The Impact of Sex on the Response to Proton Pump Inhibitor Treatment
Abstract
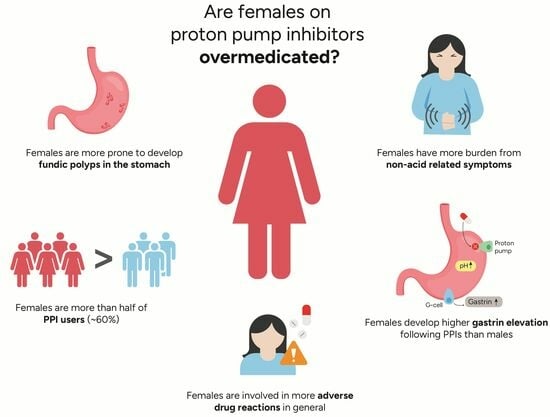
:1. Introduction
2. Association between Female Sex and Increased Gastrin Release Following PPIs
3. Role of Sex in Metabolism of PPIs
4. Is There a Need for Sex-Specific PPI Dosage?
5. Overuse or Overmedication of PPIs among Females
6. Side Effects of Secondary Gastrin Elevation
7. Other Side Effects of PPIs
8. Limitations
9. Summary and Future Directions
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Brabete, A.C.; Greaves, L.; Maximos, M.; Huber, E.; Li, A.; Le, M.L. A Sex- and Gender-Based Analysis of Adverse Drug Reactions: A Scoping Review of Pharmacovigilance Databases. Pharmaceuticals 2022, 15, 298. [Google Scholar] [CrossRef] [PubMed]
- Targownik, L.E.; Fisher, D.A.; Saini, S.D. AGA Clinical Practice Update on De-Prescribing of Proton Pump Inhibitors: Expert Review. Gastroenterology 2022, 162, 1334–1342. [Google Scholar] [CrossRef] [PubMed]
- Savarino, V.; Marabotto, E.; Zentilin, P.; Furnari, M.; Bodini, G.; De Maria, C.; Pellegatta, G.; Coppo, C.; Savarino, E. Proton pump inhibitors: Use and misuse in the clinical setting. Expert. Rev. Clin. Pharmacol. 2018, 11, 1123–1134. [Google Scholar] [CrossRef] [PubMed]
- Torres-Bondia, F.; de Batlle, J.; Galvan, L.; Buti, M.; Barbe, F.; Pinol-Ripoll, G. Evolution of the consumption trend of proton pump inhibitors in the Lleida Health Region between 2002 and 2015. BMC Public Health 2022, 22, 818. [Google Scholar] [CrossRef] [PubMed]
- Tran-Duy, A.; Spaetgens, B.; Hoes, A.W.; de Wit, N.J.; Stehouwer, C.D. Use of Proton Pump Inhibitors and Risks of Fundic Gland Polyps and Gastric Cancer: Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2016, 14, 1706–1719.e5. [Google Scholar] [CrossRef]
- Niklasson, A.; Lindstrom, L.; Simren, M.; Lindberg, G.; Bjornsson, E. Dyspeptic symptom development after discontinuation of a proton pump inhibitor: A double-blind placebo-controlled trial. Am. J. Gastroenterol. 2010, 105, 1531–1537. [Google Scholar] [CrossRef]
- Reimer, C.; Sondergaard, B.; Hilsted, L.; Bytzer, P. Proton-pump inhibitor therapy induces acid-related symptoms in healthy volunteers after withdrawal of therapy. Gastroenterology 2009, 137, 80–87.e1. [Google Scholar] [CrossRef]
- Cui, G.; Waldum, H.L. Physiological and clinical significance of enterochromaffin-like cell activation in the regulation of gastric acid secretion. World J. Gastroenterol. 2007, 13, 493–496. [Google Scholar] [CrossRef]
- Waldum, H.L.; Qvigstad, G.; Fossmark, R.; Kleveland, P.M.; Sandvik, A.K. Rebound acid hypersecretion from a physiological, pathophysiological and clinical viewpoint. Scand. J. Gastroenterol. 2010, 45, 389–394. [Google Scholar] [CrossRef]
- Ahrens, D.; Chenot, J.F.; Behrens, G.; Grimmsmann, T.; Kochen, M.M. Appropriateness of treatment recommendations for PPI in hospital discharge letters. Eur. J. Clin. Pharmacol. 2010, 66, 1265–1271. [Google Scholar] [CrossRef]
- Heidelbaugh, J.J.; Goldberg, K.L.; Inadomi, J.M. Overutilization of proton pump inhibitors: A review of cost-effectiveness and risk [corrected]. Am. J. Gastroenterol. 2009, 104 (Suppl. S2), S27–S32. [Google Scholar] [CrossRef] [PubMed]
- Helgadottir, H.; Metz, D.C.; Lund, S.H.; Gizurarson, S.; Jacobsen, E.I.; Asgeirsdottir, G.A.; Yngadottir, Y.; Bjornsson, E.S. Study of Gender Differences in Proton Pump Inhibitor Dose Requirements for GERD: A Double-Blind Randomized Trial. J. Clin. Gastroenterol. 2017, 51, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Fass, R.; Inadomi, J.; Han, C.; Mody, R.; O’Neil, J.; Perez, M.C. Maintenance of heartburn relief after step-down from twice-daily proton pump inhibitor to once-daily dexlansoprazole modified release. Clin. Gastroenterol. Hepatol. 2012, 10, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Vakil, N.; Niklasson, A.; Denison, H.; Ryden, A. Gender differences in symptoms in partial responders to proton pump inhibitors for gastro-oesophageal reflux disease. United Eur. Gastroenterol. J. 2015, 3, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Matsuhashi, N.; Kudo, M.; Yoshida, N.; Murakami, K.; Kato, M.; Sanuki, T.; Oshio, A.; Joh, T.; Higuchi, K.; Haruma, K.; et al. Factors affecting response to proton pump inhibitor therapy in patients with gastroesophageal reflux disease: A multicenter prospective observational study. J. Gastroenterol. 2015, 50, 1173–1183. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kim, N.; Kim, G.H. Sex and Gender Differences in Gastroesophageal Reflux Disease. J. Neurogastroenterol. Motil. 2016, 22, 575–588. [Google Scholar] [CrossRef] [PubMed]
- Dickson, E.J.; Stuart, R.C. Genetics of response to proton pump inhibitor therapy: Clinical implications. Am. J. Pharmacogenom. 2003, 3, 303–315. [Google Scholar] [CrossRef]
- Andersson, T.; Hassan-Alin, M.; Hasselgren, G.; Rohss, K.; Weidolf, L. Pharmacokinetic studies with esomeprazole, the (S)-isomer of omeprazole. Clin. Pharmacokinet. 2001, 40, 411–426. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F.; Bairey Merz, N.; Barnes, P.J.; Brinton, R.D.; Carrero, J.J.; DeMeo, D.L.; De Vries, G.J.; Epperson, C.N.; Govindan, R.; Klein, S.L.; et al. Sex and gender: Modifiers of health, disease, and medicine. Lancet 2020, 396, 565–582. [Google Scholar] [CrossRef]
- Shiotani, A.; Katsumata, R.; Gouda, K.; Fukushima, S.; Nakato, R.; Murao, T.; Ishii, M.; Fujita, M.; Matsumoto, H.; Sakakibara, T. Hypergastrinemia in Long-Term Use of Proton Pump Inhibitors. Digestion 2018, 97, 154–162. [Google Scholar] [CrossRef]
- Helgadottir, H.; Lund, S.H.; Gizurarson, S.; Metz, D.C.; Bjornsson, E.S. Predictors of Gastrin Elevation Following Proton Pump Inhibitor Therapy. J. Clin. Gastroenterol. 2020, 54, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Jansen, J.B.; Klinkenberg-Knol, E.C.; Meuwissen, S.G.; De Bruijne, J.W.; Festen, H.P.; Snel, P.; Luckers, A.E.; Biemond, I.; Lamers, C.B. Effect of long-term treatment with omeprazole on serum gastrin and serum group A and C pepsinogens in patients with reflux esophagitis. Gastroenterology 1990, 99, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Koop, H.; Klein, M.; Arnold, R. Serum gastrin levels during long-term omeprazole treatment. Aliment. Pharmacol. Ther. 1990, 4, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Sokic-Milutinovic, A.; Todorovic, V.; Milosavljevic, T.; Micev, M.; Drndarevic, N.; Mitrovic, O. Gastrin and antral G cells in course of Helicobacter pylori eradication: Six months follow up study. World J. Gastroenterol. 2005, 11, 4140–4147. [Google Scholar] [CrossRef]
- Veysey-Smith, R.; Moore, A.R.; Murugesan, S.V.; Tiszlavicz, L.; Dockray, G.J.; Varro, A.; Pritchard, D.M. Effects of Proton Pump Inhibitor Therapy, H. pylori Infection and Gastric Preneoplastic Pathology on Fasting Serum Gastrin Concentrations. Front. Endocrinol. 2021, 12, 741887. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.S.; Varro, A.; Lightdale, C.J.; Lertkowit, N.; Slack, K.N.; Fingerhood, M.L.; Tsai, W.Y.; Wang, T.C.; Abrams, J.A. Elevated serum gastrin is associated with a history of advanced neoplasia in Barrett’s esophagus. Am. J. Gastroenterol. 2010, 105, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Brunner, G.; Athmann, C.; Schneider, A. Long-term, open-label trial: Safety and efficacy of continuous maintenance treatment with pantoprazole for up to 15 years in severe acid-peptic disease. Aliment. Pharmacol. Ther. 2012, 36, 37–47. [Google Scholar] [CrossRef]
- Helgadottir, H.; Metz, D.C.; Yang, Y.X.; Rhim, A.D.; Bjornsson, E.S. The effects of long-term therapy with proton pump inhibitors on meal stimulated gastrin. Dig. Liver Dis. 2014, 46, 125–130. [Google Scholar] [CrossRef]
- Helgadottir, H. Proton Pump Inhibitors. Acid Rebound, Development and Predictors of Gastrin Elevation and Dosage Based in Gender. Ph.D. Thesis, University of Iceland, Reykjavík, Iceland, 2019. [Google Scholar]
- Korman, M.G.; Hansky, J.; Strickland, R.G. Progressive increase in the functional G cell mass with age in atrophic gastritis. Gut 1973, 14, 549–551. [Google Scholar] [CrossRef]
- Waldum, H.L.; Arnestad, J.S.; Brenna, E.; Eide, I.; Syversen, U.; Sandvik, A.K. Marked increase in gastric acid secretory capacity after omeprazole treatment. Gut 1996, 39, 649–653. [Google Scholar] [CrossRef]
- Prewett, E.J.; Smith, J.T.; Nwokolo, C.U.; Sawyerr, A.M.; Pounder, R.E. Twenty-four hour intragastric acidity and plasma gastrin concentration profiles in female and male subjects. Clin. Sci. 1991, 80, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Gedde-Dahl, D. Radioimmunoassay of gastrin. Fasting serum levels in humans with normal and high gastric acid secreation. Scand. J. Gastroenterol. 1974, 9, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Archimandritis, A.; Alegakis, G.; Theodoropoulos, G.; Kalos, A.; Drivas, G.; Melissinos, K. Serum gastrin concentrations in healthy males and females of various ages. Acta Hepato-Gastroenterol. 1979, 26, 58–63. [Google Scholar]
- Feldman, M.; Richardson, C.T.; Walsh, J.H. Sex-related differences in gastrin release and parietal cell sensitivity to gastrin in healthy human beings. J. Clin. Investig. 1983, 71, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Mossi, S.; Meyer-Wyss, B.; Renner, E.L.; Merki, H.S.; Gamboni, G.; Beglinger, C. Influence of Helicobacter pylori, sex, and age on serum gastrin and pepsinogen concentrations in subjects without symptoms and patients with duodenal ulcers. Gut 1993, 34, 752–756. [Google Scholar] [CrossRef] [PubMed]
- Camilo, S.M.; Almeida, E.C.; Miranzi, B.A.; Silva, J.C.; Nomelini, R.S.; Etchebehere, R.M. Endoscopic and histopathologic gastric changes in chronic users of proton-pump inhibitors. Arq. Gastroenterol. 2015, 52, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Helgadottir, H.; Lund, S.H.; Gizurarson, S.; Waldum, H.; Bjornsson, E.S. Pharmacokinetics of single and repeated oral doses of esomeprazole and gastrin elevation in healthy males and females. Scand. J. Gastroenterol. 2021, 56, 128–136. [Google Scholar] [CrossRef]
- Card, W.I.; Marks, I.N. The relationship between the acid output of the stomach following “maximal” histamine stimulation and the parietal cell mass. Clin. Sci. 1960, 19, 147–163. [Google Scholar]
- Cox, A.J. Stomach size and its relation to chronic peptic ulcer. AMA Arch. Pathol. 1952, 54, 407–422. [Google Scholar]
- Leblanc, V.; Begin, C.; Corneau, L.; Dodin, S.; Lemieux, S. Gender differences in dietary intakes: What is the contribution of motivational variables? J. Hum. Nutr. Diet. 2015, 28, 37–46. [Google Scholar] [CrossRef]
- Sadik, R.; Abrahamsson, H.; Stotzer, P.O. Gender differences in gut transit shown with a newly developed radiological procedure. Scand. J. Gastroenterol. 2003, 38, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Graff, J.; Brinch, K.; Madsen, J.L. Gastrointestinal mean transit times in young and middle-aged healthy subjects. Clin. Physiol. 2001, 21, 253–259. [Google Scholar] [CrossRef]
- Slagter, S.N.; van Waateringe, R.P.; van Beek, A.P.; van der Klauw, M.M.; Wolffenbuttel, B.H.R.; van Vliet-Ostaptchouk, J.V. Sex, BMI and age differences in metabolic syndrome: The Dutch Lifelines Cohort Study. Endocr. Connect. 2017, 6, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Ala, S.; Zanad, F.; Shiran, M.R. Population pharmacokinetics of omeprazole in a random Iranian population. Caspian J. Intern. Med. 2013, 4, 712–716. [Google Scholar] [PubMed]
- Anderson, G.D. Sex and racial differences in pharmacological response: Where is the evidence? Pharmacogenetics, pharmacokinetics, and pharmacodynamics. J. Womens Health 2005, 14, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Hagymasi, K.; Mullner, K.; Herszenyi, L.; Tulassay, Z. Update on the pharmacogenomics of proton pump inhibitors. Pharmacogenomics 2011, 12, 873–888. [Google Scholar] [CrossRef] [PubMed]
- McColl, K.E.; Kennerley, P. Proton pump inhibitors--differences emerge in hepatic metabolism. Dig. Liver Dis. 2002, 34, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Hunt, C.M.; Westerkam, W.R.; Stave, G.M. Effect of age and gender on the activity of human hepatic CYP3A. Biochem. Pharmacol. 1992, 44, 275–283. [Google Scholar] [CrossRef]
- Harris, R.Z.; Benet, L.Z.; Schwartz, J.B. Gender effects in pharmacokinetics and pharmacodynamics. Drugs 1995, 50, 222–239. [Google Scholar] [CrossRef]
- Tamminga, W.J.; Wemer, J.; Oosterhuis, B.; Weiling, J.; Wilffert, B.; de Leij, L.F.; de Zeeuw, R.A.; Jonkman, J.H. CYP2D6 and CYP2C19 activity in a large population of Dutch healthy volunteers: Indications for oral contraceptive-related gender differences. Eur. J. Clin. Pharmacol. 1999, 55, 177–184. [Google Scholar] [CrossRef]
- Wolbold, R.; Klein, K.; Burk, O.; Nussler, A.K.; Neuhaus, P.; Eichelbaum, M.; Schwab, M.; Zanger, U.M. Sex is a major determinant of CYP3A4 expression in human liver. Hepatology 2003, 38, 978–988. [Google Scholar] [CrossRef] [PubMed]
- Pregun, I.; Herszenyi, L.; Juhasz, M.; Miheller, P.; Hritz, I.; Patocs, A.; Racz, K.; Tulassay, Z. Effect of proton-pump inhibitor therapy on serum chromogranin a level. Digestion 2011, 84, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.K.; Walt, R.P.; Pounder, R.E.; Gomes, M.D.; Wood, E.C.; Logan, L.H. Optimal dose of oral omeprazole for maximal 24 hour decrease of intragastric acidity. Gut 1984, 25, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Ligumsky, M.; Lysy, J.; Siguencia, G.; Friedlander, Y. Effect of long-term, continuous versus alternate-day omeprazole therapy on serum gastrin in patients treated for reflux esophagitis. J. Clin. Gastroenterol. 2001, 33, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Sanduleanu, S.; Stridsberg, M.; Jonkers, D.; Hameeteman, W.; Biemond, I.; Lundqvist, G.; Lamers, C.; Stockbrugger, R.W. Serum gastrin and chromogranin A during medium- and long-term acid suppressive therapy: A case-control study. Aliment. Pharmacol. Ther. 1999, 13, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Bonapace, E.S.; Fisher, R.S.; Parkman, H.P. Does fasting serum gastrin predict gastric acid suppression in patients on proton-pump inhibitors? Dig. Dis. Sci. 2000, 45, 34–39. [Google Scholar] [CrossRef]
- Bjornsson, E.; Abrahamsson, H.; Simren, M.; Mattsson, N.; Jensen, C.; Agerforz, P.; Kilander, A. Discontinuation of proton pump inhibitors in patients on long-term therapy: A double-blind, placebo-controlled trial. Aliment. Pharmacol. Ther. 2006, 24, 945–954. [Google Scholar] [CrossRef]
- Hendricks, E.; Ajmeri, A.N.; Singh, M.M.; Mongalo, M.; Goebel, L.J. A Randomized Open-Label Study of Two Methods of Proton Pump Inhibitors Discontinuation. Cureus 2021, 13, e15022. [Google Scholar] [CrossRef]
- Inadomi, J.M.; McIntyre, L.; Bernard, L.; Fendrick, A.M. Step-down from multiple- to single-dose proton pump inhibitors (PPIs): A prospective study of patients with heartburn or acid regurgitation completely relieved with PPIs. Am. J. Gastroenterol. 2003, 98, 1940–1944. [Google Scholar] [CrossRef]
- Cote, G.A.; Ferreira, M.R.; Rozenberg-Ben-Dror, K.; Howden, C.W. Programme of stepping down from twice daily proton pump inhibitor therapy for symptomatic gastro-oesophageal reflux disease associated with a formulary change at a VA medical center. Aliment. Pharmacol. Ther. 2007, 25, 709–714. [Google Scholar] [CrossRef]
- Halfdanarson, O.O.; Pottegard, A.; Bjornsson, E.S.; Lund, S.H.; Ogmundsdottir, M.H.; Steingrimsson, E.; Ogmundsdottir, H.M.; Zoega, H. Proton-pump inhibitors among adults: A nationwide drug-utilization study. Therap. Adv. Gastroenterol. 2018, 11, 1756284818777943. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, V.; Tzadok, R.; Chodick, G.; Kariv, R. Proton pump inhibitors long term use-trends and patterns over 15 years of a large health maintenance organization. Pharmacoepidemiol. Drug Saf. 2021, 30, 1576–1587. [Google Scholar] [CrossRef] [PubMed]
- Othman, F.; Card, T.R.; Crooks, C.J. Proton pump inhibitor prescribing patterns in the UK: A primary care database study. Pharmacoepidemiol. Drug Saf. 2016, 25, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Pottegard, A.; Broe, A.; Hallas, J.; de Muckadell, O.B.; Lassen, A.T.; Lodrup, A.B. Use of proton-pump inhibitors among adults: A Danish nationwide drug utilization study. Therap. Adv. Gastroenterol. 2016, 9, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Shanika, L.G.T.; Reynolds, A.; Pattison, S.; Braund, R. Proton pump inhibitor use: Systematic review of global trends and practices. Eur. J. Clin. Pharmacol. 2023, 79, 1159–1172. [Google Scholar] [CrossRef]
- Maes, M.L.; Fixen, D.R.; Linnebur, S.A. Adverse effects of proton-pump inhibitor use in older adults: A review of the evidence. Ther. Adv. Drug Saf. 2017, 8, 273–297. [Google Scholar] [CrossRef]
- McColl, K.E.; Gillen, D. Evidence that proton-pump inhibitor therapy induces the symptoms it is used to treat. Gastroenterology 2009, 137, 20–22. [Google Scholar] [CrossRef]
- Moayyedi, P.; Mason, J. Clinical and economic consequences of dyspepsia in the community. Gut 2002, 50 (Suppl. S4), iv10–iv12. [Google Scholar] [CrossRef]
- Bytzer, P.; van Zanten, S.V.; Mattsson, H.; Wernersson, B. Partial symptom-response to proton pump inhibitors in patients with non-erosive reflux disease or reflux oesophagitis—A post hoc analysis of 5796 patients. Aliment. Pharmacol. Ther. 2012, 36, 635–643. [Google Scholar] [CrossRef]
- Lundell, L.; Hatlebakk, J.; Galmiche, J.P.; Attwood, S.E.; Ell, C.; Fiocca, R.; Persson, T.; Nagy, P.; Eklund, S.; Lind, T. Long-term effect on symptoms and quality of life of maintenance therapy with esomeprazole 20 mg daily: A post hoc analysis of the LOTUS trial. Curr. Med. Res. Opin. 2015, 31, 65–73. [Google Scholar] [CrossRef]
- Koggel, L.M.; Lantinga, M.A.; Buchner, F.L.; Drenth, J.P.H.; Frankema, J.S.; Heeregrave, E.J.; Heringa, M.; Numans, M.E.; Siersema, P.D. Predictors for inappropriate proton pump inhibitor use: Observational study in primary care. Br. J. Gen. Pract. 2022, 72, e899–e906. [Google Scholar] [CrossRef] [PubMed]
- Voukelatou, P.; Vrettos, I.; Emmanouilidou, G.; Dodos, K.; Skotsimara, G.; Kontogeorgou, D.; Kalliakmanis, A. Predictors of Inappropriate Proton Pump Inhibitors Use in Elderly Patients. Curr. Gerontol. Geriatr. Res. 2019, 2019, 7591045. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.A.R.; Hassan, J.; Egan, L.J. Review of recent evidence on the management of heartburn in pregnant and breastfeeding women. BMC Gastroenterol. 2022, 22, 219. [Google Scholar] [CrossRef] [PubMed]
- Waldum, H.L.; Hauso, O.; Brenna, E.; Qvigstad, G.; Fossmark, R. Does long-term profound inhibition of gastric acid secretion increase the risk of ECL cell-derived tumors in man? Scand. J. Gastroenterol. 2016, 51, 767–773. [Google Scholar] [CrossRef]
- Freeman, H.J. Proton pump inhibitors and an emerging epidemic of gastric fundic gland polyposis. World J. Gastroenterol. 2008, 14, 1318–1320. [Google Scholar] [CrossRef]
- Gao, W.; Huang, Y.; Lu, S.; Li, C. The clinicopathological characteristics of gastric polyps and the relationship between fundic gland polyps, Helicobacter pylori infection, and proton pump inhibitors. Ann. Palliat. Med. 2021, 10, 2108–2114. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, S.; Osawa, H.; Hayashi, Y.; Sakamoto, H.; Miura, Y.; Lefor, A.K.; Yamamoto, H. Changes in gastric morphology during long-term use of vonoprazan compared to proton pump inhibitors. Singapore Med. J. 2022, 63, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Sano, W.; Inoue, F.; Hirata, D.; Iwatate, M.; Hattori, S.; Fujita, M.; Sano, Y. Sporadic fundic gland polyps with dysplasia or carcinoma: Clinical and endoscopic characteristics. World J. Gastrointest. Oncol. 2021, 13, 662–672. [Google Scholar] [CrossRef]
- Murphy, G.; Abnet, C.C.; Choo-Wosoba, H.; Vogtmann, E.; Weinstein, S.J.; Taylor, P.R.; Mannisto, S.; Albanes, D.; Dawsey, S.M.; Rehfeld, J.F.; et al. Serum gastrin and cholecystokinin are associated with subsequent development of gastric cancer in a prospective cohort of Finnish smokers. Int. J. Epidemiol. 2017, 46, 914–923. [Google Scholar] [CrossRef]
- Abrahami, D.; McDonald, E.G.; Schnitzer, M.E.; Barkun, A.N.; Suissa, S.; Azoulay, L. Proton pump inhibitors and risk of gastric cancer: Population-based cohort study. Gut 2022, 71, 16–24. [Google Scholar] [CrossRef]
- Snir, Y.; Leibovitzh, H.; Leibovici-Weissman, Y.; Vilkin, A.; Cohen, A.D.; Shochat, T.; Niv, Y.; Dotan, I.; Feldhamer, I.; Boltin, D.; et al. Dose-dependent association of proton pump inhibitors use with gastric intestinal metaplasia among Helicobacter pylori-positive patients. United Eur. Gastroenterol. J. 2021, 9, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Poly, T.N.; Walther, B.A.; Dubey, N.K.; Anggraini Ningrum, D.N.; Shabbir, S.A.; Jack Li, Y.C. Adverse outcomes of long-term use of proton pump inhibitors: A systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 2018, 30, 1395–1405. [Google Scholar] [CrossRef] [PubMed]
- Jaynes, M.; Kumar, A.B. The risks of long-term use of proton pump inhibitors: A critical review. Ther. Adv. Drug Saf. 2019, 10, 2042098618809927. [Google Scholar] [CrossRef] [PubMed]
- Luk, C.P.; Parsons, R.; Lee, Y.P.; Hughes, J.D. Proton pump inhibitor-associated hypomagnesemia: What do FDA data tell us? Ann. Pharmacother. 2013, 47, 773–780. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, L.; Jia, M.; Wang, J.; Shen, Z.; Wang, S.; Zhang, X.; Xu, J.; Zheng, Z.; Lv, X.; et al. Syndrome of inappropriate antidiuretic hormone secretion is associated with different proton pump inhibitor use: A pharmacovigilance study. BMC Nephrol. 2022, 23, 191. [Google Scholar] [CrossRef]
- Yang, Y.X.; Metz, D.C. Safety of proton pump inhibitor exposure. Gastroenterology 2010, 139, 1115–1127. [Google Scholar] [CrossRef]



| Author, Year | N (M/F) | Subjects | Sex Difference | Baseline Gastrin (M vs. F) | Comments |
|---|---|---|---|---|---|
| Gedde-Dahl, 1974 [33] | 298 (180/118) | Patients | No | 67 (45) vs. 65 (40) (mean (SD)) | Patients with different diseases undergoing pentagastrin test |
| Archimandritis et al., 1979 [34] | 80 (43/37) | Healthy volunteers | No | 54 (3) vs. 56.5 (3) pg/cc (mean (SEM)) | No gender difference found 10 and 40 min after a meal |
| Feldman et al., 1983 [35] | 41 (26/15) | Healthy volunteers | Yes | - | Females had higher basal and meal-stimulated gastrin levels, with average rises of 19 (2) vs. 53 (10) pg/mL (p < 0.001). |
| Prewett et al., 1991 [32] | 131 (96/35) | Healthy volunteers | Yes | 185 vs. 407 | Gastrin values are 24 h integrated plasma gastrin concentrations (pmol⋅h⋅L−1). |
| Mossi et al., 1993 [36] | 62 (30/32) | Healthy volunteers, H. pylori (-) | No | 73 (5) vs. 74 (5) pg/mL (mean (SEM)) | No association between sex or age and baseline gastrin levels |
| Jansen et al., 1990 [22] | 32 (18/14) | EE patients | No | Baseline (p = NS) | At all-time intervals, females had higher fasting gastrin levels than males. Eight patients reached gastrin levels > 6 times the upper limit of normal range during follow-up (5 females). |
| on PPIs | Yes | 18 months (p < 0.01) | |||
| Yes | 21 months (p < 0.05) | ||||
| Wang et al., 2010 [26] | 95 (67/28) | BE and GERD patients on chronic PPIs | No | 65 vs. 80 pM (mean) 40 vs. 47 pM (median) | No association between sex or age and baseline gastrin levels |
| Camilo et al., 2015 [37] | 81 (13/68) | Chronic PPI users | - | - | Females were the only patients with gastrin levels > 115 pg/mL. |
| Shiotani et al., 2018 [20] | 199 (143/56) | CV patients on PPIs, prophylaxis with aspirin | Yes | 214 vs. 357 pg/mL | The F gender was associated with hypergastrinemia in a multiple logistic regression analysis, adjusted also for PPI use (vs. H2RAs and controls) and corpus atrophy. |
| Helgadottir et al., 2020 [21] | 157 (79/78) | GERD patients on long-term PPIs | Yes | 60 (42–90) vs. 92 (53–118) pg/mL (median (IQR)) | Gastrin elevation was significantly associated with the F sex and PPI dosage. |
| Helgadottir et al., 2021 [38] | 29 (14/15) | Healthy volunteers on short-term PPIs | Yes | Day 0 (12 vs. 7 pM) | Females had significantly higher baseline gastrin levels than males, but there was no significant difference between the sexes at the end of treatment (day 5). |
| No | Day 5 (15 vs. 15 pM) |
| Females have a lower number of parietal cells [39]. |
| Females are less sensitive to gastrin effects on parietal cells [35]. |
| Smaller stomachs of females, with more postprandial distension [40] |
| Gender differences in dietary intakes, lower energy density in females than in males [41] |
| Gender differences in gastric emptying, with slower gastric emptying in females [42,43] |
| Gender difference in body mass index (BMI) [44] |
| Sex hormones (unlikely, as no fluctuations in gastrin release throughout one menstrual cycle were observed in six females) [35] |
| Difference in metabolism of PPIs [45,46] |
| Author, Year | N (M/F) | Subjects | Type of Study | Sex Variable Mentioned | Type of PPI Deprescribing | Predictors and Other Comments |
|---|---|---|---|---|---|---|
| Inadomi et al., 2003 [60] | 117 (112/5) | Patients with heartburn or acid regurgitatio | Prospective study | No | Step-down from multiple- to single-dose | Only the duration of PPI use before study predicted successful step-down (OR 0.66). |
| Bjornsson et al., 2006 [58] | 96 (44/52) | Patients without history of PUD or EE | Double-blind, placebo-controlled trial | No | Step-off | GERD as PPI indication (OR 8.050) and serum gastrin (OR 1.018) predicted the need for reinstitution of PPIs after discontinuation. |
| Cote et al., 2007 [61] | 223 | GERD patients | Retrospective study | Very few females (~1%) | Step-down from BID to SID | Dose reduction was more successful in those without EE. |
| Fass et al., 2012 [13] | 142 (62/80) | Symptomatic GERD patients | Single-blind trial | Yes | Step-down from BID to SID modified release PPI | No predictor was significant (age, sex, BMI, and baseline symptom scores). But OR for females not remaining well controlled was 0.499, NS. |
| Helgadottir et al., 2017 [12] | 100 (51/49) | EE patients | Double-blind randomized trial | Yes | 50% dose reduction | Successful step-down was predicted only by female sex with OR 1.3 (p = 0.048). Baseline fasting s-gastrin was NS (p = 0.49). |
| Hendricks et al., 2021 [59] | 33 (19/15) | Patients with a clinical diagnosis of GERD | Randomized open-label trial | Yes | Step-off | Sex was not associated with resuming PPIs. H2RA use was associated with successful discontinuation of PPIs with HR 0.21 (p = 0.002). |
| Sex | Diagnosis | PPI Overmedication? |
|---|---|---|
| Female > Male | Non-erosive reflux disease Heartburn or regurgitation Extra-esophageal symptoms Comorbid anxiety or depression | Females at risk for overmedication because of partial symptom response and unnecessary PPI dose escalation for symptoms that are not related to acid reflux |
| Male > Female | Reflux esophagitis Barrett’s esophagus Esophageal adenocarcinoma | Females at risk for overmedication because they might be more sensitive to PPIs than males and could remain symptom control on lower PPI doses |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Helgadottir, H.; Björnsson, E.S. The Impact of Sex on the Response to Proton Pump Inhibitor Treatment. Pharmaceuticals 2023, 16, 1722. https://doi.org/10.3390/ph16121722
Helgadottir H, Björnsson ES. The Impact of Sex on the Response to Proton Pump Inhibitor Treatment. Pharmaceuticals. 2023; 16(12):1722. https://doi.org/10.3390/ph16121722
Chicago/Turabian StyleHelgadottir, Holmfridur, and Einar S. Björnsson. 2023. "The Impact of Sex on the Response to Proton Pump Inhibitor Treatment" Pharmaceuticals 16, no. 12: 1722. https://doi.org/10.3390/ph16121722
APA StyleHelgadottir, H., & Björnsson, E. S. (2023). The Impact of Sex on the Response to Proton Pump Inhibitor Treatment. Pharmaceuticals, 16(12), 1722. https://doi.org/10.3390/ph16121722