Added Value of Scintillating Element in Cerenkov-Induced Photodynamic Therapy
Abstract
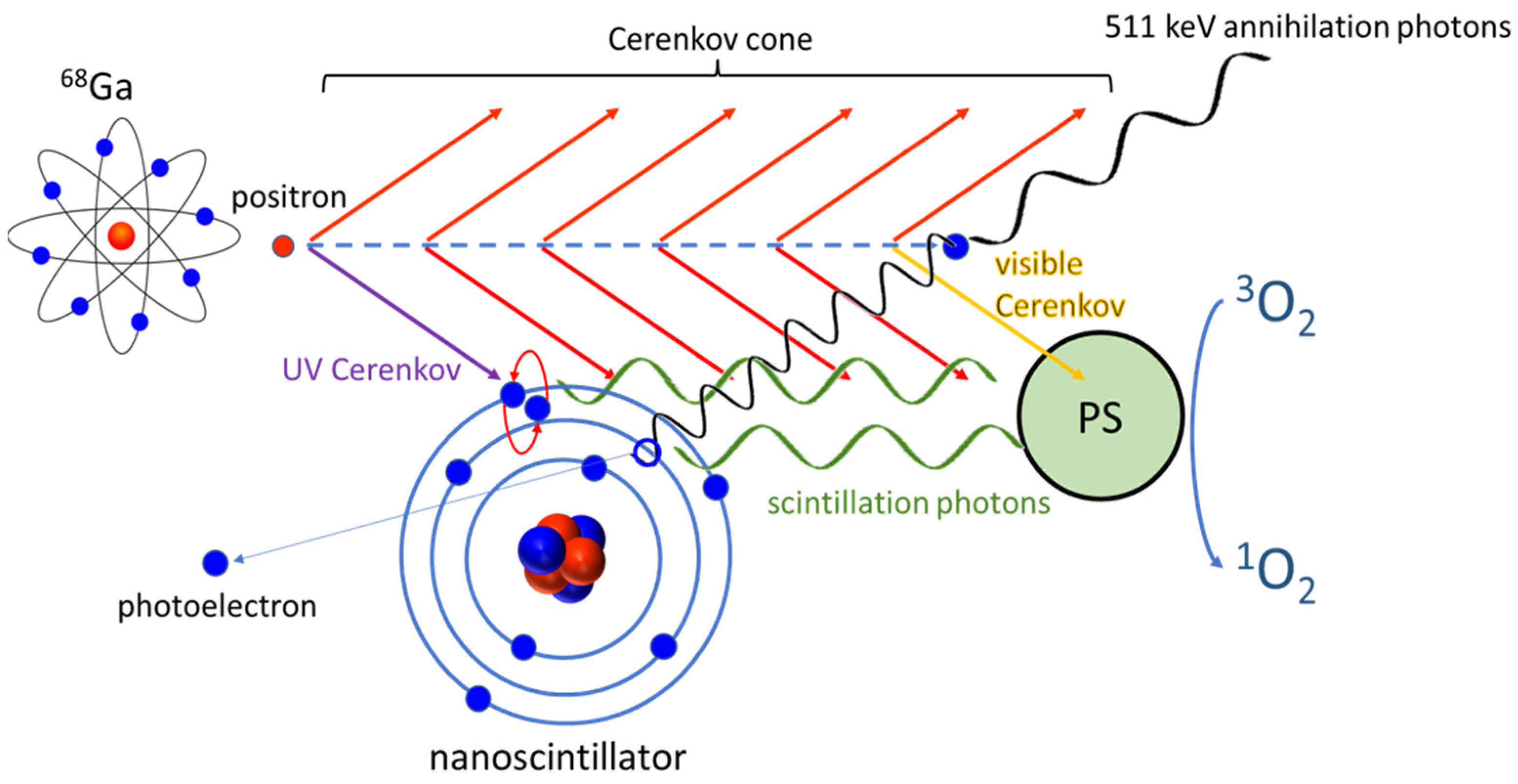
:1. Introduction
2. Results
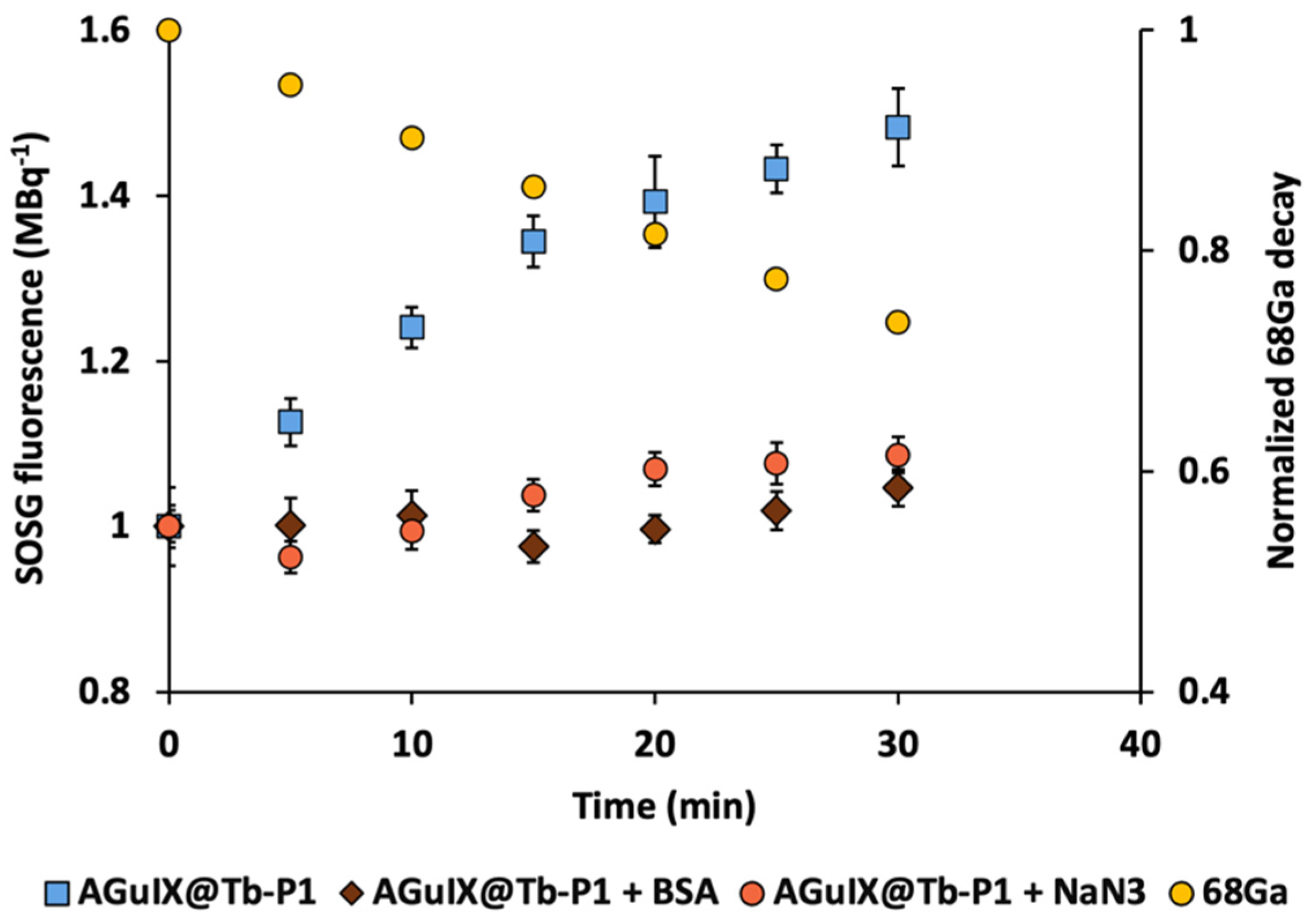
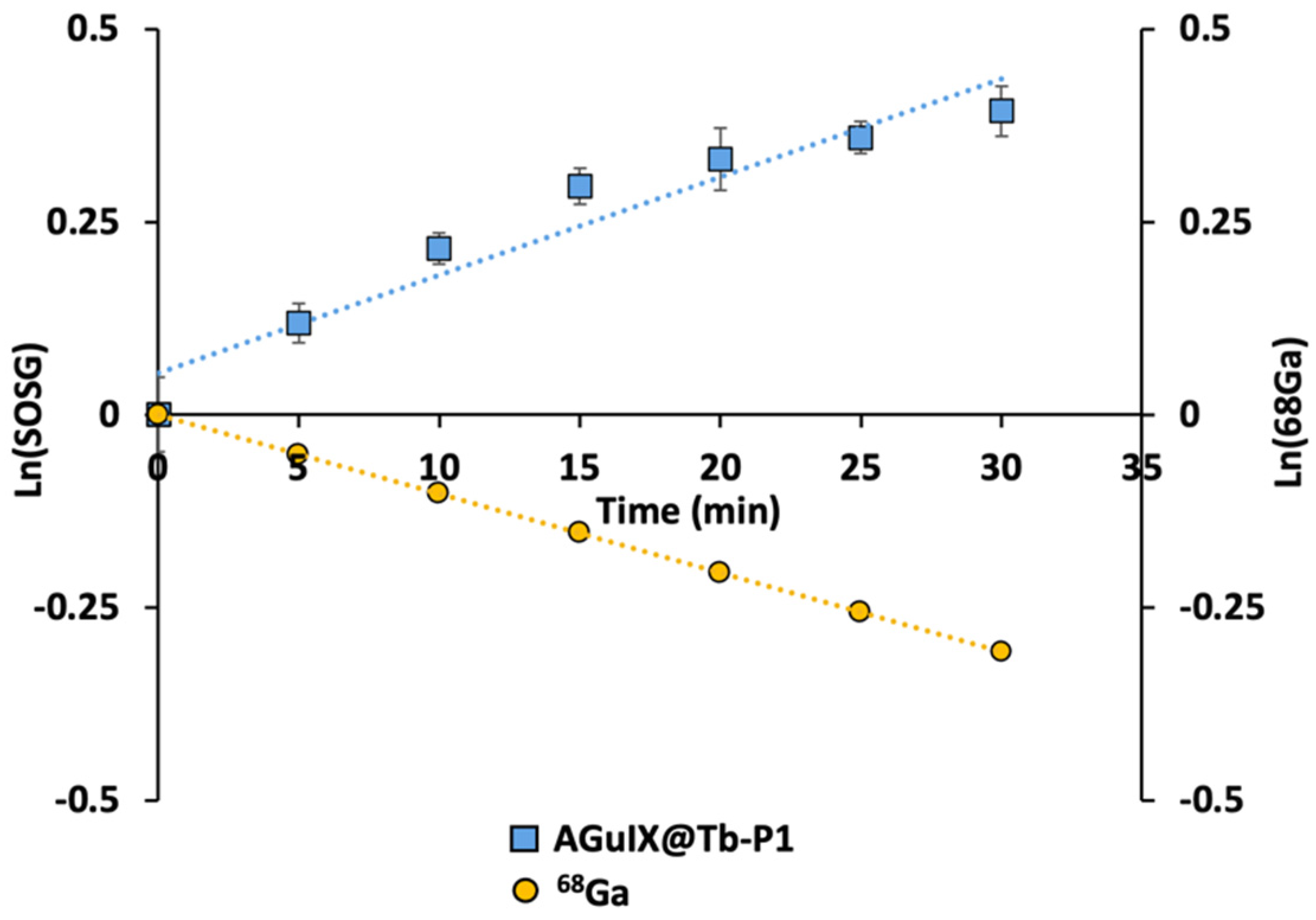
2.1. Singlet Oxygen Production with 68Ga Irradiation
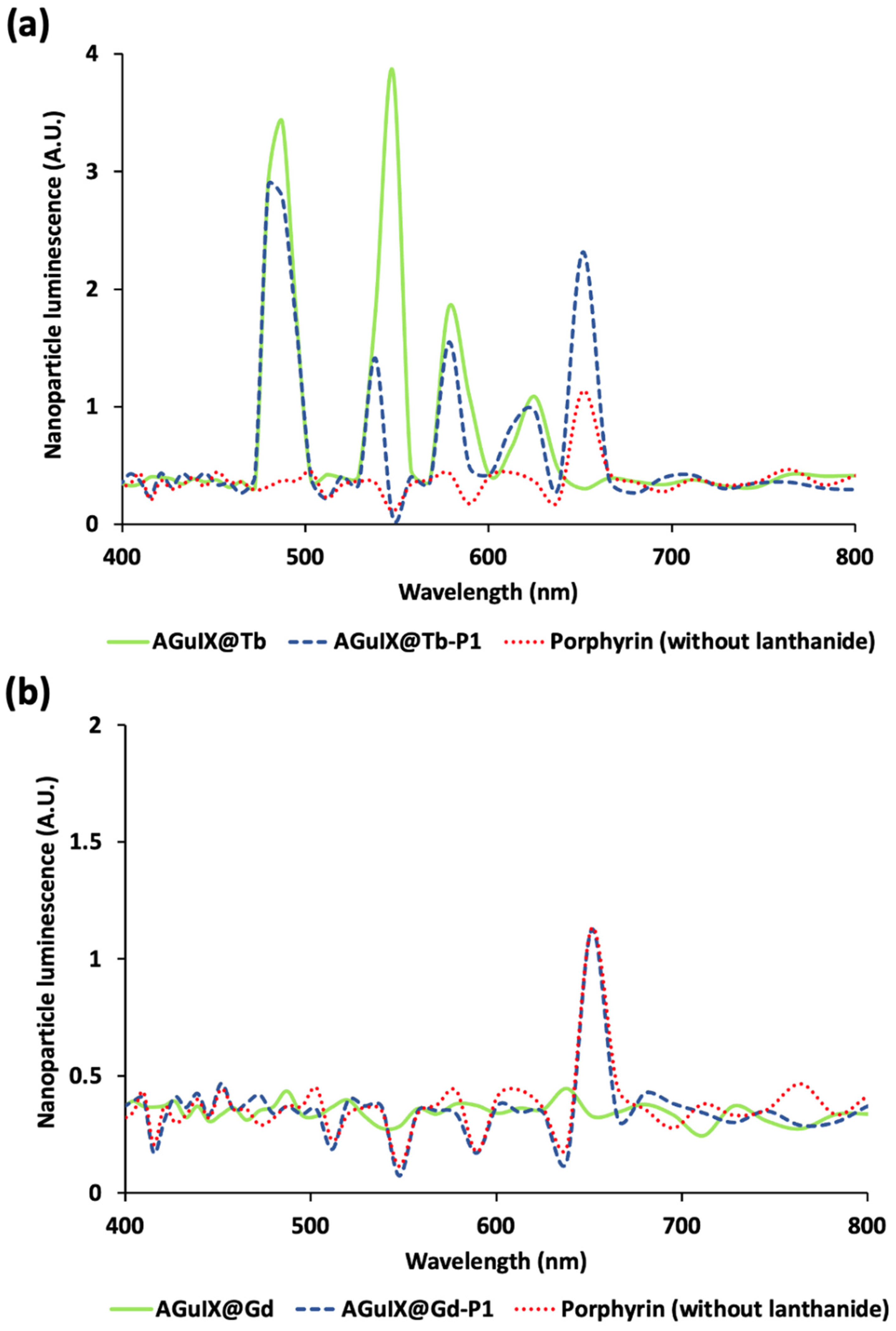
2.2. Monte Carlo Simulations
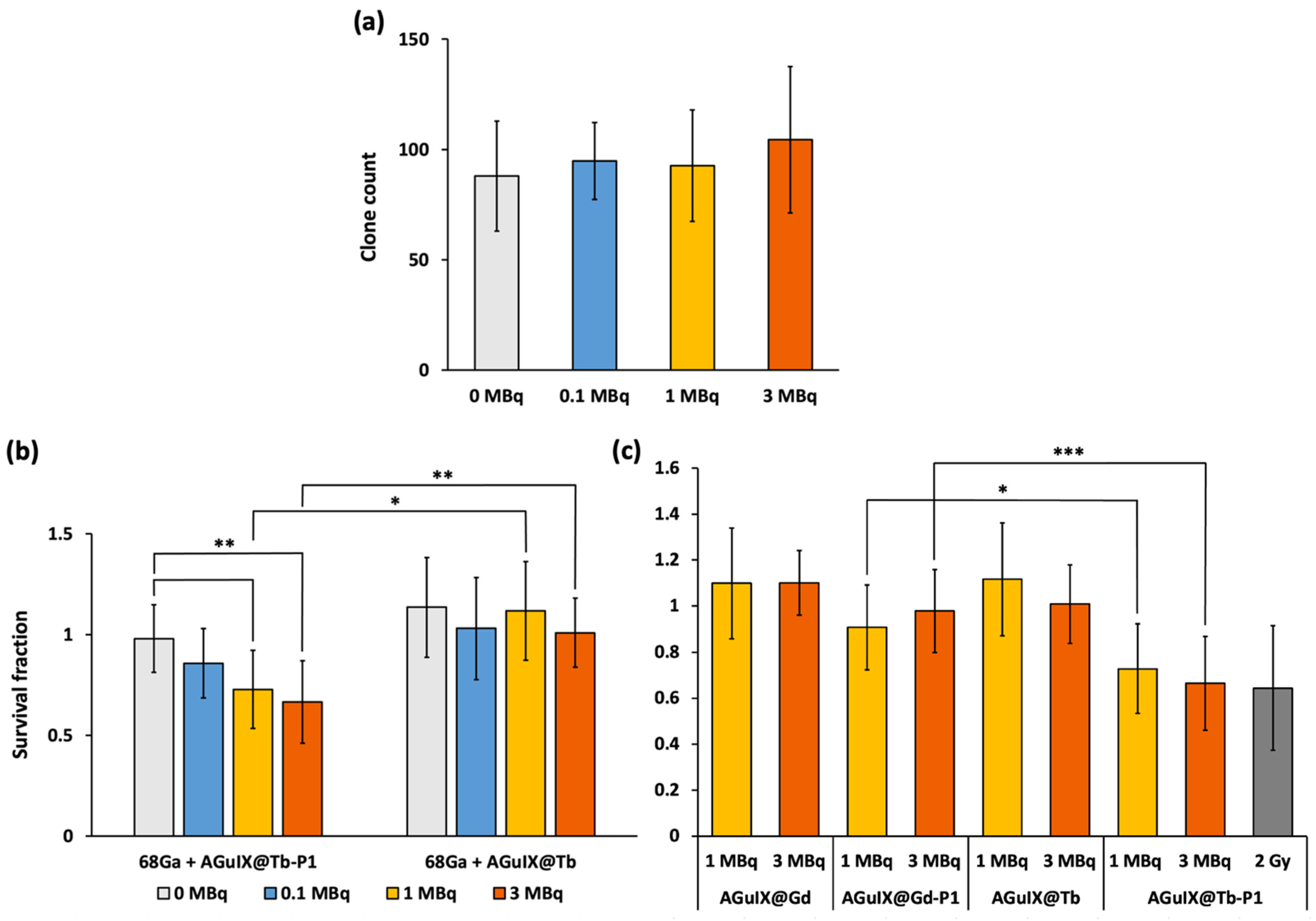
2.3. Cerenkov-Induced Photodynamic Effect on U-251 MG Cell Survival
3. Discussion
3.1. Scintillator Luminescence Increases Porphyrin Excitation
3.2. Low activity Concentration Yields Singlet Oxygen Production
3.3. 68Ga Irradiation of AGuIX@Tb-P1 Induces an Effective Photodynamic Effect
4. Materials and Methods
4.1. Reagents
4.2. Nanoparticle Solution Preparation
4.3. Radiosynthesis of 68Ga-Citrate
4.4. Singlet Oxygen Production with 68Ga Irradiation
4.5. Monte Carlo Simulations
4.5.1. General Parameters
4.5.2. Geometry
4.5.3. Source
4.5.4. Recorded Data
4.5.5. Simulation Scenarios
- (i)
- Nanoparticles were replaced by water to observe the raw Cerenkov spectrum.
- (ii)
- We used nanoparticles without Tb or Gd but with P1. That allowed the observation of direct PS activation through the Cerenkov visible part.
- (iii)
- We added Tb to the nanoparticle to observe scintillation and nanoscintillator fluorescence (due to Cerenkov UV photons) contributions.
- (iv)
- At last, we replaced Tb with Gd to validate the overall simulation results. Gd was not expected to transfer energy to the PS, and we expected to obtain a similar P1 fluorescence as in ii.
4.6. Biological Experiments
4.6.1. Cell Culture
4.6.2. Anchorage-Dependent Clonogenic Assay
4.6.3. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Davis, M.E. Glioblastoma: Overview of Disease and Treatment. Clin. J. Oncol. Nurs. 2016, 20, S2–S8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNamara, C.; Mankad, K.; Thust, S.; Dixon, L.; Limback-Stanic, C.; D’Arco, F.; Jacques, T.S.; Löbel, U. 2021 WHO Classification of Tumours of the Central Nervous System: A Review for the Neuroradiologist. Neuroradiology 2022, 64, 1919–1950. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz, J.V.R.; Batista, C.; Afonso, B.d.H.; Alexandre-Moreira, M.S.; Dubois, L.G.; Pontes, B.; Moura Neto, V.; Mendes, F.d.A. Obstacles to Glioblastoma Treatment Two Decades after Temozolomide. Cancers 2022, 14, 3203. [Google Scholar] [CrossRef] [PubMed]
- Calixto, G.; Bernegossi, J.; de Freitas, L.; Fontana, C.; Chorilli, M. Nanotechnology-Based Drug Delivery Systems for Photodynamic Therapy of Cancer: A Review. Molecules 2016, 21, 342. [Google Scholar] [CrossRef] [PubMed]
- Larue, L.; Ben Mihoub, A.; Youssef, Z.; Colombeau, L.; Acherar, S.; André, J.C.; Arnoux, P.; Baros, F.; Vermandel, M.; Frochot, C. Using X-Rays in Photodynamic Therapy: An Overview. Photochem. Photobiol. Sci. 2018, 17, 1612–1650. [Google Scholar] [CrossRef] [PubMed]
- Dhaini, B.; Kenzhebayeva, B.; Ben-Mihoub, A.; Gries, M.; Acherar, S.; Baros, F.; Thomas, N.; Daouk, J.; Schohn, H.; Hamieh, T.; et al. Peptide-Conjugated Nanoparticles for Targeted Photodynamic Therapy. Nanophotonics 2021, 10, 3089–3134. [Google Scholar] [CrossRef]
- Maeda, H. Toward a Full Understanding of the EPR Effect in Primary and Metastatic Tumors as Well as Issues Related to Its Heterogeneity. Adv. Drug Deliv. Rev. 2015, 91, 3–6. [Google Scholar] [CrossRef]
- Maeda, H.; Sano, Y.; Takeshita, J.; Iwai, Z.; Kosaka, H.; Marubayashi, T.; Matsukado, Y. A Pharmacokinetic Simulation Model for Chemotherapy of Brain Tumor with an Antitumor Protein Antibiotic, Neocarzinostatin. Theoretical Considerations behind a Two-Compartment Model for Continuous Infusion via an Internal Carotid Artery. Cancer Chemother. Pharmacol. 1981, 5, 243–249. [Google Scholar] [CrossRef]
- Beck, T.J.; Kreth, F.W.; Beyer, W.; Mehrkens, J.H.; Obermeier, A.; Stepp, H.; Stummer, W.; Baumgartner, R. Interstitial Photodynamic Therapy of Nonresectable Malignant Glioma Recurrences Using 5-Aminolevulinic Acid Induced Protoporphyrin IX. Lasers Surg. Med. 2007, 39, 386–393. [Google Scholar] [CrossRef]
- Krishnamurthy, S.; Powers, S.K.; Witmer, P.; Brown, T. Optimal Light Dose for Interstitial Photodynamic Therapy in Treatment for Malignant Brain Tumors. Lasers Surg. Med. 2000, 27, 224–234. [Google Scholar] [CrossRef]
- Bhandari, C.; Guirguis, M.; Savan, N.A.; Shrivastava, N.; Oliveira, S.; Hasan, T.; Obaid, G. What NIR Photodynamic Activation Offers Molecular Targeted Nanomedicines: Perspectives into the Conundrum of Tumor Specificity and Selectivity. Nano Today 2021, 36, 101052. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, J. Using Nanoparticles to Enable Simultaneous Radiation and Photodynamic Therapies for Cancer Treatment. J. Nanosci. Nanotechnol. 2006, 6, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.-D.; Hao, X.-Y.; Li, H.-C.; Ke, M.-R.; Zheng, B.-Y.; Huang, J.-D. Progress in the Development of Nanosensitizers for X-Ray-Induced Photodynamic Therapy. Drug Discov. Today 2018, 23, 1791–1800. [Google Scholar] [CrossRef]
- Kamkaew, A.; Chen, F.; Zhan, Y.; Majewski, R.L.; Cai, W. Scintillating Nanoparticles as Energy Mediators for Enhanced Photodynamic Therapy. ACS Nano 2016, 10, 3918–3935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dothager, R.S.; Goiffon, R.J.; Jackson, E.; Harpstrite, S.; Piwnica-Worms, D. Cerenkov Radiation Energy Transfer (CRET) Imaging: A Novel Method for Optical Imaging of PET Isotopes in Biological Systems. PLOS ONE 2010, 5, e13300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Zhang, X.; Xing, B.; Han, P.; Gambhir, S.S.; Cheng, Z. Radiation-Luminescence-Excited Quantum Dots for in Vivo Multiplexed Optical Imaging. Small 2010, 6, 1087–1091. [Google Scholar] [CrossRef]
- Jelley, J.V. Cerenkov Radiation and Its Applications; Pergamon Press: Oxford, UK, 1958. [Google Scholar]
- Ran, C.; Zhang, Z.; Hooker, J.; Moore, A. In Vivo Photoactivation without “Light”: Use of Cherenkov Radiation to Overcome the Penetration Limit of Light. Mol. Imaging Biol. 2012, 14, 156–162. [Google Scholar] [CrossRef] [Green Version]
- Cline, B.; Delahunty, I.; Xie, J. Nanoparticles to Mediate X-ray-Induced Photodynamic Therapy and Cherenkov Radiation Photodynamic Therapy. WIREs Nanomed. Nanobiotechnol. 2019, 11, e1541. [Google Scholar] [CrossRef]
- Duan, D.; Liu, H.; Xu, Y.; Han, Y.; Xu, M.; Zhang, Z.; Liu, Z. Activating TiO2 Nanoparticles: Gallium-68 Serves as a High-Yield Photon Emitter for Cerenkov-Induced Photodynamic Therapy. ACS Appl. Mater. Interfaces 2018, 10, 5278–5286. [Google Scholar] [CrossRef]
- Kotagiri, N.; Cooper, M.L.; Rettig, M.; Egbulefu, C.; Prior, J.; Cui, G.; Karmakar, P.; Zhou, M.; Yang, X.; Sudlow, G.; et al. Radionuclides Transform Chemotherapeutics into Phototherapeutics for Precise Treatment of Disseminated Cancer. Nat. Commun. 2018, 9, 275. [Google Scholar] [CrossRef] [Green Version]
- Ni, D.; Ferreira, C.A.; Barnhart, T.E.; Quach, V.; Yu, B.; Jiang, D.; Wei, W.; Liu, H.; Engle, J.W.; Hu, P.; et al. Magnetic Targeting of Nanotheranostics Enhances Cerenkov Radiation-Induced Photodynamic Therapy. J. Am. Chem. Soc. 2018, 140, 14971–14979. [Google Scholar] [CrossRef] [PubMed]
- Hartl, B.A.; Hirschberg, H.; Marcu, L.; Cherry, S.R. Activating Photodynamic Therapy in Vitro with Cerenkov Radiation Generated from Yttrium-90. J. Environ. Pathol. Toxicol. Oncol. Off. Organ Int. Soc. Environ. Toxicol. Cancer 2016, 35, 185–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotagiri, N.; Sudlow, G.P.; Akers, W.J.; Achilefu, S. Breaking the Depth Dependency of Phototherapy with Cerenkov Radiation and Low-Radiance-Responsive Nanophotosensitizers. Nat. Nanotechnol. 2015, 10, 370–379. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, Y.; Nagaya, T.; Sato, K.; Okuyama, S.; Ogata, F.; Wong, K.; Adler, S.; Choyke, P.L.; Kobayashi, H. Cerenkov Radiation-Induced Photoimmunotherapy with 18F-FDG. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2017, 58, 1395–1400. [Google Scholar] [CrossRef] [Green Version]
- Pratx, G.; Kapp, D.S. Is Cherenkov Luminescence Bright Enough for Photodynamic Therapy? Nat. Nanotechnol. 2018, 13, 354. [Google Scholar] [CrossRef] [PubMed]
- Daouk, J.; Dhaini, B.; Petit, J.; Frochot, C.; Barberi-Heyob, M.; Schohn, H. Can Cerenkov Light Really Induce an Effective Photodynamic Therapy? Radiation 2020, 1, 5–17. [Google Scholar] [CrossRef]
- Daouk, J.; Iltis, M.; Dhaini, B.; Béchet, D.; Arnoux, P.; Rocchi, P.; Delconte, A.; Habermeyer, B.; Lux, F.; Frochot, C.; et al. Terbium-Based AGuIX-Design Nanoparticle to Mediate X-Ray-Induced Photodynamic Therapy. Pharmaceuticals 2021, 14, 396. [Google Scholar] [CrossRef]
- Sancey, L.; Lux, F.; Kotb, S.; Roux, S.; Dufort, S.; Bianchi, A.; Crémillieux, Y.; Fries, P.; Coll, J.-L.; Rodriguez-Lafrasse, C.; et al. The Use of Theranostic Gadolinium-Based Nanoprobes to Improve Radiotherapy Efficacy. Br. J. Radiol. 2014, 87, 20140134. [Google Scholar] [CrossRef]
- Agostinelli, S.; Allison, J.; Amako, K.; Apostolakis, J.; Araujo, H.; Arce, P.; Asai, M.; Axen, D.; Banerjee, S.; Barrand, G.; et al. Geant4—A Simulation Toolkit. Nucl. Instrum. Methods Phys. Res. Sect. Accel. Spectrometers Detect. Assoc. Equip. 2003, 506, 250–303. [Google Scholar] [CrossRef]
- Trigila, C.; Moghe, E.; Roncali, E. Technical Note: Standalone Application to Generate Custom Reflectance Look-Up Table for Advanced Optical Monte Carlo Simulation in GATE/Geant4. Med. Phys. 2021, 48, 2800–2808. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.S.; Sun, C.; Pratx, G. Radioluminescence in Biomedicine: Physics, Applications, and Models. Phys. Med. Biol. 2019, 64, 04TR01. [Google Scholar] [CrossRef] [PubMed]
- Setsukinai, K.; Urano, Y.; Kakinuma, K.; Majima, H.J.; Nagano, T. Development of Novel Fluorescence Probes That Can Reliably Detect Reactive Oxygen Species and Distinguish Specific Species. J. Biol. Chem. 2003, 278, 3170–3175. [Google Scholar] [CrossRef] [Green Version]
- Price, M.; Reiners, J.J.; Santiago, A.M.; Kessel, D. Monitoring Singlet Oxygen and Hydroxyl Radical Formation with Fluorescent Probes during Photodynamic Therapy. Photochem. Photobiol. 2009, 85, 1177–1181. [Google Scholar] [CrossRef] [Green Version]
- Misawa, M.; Takahashi, J. Generation of Reactive Oxygen Species Induced by Gold Nanoparticles under X-Ray and UV Irradiations. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 604–614. [Google Scholar] [CrossRef] [PubMed]
- Verry, C.; Sancey, L.; Dufort, S.; Duc, G.L.; Mendoza, C.; Lux, F.; Grand, S.; Arnaud, J.; Quesada, J.L.; Villa, J.; et al. Treatment of Multiple Brain Metastases Using Gadolinium Nanoparticles and Radiotherapy: NANO-RAD, a Phase I Study Protocol. BMJ Open 2019, 9, e023591. [Google Scholar] [CrossRef]
- Sun, H.; Cai, H.; Xu, C.; Zhai, H.; Lux, F.; Xie, Y.; Feng, L.; Du, L.; Liu, Y.; Sun, X.; et al. AGuIX Nanoparticles Enhance Ionizing Radiation-Induced Ferroptosis on Tumor Cells by Targeting the NRF2-GPX4 Signaling Pathway. J. Nanobiotechnol. 2022, 20, 449. [Google Scholar] [CrossRef]
- Ianzini, F.; Mackey, M.A. Spontaneous Premature Chromosome Condensation and Mitotic Catastrophe Following Irradiation of HeLa S3 Cells. Int. J. Radiat. Biol. 1997, 72, 409–421. [Google Scholar] [CrossRef]
- Robertson, C.A.; Evans, D.H.; Abrahamse, H. Photodynamic Therapy (PDT): A Short Review on Cellular Mechanisms and Cancer Research Applications for PDT. J. Photochem. Photobiol. B 2009, 96, 1–8. [Google Scholar] [CrossRef]
- Yu, B.; Ni, D.; Rosenkrans, Z.T.; Barnhart, T.E.; Wei, H.; Ferreira, C.A.; Lan, X.; Engle, J.W.; He, Q.; Yu, F.; et al. A Missile-Detonation Strategy to Precisely Supply and Efficiently Amplify Cerenkov Radiation Energy for Cancer Theranostics. Adv. Mater. Deerfield Beach Fla 2019, 31, e1904894. [Google Scholar] [CrossRef]
- Paquot, H.; Daouk, J.; Chateau, A.; Rétif, P.; Barberi-Heyob, M.; Pinela, S. Radiation-Induced Mitotic Catastrophe Enhanced by Gold Nanoparticles: Assessment with a Specific Automated Image Processing Workflow. Radiat. Res. 2019, 192, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Chauvin, M.; Borys, D.; Botta, F.; Bzowski, P.; Dabin, J.; Denis-Bacelar, A.M.; Desbrée, A.; Falzone, N.; Lee, B.Q.; Mairani, A.; et al. OpenDose: Open-Access Resource for Nuclear Medicine Dosimetry. J. Nucl. Med. 2020, 61, 1514–1519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mignot, A.; Truillet, C.; Lux, F.; Sancey, L.; Louis, C.; Denat, F.; Boschetti, F.; Bocher, L.; Gloter, A.; Stéphan, O.; et al. A Top-Down Synthesis Route to Ultrasmall Multifunctional Gd-Based Silica Nanoparticles for Theranostic Applications. Chem. Eur. J. 2013, 19, 6122–6136. [Google Scholar] [CrossRef] [PubMed]
- Ovdiichuk, O.; Roeder, E.; Billotte, S.; Veran, N.; Collet, C. Fully Automated Macro- and Microfluidic Production of [68Ga]Ga-Citrate on MAIO® and IMiDEVTM Modules. Molecules 2022, 27, 994. [Google Scholar] [CrossRef]
- Jensen, S.B.; Nielsen, K.M.; Mewis, D.; Kaufmann, J. Fast and Simple One-Step Preparation of 68Ga Citrate for Routine Clinical PET. Nucl. Med. Commun. 2013, 34, 806–812. [Google Scholar] [CrossRef]
- Bose, B.; Dube, A. Interaction of Chlorin P6 with Bovine Serum Albumin and Photodynamic Oxidation of Protein. J. Photochem. Photobiol. B 2006, 85, 49–55. [Google Scholar] [CrossRef]
- Mansuy, C.; Nedelec, J.-M.; Dujardin, C.; Mahiou, R. Concentration Effect on the Scintillation Properties of Sol-Gel Derived LuBO3 Doped with Eu3+ and Tb3+. Opt. Mater. 2007, 29, 697–702. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schneller, P.; Collet, C.; Been, Q.; Rocchi, P.; Lux, F.; Tillement, O.; Barberi-Heyob, M.; Schohn, H.; Daouk, J. Added Value of Scintillating Element in Cerenkov-Induced Photodynamic Therapy. Pharmaceuticals 2023, 16, 143. https://doi.org/10.3390/ph16020143
Schneller P, Collet C, Been Q, Rocchi P, Lux F, Tillement O, Barberi-Heyob M, Schohn H, Daouk J. Added Value of Scintillating Element in Cerenkov-Induced Photodynamic Therapy. Pharmaceuticals. 2023; 16(2):143. https://doi.org/10.3390/ph16020143
Chicago/Turabian StyleSchneller, Perrine, Charlotte Collet, Quentin Been, Paul Rocchi, François Lux, Olivier Tillement, Muriel Barberi-Heyob, Hervé Schohn, and Joël Daouk. 2023. "Added Value of Scintillating Element in Cerenkov-Induced Photodynamic Therapy" Pharmaceuticals 16, no. 2: 143. https://doi.org/10.3390/ph16020143
APA StyleSchneller, P., Collet, C., Been, Q., Rocchi, P., Lux, F., Tillement, O., Barberi-Heyob, M., Schohn, H., & Daouk, J. (2023). Added Value of Scintillating Element in Cerenkov-Induced Photodynamic Therapy. Pharmaceuticals, 16(2), 143. https://doi.org/10.3390/ph16020143







