Inducing the Abscopal Effect in Liver Cancer Treatment: The Impact of Microwave Ablation Power Levels and PD-1 Antibody Therapy
Abstract
:1. Introduction
2. Results
2.1. Enhanced Efficacy of High-Power MWA with Anti-PD-1 Treatment in Suppressing Distant Tumor Growth
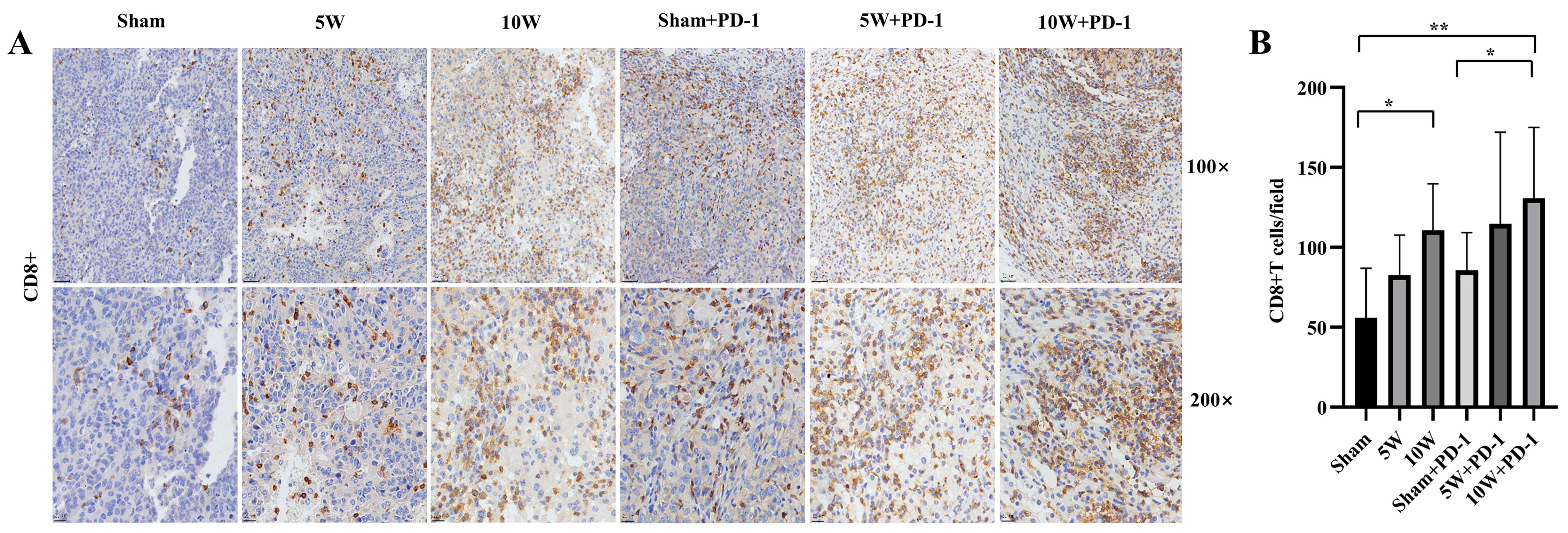
2.2. Enhanced CD8+ T-Cell Infiltration in Distant Tumors with High-Power MWA and Anti-PD-1 Therapy
2.3. Combination Therapy Significantly Diminishes Treg Infiltration in Distant Tumors
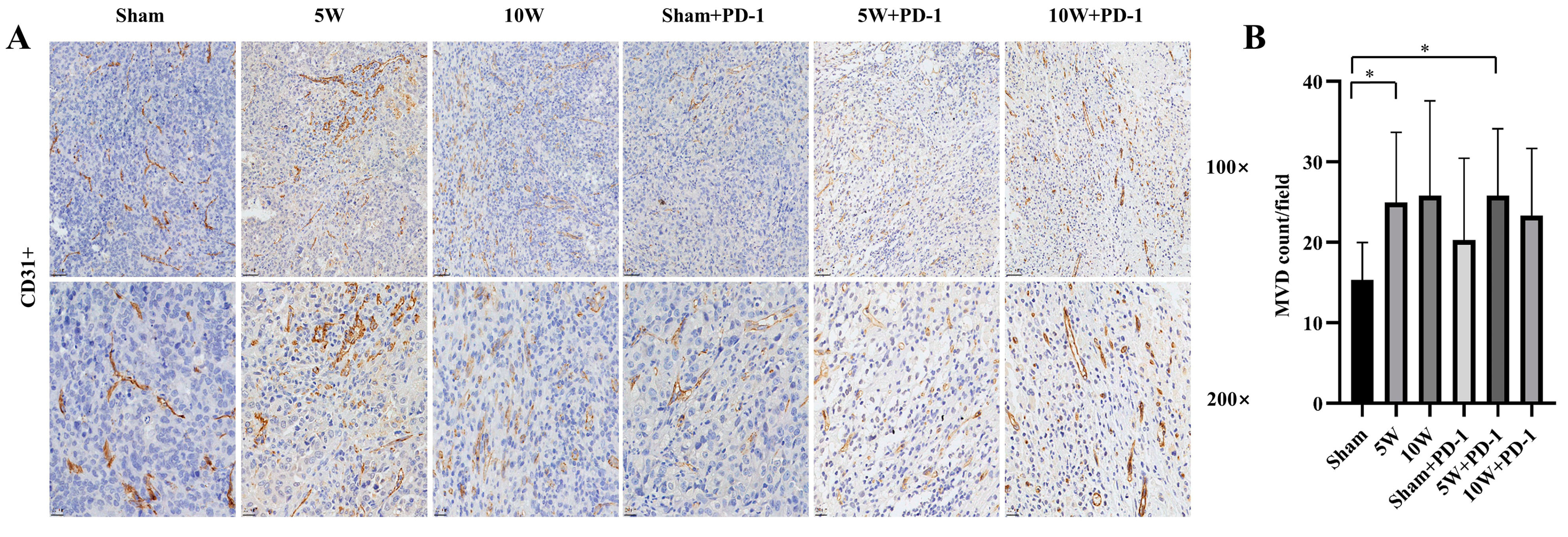
2.4. Low-Power MWA Increases Tumor Microvessel Density in Distant Tumors
2.5. Enhanced TNF-α Levels in Peripheral Blood Achieved with Combined High-Power MWA and Anti-PD-1 Therapy
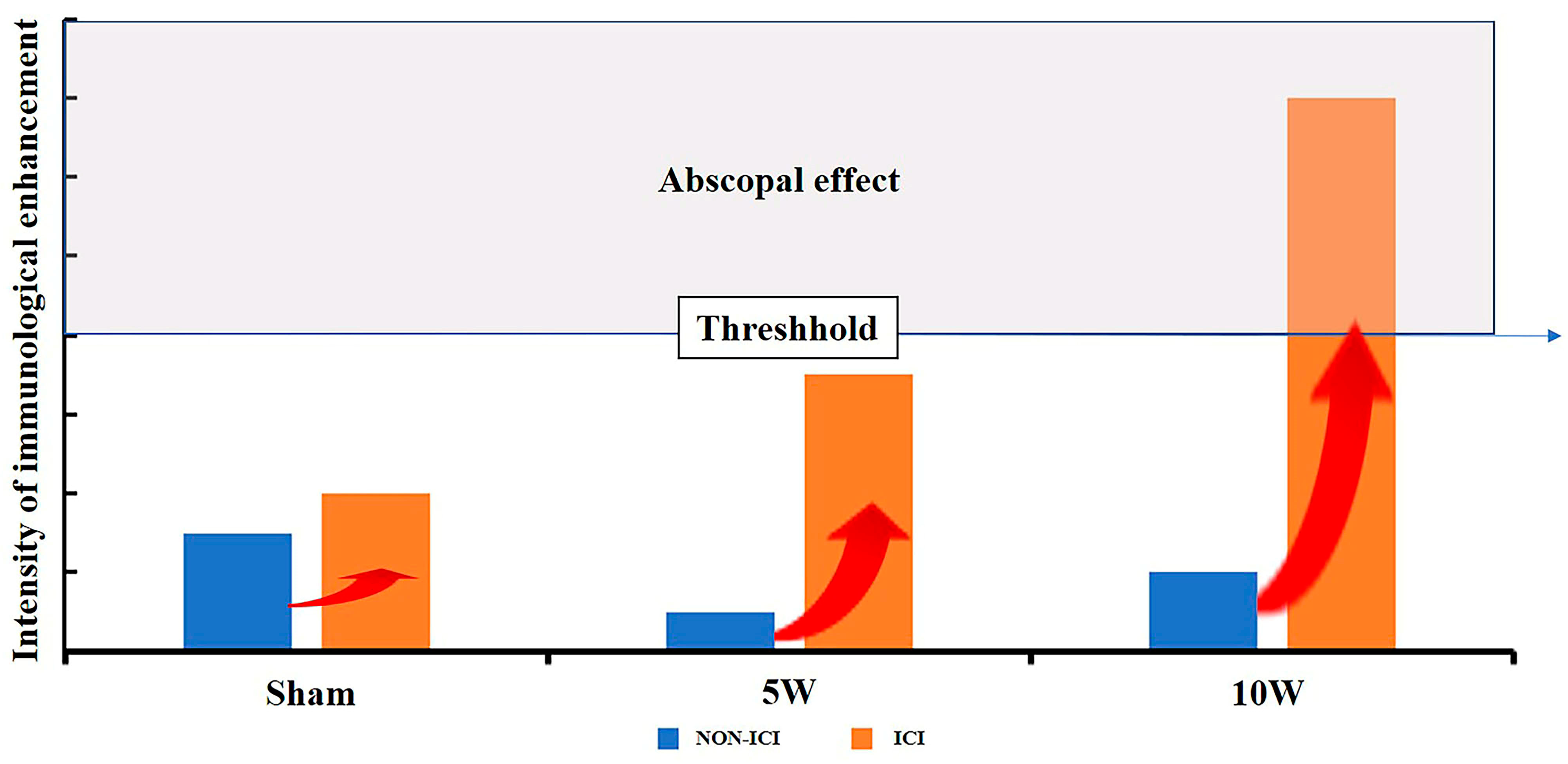
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Cell Line and Culture
4.3. Animal Model and Treatments
4.4. Tumor Evaluation
4.5. Histopathologic Examination
4.6. Enzyme-Linked Immunosorbent Assay
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: Globocan Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sun, J.; Wang, K.; Zhao, H.; Zhang, X.; Ren, Z. First- and Second-Line Treatments for Patients with Advanced Hepa-tocellular Carcinoma in China: A Systematic Review. Curr. Oncol. 2022, 29, 7305–7326. [Google Scholar] [CrossRef] [PubMed]
- Violi, N.V.; Duran, R.; Guiu, B.; Cercueil, J.-P.; Aubé, C.; Digklia, A.; Pache, I.; Deltenre, P.; Knebel, J.-F.; Denys, A. Efficacy of microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma in patients with chronic liver disease: A randomised controlled phase 2 trial. Lancet Gastroenterol. Hepatol. 2018, 3, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.F.; Dupuy, D.E. Thermal ablation of tumours: Biological mechanisms and advances in therapy. Nat. Rev. Cancer 2014, 14, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Biederman, D.M.; Titano, J.J.; Bishay, V.L.; Durrani, R.J.; Dayan, E.; Tabori, N.; Patel, R.S.; Nowakowski, F.S.; Fischman, A.M.; Kim, E. Radiation Segmentectomy versus TACE Combined with Microwave Ablation for Unresectable Solitary Hepatocellular Carcinoma Up to 3 cm: A Propensity Score Matching Study. Radiology 2017, 283, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.-Q.; Peng, L.-H.; Ma, L.-J.; Liu, D.-B.; Zhang, S.; Luo, S.-Z.; Rao, J.-H.; Zhu, H.-W.; Yang, S.-X.; Xi, S.-J.; et al. Heterogeneous immunogenomic features and distinct escape mechanisms in multifocal hepatocellular carcinoma. J. Hepatol. 2020, 72, 896–908. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso, A.; Del Prete, V.; Antonino, M.; Crucinio, N.; Neve, V.; Di Leo, A.; Carr, B.I.; Barone, M. Post-Recurrence Survival in Hepatocellular Carcinoma after Percutaneous Radiofrequency Ablation. Dig. Liver Dis. 2014, 46, 1014–1019. [Google Scholar] [CrossRef]
- Rodríguez-Ruiz, M.E.; Vanpouille-Box, C.; Melero, I.; Formenti, S.C.; Demaria, S. Immunological Mechanisms Responsible for Radiation-Induced Abscopal Effect. Trends Immunol. 2018, 39, 644–655. [Google Scholar] [CrossRef]
- Ngwa; Irabor, O.C.; Ngwa, W.; Irabor, O.C.; Schoenfeld, J.D.; Hesser, J.; Demaria, S.; Formenti, S.C. Using Immunotherapy to Boost the Abscopal Effect. Nat. Rev. Cancer 2018, 18, 313–322. [Google Scholar] [CrossRef]
- Slovak, R.; Ludwig, J.M.; Gettinger, S.N.; Herbst, R.S.; Kim, H.S. Immuno-Thermal Ablations—Boosting the Anticancer Immune Response. J. Immunother. Cancer 2017, 5, 78. [Google Scholar] [CrossRef]
- Ablin, R.; Soanes, W.; Gonder, M. Prospects for cryo-immunotherapy in cases of metastasizing carcinoma of the prostate. Cryobiology 1971, 8, 271–279. [Google Scholar] [CrossRef]
- Tan, L.; Chen, S.; Wei, G.; Li, Y.; Liao, J.; Jin, H.; Zou, Y.; Huang, M.; Peng, Z.; Guo, Y.; et al. Sublethal Heat Treatment of Hepatocellular Carcinoma Promotes Intrahepatic Metastasis and Stemness in a Vegfr1-Dependent Manner. Cancer Lett. 2019, 460, 29–40. [Google Scholar] [CrossRef]
- Postow, M.A.; Callahan, M.K.; Wolchok, J.D. Immune Checkpoint Blockade in Cancer Therapy. J. Clin. Oncol. 2015, 33, 1974–1982. [Google Scholar] [CrossRef]
- Finn, R.S.; Ikeda, M.; Zhu, A.X.; Sung, M.W.; Baron, A.D.; Kudo, M.; Okusaka, T.; Kobayashi, M.; Kumada, H.; Kaneko, S.; et al. Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients with Unresectable Hepatocellular Carcinoma. J. Clin. Oncol. 2020, 38, 2960–2970. [Google Scholar] [CrossRef]
- Dong, H.; Strome, S.E.; Salomao, D.R.; Tamura, H.; Hirano, F.; Flies, D.B.; Roche, P.C.; Lu, J.; Zhu, G.; Tamada, K.; et al. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat. Med. 2002, 8, 793–800. [Google Scholar] [CrossRef]
- Herbst, R.S.; Soria, J.C.; Kowanetz, M.; Fine, G.D.; Hamid, O.; Gordon, M.S.; Sosman, J.A.; McDermott, D.F.; Powderly, J.D.; Gettinger, S.N.; et al. Predictive Correlates of Response to the Anti-Pd-L1 Antibody Mpdl3280a in Cancer Patients. Nature 2014, 515, 563–567. [Google Scholar] [CrossRef]
- Tumeh, P.; Harview, C.; Yearly, J.; Shintaku, I.; Taylor, E.; Robert, L.; Chmielowski, B.; Spasic, M.; Henry, G.; Ciobanu, V.; et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014, 515, 568–571. [Google Scholar] [CrossRef]
- Kumagai, S.; Togashi, Y.; Kamada, T.; Sugiyama, E.; Nishinakamura, H.; Takeuchi, Y.; Vitaly, K.; Itahashi, K.; Maeda, Y.; Matsui, S.; et al. The PD-1 expression balance between effector and regulatory T cells predicts the clinical efficacy of PD-1 blockade therapies. Nat. Immunol. 2020, 21, 1346–1358. [Google Scholar] [CrossRef]
- Finn, R.S.; Ryoo, B.Y.; Merle, P.; Kudo, M.; Bouattour, M.; Lim, H.Y.; Breder, V.; Edeline, J.; Chao, Y.; Ogasawara, S.; et al. Pembrolizumab as Second-Line Therapy in Patients with Advanced Hepatocellular Carcinoma in Keynote-240: A Randomized, Double-Blind, Phase Iii Trial. J. Clin. Oncol. 2020, 38, 193–202. [Google Scholar] [CrossRef]
- Yau, T.; Park, J.W.; Finn, R.S.; Cheng, A.L.; Mathurin, P.; Edeline, J.; Kudo, M.; Harding, J.J.; Merle, P.; Rosmorduc, O.; et al. Nivolumab Versus Sorafenib in Advanced Hepatocellular Carcinoma (Checkmate 459): A Randomised, Multicentre, Open-Label, Phase 3 Trial. Lancet Oncol. 2022, 23, 77–90. [Google Scholar] [CrossRef]
- Sperandio, R.C.; Pestana, R.C.; Miyamura, B.V.; Kaseb, A.O. Hepatocellular Carcinoma Immunotherapy. Annu. Rev. Med. 2022, 73, 267–278. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef]
- Hack, S.P.; Spahn, J.; Chen, M.; Cheng, A.-L.; Kaseb, A.; Kudo, M.; Lee, H.C.; Yopp, A.; Chow, P.; Qin, S. IMbrave 050: A Phase III trial of atezolizumab plus bevacizumab in high-risk hepatocellular carcinoma after curative resection or ablation. Futur. Oncol. 2020, 16, 975–989. [Google Scholar] [CrossRef]
- Llovet, J.M.; Kudo, M.; Cheng, A.; Finn, R.S.; Galle, P.R.; Kaneko, S.; Meyer, T.; Qin, S.; Dutcus, C.E.; Chen, E.; et al. Lenvatinib (Len) Plus Pembrolizumab (Pembro) for the First-Line Treatment of Patients (Pts) with Advanced Hepatocellular Carci-Noma (Hcc): Phase 3 Leap-002 Study. J. Clin. Oncol. 2019, 37, TPS4152. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Chan, S.L.; Furuse, J.; Galle, P.R.; Kelley, R.K.; Qin, S.; Armstrong, J.; Darilay, A.; Vlahovic, G.; Negro, A.; et al. A Randomized, Multicenter Phase 3 Study of Durvalumab (D) and Tremelimumab (T) as First-Line Treatment in Patients with Un-Resectable Hepatocellular Carcinoma (Hcc): Himalaya Study. J. Clin. Oncol. 2018, 36, TPS4144. [Google Scholar] [CrossRef]
- Sangro, B.; Kudo, M.; Qin, S.; Ren, Z.; Chan, S.; Joseph, E.; Arai, Y.; Mann, H.; Morgan, S.R.; Cohen, G.L.; et al. P-347. A Phase 3, Randomized, Double-Blind, Placebo-Controlled Study of Transarterial Chemoembolization Combined with Dur-valumab or Durvalumab Plus Bevacizumab Therapy in Patients with Locoregional Hepatocellular Carcinoma: Emerald-1. Ann. Oncol. 2020, 31, S202–S203. [Google Scholar] [CrossRef]
- Huang, S.; Li, T.; Chen, Y.; Liu, J.; Wang, Y.; Yang, C.; Wang, C.; Ju, S.; Bai, Y.; Yao, W.; et al. Microwave ablation combined with anti-PD-1 therapy enhances systemic antitumor immunity in a multitumor murine model of Hepa1-6. Int. J. Hyperth. 2022, 39, 278–286. [Google Scholar] [CrossRef]
- Velez, E.; Goldberg, S.N.; Kumar, G.; Wang, Y.; Gourevitch, S.; Sosna, J.; Moon, T.; Brace, C.L.; Ahmed, M. Hepatic Thermal Ablation: Effect of Device and Heating Parameters on Local Tissue Reactions and Distant Tumor Growth. Radiology 2016, 281, 782–792. [Google Scholar] [CrossRef]
- Reck, M.; Remon, J.; Hellmann, M.D. First-Line Immunotherapy for Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2022, 40, 586–597. [Google Scholar] [CrossRef]
- Carlino, M.S.; Larkin, J.; Long, G.V. Immune Checkpoint Inhibitors in Melanoma. Lancet 2021, 398, 1002–1014. [Google Scholar] [CrossRef]
- Morita, M.; Nishida, N.; Aoki, T.; Chishina, H.; Takita, M.; Ida, H.; Hagiwara, S.; Minami, Y.; Ueshima, K.; Kudo, M. Role of β-Catenin Activation in the Tumor Immune Microenvironment and Immunotherapy of Hepatocellular Carcinoma. Cancers 2023, 15, 2311. [Google Scholar] [CrossRef]
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.Y.; Choo, S.P.; Trojan, J.; Welling, T.H.R.; et al. Nivolumab in Patients with Advanced Hepatocellular Carcinoma (Checkmate 040): An Open-Label, Non-Comparative, Phase 1/2 Dose Escalation and Expansion Trial. Lancet 2017, 389, 2492–2502. [Google Scholar] [CrossRef]
- Shi, L.R.; Chen, L.J.; Wu, C.P.; Zhu, Y.B.; Xu, B.; Zheng, X.; Sun, M.F.; Wen, W.; Dai, X.C.; Yang, M.; et al. Pd-1 Blockade Boosts Radiofrequency Ablation-Elicited Adaptive Immune Responses against Tumor. Clin. Cancer Res. 2016, 22, 1173–1184. [Google Scholar] [CrossRef]
- Yoo, G.S.; Ahn, W.-G.; Kim, S.-Y.; Kang, W.; Choi, C.; Park, H.C. Radiation-induced abscopal effect and its enhancement by programmed cell death 1 blockade in the hepatocellular carcinoma: A murine model study. Clin. Mol. Hepatol. 2021, 27, 144–156. [Google Scholar] [CrossRef]
- Tan, J.; Liu, T.; Fan, W.; Wei, J.; Zhu, B.; Liu, Y.; Liu, L.; Zhang, X.; Chen, S.; Lin, H.; et al. Anti-PD-L1 antibody enhances curative effect of cryoablation via antibody-dependent cell-mediated cytotoxicity mediating PD-L1highCD11b+ cells elimination in hepatocellular carcinoma. Acta Pharm. Sin. B 2023, 13, 632–647. [Google Scholar] [CrossRef]
- Izzo, F.; Granata, V.; Grassi, R.; Fusco, R.; Palaia, R.; Delrio, P.; Carrafiello, G.; Azoulay, D.; Petrillo, A.; Curley, S.A. Radiof-requency Ablation and Microwave Ablation in Liver Tumors: An Update. Oncologist 2019, 24, e990–e1005. [Google Scholar] [CrossRef]
- Outh-Gauer, S.; Alt, M.; Le Tourneau, C.; Augustin, J.; Broudin, C.; Gasne, C.; Denize, T.; Mirghani, H.; Fabre, E.; Ménard, M.; et al. Immunotherapy in Head and Neck Cancers: A New Challenge for Immunologists, Pathologists and Clinicians. Cancer Treat Rev. 2018, 65, 54–64. [Google Scholar] [CrossRef]
- Liu, J.; Sun, M.; Fang, K.; Wang, J.; Ma, B.; Song, L.; Liu, T.; Tang, M.; Wang, K.; Xia, Y. Effect of Different Liver Resection Modalities on the Prognosis of Patients with Hepatocellular Carcinoma on the Left Lateral Lobe. J. Hepatocell. Carcinoma 2023, 10, 997–1007. [Google Scholar] [CrossRef]
- Baker, S.; Dahele, M.; Lagerwaard, F.J.; Senan, S. A critical review of recent developments in radiotherapy for non-small cell lung cancer. Radiat. Oncol. 2016, 11, 1–14. [Google Scholar] [CrossRef]
- Kim, D.-H. Combination of interventional oncology local therapies and immunotherapy for the treatment of hepatocellular carcinoma. J. Liver Cancer 2022, 22, 93–102. [Google Scholar] [CrossRef]
- Tranberg, K.-G. Local Destruction of Tumors and Systemic Immune Effects. Front. Oncol. 2021, 11, 708810. [Google Scholar] [CrossRef]
- Yu, L.; Xie, H.; Wang, L.; Cheng, M.; Liu, J.; Xu, J.; Wei, Z.; Ye, X.; Xie, Q.; Liang, J. Microwave ablation induces abscopal effect via enhanced systemic antitumor immunity in colorectal cancer. Front. Oncol. 2023, 13, 1174713. [Google Scholar] [CrossRef]
- Liang, P.; Dong, B.; Yu, X.; Yu, D.; Wang, Y.; Feng, L.; Xiao, Q. Prognostic Factors for Survival in Patients with Hepatocellular Carcinoma after Percutaneous Microwave Ablation. Radiology 2005, 235, 299–307. [Google Scholar] [CrossRef]
- Takaki, H.; Cornelis, F.; Kako, Y.; Kobayashi, K.; Kamikonya, N.; Yamakado, K. Thermal ablation and immunomodulation: From preclinical experiments to clinical trials. Diagn. Interv. Imaging 2017, 98, 651–659. [Google Scholar] [CrossRef]
- Zhu, J.; Yu, M.; Chen, L.; Kong, P.; Li, L.; Ma, G.; Ge, H.; Cui, Y.; Li, Z.; Pan, H.; et al. Enhanced antitumor efficacy through microwave ablation in combination with immune checkpoints blockade in breast cancer: A pre-clinical study in a murine model. Diagn. Interv. Imaging 2018, 99, 135–142. [Google Scholar] [CrossRef]
- De Bethlenfalva-Hora, C.E.; Mertens, J.C.; Piguet, A.-C.; Kettenbach, J.; Schmitt, J.; Terracciano, L.; Weimann, R.; Dufour, J.-F.; Geier, A. Radiofrequency ablation suppresses distant tumour growth in a novel rat model of multifocal hepatocellular carcinoma. Clin. Sci. 2014, 126, 243–252. [Google Scholar] [CrossRef]
- Mole, R.H. Whole Body Irradiation; Radiobiology or Medicine? Br. J. Radiol. 1953, 26, 234–241. [Google Scholar] [CrossRef]
- Ghaffari-Nazari, H.; Alimohammadi, M.; Alimohammadi, R.; Rostami, E.; Bakhshandeh, M.; Webster, T.J.; Chalbatani, G.M.; Tavakkol-Afshari, J.; Jalali, S.A. Radiation dose and schedule influence the abscopal effect in a bilateral murine CT26 tumor model. Int. Immunopharmacol. 2022, 108, 108737. [Google Scholar] [CrossRef]
- Yu, Z.; Geng, J.; Zhang, M.; Zhou, Y.; Fan, Q.; Chen, J. Treatment of osteosarcoma with microwave thermal ablation to induce immunogenic cell death. Oncotarget 2014, 5, 6526–6539. [Google Scholar] [CrossRef]
- O’Malley, D.M.; Neffa, M.; Monk, B.J.; Melkadze, T.; Huang, M.; Kryzhanivska, A.; Bulat, I.; Meniawy, T.M.; Bagameri, A.; Wang, E.W.; et al. Dual PD-1 and CTLA-4 Checkpoint Blockade Using Balstilimab and Zalifrelimab Combination as Second-Line Treatment for Advanced Cervical Cancer: An Open-Label Phase II Study. J. Clin. Oncol. 2022, 40, 762–771. [Google Scholar] [CrossRef]
- Ahmadzadeh, M.; Johnson, L.A.; Heemskerk, B.; Wunderlich, J.R.; Dudley, M.E.; White, D.E.; Rosenberg, S.A. Tumor antigen–specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood 2009, 114, 1537–1544. [Google Scholar] [CrossRef]
- Luheshi, N.; Davies, G.; Poon, E.; Wiggins, K.; McCourt, M.; Legg, J. Th1 cytokines are more effective than Th2 cytokines at licensing anti-tumour functions in CD40-activated human macrophages in vitro. Eur. J. Immunol. 2014, 44, 162–172. [Google Scholar] [CrossRef]
- Osada, S.; Imai, H.; Tomita, H.; Tokuyama, Y.; Okumura, N.; Matsuhashi, N.; Sakashita, F.; Nonaka, K. Serum cytokine levels in response to hepatic cryoablation. J. Surg. Oncol. 2007, 95, 491–498. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, J.; Xu, D.; Gao, X.-M.; Zhang, Z.; Hsu, J.L.; Li, C.-W.; Lim, S.-O.; Sheng, Y.-Y.; Zhang, Y.; et al. Disruption of tumour-associated macrophage trafficking by the osteopontin-induced colony-stimulating factor-1 signalling sensitises hepatocellular carcinoma to anti-PD-L1 blockade. Gut 2019, 68, 1653–1666. [Google Scholar] [CrossRef]
- Balkwill, F. Tumour necrosis factor and cancer. Nat. Rev. Cancer 2009, 9, 361–371. [Google Scholar] [CrossRef]
- Guo, R.Q.; Peng, J.Z.; Li, Y.M.; Li, X.G. Microwave Ablation Combined with Anti-Pd-1/Ctla-4 Therapy Induces an An-titumor Immune Response to Renal Cell Carcinoma in a Murine Model. Cell Cycle 2023, 22, 242–254. [Google Scholar] [CrossRef]
- Wang, M.; Duan, Y.; Yang, M.; Guo, Y.; Li, F.; Wang, J.; Si, T. The analysis of immunogenic cell death induced by ablation at different temperatures in hepatocellular carcinoma cells. Front. Cell Dev. Biol. 2023, 11, 1146195. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Q.; Muktiali, M.; Ren, B.; Hu, Y.; Li, D.; Li, Z.; Li, D.; Xie, Y.; Tao, M.; et al. Effect of microwave ablation treatment of hepatic malignancies on serum cytokine levels. BMC Cancer 2020, 20, 1–10. [Google Scholar] [CrossRef]
- Poon, R.T.; Ng, I.O.; Lau, C.; Yu, W.C.; Yang, Z.F.; Fan, S.T.; Wong, J. Tumor Microvessel Density as a Predictor of Re-currence after Resection of Hepatocellular Carcinoma: A Prospective Study. J. Clin. Oncol. 2002, 20, 1775–1785. [Google Scholar] [CrossRef]
- Ahmed, M.; Kumar, G.; Gourevitch, S.; Levchenko, T.; Galun, E.; Torchilin, V.; Goldberg, S.N. Radiofrequency ablation (RFA)-induced systemic tumor growth can be reduced by suppression of resultant heat shock proteins. Int. J. Hyperth. 2018, 34, 934–942. [Google Scholar] [CrossRef]
- Chen, Y.; Zeng, L.; Zhu, H.; Wu, Q.; Liu, R.; Liang, Q.; Chen, B.; Dai, H.; Tang, K.; Liao, C.; et al. Ferritin Nanocaged Doxorubicin Potentiates Chemo—Immunotherapy against Hepatocellular Carcinoma via Immunogenic Cell Death. Small Methods 2023, 7, e2201086. [Google Scholar] [CrossRef]
- Zhong, X.; Zhou, Y.; Cao, Y.; Ding, J.; Wang, P.; Luo, Y.; Liu, H.; Zhu, Z.; Jing, X. Enhanced antitumor efficacy through microwave ablation combined with a dendritic cell-derived exosome vaccine in hepatocellular carcinoma. Int. J. Hyperth. 2020, 37, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Weidner, N.; Folkman, J.; Pozza, F.; Bevilacqua, P.; Allred, E.N.; Moore, D.H.; Meli, S.; Gasparini, G. Tumor Angiogenesis: A New Significant and Independent Prognostic Indicator in Early-Stage Breast Carcinoma. JNCI J. Natl. Cancer Inst. 1992, 84, 1875–1887. [Google Scholar] [CrossRef]



| Sham MWA | Low-Power MWA | High-Power MWA | |
|---|---|---|---|
| PBS | Sham | 5W | 10W |
| Anti-PD-1 | Sham + PD-1 | 5W + PD-1 | 10W + PD-1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, C.; Zhang, G.; Huang, R.; Zeng, L.; Chen, B.; Dai, H.; Tang, K.; Lin, R.; Huang, Y. Inducing the Abscopal Effect in Liver Cancer Treatment: The Impact of Microwave Ablation Power Levels and PD-1 Antibody Therapy. Pharmaceuticals 2023, 16, 1672. https://doi.org/10.3390/ph16121672
Liao C, Zhang G, Huang R, Zeng L, Chen B, Dai H, Tang K, Lin R, Huang Y. Inducing the Abscopal Effect in Liver Cancer Treatment: The Impact of Microwave Ablation Power Levels and PD-1 Antibody Therapy. Pharmaceuticals. 2023; 16(12):1672. https://doi.org/10.3390/ph16121672
Chicago/Turabian StyleLiao, Changli, Guiyuan Zhang, Ruotong Huang, Linyuan Zeng, Bin Chen, Haitao Dai, Keyu Tang, Run Lin, and Yonghui Huang. 2023. "Inducing the Abscopal Effect in Liver Cancer Treatment: The Impact of Microwave Ablation Power Levels and PD-1 Antibody Therapy" Pharmaceuticals 16, no. 12: 1672. https://doi.org/10.3390/ph16121672
APA StyleLiao, C., Zhang, G., Huang, R., Zeng, L., Chen, B., Dai, H., Tang, K., Lin, R., & Huang, Y. (2023). Inducing the Abscopal Effect in Liver Cancer Treatment: The Impact of Microwave Ablation Power Levels and PD-1 Antibody Therapy. Pharmaceuticals, 16(12), 1672. https://doi.org/10.3390/ph16121672





