MD Simulations to Calculate NMR Relaxation Parameters of Vanadium(IV) Complexes: A Promising Diagnostic Tool for Cancer and Alzheimer’s Disease
Abstract
:1. Introduction
2. Results and Discussion
2.1. Calculating Ti and Ri from MD Simulation Data
2.2. Validation of the Ti and Ri Calculations from MD Simulation Data
2.3. MD Simulation of VC1 and VC2 in Water Only and in the Presence of the Respective Protein Targets
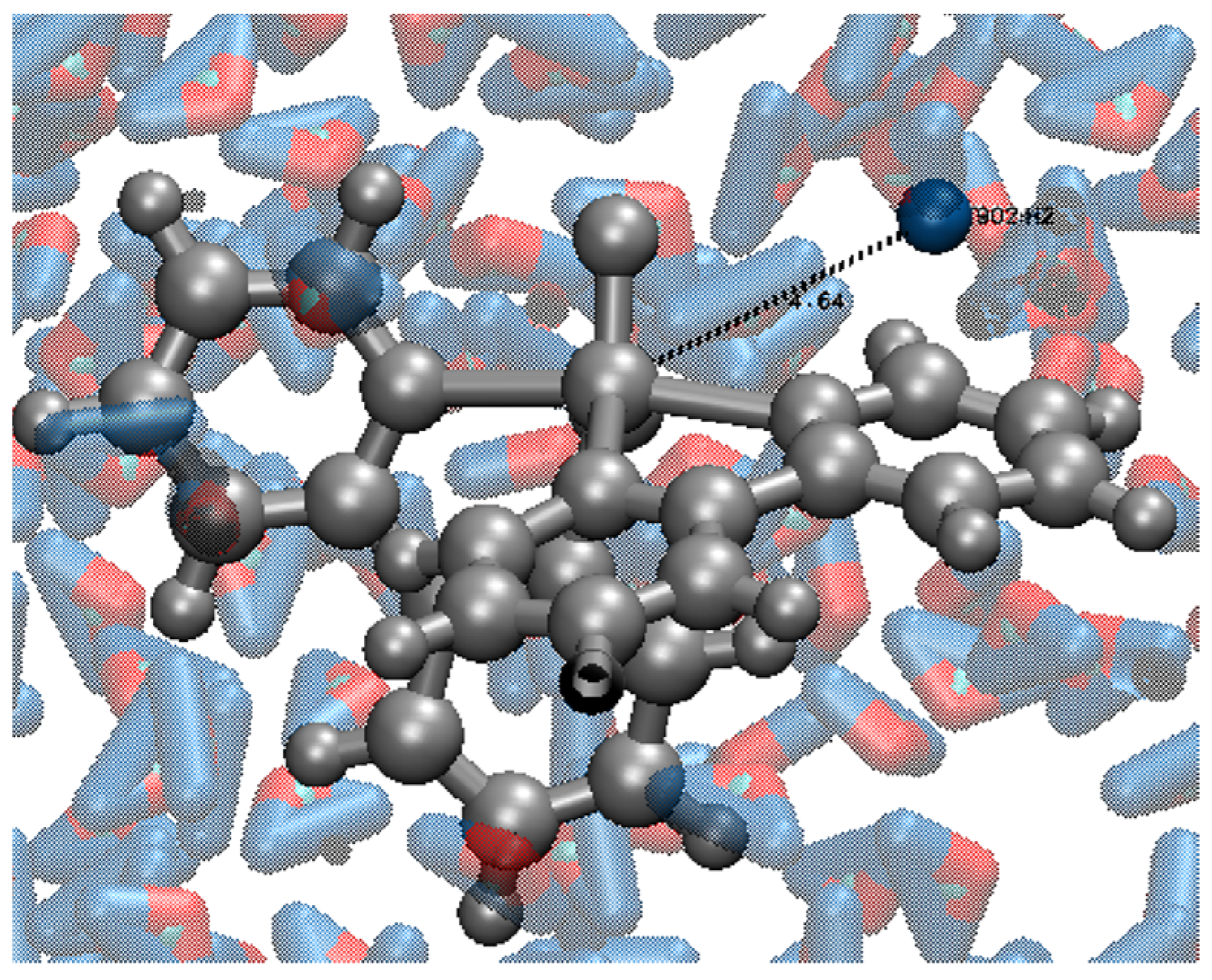
2.4. OWSCA Conformational Selecting and Calculated Ti and Ri Values
3. Materials and Methods
3.1. Systems Descriptions and Docking Studies
3.2. Molecular Dynamics Simulations
3.3. Conformational Selections of V(IV) Complexes Using the Optimal Wavelet Signal Compression Algorithm and MD Data for T1 and R1 Estimation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Cancer Institute. Cancer Statistics Page. Available online: https://seer.cancer.gov/statfacts/html (accessed on 21 November 2023).
- Association, A. 2023 Alzheimer’s disease facts and figures. Alzheimer’s Dement 2023, 19, 1598–1695. [Google Scholar] [CrossRef]
- Zabłocka, A.; Kazana, W.; Sochocka, M.; Stańczykiewicz, B.; Janusz, M.; Leszek, J.; Orzechowska, B. Inverse Correlation between Alzheimer’s Disease and Cancer: Short Overview. Mol. Neurobiol. 2021, 58, 6335–6349. [Google Scholar] [CrossRef] [PubMed]
- Fowler, M.E.; Wright, N.C.; Triebel, K.; Rocque, G.B.; Irvin, M.R.; Kennedy, R.E. The Relationship between Prior Cancer Diagnosis and All-Cause Dementia Progression among US Adults. J. Alzheimer’s Dis. 2022, 88, 521–535. [Google Scholar] [CrossRef] [PubMed]
- Ospina-Romero, M.; Glymour, M.M.; Hayes-Larson, E.; Mayeda, E.R.; Graff, R.E.; Brenowitz, W.D.; Ackley, S.F.; Witte, J.S.; Kobayashi, L.C. Association Between Alzheimer Disease and Cancer With Evaluation of Study Biases: A Systematic Review and Meta-analysis. JAMA Netw. Open 2020, 3, e2025515. [Google Scholar] [CrossRef] [PubMed]
- Porsteinsson, A.P.; Isaacson, R.S.; Knox, S.; Sabbagh, M.N.; Rubino, I. Diagnosis of Early Alzheimer’s Disease: Clinical Practice in 2021. J. Prev. Alzheimers Dis. 2021, 3, 371–386. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Gole, J.; Gore, A.; He, Q.; Lu, M.; Min, J.; Yuan, Z.; Yang, X.; Jiang, Y.; Zhang, T.; et al. Non-invasive early detection of cancer four years before conventional diagnosis using a blood test. Nat. Commun. 2020, 11, 3475. [Google Scholar] [CrossRef]
- Winblad, B.; Amouyel, P.; Andrieu, S.; Ballard, C.; Brayne, C.; Brodaty, H.; Cedazo-Minguez, A.; Dubois, B.; Edvardsson, D.; Feldman, H.; et al. Defeating Alzheimer’s disease and other dementias: A priority for European science and society. Lancet Neurol. 2016, 5, 455–532. [Google Scholar] [CrossRef]
- Rajasekhar, K.; Govindaraju, T. Current progress, challenges and future prospects of diagnostic and therapeutic interventions in Alzheimer’s disease. RSC Adv. 2018, 8, 23780–23804. [Google Scholar] [CrossRef]
- Fitzgerald, R.C.; Antoniou, A.C.; Fruk, L.; Rosenfeld, N. The future of early cancer detection. Nat. Med. 2022, 28, 666–677. [Google Scholar] [CrossRef]
- Zhang, L.; Sanagapalli, S.; Stoita, A. Challenges in diagnosis of pancreatic cancer. World J. Gastroenterol. 2018, 19, 2047–2060. [Google Scholar] [CrossRef]
- Unger-Saldaña, K. Challenges to the early diagnosis and treatment of breast cancer in developing 335 countries. World J. Clin. Oncol. 2014, 3, 465–477. [Google Scholar] [CrossRef]
- Barisano, G.; Sepehrband, F.; Ma, S.; Jann, K.; Cabeen, R.; Wang, D.J.; Toga, A.W.; Law, M. Clinical 7 T MRI: Are we there yet? A review about magnetic resonance imaging at ultra-high field. Br. J. Radiol. 2019, 1094, 492. [Google Scholar] [CrossRef] [PubMed]
- Boldrini, L.; Cusumano, D.; Cellini, F.; Azario, L.; Mattiucci, G.C.; Valentini, V. Online adaptive magnetic resonance guided radiotherapy for pancreatic cancer: State of the art, pearls and pitfalls. Radiat. Oncol. 2019, 14, 71. [Google Scholar] [CrossRef]
- Deininger-Czermak, E.; Villefort, C.; Knebel Doeberitz, N.; Franckenberg, S.; Kälin, P.; Kenkel, D.; Gascho, D.; Piccirelli, M.; Finkenstaedt, T.; Thali, M.J.; et al. Comparison of MR Ultrashort Echo Time and Optimized 3D-Multiecho In-Phase Sequence to Computed Tomography for Assessment of the Osseous Craniocervical Junction. J. Magn. Reson. Imaging 2020, 4, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Geraldes, C.F.G.C. Introduction to Infrared and Raman-Based Biomedical Molecular Imaging and Comparison with Other Modalities. Molecules 2020, 23, 5547. [Google Scholar] [CrossRef]
- Pinto, S.M.; Tomé, V.; Calvete, M.J.F.; Castro, M.M.C.; Tóth, E.; Geraldes, C.F. Metal-based redox-responsive MRI contrast agents. Coord. Chem. Rev. 2019, 1, 1–31. [Google Scholar] [CrossRef]
- Botta, M.; Carniato, F.; Esteban-Gómez, D.; Platas-Iglesias, C.; Tei, L. Mn(II) compounds as an alternative to Gd-based MRI probes. Future Med. Chem. 2019, 12, 608. [Google Scholar] [CrossRef]
- Orts-Arroyo, M.; Ten-Esteve, A.; Ginés-Cárdenas, S.; Castro, I.; Martí-Bonmatí, L.; Martínez- 351 Lillo, J. A Gadolinium(III) Complex Based on the Thymine Nucleobase with Properties Suitable for Magnetic Resonance Imaging. Int. J. Mol. Sci. 2021, 22, 4586. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, J.; Ramalho, M.; Jay, M.; Burke, L.M.; Semelka, R.C. Gadolinium toxicity and treatment. Magn. Reson. Imaging 2016, 34, 1394–1398. [Google Scholar] [CrossRef]
- Gupta, A.; Caravan, P.; Price, W.S.; Platas-Iglesias, C.; Gale, E.M. Applications for Transition-Metal Chemistry in Contrast-Enhanced Magnetic Resonance Imaging. Inorg. Chem. 2020, 10, 6648–6678. [Google Scholar] [CrossRef]
- Mustafi, D.; Peng, B.; Foxley, S.; Makinen, M.W.; Karczmar, G.S.; Zamora, M.; Ejnik, J.; Martin, H. New vanadium-based magnetic resonance imaging probes: Clinical potential for early detection of cancer. JBIC J. Biol. Inorg. Chem. 2009, 14, 1187–1197. [Google Scholar] [CrossRef]
- Ahmed, T.T.; Alajrawy, O.I. Oxovanadinum (IV) complexes with bidentate ligands synthesis, characterization, and comparison between experimental and theoretical. Mater. Today Proc. 2023, 80, 3823–3836. [Google Scholar] [CrossRef]
- Swamy, S.J.; Reddy, A.D.; Bhaskar, K. Synthesis and spectral studies of some oxovanadium(IV) and vanadium(IV) complexes. IJC-A 2001, 40, 1166–1171. [Google Scholar]
- Rehder, D. Vanadium. Its Role for Humans. In Interrelations between Essential Metal Ions and Human Diseases; Sigel, A., Sigel, H., Sigel, R.K.O., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 139–169. [Google Scholar] [CrossRef]
- Orvig, C.; Thompson, K.H.; Battell, M.; McNeill, J.H. Vanadium Compounds as Insulin Mimics. Met. Ions Biol. Syst. 1995, 31, 575–594. [Google Scholar]
- Sharfalddin, A.A.; Al-Younis, I.M.; Mohammed, H.A.; Dhahri, M.; Mouffouk, F.; Abu Ali, H.; Anwar, J.; Qureshi, K.A.; Hussien, M.A.; Alghrably, M.; et al. Therapeutic Properties of Vanadium Complexes. Inorganics 2022, 10, 244. [Google Scholar] [CrossRef]
- Ferretti, V.A.; León, I.E. An Overview of Vanadium and Cell Signaling in Potential Cancer Treatments. Inorganics 2022, 10, 47. [Google Scholar] [CrossRef]
- Turtoi, M.; Anghelache, M.; Patrascu, A.A.; Deleanu, M.; Voicu, G.; Raduca, M.; Safciuc, F.; Manduteanu, I.; Calin, M.; Popescu, D.-L. Antitumor Properties of a New Macrocyclic Tetranuclear Oxidovanadium(V) Complex with 3-Methoxysalicylidenvaline Ligand. Biomedicines 2022, 10, 1217. [Google Scholar] [CrossRef]
- Turtoi, M.; Anghelache, M.; Patrascu, A.A.; Maxim, C.; Manduteanu, I.; Calin, M.; Popescu, D.-L. Synthesis, Characterization, and In Vitro Insulin-Mimetic Activity Evaluation of Valine Schiff Base Coordination Compounds of Oxidovanadium(V). Biomedicines 2021, 9, 562. [Google Scholar] [CrossRef]
- Dong, Y.; Stewart, T.; Zhang, Y.; Shi, M.; Tan, C.; Li, X.; Yuan, L.; Mehrotra, A.; Zhang, J.; Yang, X. Anti-diabetic vanadyl complexes reduced Alzheimer’s disease pathology independent of amyloid plaque deposition. Sci. China Life Sci. 2019, 62, 126–139. [Google Scholar] [CrossRef]
- Del Carpio, E.; Hernández, L.; Ciangherotti, C.; Coa, V.V.; Jiménez, L.; Lubes, V.; Lubes, G. Vanadium: History, chemistry, interactions with amino acids and potential therapeutic applications. Coord. Chem. Rev. 2018, 372, 117–140. [Google Scholar] [CrossRef]
- Rehder, D. The potentiality of vanadium in medicinal applications. Future Med. Chem. 2012, 4, 1823–1837. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, J.C.; Etcheverry, S.; Gambino, D. Vanadium compounds in medicine. Coord. Chem. Rev. 2015, 15, 24–48. [Google Scholar] [CrossRef] [PubMed]
- Semiz, S. Vanadium as potential therapeutic agent for COVID-19: A focus on its antiviral, antiinflamatory, and antihyperglycemic effects. J. Trace Elem. Med. Biol. 2022, 69, 126887. [Google Scholar] [CrossRef] [PubMed]
- Vlasiou, M.C.; Pafti, K.S. Screening possible drug molecules for COVID-19. The example of vanadium (III/IV/V) complex molecules with computational chemistry and molecular docking. Comput. Toxicol. 2021, 18, 100157. [Google Scholar] [CrossRef]
- Scior, T.; Abdallah, H.H.; Mustafa, S.F.Z.; Guevara-García, J.A.; Rehder, D. Are vanadium complexes druggable against the main protease Mpro of SARS-CoV-2?—A computational approach. Inorganica Chim. Acta 2021, 519, 120287. [Google Scholar] [CrossRef]
- Tavares, C.A.; Santos, T.M.R.; Cunha, E.F.F.; Ramalho, T.C. Molecular Dynamics-Assisted Interaction of Vanadium Complex AMPK: From Force Field Development to Biological Application 361 for Alzheimer’s Treatment. J. Phys. Chem. B 2023, 127, 495–504. [Google Scholar] [CrossRef]
- Santos, T.M.R.; Tavares, C.A.; Cunha, E.F.F.; Ramalho, T.C. Vanadium complex as a potential modulator of the autophagic mechanism through proteins PI3K and ULK1: Development, validation and biological implications of a specific force field for [VO(bpy)2Cl]. J. Bio. Struct. Dyn 2023, 1–15. [Google Scholar] [CrossRef]
- Ramalho, T.C.; Taft, C.A. Thermal and solvent effects on the NMR and UV parameters of some bioreductive drugs. J. Chem. Phys. 2005, 123, 054319. [Google Scholar] [CrossRef]
- Gonçalves, A.S.; França, T.C.C.; Caetano, M.S.; Ramalho, T.C. Reactivation steps by 2-PAM of tabun-inhibited human acetylcholinesterase: Reducing the computational cost in hybrid QM/MM methods. J. Biomol. Struct. Dyn. 2014, 32, 301–307. [Google Scholar] [CrossRef]
- Martins, T.L.C.; Ramalho, T.C.; Figueroa-Villar, J.D.; Flores, A.F.; Pereira, C.M.P. Theoretical and experimental 13C and 15N NMR investigation of guanylhydrazones in solution. Magn. Reson. Chem. 2003, 41, 983–988. [Google Scholar] [CrossRef]
- Chen, P.; Hologne, M.; Walker, O.; Hening, J. Ab Initio Prediction of NMR Spin Relaxation Parameters from Molecular Dynamics Simulations. J. Chem. Theory Comput. 2018, 14, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Villa, A.; Stock, G. What NMR Relaxation Can Tell Us about the Internal Motion of an RNA Hairpin: A Molecular Dynamics Simulation Study. J. Chem. Theory Comput. 2006, 2, 1228–1236. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, M.A.; Santos, L.S.; Prata, D.M.; Peixoto, F.C.; Ramalho, T.C. NMR relaxation and relaxivity parameters of MRI probes revealed by optimal wavelet signal compression of molecular dynamics simulations. Int. J. Quantum Chem. 2019, 119, e25896. [Google Scholar] [CrossRef]
- Rohrer, M.; Bauer, H.; Mintorovitch, J.; Requardt, M.; Weinmann, H. Comparison of Magnetic Properties of MRI Contrast Media Solutions at Different Magnetic Field Strengths. Investig. Radiol. 2005, 40, 715–724. [Google Scholar] [CrossRef]
- Lino, J.B.; Gonçalves, M.A.; Santos, L.S.; Ramalho, T.C. Value of NMR relaxation parameters of diamagnetic molecules for quantum information processing: Optimizing the coherent phase. Theor. Chem. Acc. 2021, 140, 8. [Google Scholar] [CrossRef]
- Pierre, V.C.; Allen, M.J. Iron-oxide Nanoparticle-based Contrast Agents. In Contrast Agents for MRI: Experimental Methods; Ashbrook, S., Balcom, B., Furó, I., Kainosho, M., Liu, M., Eds.; RSC: London, UK, 2018; p. 318. [Google Scholar]
- Xu, F.; Cheng, C.; Chen, D.; Gu, H. Magnetite Nanocrystal Clusters with Ultra-High Sensitivity in Magnetic Resonance Imaging. ChemPhysChem 2012, 13, 336–341. [Google Scholar] [CrossRef]
- Paquet, C.; de Haan, H.W.; Leek, D.M.; Lin, H.-Y.; Xiang, B.; Tian, G.; Kell, A.; Simard, B. Clusters of superparamagnetic iron oxide nanoparticles encapsulated in a hydrogel: A particle architecture generating a synergistic enhancement of the T2 relaxation. ACS Nano 2011, 5, 3104–3112. [Google Scholar] [CrossRef]
- Devra, A.; Prabhu, P.; Singh, H.; Dorai, A.; Dorai, K. Efficient experimental design of high-fidelity three-qubit quantum gates via genetic programming. Quantum Inf. Process. 2018, 17, 67. [Google Scholar] [CrossRef]
- Kastrup, A.; Glover, G.H. Neuroimaging at 1.5 T and 3.0 T: Comparison of oxygenation-sensitive magnetic resonance imaging. Magn. Reson. Med. 2001, 4, 595–604. [Google Scholar] [CrossRef]
- Uggeri, F.; Aime, S.; Anelli, P.L.; Botta, M.; Brocchetta, M.; de Haeen, C.; Ermondi, G.; Grandi, M.; Paoli, P. Novel Contrast Agents for Magnetic Resonance Imaging. Synthesis and Characterization of the Ligand BOPTA and Its Ln(III) Complexes (Ln = Gd, La, Lu). X-ray Structure of Disodium (TPS-9-145337286-C-S)-[4-Carboxy-5,8,11-tris(carboxymethyl)-1-phenyl-2-oxa-5,8,11-triazatridecan-13-oato(5-)]gadolinate(2-) in a Mixture with Its Enantiomer. Inorg. Chem. 1995, 34, 633–643. [Google Scholar] [CrossRef]
- Klemm, P.J.; Floyd, W.C.; Smiles, D.E.; Fréchet, J.M.J.; Raymond, K.N. Improving T1 and T2 magnetic resonance imaging contrast agents through the conjugation of an esteramide dendrimer to high-water-coordination Gd(III) hydroxypyridinone complexes. Contrast Media Mol. Imaging 2012, 7, 95–99. [Google Scholar] [CrossRef]
- Lagostina, V.; Carniato, F.; Esteban-Gómez, D.; Platas-Iglesias, C.; Chiesa, M.; Botta, M. Magnetic and relaxation properties of vanadium(iv) complexes: An integrated 1H relaxometric, EPR and computational study. Inorg. Chem. Front. 2023, 10, 1999–2013. [Google Scholar] [CrossRef]
- Tang, X.; Cai, F.; Ding, D.; Zhang, L.; Cai, X.; Fang, Q. Magnetic resonance imaging relaxation time in Alzheimer’s disease. Brain Res. Bull. 2018, 140, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Deoni, S.C.L. Quantitative relaxometry of the brain. Top. Magn. Reason. Imaging 2010, 294, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zhou, X.E.; Novick, S.J.; Shaw, S.J.; Li, Y.; Brunzelle, J.S.; Hitoshi, Y.; Griffin, P.R.; Xu, H.E.; Melcher, K. Structures of AMP-activated protein kinase bound to novel pharmacological activators in phosphorylated, non-phosphorylated, and nucleotide-free states. J. Biol. Chem. 2019, 3, 953–967. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, M.B.; Novotny, C.J.; Shokat, K.M. Structure of the Human Autophagy Initiating Kinase ULK1 in Complex with Potent Inhibitors. ACS Chem. Biol. 2015, 10, 257–261. [Google Scholar] [CrossRef] [PubMed]
- BIOVIA. Discovery Studio Visualizer; Dassault Systemes: San Diego, CA, USA, 2021. [Google Scholar]
- Thomsen, R.; Christensen, M.H. MolDock: A new technique for high-accuracy molecular docking. J. Med. Chem. 2006, 49, 3315–3321. [Google Scholar] [CrossRef]
- Thabah, D.; Syiem, D.; Pakyntein, C.; Banerjee, S.; Kharshiing, C.E.; Bhattacharjee, A. Potentilla fulgens upregulate GLUT4, AMPK, AKT and insulin in alloxan-induced diabetic mice: An in 385 vivo and in silico study. Arch. Physiol. Biochem. 2021, 129, 1071–1083. [Google Scholar] [CrossRef]
- Zhang, H.-R.; Gao, C.-L.; Zhang, L.-C.; Yu, R.-L.; Kang, C.-M. Homology modeling, virtual screening and MD simulations for the identification of NUAK1 and ULK1 potential dual inhibitors. New J. Chem. 2022, 46, 4103–4113. [Google Scholar] [CrossRef]
- Gonçalves, M.A.; Santos, L.S.; Prata, D.M.; Peixoto, F.C.; Da Cunha, E.F.F.; Ramalho, T.C. Optimal wavelet signal compression as an efficient alternative to investigate molecular dynamics simulations: Application to thermal and solvent effects of MRI probes. Theor. Chem. Acc. 2017, 2, 136. [Google Scholar] [CrossRef]
- Misiti, M.; Misiti, Y.; Oppenheim, G.; Poggi, J.M. Wavelets and Their Applications; ISTE DSP Series; ISTE Ltd.: London, UK, 2007; ISBN 9781905209316. [Google Scholar]
- Gonçalves, M.A.; Nunes, C.A.; Sáfadi, T.; Ramalho, T.C. Optimal Wavepress is a User-Friendly Toolkit for Computational Chemistry, Drug Design and Material Science. J. Braz. Chem. Soc. 2023, 34, 1457–1463. [Google Scholar] [CrossRef]
- Gonçalves, M.A.; Gonçalves, A.S.; Franca, T.C.C.; Santana, M.S.; da Cunha, E.F.F.; Ramalho, T.C. Improved Protocol for the Selection of Structures from Molecular Dynamics of Organic Systems in Solution: The Value of Investigating Different Wavelet Families. J. Chem. Theory Comput. 2022, 18, 5810–5818. [Google Scholar] [CrossRef] [PubMed]
- Giacoppo, J.O.; França, T.C.; Kuča, K.; da Cunha, E.F.; Abagyan, R.; Mancini, D.T.; Ramalho, T.C. Molecular modeling and in vitro reactivation study between the oxime BI-6 and acetylcholinesterase inhibited by different nerve agents. J. Biomol. Struct. Dyn. 2015, 33, 2048–2058. [Google Scholar] [CrossRef]






| System | Theoretical | Experimental | ||||||
|---|---|---|---|---|---|---|---|---|
| T1 | R1 | T2 | R2 | T1 | R1 | T2 | R2 | |
| Magnetite | 0.028 | 35.72 | 0.018 | 55.55 | 0.032 | 31.25 [48] | 0.020 | 50.50 [49,50] |
| TCE(C-C) | 8.98 | 0.11 | 1.17 | 0.85 | 8.90 [51] | 0.11 | 1.18 [51] | 0.85 |
| TFE(3F-4F) | 5.35 | 0.18 | 0.12 | 8.33 | 5.37 [51] | 0.19 | 0.14 [51] | 7.14 |
| TFE(5F-3F) | 5.52 | 0.10 | 0.10 | 10.00 | 5.56 [51] | 0.18 | 0.12 [51] | 8.33 |
| System | T1 | T2 | R1 | R2 | R1/R2 |
|---|---|---|---|---|---|
| VC1 + WAT | 0.084 | 0.056 | 11.905 | 17.921 | 0.664 |
| VC1 + AMPK + WAT | 0.074 | 0.050 | 13.514 | 20.121 | 0.672 |
| VC2 + WAT | 0.086 | 0.058 | 11.630 | 17.331 | 0.671 |
| VC2 + ULK1 + WAT | 1.046 | 0.702 | 0.956 | 1.424 | 0.671 |
| Gd-DOTA (DOTAREM) | 0.032 [52] | 0.025 [52,53] | 31.250 | 40.000 | 0.781 |
| Gd-DTPA (MAGNEVIST) | - | 0.020 [46,50,54] | - | 50.000 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, R.M.; Tavares, C.A.; Santos, T.M.R.; Rasouli, H.; Ramalho, T.C. MD Simulations to Calculate NMR Relaxation Parameters of Vanadium(IV) Complexes: A Promising Diagnostic Tool for Cancer and Alzheimer’s Disease. Pharmaceuticals 2023, 16, 1653. https://doi.org/10.3390/ph16121653
Santos RM, Tavares CA, Santos TMR, Rasouli H, Ramalho TC. MD Simulations to Calculate NMR Relaxation Parameters of Vanadium(IV) Complexes: A Promising Diagnostic Tool for Cancer and Alzheimer’s Disease. Pharmaceuticals. 2023; 16(12):1653. https://doi.org/10.3390/ph16121653
Chicago/Turabian StyleSantos, Rodrigo Mancini, Camila Assis Tavares, Taináh Martins Resende Santos, Hassan Rasouli, and Teodorico Castro Ramalho. 2023. "MD Simulations to Calculate NMR Relaxation Parameters of Vanadium(IV) Complexes: A Promising Diagnostic Tool for Cancer and Alzheimer’s Disease" Pharmaceuticals 16, no. 12: 1653. https://doi.org/10.3390/ph16121653







