Preliminary Evidence of Efficacy, Safety, and Treatment Satisfaction with Tirbanibulin 1% Ointment: A Clinical Perspective on Actinic Keratoses
Abstract
1. Introduction
2. Results
2.1. Demographic Characteristics of Enrolled Patients
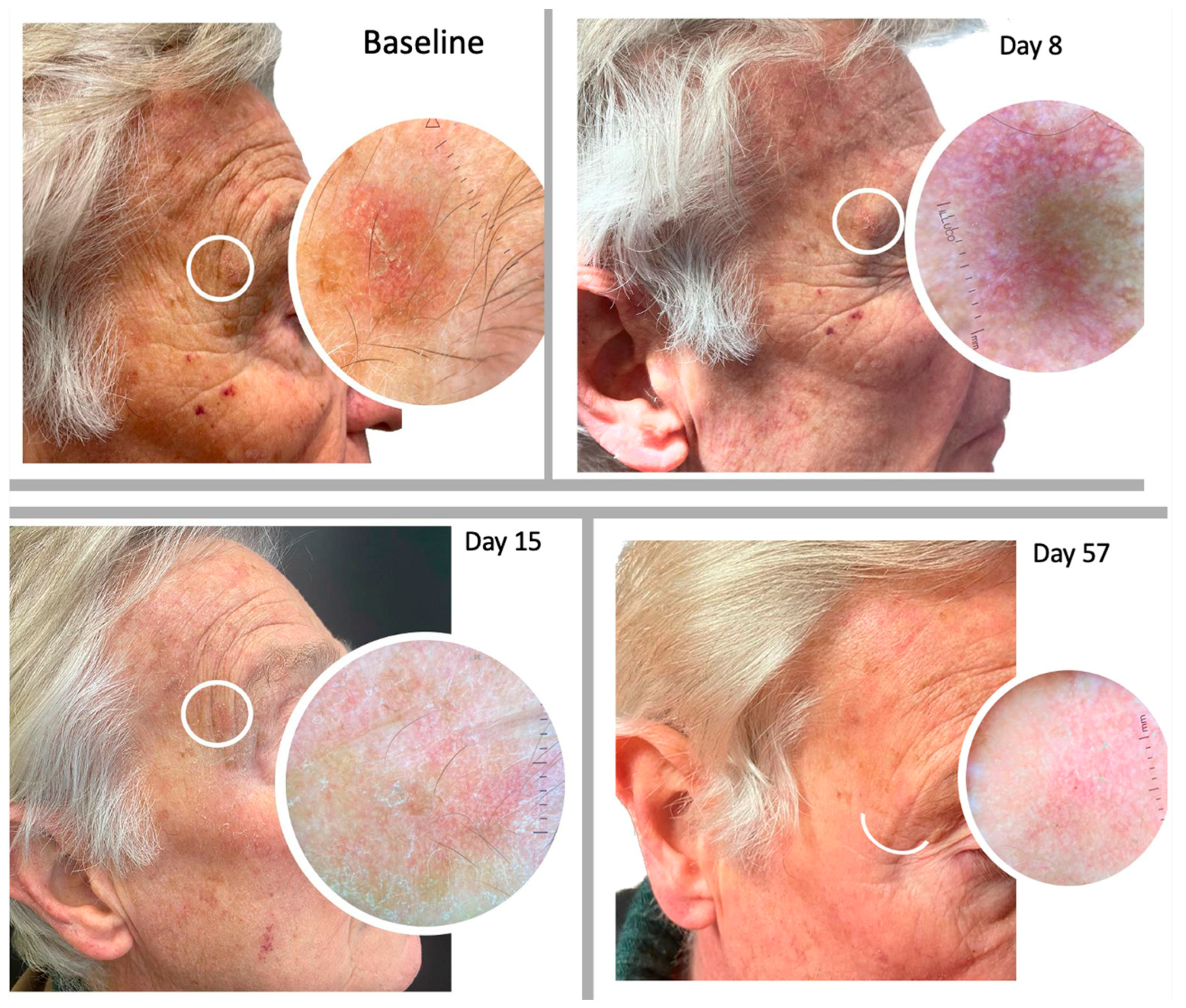
2.2. Safety in Term of LSR
2.3. Efficacy
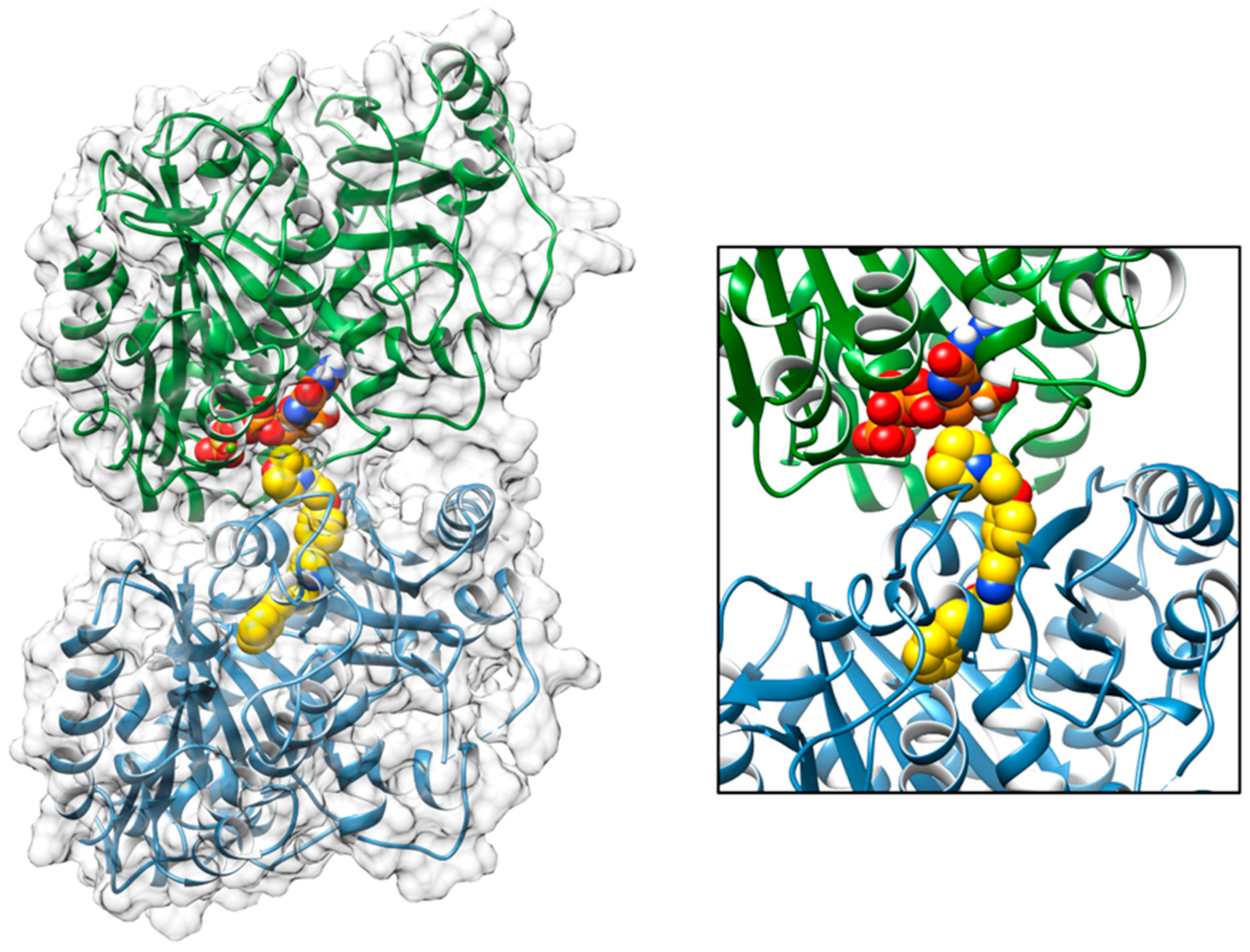
2.4. Molecular Docking Tirbanibulin
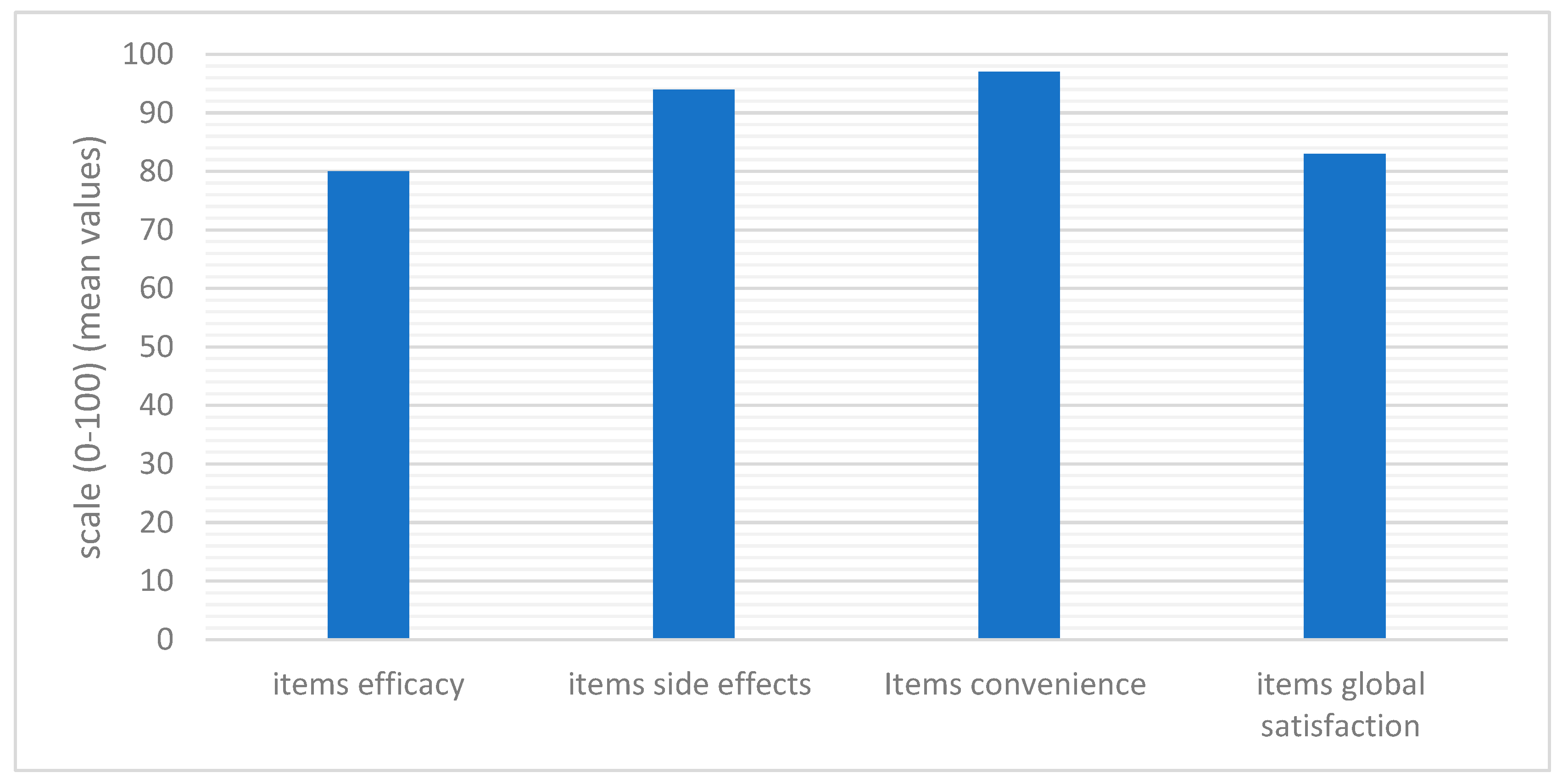
2.5. Treatment Satisfaction
3. Materials and Methods
3.1. Enrolled Patients and Study Design
3.2. Primary and Secondary Endpoints
3.3. Score Evaluation: Efficacy, Safety, and Treatment Satisfaction
3.4. Molecular Docking Simulation of the Interaction of Tirbanibulin
3.5. Statistical Analysis
4. Discussion
5. Conclusions
6. Future Directions
7. Limitation of the Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Wood, D.L.A.; Lachner, N.; Tan, J.M.; Tang, S.; Angel, N.; Laino, A.; Linedale, R.; Lê Cao, K.A.; Morrison, M.; Frazer, I.; et al. A Natural History of Actinic Keratosis and Cutaneous Squamous Cell Carcinoma Microbiomes. mBio 2018, 9, e01432-18. [Google Scholar] [CrossRef] [PubMed]
- Padilla, R.S.; Sebastian, S.; Jiang, Z.; Nindl, I.; Larson, R. Gene expression patterns of normal human skin, actinic keratosis, and squamous cell carcinoma: A spectrum of disease progression. Arch Dermatol. 2010, 146, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Gutzmer, R.; Wiegand, S.; Kölbl, O.; Wermker, K.; Heppt, M.; Berking, C. Actinic Keratosis and Cutaneous Squamous Cell Carcinoma. Dtsch Arztebl. Int. 2019, 116, 616–626. [Google Scholar] [CrossRef] [PubMed]
- Fernandez Figueras, M.T. From actinic keratosis to squamous cell carcinoma: Pathophysiology revisited. J. Eur. Acad. Dermatol. Venereol. 2017, 31 (Suppl. 2), 5–7. [Google Scholar] [CrossRef] [PubMed]
- Werner, R.N.; Stockfleth, E.; Connolly, S.M.; Correia, O.; Erdmann, R.; Foley, P.; Gupta, A.K.; Jacobs, A.; Kerl, H.; Lim, H.W.; et al. Evidence- and consensus-based (S3) Guidelines for the Treatment of Actinic Keratosis—International League of Dermatological Societies in cooperation with the European Dermatology Forum—Short version. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 2069–2079. [Google Scholar] [CrossRef] [PubMed]
- Werner, R.N.; Sammain, A.; Erdmann, R.; Hartmann, V.; Stockfleth, E.; Nast, A. The natural history of actinic keratosis: A systematic review. Br. J. Dermatol. 2013, 169, 502–518. [Google Scholar] [CrossRef] [PubMed]
- Reinehr, C.P.H.; Bakos, R.M. Actinic keratoses: Review of clinical, dermoscopic, and therapeutic aspects. An. Bras. Dermatol. 2019, 94, 637–657. [Google Scholar] [CrossRef] [PubMed]
- Blauvelt, A.; Kempers, S.; Lain, E.; Schlesinger, T.; Tyring, S.; Forman, S.; Ablon, G.; Martin, G.; Wang, H.; Cutler, D.L.; et al. Phase 3 Trials of Tirbanibulin Ointment for Actinic Keratosis. N. Engl. J. Med. 2021, 384, 512–520. [Google Scholar] [CrossRef]
- Röwert-Huber, J.; Patel, M.J.; Forschner, T.; Ulrich, C.; Eberle, J.; Kerl, H.; Sterry, W.; Stockfleth, E. Actinic keratosis is an early in situ squamous cell carcinoma: A proposal for reclassification. Br. J. Dermatol. 2007, 156 (Suppl. 3), 8–12, Erratum in Br. J. Dermatol. 2007, 157, 431. [Google Scholar] [CrossRef]
- Campione, E.; Di Prete, M.; Di Raimondo, C.; Costanza, G.; Palumbo, V.; Garofalo, V.; Mazzilli, S.; Franceschini, C.; Dika, E.; Bianchi, L.; et al. Topical Treatment of Actinic Keratosis and Metalloproteinase Expression: A Clinico-Pathological Retrospective Study. Int. J. Mol. Sci. 2022, 23, 11351. [Google Scholar] [CrossRef]
- Chetty, P.; Choi, F.; Mitchell, T. Primary care review of actinic keratosis and its therapeutic options: A global perspective. Dermatol. Ther. 2015, 5, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Zalaudek, I.; Argenziano, G. Dermoscopy of actinic keratosis, intraepidermal carcinoma and squamous cell carcinoma. Curr. Probl. Dermatol. 2015, 46, 70–76. [Google Scholar] [PubMed]
- Longo, C.; Casari, A.; Pepe, P.; Moscarella, E.; Zalaudek, I.; Argenziano, G.; Pellacani, G. Confocal microscopy insights into the treatment and cellular immune response of Basal cell carcinoma to photodynamic therapy. Dermatology 2012, 225, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Campione, E.; Ventura, A.; Diluvio, L.; Mazzeo, M.; Mazzilli, S.; Garofalo, V.; Di Prete, M.; Bianchi, L. Current developments in pharmacotherapy for actinic keratosis. Expert Opin. Pharmacother. 2018, 19, 1693–1704. [Google Scholar] [CrossRef] [PubMed]
- Richard, M.A.; Amici, J.M.; Basset-Seguin, N.; Claudel, J.P.; Cribier, B.; Dreno, B. Management of actinic keratosis at specific body sites in patients at high risk of carcinoma lesions: Expert consensus from the AKTeam™ of expert clinicians. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Eisen, D.B.; Asgari, M.M.; Bennett, D.D.; Connolly, S.M.; Dellavalle, R.P.; Freeman, E.E.; Goldenberg, G.; Leffell, D.J.; Peschin, S.; Sligh, J.E.; et al. Guidelines of Care for the Management of Actinic Keratosis: Executive Summary. J. Am. Acad. Dermatol. 2021, 85, 945–955. [Google Scholar] [CrossRef] [PubMed]
- Conforti, C.; Giuffrida, R.; Dianzani, C.; Guarneri, F.; Marangi, G.F.; Neagu, N.; Persichetti, P.; Zalaudek, I.; di Meo, N. Effectiveness and tolerability of treatment for isolated actinic keratoses: A retrospective comparison between cryotherapy, CO2 laser and 5-fluorouracil 0.5%/salicylic acid 10. Dermatol. Ther. 2021, 34, e14846. [Google Scholar] [CrossRef]
- Dirschka, T.; Radny, P.; Dominicus, R.; Mensing, H.; Brüning, H.; Jenne, L.; Karl, L.; Sebastian, M.; Oster-Schmidt, C.; Klövekorn, W.; et al. Photodynamic therapy with BF-200 ALA for the treatment of actinic keratosis: Results of a prospective, randomized, double-blind, placebo controlled phase III study. Br. J. Dermatol. 2010, 166, 137–146. [Google Scholar] [CrossRef]
- Papadavid, E.; Stratigos, A.J.; Falagas, M.E. Imiquimod: An immune response modifier in the treatment of precancerous skin lesions and skin cancer. Expert Opin. Pharmacother. 2007, 8, 1743–1755. [Google Scholar] [CrossRef]
- Bobyr, I.; Campanati, A.; Giacchetti, A.; Offidani, A. Fluorescent photodiagnostic evaluation of field cancerization treated with a medical device containing piroxicam 0.8% and sunscreen SPF 50+ for actinic keratosis. Photodermatol. Photoimmunol. Photomed. 2019, 35, 277–279. [Google Scholar] [CrossRef]
- Puviani, M.; Galloni, C.; Marchetti, S.; Sergio Pavone, P.; Lovati, S.; Pistone, G.; Caputo, V.; Tilotta, G.; Scarcella, G.; Campione, E.; et al. Efficacy of a film-forming medical device containing sunscreen (50+) and piroxicam 0.8% in actinic keratosis and field cancerization: A multicenter, assessor-blinded, 3 month trial. Curr. Med. Res. Opin. 2017, 33, 1255–1259. [Google Scholar] [CrossRef]
- Babino, G.; Diluvio, L.; Bianchi, L.; Orlandi, A.; Di Prete, M.; Chimenti, S.; Milani, M.; Campione, E. Long-term use of a new topical formulation containing piroxicam 0.8% and sunscreen: Efficacy and tolerability on actinic keratosis. A proof of concept study. Curr. Med. Res. Opin. 2016, 32, 1345–1349. [Google Scholar] [CrossRef]
- Campione, E.; Paternò, E.J.; Candi, E.; Falconi, M.; Costanza, G.; Diluvio, L.; Terrinoni, A.; Bianchi, L.; Orlandi, A. The relevance of piroxicam for the prevention and treatment of nonmelanoma skin cancer and its precursors. Drug Des. Devel. Ther. 2015, 9, 5843–5850. [Google Scholar] [CrossRef] [PubMed]
- Segura, S.; Gadea, A.; Nonell, L.; Andrades, E.; Sánchez, S.; Pujol, R.; Hernández-Muñoz, I.; Toll, A. Identification of differentially expressed genes in actinic keratosis samples treated with ingenol mebutate gel. PLoS ONE 2020, 15, e0232146. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.C.; Martin, A.J.; Choy, B.; Fernández-Peñas, P.; Dalziell, R.A.; McKenzie, C.A.; Scolyer, R.A.; Dhillon, H.M.; Vardy, J.L.; Kricker, A.; et al. A Phase 3 Randomized Trial of Nicotinamide for Skin-Cancer Chemoprevention. N. Engl. J. Med. 2015, 373, 1618–1626. [Google Scholar] [CrossRef]
- Fania, L.; Mazzanti, C.; Campione, E.; Candi, E.; Abeni, D.; Dellambra, E. Role of Nicotinamide in Genomic Stability and Skin Cancer Chemoprevention. Int. J. Mol. Sci. 2019, 20, 5946. [Google Scholar] [CrossRef] [PubMed]
- Cosio, T.; Di Prete, M.; Campione, E. Arsenic Trioxide, Itraconazole, All-Trans Retinoic Acid and Nicotinamide: A Proof of Concept for Combined Treatments with Hedgehog Inhibitors in Advanced Basal Cell Carcinoma. Biomedicines 2020, 8, 156. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, T.; Stockfleth, E.; Grada, A.; Berman, B. Tirbanibulin for Actinic Keratosis: Insights into the Mechanism of Action. Clin. Cosmet. Investig. Dermatol. 2022, 15, 2495–2506. [Google Scholar] [CrossRef]
- Dianzani, C.; Conforti, C.; Giuffrida, R.; Corneli, P.; di Meo, N.; Farinazzo, E.; Moret, A.; Magaton Rizzi, G.; Zalaudek, I. Current therapies for actinic keratosis. Int. J. Dermatol. 2020, 59, 677–684. [Google Scholar] [CrossRef]
- Dao, D.D.; Sahni, V.N.; Sahni, D.R.; Balogh, E.A.; Grada, A.; Feldman, S.R. 1% Tirbanibulin Ointment for the Treatment of Actinic Keratoses. Ann. Pharmacother. 2022, 56, 494–500. [Google Scholar] [CrossRef]
- de Oliveira, E.C.V.; da Motta, V.R.V.; Pantoja, P.C.; Ilha, C.S.O.; Magalhães, R.F.; Galadari, H.; Leonardi, G.R. Actinic keratosis—Review for clinical practice. Int. J. Dermatol. 2019, 58, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Yang, J.; Yan, W.; Yu, Y.; Zheng, Y.; Ye, H.; Chen, Q.; Chen, L. Reversible binding of the anticancer drug KXO1 (tirbanibulin) to the colchicine-binding site of β-tubulin explains KXO1’s low clinical toxicity. J. Biol. Chem. 2019, 294, 18099–18108. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, L.; Kahl, P.; Majores, M.; Bierhoff, E.; Stockfleth, E.; Dirschka, T. Actinic keratosis: Correlation between clinical and histological classification systems. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1303–1307. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Lim-Wilby, M. Molecular docking. Methods Mol. Biol. 2008, 443, 365–382. [Google Scholar] [CrossRef]
- Kirchberger, M.C.; Gfesser, M.; Erdmann, M.; Schliep, S.; Berking, C.; Heppt, M.V. Tirbanibulin 1% Ointment Significantly Reduces the Actinic Keratosis Area and Severity Index in Patients with Actinic Keratosis: Results from a Real-World Study. J. Clin. Med. 2023, 12, 4837. [Google Scholar] [CrossRef]
- Lacarrubba, F.; Verzì, A.E.; Polita, M.; Aleo, A.; Micali, G. Line-field confocal optical coherence tomography in the treatment monitoring of actinic keratosis with tirbanibulin: A pilot study. J. Eur. Acad. Dermatol. Venereol. 2023, 37, e1131–e1133. [Google Scholar] [CrossRef]
- Dréno, B.; Amici, J.M.; Basset-Seguin, N.; Cribier, B.; Claudel, J.P.; Richard, M.A.; AKTeam™. Management of actinic keratosis: A practical report and treatment algorithm from AKTeam™ expert clinicians. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 1141–1149. [Google Scholar] [CrossRef]




| Median Age (Years) | 75 (60–93) |
|---|---|
| Gender | |
| Female | 12 (40%) |
| Male | 18 (60%) |
| Skin phototypes according Fitzpatrick classification | |
| I | 12 (40%) |
| II | 16 (53%) |
| III | 2 (7%) |
| Prior therapies (26 patients) | |
| Curettage/cryosurgery | 6 (24%) |
| Medical device Piroxicam 0.8% and sunfilters | 7 (27%) |
| Diclofenac 3% | 6 (24%) |
| 5-Fluorouracil 5% | 2 (8%) |
| 5-Fluorouracil 0.5%+ salicylic acid 10% | 2 (8%) |
| Imiquimod | 4 (15%) |
| Laser-assisted photodynamic therapy | 5 (19%) |
| 5-Aminolevulinic acid patch | 1 (4%) |
| Acetylsalicylic Acid | 20.75% |
| Thiazide Diuretics | 11.30% |
| Angiotensin II Receptor Blockers | 11.30% |
| Statins | 11.30% |
| Antiplatelet Agents | 9.43% |
| Metformin | 7.54% |
| Levothyroxine | 7.54% |
| Potassium-Sparing Diuretics | 5.66% |
| Beta-Blockers | 3.77% |
| Calcium Channel Blockers | 3.77% |
| Incretin | 1.88% |
| Insulin | 1.88% |
| Carbamazepine | 1.88% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campione, E.; Rivieccio, A.; Gaeta Shumak, R.; Costanza, G.; Cosio, T.; Lambiase, S.; Garofalo, V.; Artosi, F.; Lozzi, F.; Freni, C.; et al. Preliminary Evidence of Efficacy, Safety, and Treatment Satisfaction with Tirbanibulin 1% Ointment: A Clinical Perspective on Actinic Keratoses. Pharmaceuticals 2023, 16, 1686. https://doi.org/10.3390/ph16121686
Campione E, Rivieccio A, Gaeta Shumak R, Costanza G, Cosio T, Lambiase S, Garofalo V, Artosi F, Lozzi F, Freni C, et al. Preliminary Evidence of Efficacy, Safety, and Treatment Satisfaction with Tirbanibulin 1% Ointment: A Clinical Perspective on Actinic Keratoses. Pharmaceuticals. 2023; 16(12):1686. https://doi.org/10.3390/ph16121686
Chicago/Turabian StyleCampione, Elena, Antonia Rivieccio, Ruslana Gaeta Shumak, Gaetana Costanza, Terenzio Cosio, Sara Lambiase, Virginia Garofalo, Fabio Artosi, Flavia Lozzi, Claudia Freni, and et al. 2023. "Preliminary Evidence of Efficacy, Safety, and Treatment Satisfaction with Tirbanibulin 1% Ointment: A Clinical Perspective on Actinic Keratoses" Pharmaceuticals 16, no. 12: 1686. https://doi.org/10.3390/ph16121686
APA StyleCampione, E., Rivieccio, A., Gaeta Shumak, R., Costanza, G., Cosio, T., Lambiase, S., Garofalo, V., Artosi, F., Lozzi, F., Freni, C., Romeo, A., Dika, E., Falconi, M., & Bianchi, L. (2023). Preliminary Evidence of Efficacy, Safety, and Treatment Satisfaction with Tirbanibulin 1% Ointment: A Clinical Perspective on Actinic Keratoses. Pharmaceuticals, 16(12), 1686. https://doi.org/10.3390/ph16121686












