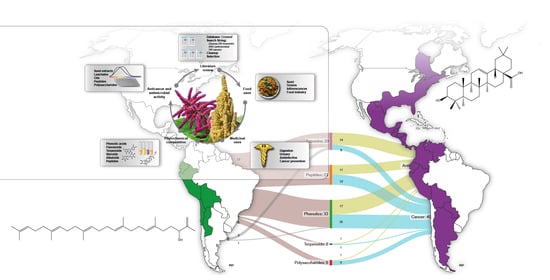
Chenopodium quinoa Willd. and Amaranthus hybridus L.: Ancestral Andean Food Security and Modern Anticancer and Antimicrobial Activity
Abstract
:1. Introduction
2. Method
3. Background
4. Nutritional Properties and Food Uses
5. Medicinal Uses
6. Phytochemical Composition
7. Biological Activity
7.1. Overview
7.2. Antimicrobial Activity
7.3. Anticancer Activity
7.3.1. Anticancer Activity of C. quinoa
7.3.2. Anticancer Activity of A. hybridus
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jaikishun, S.; Li, W.; Yang, Z.; Song, S. Quinoa: In Perspective of Global Challenges. Agronomy 2019, 9, 176. [Google Scholar] [CrossRef]
- Bazile, D.; Jacobsen, S.-E.; Verniau, A. The Global Expansion of Quinoa: Trends and Limits. Front. Plant Sci. 2016, 7, 622. [Google Scholar] [CrossRef] [PubMed]
- Bellemare, M.F.; Fajardo-Gonzalez, J.; Gitter, S.R. Foods and Fads: The Welfare Impacts of Rising Quinoa Prices in Peru. World Dev. 2018, 112, 163–179. [Google Scholar] [CrossRef]
- Schmidt, D.; Verruma-Bernardi, M.R.; Forti, V.A.; Borges, M.T.M.R. Quinoa and Amaranth as Functional Foods: A Review. Food Rev. Int. 2023, 39, 2277–2296. [Google Scholar] [CrossRef]
- Joshi, R.P.; Jain, A.K.; Malhotra, N.; Kumari, M. Chapter 4—Origin, Domestication, and Spread. In Millets and Pseudo Cereals; Singh, M., Sood, S., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2021; pp. 33–38. ISBN 978-0-12-820089-6. [Google Scholar]
- Fernández-Ríos, A.; Laso, J.; Hoehn, D.; Amo-Setién, F.J.; Abajas-Bustillo, R.; Ortego, C.; Fullana-i-Palmer, P.; Bala, A.; Batlle-Bayer, L.; Balcells, M.; et al. A Critical Review of Superfoods from a Holistic Nutritional and Environmental Approach. J. Clean. Prod. 2022, 379, 134491. [Google Scholar] [CrossRef]
- Serwecińska, L. Antimicrobials and Antibiotic-Resistant Bacteria: A Risk to the Environment and to Public Health. Water 2020, 12, 3313. [Google Scholar] [CrossRef]
- Urban-Chmiel, R.; Marek, A.; Stępień-Pyśniak, D.; Wieczorek, K.; Dec, M.; Nowaczek, A.; Osek, J. Antibiotic Resistance in Bacteria—A Review. Antibiotics 2022, 11, 1079. [Google Scholar] [CrossRef]
- Chokshi, A.; Sifri, Z.; Cennimo, D.; Horng, H. Global Contributors to Antibiotic Resistance. J. Glob. Infect. Dis. 2019, 11, 36. [Google Scholar] [CrossRef]
- Miethke, M.; Pieroni, M.; Weber, T.; Brönstrup, M.; Hammann, P.; Halby, L.; Arimondo, P.B.; Glaser, P.; Aigle, B.; Bode, H.B.; et al. Towards the Sustainable Discovery and Development of New Antibiotics. Nat. Rev. Chem. 2021, 5, 726–749. [Google Scholar] [CrossRef]
- Calixto, J.B. The Role of Natural Products in Modern Drug Discovery. An. Acad. Bras. Ciênc. 2019, 91, e20190105. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Hashim, D.; Boffetta, P.; La Vecchia, C.; Rota, M.; Bertuccio, P.; Malvezzi, M.; Negri, E. The Global Decrease in Cancer Mortality: Trends and Disparities. Ann. Oncol. 2016, 27, 926–933. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Huang, M.; Lu, J.-J.; Ding, J. Natural Products in Cancer Therapy: Past, Present and Future. Nat. Prod. Bioprospect. 2021, 11, 5–13. [Google Scholar] [CrossRef]
- Masrour-Roudsari, J.; Ebrahimpour, S. Causal Role of Infectious Agents in Cancer: An Overview. Casp. J. Intern. Med. 2017, 8, 153–158. [Google Scholar] [CrossRef]
- Benharroch, D.; Osyntsov, L. Infectious Diseases Are Analogous with Cancer. Hypothesis and Implications. J. Cancer 2012, 3, 117. [Google Scholar] [CrossRef]
- Digital Science Dimensions. Available online: https://app.dimensions.ai/ (accessed on 25 May 2022).
- Paul, J.; Lim, W.M.; O’Cass, A.; Hao, A.W.; Bresciani, S. Scientific Procedures and Rationales for Systematic Literature Reviews (SPAR-4-SLR). Int. J. Consum. Stud. 2021, 45, O1–O16. [Google Scholar] [CrossRef]
- Visser, M.; van Eck, N.J.; Waltman, L. Large-Scale Comparison of Bibliographic Data Sources: Scopus, Web of Science, Dimensions, Crossref, and Microsoft Academic. Quant. Sci. Stud. 2021, 2, 20–41. [Google Scholar] [CrossRef]
- Australian Bureau of Statistics Australian and New Zealand Standard Research Classification (ANZSRC). Available online: https://www.abs.gov.au/statistics/classifications/australian-and-new-zealand-standard-research-classification-anzsrc/latest-release (accessed on 24 July 2022).
- International Plant Names Index the International Plant Names Index and World Checklist of Vascular Plants. Available online: https://powo.science.kew.org/ (accessed on 18 October 2023).
- POWO Chenopodium quinoa Willd. Available online: http://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:165175-1 (accessed on 28 September 2023).
- Fuentes, F.F.; Martinez, E.A.; Hinrichsen, P.V.; Jellen, E.N.; Maughan, P.J. Assessment of Genetic Diversity Patterns in Chilean Quinoa (Chenopodium quinoa Willd.) Germplasm Using Multiplex Fluorescent Microsatellite Markers. Conserv. Genet. 2009, 10, 369–377. [Google Scholar] [CrossRef]
- Joshi, D.C.; Sood, S.; Hosahatti, R.; Kant, L.; Pattanayak, A.; Kumar, A.; Yadav, D.; Stetter, M.G. From Zero to Hero: The Past, Present and Future of Grain Amaranth Breeding. Theor. Appl. Genet. 2018, 131, 1807–1823. [Google Scholar] [CrossRef]
- POWO. Amaranthus hybridus L. Available online: http://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:10648-2 (accessed on 28 September 2023).
- Song, L.M.; Yu, Y.; Du, L.D.; Ji, X.Y.; Gao, H.; Cai, Y.Q.; Li, C.J.; Xue, P. Does Saponin in Quinoa Really Embody the Source of Its Bitterness? Food Chem. 2024, 437, 137872. [Google Scholar] [CrossRef]
- Gómez Prado, L.; Aguilar Castellanos, E. Guía de Cultivo de La Quínoa, 2nd ed.; Universidad Agraria Nacional La Molina: Lima, Perú, 2016; ISBN 978-92-5-309069-3. [Google Scholar]
- Toderich, K.N.; Mamadrahimov, A.A.; Khaitov, B.B.; Karimov, A.A.; Soliev, A.A.; Nanduri, K.R.; Shuyskaya, E.V. Differential Impact of Salinity Stress on Seeds Minerals, Storage Proteins, Fatty Acids, and Squalene Composition of New Quinoa Genotype, Grown in Hyper-Arid Desert Environments. Front. Plant Sci. 2020, 11, 607102. [Google Scholar] [CrossRef]
- Aguirre Mendoza, Z.; Jaramillo Díaz, N.; Quizhpe Coronel, W. Arvenses Asociadas a Cultivos y Pastizales del Ecuador; Universidad Nacional de Loja: Loja, Ecuador, 2019; ISBN 978-9978-355-40-4. [Google Scholar]
- Calizaya, F.; Gómez, L.; Zegarra, J.; Pozo, M.; Mindani, C.; Caira, C.; Calizaya, E. Unveiling Ancestral Sustainability: A Comprehensive Study of Economic, Environmental, and Social Factors in Potato and Quinoa Cultivation in the Highland Aynokas of Puno, Peru. Sustainability 2023, 15, 13163. [Google Scholar] [CrossRef]
- Taaime, N.; Rafik, S.; El Mejahed, K.; Oukarroum, A.; Choukr-Allah, R.; Bouabid, R.; El Gharous, M. Worldwide Development of Agronomic Management Practices for Quinoa Cultivation: A Systematic Review. Front. Agron. 2023, 5, 1215441. [Google Scholar] [CrossRef]
- López, M.L.; Recalde, M.A. The First Quinoa (Chenopodium quinoa Willd) Macrobotanical Remains at Sierras Del Norte (Central Argentina) and Their Implications in Pre-Hispanic Subsistence Practices. J. Archaeol. Sci. Rep. 2016, 8, 426–433. [Google Scholar] [CrossRef]
- Lost Crops of the Incas: Little-Known Plants of the Andes with Promise for Worldwide Cultivation; National Academies Press: Washington, DC, USA, 1989; ISBN 978-0-309-04264-2.
- Bermejo, J.E.H.; León, J. Neglected Crops: 1492 from a Different Perspective; FAO: Rome, Italy, 1994; ISBN 978-92-5-103217-6. [Google Scholar]
- Rodríguez Gómez, M.J.; Matías Prieto, J.; Cruz Sobrado, V.; Calvo Magro, P. Nutritional Characterization of Six Quinoa (Chenopodium quinoa Willd) Varieties Cultivated in Southern Europe. J. Food Compos. Anal. 2021, 99, 103876. [Google Scholar] [CrossRef]
- Abd El-Hakim, A.F.; Mady, E.; Abou Tahoun, A.M.; Ghaly, M.S.A.; Eissa, M.A. Seed Quality and Protein Classification of Some Quinoa Varieties. J. Ecol. Eng. 2022, 23, 24–33. [Google Scholar] [CrossRef]
- Shi, D.; Fidelis, M.; Ren, Y.; Stone, A.K.; Ai, Y.; Nickerson, M.T. The Functional Attributes of Peruvian (Kankolla and Blanca Juli Blend) and Northern Quinoa (NQ94PT) Flours and Protein Isolates, and Their Protein Quality. Food Res. Int. 2020, 128, 108799. [Google Scholar] [CrossRef]
- Baraniak, J.; Kania-Dobrowolska, M. The Dual Nature of Amaranth—Functional Food and Potential Medicine. Foods 2022, 11, 618. [Google Scholar] [CrossRef]
- Aja, P.M.; Ale, B.A.; Ekpono, E.U.; Nwite, I.; Aja, L.; Asouzu, N.C.; Njoku, A. Amino Acid Profiles of Solanum Aethiopicum, Amaranthus hybridus, and Telfairia Occidentalis, Common Leafy Vegetables in Nigeria. Sci. Prog. 2021, 104, 00368504211032079. [Google Scholar] [CrossRef]
- Tang, Y.; Tsao, R. Phytochemicals in Quinoa and Amaranth Grains and Their Antioxidant, Anti-inflammatory, and Potential Health Beneficial Effects: A Review. Mol. Nutr. Food Res. 2017, 61, 1600767. [Google Scholar] [CrossRef]
- Santiago-Saenz, Y.O.; Hernández-Fuentes, A.D.; López-Palestina, C.U.; Garrido-Cauich, J.H.; Alatorre-Cruz, J.M.; Monroy-Torres, R.; Santiago-Saenz, Y.O.; Hernández-Fuentes, A.D.; López-Palestina, C.U.; Garrido-Cauich, J.H.; et al. Nutritional Importance and Biological Activity of Bioactive Compounds from Quelites Consumed in Mexico. Rev. Chil. Nutr. 2019, 46, 593–605. [Google Scholar] [CrossRef]
- Reynaga Nava, A. Caracterización Fisica-Quimica de Trece Ecotipos de Quinua Real (Chenopodium quinoa Willd.) del Altiplano Sur de Bolivia Con Fines Agroindustriales; Reynaga Nava, Arturo Edgar: Murillo, Bolivia, 2011; ISBN 978-99954-2-124-3. [Google Scholar]
- Akubugwo, I.E.; Obasi, N.A.; Chinyere, G.C.; Ugbogu, A.E. Nutritional and Chemical Value of Amaranthus hybridus L. Leaves from Afikpo, Nigeria. Afr. J. Biotechnol. 2007, 6, 2833–2839. [Google Scholar] [CrossRef]
- Yaméogo, C.W.; Garanet, F. Minerals Composition of Solanum aethiopicum L. and Amaranthus hybridus L. Leaves from Burkina Faso. Eur. J. Nutr. Food Saf. 2023, 15, 35–41. [Google Scholar] [CrossRef]
- FAO. International Year of Quinoa 2013. Available online: https://www.fao.org/quinoa-2013/ (accessed on 26 October 2022).
- FAO. La Quinua: Cultivo Milenario Para Contribuir a la Seguridad Alimentaria Mundial; FAO: Rome, Italy, 2011; p. 66. [Google Scholar]
- Bailon-Moscoso, N.; Tinitana, F.; Martínez-Espinosa, R.; Jaramillo-Velez, A.; Palacio-Arpi, A.; Aguilar-Hernandez, J.; Romero-Benavides, J.C. Cytotoxic, Antioxidative, Genotoxic and Antigenotoxic Effects of Horchata, Beverage of South Ecuador. BMC Complement. Altern. Med. 2017, 17, 539. [Google Scholar] [CrossRef]
- Rojas-Le-Fort, M.; Valdivieso-López, I.P.; Duarte-Casar, R. Representations of Ecuadorian Cuisine in the Coast and the Highlands Regions through the Free Listing Technique. Discov. Food 2023, 3, 20. [Google Scholar] [CrossRef]
- de la Torre, L.; Navarrete, H.; Muriel, P.; Macía, M.; Balslev, H. Enciclopedia de Las Plantas Útiles del Ecuador; Herbario QCA de la Escuela de Ciencias Biológicas de la Pontificia Universidad Católica del Ecuador: Quito, Ecuador, 2008; ISBN 978-9978-77-135-8. [Google Scholar]
- Filho, A.M.M.; Pirozi, M.R.; Borges, J.T.D.S.; Pinheiro Sant’Ana, H.M.; Chaves, J.B.P.; Coimbra, J.S.D.R. Quinoa: Nutritional, Functional, and Antinutritional Aspects. Crit. Rev. Food Sci. Nutr. 2017, 57, 1618–1630. [Google Scholar] [CrossRef]
- Alandia, G.; Odone, A.; Rodriguez, J.P.; Bazile, D.; Condori, B. Quinoa—Evolution and Future Perspectives. In The Quinoa Genome; Schmöckel, S.M., Ed.; Compendium of Plant Genomes; Springer International Publishing: Cham, Switzerland, 2021; pp. 179–195. ISBN 978-3-030-65237-1. [Google Scholar]
- Salcedo, S.; Santivañez, T. Recetario Internacional de la Quinua: Tradición y Vanguardia; FAO: Rome, Iatly, 2014; ISBN 978-92-5-308058-8. [Google Scholar]
- Ayala Macedo, G. Consumption of Quinoa in Peru. Food Rev. Int. 2003, 19, 221–227. [Google Scholar] [CrossRef]
- Hançer, Ç.K.; Sevgi, E.; Altinbaşak, B.B.; Çakir, E.A.; Akkaya, M. Traditional Knowledge of Wild Edible Plants of Biga (Çanakkale), Turkey. Acta Soc. Bot. Pol. 2020, 89, 8914. [Google Scholar] [CrossRef]
- Hilou, A.; Ouedraogo, I.; Sombié, P.A.E.D.; Guenné, S.; Paré, D.; Compaoré, M. Leafy Amaranthus Consumption Patterns in Ouagadougou, Burkina Faso. Afr. J. Food Agric. Nutr. Dev. 2016, 16, 11248–11264. [Google Scholar] [CrossRef]
- Franco, A.; Arias Giraldo, S.; Anaya García, S.E.; Muñoz Quintero, D. Perspectivas tecnológicas y nutricionales de la quinua (Chenopodium quinoa): Un pseudocereal andino funcional. Rev. Española Nutr. Comunitaria = Span. J. Community Nutr. 2021, 27, 12. [Google Scholar]
- Melini, F.; Melini, V.; Galfo, M. A Cross-Sectional Survey of the Nutritional Quality of Quinoa Food Products Available in the Italian Market. Foods 2023, 12, 1562. [Google Scholar] [CrossRef]
- Balakrishnan, G.; Schneider, R.G. The Role of Amaranth, Quinoa, and Millets for the Development of Healthy, Sustainable Food Products—A Concise Review. Foods 2022, 11, 2442. [Google Scholar] [CrossRef] [PubMed]
- Aderibigbe, O.R.; Ezekiel, O.O.; Owolade, S.O.; Korese, J.K.; Sturm, B.; Hensel, O. Exploring the Potentials of Underutilized Grain Amaranth (Amaranthus spp.) along the Value Chain for Food and Nutrition Security: A Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Repo-Carrasco-Valencia, R.; Basilio-Atencio, J.; Luna-Mercado, G.I.; Pilco-Quesada, S.; Vidaurre-Ruiz, J. Andean Ancient Grains: Nutritional Value and Novel Uses. Biol. Life Sci. Forum 2022, 8, 15. [Google Scholar] [CrossRef]
- Haros, C.M.; Martínez, M.L.; Agostini, B.V.; Muñoz, L.A. Food Uses of Selected Ancient Grains. In Latin-American Seeds; CRC Press: Boca Raton, FL, USA, 2023; ISBN 978-1-00-308842-4. [Google Scholar]
- Mouritsen, O.G.; Rhatigan, P.; Pérez-Lloréns, J.L. The Rise of Seaweed Gastronomy: Phycogastronomy. Bot. Mar. 2019, 62, 195–209. [Google Scholar] [CrossRef]
- Bakouri, H.; Ziane, A.; Guemra, K. Development of Multifunctional Packaging Films Based on Arginine-Modified Chitosan/Gelatin Matrix and Betacyanins from Weed Amaranth (A. Hybridus). Int. J. Biol. Macromol. 2023, 230, 123181. [Google Scholar] [CrossRef]
- Alak, G.; Guler, K.; Ucar, A.; Parlak, V.; Kocaman, E.M.; Yanık, T.; Atamanalp, M. Quinoa as Polymer in Edible Films with Essential Oil: Effects on Rainbow Trout Fillets Shelf Life. J. Food Process. Preserv. 2019, 43, e14268. [Google Scholar] [CrossRef]
- Sharma, A.; Alam, M.; Pant, K.; Nanda, V. Amaranth & Quinoa Sprouts. In Advances in Plant Sprouts: Phytochemistry and Biofunctionalities; Majid, I., Kehinde, B.A., Dar, B., Nanda, V., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 127–151. ISBN 978-3-031-40916-5. [Google Scholar]
- Janmohammadi, H.; Hosseintabar-Ghasemabad, B.; Oliyai, M.; Alijani, S.; Gorlov, I.F.; Slozhenkina, M.I.; Mosolov, A.A.; Suarez Ramirez, L.; Seidavi, A.; Laudadio, V.; et al. Effect of Dietary Amaranth (Amaranthus hybridus Chlorostachys) Supplemented with Enzyme Blend on Egg Quality, Serum Biochemistry and Antioxidant Status in Laying Hens. Antioxidants 2023, 12, 456. [Google Scholar] [CrossRef]
- Asher, A.; Galili, S.; Whitney, T.; Rubinovich, L. The Potential of Quinoa (Chenopodium quinoa) Cultivation in Israel as a Dual-Purpose Crop for Grain Production and Livestock Feed. Sci. Hortic. 2020, 272, 109534. [Google Scholar] [CrossRef]
- Jan, N.; Hussain, S.Z.; Naseer, B.; Bhat, T.A. Amaranth and Quinoa as Potential Nutraceuticals: A Review of Anti-Nutritional Factors, Health Benefits and Their Applications in Food, Medicinal and Cosmetic Sectors. Food Chem. X 2023, 18, 100687. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Li, S.; Roy, D.; Guo, Q.; Ye, A. Modifying Quinoa Protein for Enhanced Functional Properties and Digestibility: A Review. Curr. Res. Food Sci. 2023, 7, 100604. [Google Scholar] [CrossRef] [PubMed]
- Cerdá-Bernad, D.; Valero-Cases, E.; Pastor, J.-J.; Frutos, M.J.; Pérez-Llamas, F. Probiotic Red Quinoa Drinks for Celiacs and Lactose Intolerant People: Study of Functional, Physicochemical and Probiotic Properties during Fermentation and Gastrointestinal Digestion. Int. J. Food Sci. Nutr. 2022, 73, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Granda, L.; Rosero, M.; Rosero, A. Plantas Medicinales de La Región Andina Tropical. Quinoa (Chenopodium quinoa Willd.) y Coca (Erythroxylum sp.), Tesoros Milenarios Para Tratamiento Medicinal. In Etnobotánica y Fitoterapia en América; Mendel University in Brno: Brno, Czech Republic, 2015; ISBN 978-80-7509-349-3. [Google Scholar]
- WHO Anatomical Therapeutic Chemical (ATC) Classification. Available online: https://www.who.int/tools/atc-ddd-toolkit/atc-classification (accessed on 8 June 2021).
- Tinitana, F.; Rios, M.; Romero-Benavides, J.C.; de la Cruz Rot, M.; Pardo-de-Santayana, M. Medicinal Plants Sold at Traditional Markets in Southern Ecuador. J. Ethnobiol. Ethnomed. 2016, 12, 29. [Google Scholar] [CrossRef] [PubMed]
- Ruth, O.N.; Unathi, K.; Nomali, N.; Chinsamy, M. Underutilization Versus Nutritional-Nutraceutical Potential of the Amaranthus Food Plant: A Mini-Review. Appl. Sci. 2021, 11, 6879. [Google Scholar] [CrossRef]
- Kongdang, P.; Dukaew, N.; Pruksakorn, D.; Koonrungsesomboon, N. Biochemistry of Amaranthus Polyphenols and Their Potential Benefits on Gut Ecosystem: A Comprehensive Review of the Literature. J. Ethnopharmacol. 2021, 281, 114547. [Google Scholar] [CrossRef]
- Stigger, A.L.; Marcolongo-Pereira, C.; de Lourdes Adrien, M.; Santos, B.L.; Fiss, L.; Vargas, S.F., Jr.; Grecco, F.B.; Schild, A.L. Intoxicação espontânea por Amaranthus hybridus (Amaranthaceae) em bovinos no sul do Rio Grande do Sul. Pesq. Vet. Bras. 2013, 33, 1004–1008. [Google Scholar] [CrossRef]
- Rios, M.; Tinitana, F.; Jarrín-V, P.; Donoso, N.; Romero-Benavides, J.C. “Horchata” Drink in Southern Ecuador: Medicinal Plants and People’s Wellbeing. J. Ethnobiol. Ethnomed. 2017, 13, 18. [Google Scholar] [CrossRef]
- Peralta, I.E.; Villacrés, E.; Mazón, N.; Rivera, M.M.; Subía, G.C. El Ataco, Sangorache o Amaranto Negro (Amaranthus hybridus L.) en Ecuador; Publicación Miscelánea; INIAP: Quito, Ecuador, 2008; p. 63. [Google Scholar]
- Ibarra-Morales, A.; Pino, I.S.; Solís-Fernández, K.Z. El amaranto en la región maya. Ecofronteras 2021, 25, 71. [Google Scholar]
- Qian, G.; Li, X.; Zhang, H.; Zhang, H.; Zhou, J.; Ma, X.; Sun, W.; Yang, W.; He, R.; Wahab, A.; et al. Metabolomics Analysis Reveals the Accumulation Patterns of Flavonoids and Phenolic Acids in Quinoa (Chenopodium quinoa Willd.) Grains of Different Colors. Food Chem. X 2023, 17, 100594. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, B.; Li, X.; Chen, P.X.; Zhang, H.; Liu, R.; Tsao, R. Bound Phenolics of Quinoa Seeds Released by Acid, Alkaline, and Enzymatic Treatments and Their Antioxidant and α-Glucosidase and Pancreatic Lipase Inhibitory Effects. J. Agric. Food Chem. 2016, 64, 1712–1719. [Google Scholar] [CrossRef]
- Zhengkang, H.; Wang, G.; Yao, W.; Zhu, W. Isoflavonic Phytoestrogens—New Prebiotics for Farm Animals: A Review on Research in China. Curr. Issues Intest. Microbiol. 2006, 7, 53–60. [Google Scholar] [PubMed]
- Ekeke, C.; Manga, T.T.; Mensah, S.I. Comparative Phytochemical, Morphological and Anatomical Studies of Amaranthus hybridus L. and Amaranthus spinosus L. (Amaranthaceae). Res. J. Med. Plants 2019, 13, 53–63. [Google Scholar] [CrossRef]
- Medoua, G.N.; Oldewage-Theron, W.H. Effect of Drying and Cooking on Nutritional Value and Antioxidant Capacity of Morogo (Amaranthus hybridus) a Traditional Leafy Vegetable Grown in South Africa. J. Food Sci. Technol. 2014, 51, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Barthomeuf, C.; Debiton, E.; Mshvildadze, V.; Kemertelidze, E.; Balansard, G. In Vitro Activity of Hederacolchisid A1 Compared with Other Saponins from Hedera Colchica Against Proliferation of Human Carcinoma and Melanoma Cells. Planta Med. 2002, 68, 672–675. [Google Scholar] [CrossRef] [PubMed]
- Ahumada, A.; Ortega, A.; Chito, D.; Benítez, R. Saponinas de quinua (Chenopodium quinoa Willd.): Un subproducto con alto potencial biológico. Rev. Colomb. Cienc. Químico-Farm. 2016, 45, 438–469. [Google Scholar] [CrossRef]
- El Hazzam, K.; Hafsa, J.; Sobeh, M.; Mhada, M.; Taourirte, M.; EL Kacimi, K.; Yasri, A. An Insight into Saponins from Quinoa (Chenopodium quinoa Willd): A Review. Molecules 2020, 25, 1059. [Google Scholar] [CrossRef]
- Fanali, C.; Beccaria, M.; Salivo, S.; Tranchida, P.; Tripodo, G.; Farnetti, S.; Dugo, L.; Dugo, P.; Mondello, L. Non-Polar Lipids Characterization of Quinoa (Chenopodium quinoa) Seed by Comprehensive Two-Dimensional Gas Chromatography with Flame Ionization/Mass Spectrometry Detection and Non-Aqueous Reversed-Phase Liquid Chromatography with Atmospheric Pressure Chemical Ionization Mass Spectrometry Detection. J. Sep. Sci. 2015, 38, 3151–3160. [Google Scholar] [CrossRef]
- Ejoh, S.I.; Wireko-Manu, F.D.; Page, D.; Renard, C.M. Traditional Green Leafy Vegetables as Underutilised Sources of Micronutrients in a Rural Farming Community in South-West Nigeria I: Estimation of Vitamin C, Carotenoids and Mineral Contents. S. Afr. J. Clin. Nutr. 2021, 34, 40–45. [Google Scholar] [CrossRef]
- Nana, F.W.; Hilou, A.; Millogo, J.F.; Nacoulma, O.G. Phytochemical Composition, Antioxidant and Xanthine Oxidase Inhibitory Activities of Amaranthus cruentus L. and Amaranthus hybridus L. Extracts. Pharmaceuticals 2012, 5, 613–628. [Google Scholar] [CrossRef]
- Escribano, J.; Cabanes, J.; Jiménez-Atiénzar, M.; Ibañez-Tremolada, M.; Gómez-Pando, L.R.; García-Carmona, F.; Gandía-Herrero, F. Characterization of Betalains, Saponins and Antioxidant Power in Differently Colored Quinoa (Chenopodium quinoa) Varieties. Food Chem. 2017, 234, 285–294. [Google Scholar] [CrossRef]
- Ďúranová, H.; Fialková, V.; Bilčíková, J.; Lukáč, N.; Kňažická, Z. Lunasin and Its Versatile Health-Promoting Actions. J. Microbiol. Biotechnol. Food Sci. 2019, 8, 1106–1110. [Google Scholar] [CrossRef]
- Maldonado-Cervantes, E.; Jeong, H.J.; León-Galván, F.; Barrera-Pacheco, A.; De León-Rodríguez, A.; de Mejia, E.G.; de Lumen, B.O.; de la Rosa, A.P.B. Amaranth Lunasin-like Peptide Internalizes into the Cell Nucleus and Inhibits Chemical Carcinogen-Induced Transformation of NIH-3T3 Cells. Peptides 2010, 31, 1635–1642. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.H.; Javaid, A. Anticancer, Antimicrobial and Antioxidant Compounds of Quinoa Inflorescence. Adv. Life Sci. 2020, 8, 68–72. [Google Scholar]
- Khan, I.H.; Javaid, A. Identification of Pharmaceutically Important Constituents of Quinoa Root. Jordan J. Pharm. Sci. 2023, 16, 96–102. [Google Scholar] [CrossRef]
- Jy, C.; Jh, M.; Ky, S.; Kh, P. Antimicrobial Activity of 4-Hydroxybenzoic Acid and Trans 4-Hydroxycinnamic Acid Isolated and Identified from Rice Hull. Biosci. Biotechnol. Biochem. 1998, 62, 2273–2276. [Google Scholar] [CrossRef]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simões, M. Antibacterial Activity and Mode of Action of Ferulic and Gallic Acids against Pathogenic Bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef]
- Wu, M.; Tian, L.; Fu, J.; Liao, S.; Li, H.; Gai, Z.; Gong, G. Antibacterial Mechanism of Protocatechuic Acid against Yersinia Enterocolitica and Its Application in Pork. Food Control 2022, 133, 108573. [Google Scholar] [CrossRef]
- Ojha, D.; Patil, K.N. P-Coumaric Acid Inhibits the Listeria Monocytogenes RecA Protein Functions and SOS Response: An Antimicrobial Target. Biochem. Biophys. Res. Commun. 2019, 517, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Jan, R.; Khan, M.; Asaf, S.; Lubna; Asif, S.; Kim, K.-M. Bioactivity and Therapeutic Potential of Kaempferol and Quercetin: New Insights for Plant and Human Health. Plants 2022, 11, 2623. [Google Scholar] [CrossRef] [PubMed]
- Ming, D.; Wang, D.; Cao, F.; Xiang, H.; Mu, D.; Cao, J.; Li, B.; Zhong, L.; Dong, X.; Zhong, X.; et al. Kaempferol Inhibits the Primary Attachment Phase of Biofilm Formation in Staphylococcus aureus. Front. Microbiol. 2017, 8, 2263. [Google Scholar] [CrossRef]
- Imran, M.; Saeed, F.; Hussain, G.; Imran, A.; Mehmood, Z.; Gondal, T.A.; El-Ghorab, A.; Ahmad, I.; Pezzani, R.; Arshad, M.U.; et al. Myricetin: A Comprehensive Review on Its Biological Potentials. Food Sci. Nutr. 2021, 9, 5854–5868. [Google Scholar] [CrossRef]
- Sharma, A.; Kathuria, D.; Kolita, B.; Gohain, A.; Das, A.K.; Bhardwaj, G.; Simal-Gandara, J. Greener Approach for the Isolation of Oleanolic Acid from Nepeta Leucophylla Benth. Its Derivatization and Their Molecular Docking as Antibacterial and Antiviral Agents. Heliyon 2023, 9, e18639. [Google Scholar] [CrossRef]
- Ndukwe, G.I.; Clark, P.D.; Jack, I.R. In Vitro Antioxidant and Antimicrobial Potentials of Three Extracts of Amaranthus hybridus L. Leaf and Their Phytochemicals. Eur. Chem. Bull. 2020, 9, 164–173. [Google Scholar] [CrossRef]
- Al-Mamun, M.A.; Husna, J.; Khatun, M.; Hasan, R.; Kamruzzaman, M.; Hoque, K.M.F.; Reza, M.A.; Ferdousi, Z. Assessment of Antioxidant, Anticancer and Antimicrobial Activity of Two Vegetable Species of Amaranthus in Bangladesh. BMC Complement. Altern. Med. 2016, 16, 157. [Google Scholar] [CrossRef]
- Maiyo, Z.C.; Ngure, R.M.; Matasyoh, J.C.; Chepkorir, R. Phytochemical Constituents and Antimicrobial Activity of Leaf Extracts of Three Amaranthus Plant Species. Afr. J. Biotechnol. 2010, 9, 3178–3182. [Google Scholar]
- Okoye, E.I. Qualitative and Quantitative Phytochemical Analysis and Antimicrobial Screening of Solvent Extracts of Amaranthus hybridus (Stem and Leaves). Chem. Res. J. 2018, 3, 9–13. [Google Scholar]
- Yong, Y.Y.; Dykes, G.; Lee, S.M.; Choo, W.S. Comparative Study of Betacyanin Profile and Antimicrobial Activity of Red Pitahaya (Hylocereus polyrhizus) and Red Spinach (Amaranthus dubius). Plant Foods Hum. Nutr. 2017, 72, 41–47. [Google Scholar] [CrossRef]
- Yelfi, Y.; Susilo, H.; Kurnia, N.M. Mouthwash of Amaranthus hybridus L. Leaf Extract with Ethyl Acetate as A Streptococcus Mutans Antibacterial. J. Ilm. Farm. Farmasyifa 2022, 5, 79–90. [Google Scholar] [CrossRef]
- Guo, L.; Wang, Y.; Bi, X.; Duo, K.; Sun, Q.; Yun, X.; Zhang, Y.; Fei, P.; Han, J. Antimicrobial Activity and Mechanism of Action of the Amaranthus tricolor Crude Extract against Staphylococcus aureus and Potential Application in Cooked Meat. Foods 2020, 9, 359. [Google Scholar] [CrossRef]
- Jimoh, M.O.; Afolayan, A.J.; Lewu, F.B. Toxicity and Antimicrobial Activities of Amaranthus caudatus L. (Amaranthaceae) Harvested from Formulated Soils at Different Growth Stages. J. Evid. Based Integr. Med. 2020, 25, 2515690X20971578. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Rizwana; Tripathi, A.D.; Kumar, T.; Sharma, K.P.; Patel, S.K.S. Nutritional and Functional New Perspectives and Potential Health Benefits of Quinoa and Chia Seeds. Antioxidants 2023, 12, 1413. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Zhang, X.; Shi, J.; Cui, K.; Yang, R.; Liu, F.; Shan, S.; Israr, G.; Li, Z. Terpenoids of Quinoa Bran Suppresses Colorectal Cancer by Inducing Cell Apoptosis. Food Biosci. 2023, 53, 102615. [Google Scholar] [CrossRef]
- Bailon-Moscoso, N.; Coronel-Hidalgo, J.; Duarte-Casar, R.; Guamán-Ortiz, L.M.; Figueroa, J.G.; Romero-Benavides, J.C. Exploring the Antioxidant Potential of Tragia volubilis L.: Mitigating Chemotherapeutic Effects of Doxorubicin on Tumor Cells. Antioxidants 2023, 12, 2003. [Google Scholar] [CrossRef] [PubMed]
- Gawlik-Dziki, U.; Świeca, M.; Sułkowski, M.; Dziki, D.; Baraniak, B.; Czyż, J. Antioxidant and Anticancer Activities of Chenopodium quinoa Leaves Extracts—In Vitro Study. Food Chem. Toxicol. 2013, 57, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Guo, T.; Zhou, Y.; Han, S.; Sun, S.; Luo, F. Biological Functions of Active Ingredients in Quinoa Bran: Advance and Prospective. Crit. Rev. Food Sci. Nutr. 2022, 1–15. [Google Scholar] [CrossRef]
- Shen, Y.; Zheng, L.; Peng, Y.; Zhu, X.; Liu, F.; Yang, X.; Li, H. Physicochemical, Antioxidant and Anticancer Characteristics of Seed Oil from Three Chenopodium quinoa Genotypes. Molecules 2022, 27, 2453. [Google Scholar] [CrossRef]
- Stikić, R.I.; Milinčić, D.D.; Kostić, A.Ž.; Jovanović, Z.B.; Gašić, U.M.; Tešić, Ž.L.; Djordjević, N.Z.; Savić, S.K.; Czekus, B.G.; Pešić, M.B. Polyphenolic Profiles, Antioxidant, and in Vitro Anticancer Activities of the Seeds of Puno and Titicaca Quinoa Cultivars. Cereal Chem. 2020, 97, 626–633. [Google Scholar] [CrossRef]
- Mohamed, D.A.; Fouda, K.A.; Mohamed, R.S. In Vitro Anticancer Activity of Quinoa and Safflower Seeds and Their Preventive Effects on Non-Alcoholic Fatty Liver. Pak. J. Biol. Sci. 2019, 22, 383–392. [Google Scholar] [CrossRef]
- Rotondo, R.; Ragucci, S.; Castaldo, S.; Oliva, M.A.; Landi, N.; Pedone, P.V.; Arcella, A.; Di Maro, A. Cytotoxicity Effect of Quinoin, Type 1 Ribosome-Inactivating Protein from Quinoa Seeds, on Glioblastoma Cells. Toxins 2021, 13, 684. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, J.; Zou, L.; Fu, C.; Li, P.; Zhao, G. Chemical Characterization, Antioxidant, Immune-Regulating and Anticancer Activities of a Novel Bioactive Polysaccharide from Chenopodium quinoa Seeds. Int. J. Biol. Macromol. 2017, 99, 622–629. [Google Scholar] [CrossRef]
- de Cedrón, M.G.; del Hierro, J.N.; Reguero, M.; Wagner, S.; Bouzas, A.; Quijada-Freire, A.; Reglero, G.; Martín, D.; de Molina, A.R. Saponin-Rich Extracts and Their Acid Hydrolysates Differentially Target Colorectal Cancer Metabolism in the Frame of Precision Nutrition. Cancers 2020, 12, 3399. [Google Scholar] [CrossRef]
- Shuvalov, O.; Kirdeeva, Y.; Fefilova, E.; Netsvetay, S.; Zorin, M.; Vlasova, Y.; Fedorova, O.; Daks, A.; Parfenyev, S.; Barlev, N. 20-Hydroxyecdysone Confers Antioxidant and Antineoplastic Properties in Human Non-Small Cell Lung Cancer Cells. Metabolites 2023, 13, 656. [Google Scholar] [CrossRef]
- Graf, B.L.; Poulev, A.; Kuhn, P.; Grace, M.H.; Lila, M.A.; Raskin, I. Quinoa Seeds Leach Phytoecdysteroids and other Compounds with Anti-Diabetic Properties. Food Chem. 2014, 163, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-García, C.; Sánchez-Quesada, C.; Gaforio, J.J. Dietary Flavonoids as Cancer Chemopreventive Agents: An Updated Review of Human Studies. Antioxidants 2019, 8, 137. [Google Scholar] [CrossRef] [PubMed]
- Darendelioğlu, E. Studies of Anticancer Activity of Beta-Carotene, Alpha-Tocopherol and Ascorbic Acid in SH-SY5Y Neuroblastoma Cells. J. Inst. Sci. Technol. 2019, 9, 1657–1665. [Google Scholar] [CrossRef]
- Adewale, A.; Olorunju, A. Modulatory of Effect of Fresh Amaranthus Caudatus and Amaranthus hybridus Aqueous Leaf Extracts on Detoxify Enzymes and Micronuclei Formation after Exposure to Sodium Arsenite. Phcog Res 2013, 5, 300. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.I.M.; Pieters, R.; Abdel-Aziem, S.H.; van der Walt, A.M.; Bezuidenhout, C.C.; Giesy, J.P.; Abdel-Wahhab, M.A. Protective Effects of Amaranthus hybridus against Aflatoxin B1 and Fumonisin B1-Induced Genotoxicity in H4IIE-Luc Cells. Hepatoma Res. 2015, 1, 136–146. [Google Scholar] [CrossRef]
- Nsamou, P.C.N.; Momo, A.C.T.; Tchatat, Y.B.P.; Fozin, G.R.B.; Kemka, F.X.; Ngadjui, E.; Watcho, P. The Edible Plant Amaranthus hybridus (Amaranthaceae) Prevents the Biochemical, Histopathological and Fertility Impairments in Colibri®-Treated Female Rats. Toxicol. Rep. 2022, 9, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Mabeyo, P.E.; Manoko, M.L.K.; Gruhonjic, A.; Fitzpatrick, P.A.; Landberg, G.; Erdélyi, M.; Nyandoro, S.S. Selenium Accumulating Leafy Vegetables Are a Potential Source of Functional Foods. Int. J. Food Sci. 2015, 2015, e549676. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Zhou, C.; Cai, Y.; Tang, Y.; Sun, W.; Yao, H.; Zheng, T.; Chen, H.; Xiao, Y.; Shan, Z.; et al. Purification, Characterization and Antioxidant Activities In Vitro of Polysaccharides from Amaranthus hybridus L. PeerJ 2020, 8, e9077. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Guo, H.; Teng, C.; Zhang, B.; Blecker, C.; Ren, G. Anti-Colon Cancer Activity of Novel Peptides Isolated from In Vitro Digestion of Quinoa Protein in Caco-2 Cells. Foods 2022, 11, 194. [Google Scholar] [CrossRef]
- Fan, X.; Guo, H.; Teng, C.; Yang, X.; Qin, P.; Richel, A.; Zhang, L.; Blecker, C.; Ren, G. Supplementation of Quinoa Peptides Alleviates Colorectal Cancer and Restores Gut Microbiota in AOM/DSS-Treated Mice. Food Chem. 2022, 408, 135196. [Google Scholar] [CrossRef]





| Stage | Substage | |
|---|---|---|
| 1 Assembling | 1a Identification | Domain: Phytochemistry, phytomedicine Research questions: What is the current knowledge about the antimicrobial and anticancer activity of neglected Andean grains C. quinoa and A. hybridus? Source type: Research articles, reviews and book chapters Source quality: Crossref database |
| 1b Acquisition | Search mechanism and material acquisition: Dimensions query, ordered by rank Search period: No time constraints Search keywords: (Quinoa OR Amaranth) AND (antimicrobial OR cancer) Total number of articles returned from the search: 221 | |
| 2 Arranging | 2a Organization | Organizing codes: As provided in Dimensions export |
| 2b Purification | Article type excluded (n = 121): Remove duplicates (n = 8); remove predatory titles; remove non-empirical, non-review articles. Remove articles not related to the topic or dealing with other Amaranthus species Article type included (n = 100): Triangulation with previous reviews to ensure seminal articles are included | |
| 3 Assessing | 3a Evaluation | Analysis method: Content—descriptive Agenda proposal method: Future research directions and identification of existing gaps |
| 3c Reporting | Reporting conventions: Discussion and summaries in the form of tables and figures Limitations: Discussed Sources of support: Acknowledged | |
| Criterion | Excluded Articles |
|---|---|
| Duplicate article | 8 |
| Unrelated topics | 112 |
| Corrigenda | 1 |
| Total | 121 |
| Species | Protein (% dew) | Carbohydrate (% dew) | Fiber (% dw) | Fat (% dw) | Ash (% dw) | Energy (kcal/100 g) |
|---|---|---|---|---|---|---|
| Quinoa | 14.1 | 64.2 | 7.0 | 6.1 | 2.4 | 353 |
| Amaranth | 13.6 | 65.3 | 6.7 | 7.0 | 6.7 | 365 |
| Plant Organ | Mode of Use | Effect | ATC Category | Ref. |
|---|---|---|---|---|
| Stem Leaves | NS | “Improve the quality of blood” | B | [74] |
| Leaves | Poultice | Sore throat relief Angina | A C | [49] |
| Leaves | Decoction | Urinary infections Laxative Rheumatism | G A M | [52] |
| Leaves (fresh) | Soup or main course | Scurvy and other avitaminoses | A | [49] |
| Fruit | Poultice or decoction | Wound treatment | D | [49] |
| Seed | Decoction | Liver abscesses Internal secretions Catarrhal affections | J | [49] |
| Bronchial disorders Colds Cough Tonsillitis | R | [49] | ||
| Soaked | Intermittent fevers | J | [52] | |
| NS | Colon cancer prevention | A | [74] | |
| Seed, stem, leaves | Decoction | Emmenagogue | G | [52] |
| Leaves | Pounded | Headaches | N | [52] |
| Plant Organ | Mode of Use | Effect | ATC Category | Ref. |
|---|---|---|---|---|
| All organs | Decoction | Calming Antiacne Heart conditions Antidiarrheal Anti-inflammatory | N D C A | [52,80] |
| All organs | NS | Carminative | A | [52] |
| Leaves | Decoction | Cancer prevention | L | [81] |
| Whole plant | Poultice | Skin conditions Vulnerary | D | [82] |
| Inflorescence | Decoction | NS | C R G V | [76] |
| C. quinoa | A. hybridus |
|---|---|
| Phenolic acids Flavonoids Terpenoids Steroids Alkaloids Peptides | Phenolic acids Flavonoids Tannins Steroids Carotenoids |
| No. | Compound | Biological Activity/Model | Effect | Method | Ref. |
|---|---|---|---|---|---|
| 1 | 4-hydroxybenzoic acid | Staphylococcus epidermidis | IC50: 355 µg/mL | Paper disc | [99] |
| 2 | Gallic acid | Pseudomonas aeruginosa | MIC: 100 µg/mL | Microdilution | [100] |
| 3 | Protocatechuic acid | Yersinia enterocolitica | MIC: 2.5 mg/mL | Microdilution | [101] |
| 4 | p-coumaric acid | Listeria monocytogenes | IC50: 373.4 μM | Spot-test assay | [102] |
| 5 | Ferulic acid | Escherichia coli Pseudomonas aeruginosa | MIC: 100 µg/mL | Microdilution | [100] |
| 6 | Kaempferol | Staphylococcus aureus | Bacterial film formation inhibition: 64 μg/mL causes 80% inhibition | Crystal violet staining | [103,104] |
| 7 | Myricetin | Escherichia coli | MIC50: 142 μg/mL | Broth microdilution | [105] |
| 8 | Oleanolic acid | Listeria monocytogenes | MIC50: 16 μg/mL | Broth microdilution | [106] |
| Plant Organ | Extract | Model | Method | Effect/Mechanism | Ref. | |||
|---|---|---|---|---|---|---|---|---|
| In Vitro | In Vivo | Biological Model | ||||||
| Chenopodium quinoa Willd | ||||||||
| Colon | ||||||||
| BSO | n-hexane | x | HCT116 cells | Hoechst and MTT staining | 647.4 µg/mL, apoptosis | [120] | ||
| RSO | n-hexane | x | HCT116 cells | Hoechst and MTT staining | 381.3 µg/mL, apoptosis | [120] | ||
| WSO | n-hexane | x | HCT116 cells | Hoechst and MTT staining | 281.9 µg/mL, apoptosis | [120] | ||
| Seed | Ethanol | x | HCT116 cells | MTT | IC50 110.68 µg/mL at 48 h | [121] | ||
| Seed | Protein | x | Caco-2 | HDAC1 | IC50 0.87–1.85 g/L | [135] | ||
| Seed | Protein | x | AOM/DSS-induced colorectal cancer in mice | Symptoms/ SCFA production | Symptom mitigation/partially alleviated dysbiosis | [136] | ||
| Liver | ||||||||
| Seed | Powder | x | HEPG2 | Cell line | IC50 14.6 µg/mL | [122] | ||
| Seed | Petroleum ether, ultrasound-assisted extraction | x | SMMC 7721 | MTT | 121.4 µg/mL (24 h) and 53.4 µg/mL (48 h), inhibition of cell proliferation | [124] | ||
| Brain | ||||||||
| Seed | NS | x | U87 Mg | MTT | 50 ± 5.0 nM, cytotoxicity | [123] | ||
| Seed | NS | x | GBM NULU | MTT | 6.6 ± 4.1 nM (24 h), 8.3 ± 1.6 (48 h), 2.3 ± 4.1 (72 h); cytotoxicity | [123] | ||
| Seed | NS | x | GBM ZAR | MTT | 3.4 ± 1.9 nM (24 h), 7.6 ± 2.7 nM (48 h), 3.3 ± 1.2 nM (72 h); cytotoxicity | [123] | ||
| Breast | ||||||||
| Seed | Petroleum ether, ultrasound-assisted extraction | x | MCF-7 | MTT | 83.48 µg/mL (24 h) and 64.67 µg/mL (48 h), inhibition of cell proliferation | [124] | ||
| Amarantus hybridus L. | ||||||||
| Leaves | Aqueous | x | Sodium arsenite-induced micronucleated polychromatic erythrocyte in Wistar albino rats | Hematological tests | 1 mL of 0.2 g/mL for 14 days, antigenotoxicity | [130] | ||
| Stem and leaves | NS | x | Aflatoxin and fumonisin-induced genotoxicity in H4IIE-luc cells | MTT | 40 µg/mL, antigenotoxicity | [131] | ||
| Seed | Methanolic | x | Mice treated with EAC cells | Hemocytometer EAC cell count by trypan blue and DAPI staining | 25, 50 and 100 µg/mL/d for 6 days; inhibition of cell growth | [108] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero-Benavides, J.C.; Guaraca-Pino, E.; Duarte-Casar, R.; Rojas-Le-Fort, M.; Bailon-Moscoso, N. Chenopodium quinoa Willd. and Amaranthus hybridus L.: Ancestral Andean Food Security and Modern Anticancer and Antimicrobial Activity. Pharmaceuticals 2023, 16, 1728. https://doi.org/10.3390/ph16121728
Romero-Benavides JC, Guaraca-Pino E, Duarte-Casar R, Rojas-Le-Fort M, Bailon-Moscoso N. Chenopodium quinoa Willd. and Amaranthus hybridus L.: Ancestral Andean Food Security and Modern Anticancer and Antimicrobial Activity. Pharmaceuticals. 2023; 16(12):1728. https://doi.org/10.3390/ph16121728
Chicago/Turabian StyleRomero-Benavides, Juan Carlos, Evelyn Guaraca-Pino, Rodrigo Duarte-Casar, Marlene Rojas-Le-Fort, and Natalia Bailon-Moscoso. 2023. "Chenopodium quinoa Willd. and Amaranthus hybridus L.: Ancestral Andean Food Security and Modern Anticancer and Antimicrobial Activity" Pharmaceuticals 16, no. 12: 1728. https://doi.org/10.3390/ph16121728
APA StyleRomero-Benavides, J. C., Guaraca-Pino, E., Duarte-Casar, R., Rojas-Le-Fort, M., & Bailon-Moscoso, N. (2023). Chenopodium quinoa Willd. and Amaranthus hybridus L.: Ancestral Andean Food Security and Modern Anticancer and Antimicrobial Activity. Pharmaceuticals, 16(12), 1728. https://doi.org/10.3390/ph16121728