic solid-lipid nanoparticles as an efficient non-viral gene delivery system. Colloids Surf. B Biointerfaces 2019, 184, 110533. [Google Scholar] [CrossRef] [PubMed]
- Trenkenschuh, E.; Savšek, U.; Friess, W. Formulation, process, and storage strategies for lyophilizates of lipophilic nanoparticulate systems established based on the two models paliperidone palmitate and solid lipid nanoparticles. Int. J. Pharm. 2021, 606, 120929. [Google Scholar] [CrossRef]
- Quintanar-Guerrero, D.; Fessi, H.; Allémann, E.; Doelker, E. Influence of stabilizing agents and preparative variables on the formation of poly(D,L-lactic acid) nanoparticles by an emulsification-diffusion technique. Int. J. Pharm. 1996, 143, 133–141. [Google Scholar] [CrossRef]
- Paliwal, R.; Babu, R.J.; Palakurthi, S. Nanomedicine scale-up technologies: Feasibilities and challenges. AAPS PharmSciTech 2014, 15, 1527–1534. [Google Scholar] [CrossRef]
- Colombo, A.P.; Briançon, S.; Lieto, J.; Fessi, H. Project, design, and use of a pilot plant for nanocapsule production. Drug Dev. Ind. Pharm. 2001, 27, 1063–1072. [Google Scholar] [CrossRef]
- Trenkenschuh, E.; Friess, W. Freeze-drying of nanoparticles: How to overcome colloidal instability by formulation and process optimization. Eur. J. Pharm. Biopharm. 2021, 165, 345–360. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Barua, S.; Mitragotri, S. Challenges associated with penetration of nanoparticles across cell and tissue barriers: A review of current status and future prospects. Nano Today 2014, 9, 223–243. [Google Scholar] [CrossRef]
- Luo, W.C.; O’Reilly Beringhs, A.; Kim, R.; Zhang, W.; Patel, S.M.; Bogner, R.H.; Lu, X. Impact of formulation on the quality and stability of freeze-dried nanoparticles. Eur. J. Pharm. Biopharm. 2021, 169, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Hanafy, M.S.; Xu, H.; Leal, J.; Zhai, Y.; Ghosh, D.; Williams, R.O.; David Charles Smyth, H.; Cui, Z. Aerosolizable siRNA-encapsulated solid lipid nanoparticles prepared by thin-film freeze-drying for potential pulmonary delivery. Int. J. Pharm. 2021, 596, 120215. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Hosn, R.R.; Remba, T.; Yun, D.; Li, N.; Abraham, W.; Melo, M.B.; Cortes, M.; Li, B.; Zhang, Y.; et al. Optimization of storage conditions for lipid nanoparticle-formulated self-replicating RNA vaccines. J. Control. Release 2023, 353, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Shirane, D.; Tanaka, H.; Nakai, Y.; Yoshioka, H.; Akita, H. Development of an alcohol dilution–lyophilization method for preparing lipid nanoparticles containing encapsulated siRNA. Biol. Pharm. Bull. 2018, 41, 1291–1294. [Google Scholar] [CrossRef]
- Hinrichs, W.L.J.; Manceñido, F.A.; Sanders, N.N.; Braeckmans, K.; De Smedt, S.C.; Demeester, J.; Frijlink, H.W. The choice of a suitable oligosaccharide to prevent aggregation of PEGylated nanoparticles during freeze thawing and freeze drying. Int. J. Pharm. 2006, 311, 237–244. [Google Scholar] [CrossRef]
- Endres, T.; Zheng, M.; Beck-Broichsitter, M.; Kissel, T. Lyophilised ready-to-use formulations of PEG-PCL-PEI nano-carriers for siRNA delivery. Int. J. Pharm. 2012, 428, 121–124. [Google Scholar] [CrossRef]
- Wu, J. The enhanced permeability and retention (EPR) effect: The significance of the concept and methods to enhance its application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef]
- Bahari, L.A.S.; Hamishehkar, H. The impact of variables on particle size of solid lipid nanoparticles and nanostructured lipid carriers; A comparative literature review. Adv. Pharm. Bull. 2016, 6, 143–151. [Google Scholar] [CrossRef]
- Song, L.; Guo, Y.; Roebuck, D.; Chen, C.; Yang, M.; Yang, Z.; Sreedharan, S.; Glover, C.; Thomas, J.A.; Liu, D.; et al. Terminal PEGylated DNA–gold nanoparticle conjugates offering high resistance to nuclease degradation and efficient intracellular delivery of DNA binding agents. ACS Appl. Mater. Interfaces 2015, 7, 18707–18716. [Google Scholar] [CrossRef]
- Dhiman, S.; Mishra, N.; Sharma, S. Development of PEGylated solid lipid nanoparticles of pentoxifylline for their beneficial pharmacological potential in pathological cardiac hypertrophy. Artif. Cells Nanomed. Biotechnol. 2015, 44, 1901–1908. [Google Scholar] [CrossRef]
- Pignatello, R.; Leonardi, A.; Pellitteri, R.; Carbone, C.; Caggia, S.; Graziano, A.C.E.; Cardile, V. Evaluation of new amphiphilic PEG derivatives for preparing stealth lipid nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2013, 434, 136–144. [Google Scholar] [CrossRef]
- Chuang, C.H.; Wu, P.C.; Tsai, T.H.; Fang, Y.P.; Tsai, Y.H.; Cheng, T.C.; Huang, C.C.; Huang, M.Y.; Chen, F.M.; Hsieh, Y.C.; et al. Development of pH-sensitive cationic PEGylated solid lipid nanoparticles for selective cancer-targeted therapy. J. Biomed. Nanotechnol. 2017, 13, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Fàbregas, A.; Sánchez-Hernández, N.; Ticó, J.R.; García-Montoya, E.; Pérez-Lozano, P.; Suñé-Negre, J.M.; Hernández-Munain, C.; Suñé, C.; Miñarro, M. A new optimized formulation of cationic solid lipid nanoparticles intended for gene delivery: Development, characterization and DNA binding efficiency of TCERG1 expression plasmid. Int. J. Pharm. 2014, 473, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Vighi, E.; Ruozi, B.; Montanari, M.; Battini, R.; Leo, E. pDNA condensation capacity and in vitro gene delivery properties of cationic solid lipid nanoparticles. Int. J. Pharm. 2010, 389, 254–261. [Google Scholar] [CrossRef]
- Wiemann, S.; Keck, C.M. Are lipid nanoparticles really superior? A holistic proof of concept study. Drug Deliv. Transl. Res. 2022, 12, 1433–1444. [Google Scholar] [CrossRef]

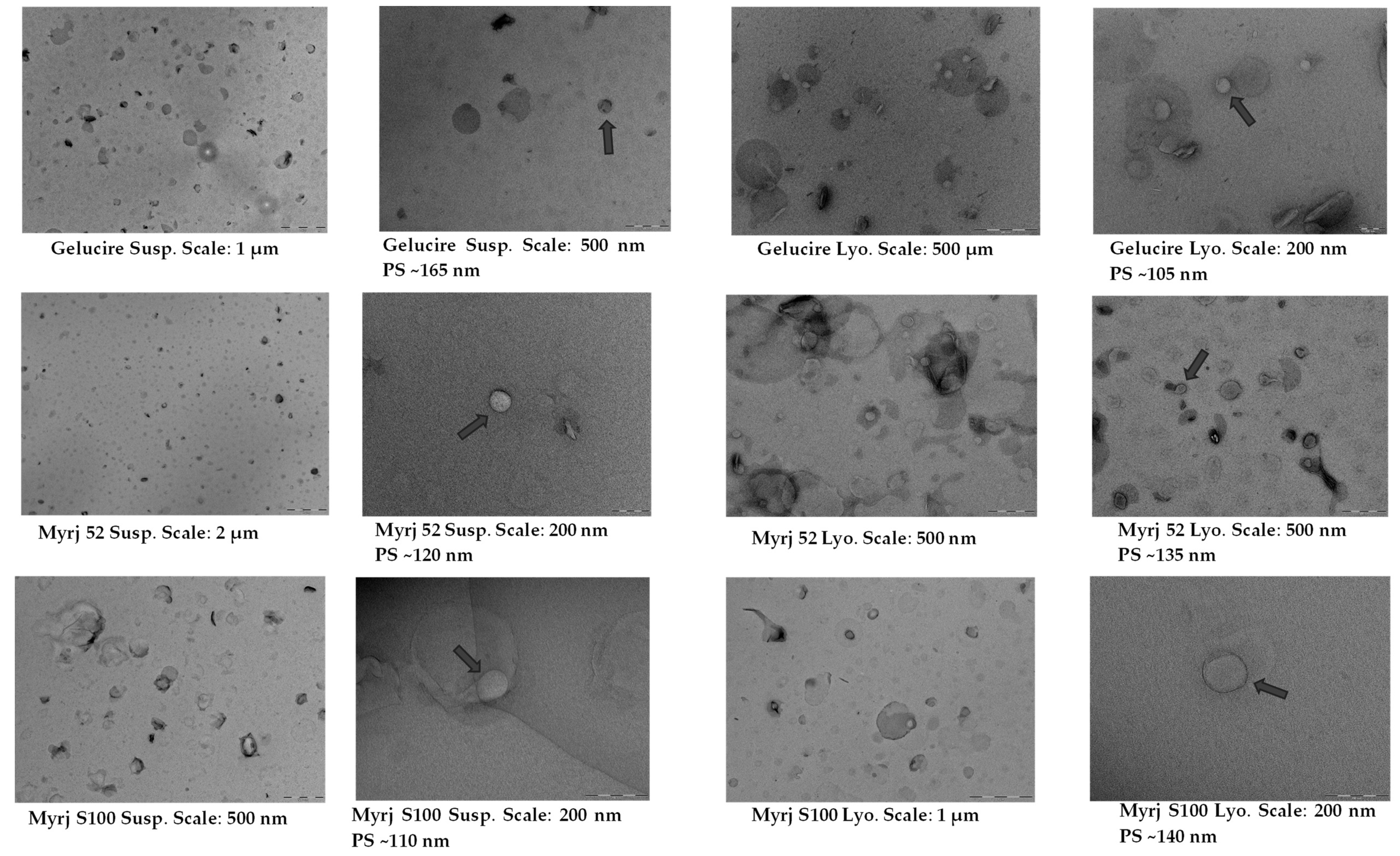




| PEG 1 | Quantity (mg) | PSD (nm) | PdI | ZP (mV) |
|---|---|---|---|---|
| Brij S100 | 50 | 348.8 | 0.252 | 23.4 |
| 150 | 243.3 | 0.118 | 23.7 | |
| 250 | 216.6 | 0.156 | 20.8 | |
| 350 | 166.2 2 | 0.177 | 23.0 | |
| Gelucire | 50 | 344.2 | 0.241 | 37.0 |
| 150 | 222.8 | 0.176 | 34.5 | |
| 250 | 226.7 | 0.159 | 38.9 | |
| 350 | 264.6 | 0.185 | 33.1 | |
| Myrj 52 | 50 | 326.4 | 0.203 | 31.9 |
| 150 | 223.7 | 0.129 | 32.3 | |
| 250 | 270.5 | 0.168 | 30.3 | |
| 350 | 246.8 | 0.153 | 28.9 | |
| Myrj S100 | 50 | 253.5 | 0.058 | 26.5 |
| 150 | 227.6 | 0.114 | 29.8 | |
| 250 | 222.8 | 0.051 | 28.0 | |
| 350 | 296.6 | 0.086 | 29.5 |
| PEG (150 mg) 1 | PSD (nm) 2 | PdI | ZP (mV) | PSD (nm) | PdI | ZP (mV) |
|---|---|---|---|---|---|---|
| Original Protocol | Modified Protocol | |||||
| Brij S100 | 243.3 | 0.118 | 23.7 | 190.90 | 0.10 | 25.10 |
| DSPE | — | — | — | 171.5 | 0.131 | 27.5 |
| Gelucire | 222.8 | 0.176 | 34.5 | 171.20 | 0.16 | 27.40 |
| Myrj 52 | 223.7 | 0.129 | 32.3 | 183.10 | 0.19 | 31.40 |
| Myrj S100 | 227.6 | 0.114 | 29.8 | 180.10 | 0.14 | 27.40 |
| CO | 237.4 | 0.163 | 34.8 | 202.10 | 0.17 | 37.40 |
| PEG (150 mg) 1 | PSD (nm) 2 | PdI | ZP (mV) | PSD (nm) | PdI | ZP (mV) |
|---|---|---|---|---|---|---|
| Suspended SLNs | Lyophilized SLNs | |||||
| Brij S100 | 190.9 | 0.096 | 25.1 | 315.1 | 0.199 | 28.0 |
| DSPE | 171.5 | 0.131 | 27.5 | 234.6 | 0.190 | 36.9 |
| Gelucire | 171.2 | 0.155 | 27.4 | 211.1 | 0.225 | 32.6 |
| Myrj 52 | 183.1 | 0.187 | 31.4 | 280 | 0.256 | 31.6 |
| Myrj S100 | 180.1 | 0.138 | 27.4 | 235.9 | 0.180 | 29.5 |
| CO | 202.1 | 0.169 | 37.4 | 235.3 | 0.194 | 44.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narváez-Narváez, D.A.; Duarte-Ruiz, M.; Jiménez-Lozano, S.; Moreno-Castro, C.; Vargas, R.; Nardi-Ricart, A.; García-Montoya, E.; Pérez-Lozano, P.; Suñé-Negre, J.M.; Hernández-Munain, C.; et al. Comparative Analysis of the Physicochemical and Biological Characteristics of Freeze-Dried PEGylated Cationic Solid Lipid Nanoparticles. Pharmaceuticals 2023, 16, 1583. https://doi.org/10.3390/ph16111583
Narváez-Narváez DA, Duarte-Ruiz M, Jiménez-Lozano S, Moreno-Castro C, Vargas R, Nardi-Ricart A, García-Montoya E, Pérez-Lozano P, Suñé-Negre JM, Hernández-Munain C, et al. Comparative Analysis of the Physicochemical and Biological Characteristics of Freeze-Dried PEGylated Cationic Solid Lipid Nanoparticles. Pharmaceuticals. 2023; 16(11):1583. https://doi.org/10.3390/ph16111583
Chicago/Turabian StyleNarváez-Narváez, David A., María Duarte-Ruiz, Sandra Jiménez-Lozano, Cristina Moreno-Castro, Ronny Vargas, Anna Nardi-Ricart, Encarna García-Montoya, Pilar Pérez-Lozano, Josep Mª Suñé-Negre, Cristina Hernández-Munain, and et al. 2023. "Comparative Analysis of the Physicochemical and Biological Characteristics of Freeze-Dried PEGylated Cationic Solid Lipid Nanoparticles" Pharmaceuticals 16, no. 11: 1583. https://doi.org/10.3390/ph16111583
APA StyleNarváez-Narváez, D. A., Duarte-Ruiz, M., Jiménez-Lozano, S., Moreno-Castro, C., Vargas, R., Nardi-Ricart, A., García-Montoya, E., Pérez-Lozano, P., Suñé-Negre, J. M., Hernández-Munain, C., Suñé, C., & Suñé-Pou, M. (2023). Comparative Analysis of the Physicochemical and Biological Characteristics of Freeze-Dried PEGylated Cationic Solid Lipid Nanoparticles. Pharmaceuticals, 16(11), 1583. https://doi.org/10.3390/ph16111583