Beyond Pain Relief: A Review on Cannabidiol Potential in Medical Therapies
Abstract
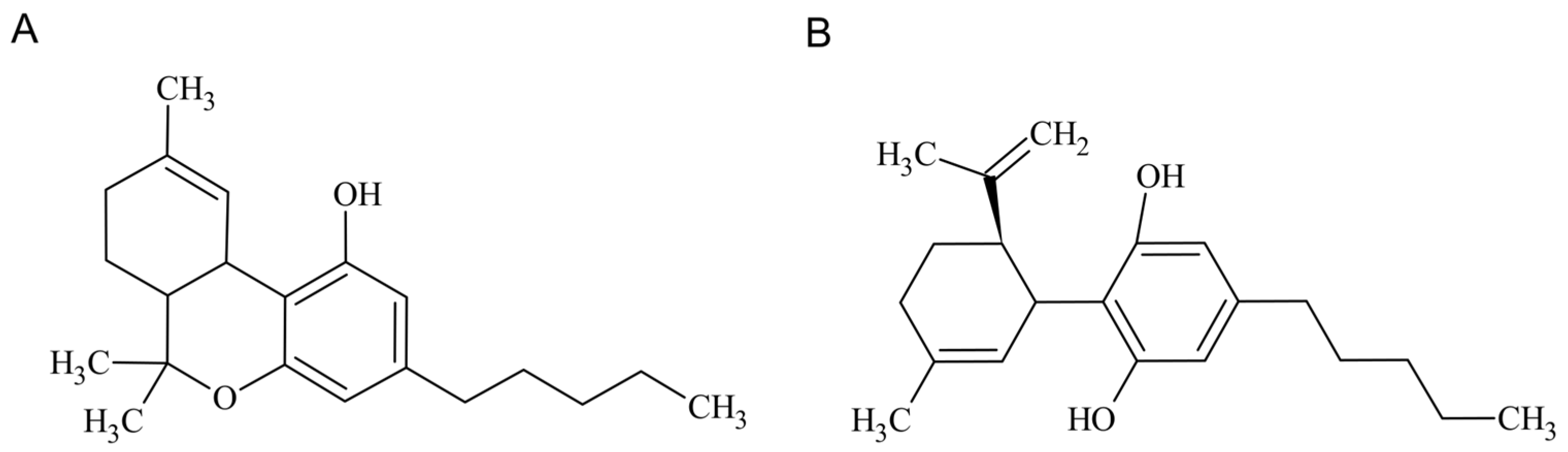
1. Introduction
2. The Endocannabinoid System
3. CBD Interaction with Other Cellular Receptors
4. CBD’s Anti-Inflammatory and Antioxidant Capacity: From Epilepsy to Depression
4.1. CBD in Epilepsy
4.2. CBD in Parkinson’s Disease
4.3. CBD in Alzheimer’s Disease
4.4. CBD in Depression
4.5. CBD use in Neurological Conditions: Clinical Trials
5. CBD in Autoimmune Diseases
5.1. CBD in Inflammatory Bowel Disease
5.2. CBD in Lupus
5.3. CBD in Rheumatoid Arthritis
5.4. CBD in Psoriasis
6. CBD in Cancer Treatment: Beyond Pain Relief
6.1. CBD Anticancer Mechanism
6.2. Combination of CBD with Chemotherapeutic Agents
6.3. The road to Clinic Application
7. Final Remarks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Atalay, S.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Properties of Cannabidiol. Antioxidants 2020, 9, 21. [Google Scholar] [CrossRef]
- Nichols, J.M.; Kaplan, B.L. Immune responses regulated by cannabidiol. Cannabis Cannabinoid Res. 2020, 5, 12–31. [Google Scholar] [CrossRef] [PubMed]
- Silote, G.P.; Sartim, A.; Sales, A.; Eskelund, A.; Guimarães, F.S.; Wegener, G.; Joca, S. Emerging evidence for the antidepressant effect of cannabidiol and the underlying molecular mechanisms. J. Chem. Neuroanat. 2019, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Franco, V.; Perucca, E. Pharmacological and Therapeutic Properties of Cannabidiol for Epilepsy. Drugs 2019, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Mandolini, G.; Lazzaretti, M.; Pigoni, A.; Oldani, L.; Delvecchio, G.; Brambilla, P. Pharmacological properties of cannabidiol in the treatment of psychiatric disorders: A critical overview. Epidemiol. Psychiatr. Sci. 2018, 27, 327–335. [Google Scholar] [CrossRef]
- Grotenhermen, F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin. Pharmacokinet. 2003, 42, 327–360. [Google Scholar] [CrossRef]
- Fraguas-Sánchez, A.; Fernández-Carballido, A.; Sofware, C.M.-S.; Torres-Suárez, A. Stability characteristics of Cannabidiol for the design of pharmacological, biochemical and pharmaceutical studies. J. Chromatogr. B 2020, 122188. [Google Scholar] [CrossRef]
- Pavlovic, R.; Nenna, G.; Calvi, L.; Panseri, S.; Borgonovo, G.; Giupponi, L.; Cannazza, G.; Giorgi, A. Quality traits of “cannabidiol oils”: Cannabinoids content, terpene fingerprint and oxidation stability of European commercially available preparations. Molecules 2018, 23, 1230. [Google Scholar] [CrossRef]
- Scheidweiler, K.B.; Andersson, M.; Swortwood, M.J.; Sempio, C.; Huestis, M.A. Long-term stability of cannabinoids in oral fluid after controlled cannabis administration. Drug Test. Anal. 2017, 9, 143–147. [Google Scholar] [CrossRef]
- Bonn-Miller, M.O.; ElSohly, M.A.; Loflin, M.J.; Chandra, S.; Vandrey, R. Cannabis and cannabinoid drug development: Evaluating botanical versus single molecule approaches. Int. Rev. Psychiatry 2018, 30, 277–284. [Google Scholar] [CrossRef]
- Mascal, M.; Hafezi, N.; Wang, D.; Hu, Y.; Serra, G.; Dallas, M.L.; Spencer, J.P. Synthetic, non-intoxicating 8, 9-dihydrocannabidiol for the mitigation of seizures. Sci. Rep. 2019, 9, 1–6. [Google Scholar] [CrossRef]
- Amendola, G.; Bocca, B.; Picardo, V.; Pelosi, P.; Battistini, B.; Ruggieri, F.; Barbini, D.A.; De Vita, D.; Madia, V.; Messore, A. Toxicological aspects of cannabinoid, pesticide and metal levels detected in light Cannabis inflorescences grown in Italy. Food Chem. Toxicol. 2021, 156, 112447. [Google Scholar] [CrossRef]
- Montoya, Z.; Conroy, M.; Vanden Heuvel, B.D.; Pauli, C.S.; Park, S.-H. Cannabis contaminants limit pharmacological use of cannabidiol. Front. Pharmacol. 2020, 11, 571832. [Google Scholar] [CrossRef]
- Sgrò, S.; Lavezzi, B.; Caprari, C.; Polito, M.; D’Elia, M.; Lago, G.; Furlan, G.; Girotti, S.; Ferri, E.N. Delta9-THC determination by the EU official method: Evaluation of measurement uncertainty and compliance assessment of hemp samples. Anal. Bioanal. Chem. 2021, 413, 3399–3410. [Google Scholar] [CrossRef]
- Żuk-Gołaszewska, K.; Gołaszewski, J. Hemp production. Sustain. Agric. Rev. 2020, 42, 1–36. [Google Scholar]
- Kennedy, M.C. Cannabis: Exercise performance and sport. A systematic review. J. Sci. Med. Sport 2017, 20, 825–829. [Google Scholar] [CrossRef]
- van Wilgen, C.P.; Keizer, D. Neuropathic pain mechanisms in patients with chronic sports injuries: A diagnostic model useful in sports medicine? Pain Med. 2011, 12, 110–117. [Google Scholar] [CrossRef]
- Jesus, C.H.A.; Redivo, D.D.B.; Gasparin, A.T.; Sotomaior, B.B.; de Carvalho, M.C.; Genaro, K.; Zuardi, A.W.; Hallak, J.E.C.; Crippa, J.A.; Zanoveli, J.M. Cannabidiol attenuates mechanical allodynia in streptozotocin-induced diabetic rats via serotonergic system activation through 5-HT1A receptors. Brain Res. 2019, 1715, 156–164. [Google Scholar] [CrossRef]
- Costa, B.; Trovato, A.E.; Comelli, F.; Giagnoni, G.; Colleoni, M. The non-psychoactive cannabis constituent cannabidiol is an orally effective therapeutic agent in rat chronic inflammatory and neuropathic pain. Eur. J. Pharmacol. 2007, 556, 75–83. [Google Scholar] [CrossRef]
- Jung, B.; Lee, J.K.; Kim, J.; Kang, E.K.; Han, S.Y.; Lee, H.Y.; Choi, I.S. Synthetic Strategies for (−)-Cannabidiol and Its Structural Analogs. Chem. –Asian J. 2019, 14, 3749–3762. [Google Scholar] [CrossRef]
- Jeong, S.; Yun, H.K.; Jeong, Y.A.; Jo, M.J.; Kang, S.H.; Kim, J.L.; Kim, D.Y.; Park, S.H.; Kim, B.R.; Na, Y.J. Cannabidiol-induced apoptosis is mediated by activation of Noxa in human colorectal cancer cells. Cancer Lett. 2019, 447, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Saleem, S.; Anwar, A. Cannabidiol: A hope to treat non-motor symptoms of Parkinson′s disease patients. Eur. Arch. Psychiatry Clin. Neurosci. 2020, 270, 135–135. [Google Scholar] [CrossRef] [PubMed]
- Hao, E.; Mukhopadhyay, P.; Cao, Z.; Erdélyi, K.; Holovac, E.; Liaudet, L.; Lee, W.-S.; Haskó, G.; Mechoulam, R.; Pacher, P. Cannabidiol protects against doxorubicin-induced cardiomyopathy by modulating mitochondrial function and biogenesis. Mol. Med. 2015, 21, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Patricio, F.; Morales-Andrade, A.A.; Patricio-Martínez, A.; Limón, I.D. Cannabidiol as a therapeutic target: Evidence of its neuroprotective and neuromodulatory function in Parkinson’s disease. Front. Pharmacol. 2020, 11, 595635. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ruiz, J.; Sagredo, O.; Pazos, M.R.; García, C.; Pertwee, R.; Mechoulam, R.; Martínez-Orgado, J. Cannabidiol for neurodegenerative disorders: Important new clinical applications for this phytocannabinoid? Br. J. Clin. Pharmacol. 2013, 75, 323–333. [Google Scholar] [CrossRef]
- Kozela, E.; Juknat, A.; Gao, F.; Kaushansky, N.; Coppola, G.; Vogel, Z. Pathways and gene networks mediating the regulatory effects of cannabidiol, a nonpsychoactive cannabinoid, in autoimmune T cells. J. Neuroinflamm. 2016, 13, 136. [Google Scholar] [CrossRef]
- Jean-Gilles, L.; Braitch, M.; Latif, M.L.; Aram, J.; Fahey, A.J.; Edwards, L.J.; Robins, R.A.; Tanasescu, R.; Tighe, P.J.; Gran, B. Effects of pro-inflammatory cytokines on cannabinoid CB 1 and CB 2 receptors in immune cells. Acta Physiol. 2015, 214, 63–74. [Google Scholar] [CrossRef]
- Srivastava, M.D.; Srivastava, B.; Brouhard, B. Δ9 tetrahydrocannabinol and cannabidiol alter cytokine production by human immune cells. Immunopharmacology 1998, 40, 179–185. [Google Scholar] [CrossRef]
- Lu, H.-C.; Mackie, K. An introduction to the endogenous cannabinoid system. Biol. Psychiatry 2016, 79, 516–525. [Google Scholar] [CrossRef]
- McPartland, J.M.; Duncan, M.; Di Marzo, V.; Pertwee, R.G. Are cannabidiol and Δ9-tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review. Br. J. Pharmacol. 2015, 172, 737–753. [Google Scholar] [CrossRef]
- Bíró, T.; Tóth, B.I.; Haskó, G.; Paus, R.; Pacher, P. The endocannabinoid system of the skin in health and disease: Novel perspectives and therapeutic opportunities. Trends Pharmacol. Sci. 2009, 30, 411–420. [Google Scholar] [CrossRef]
- Pacher, P.; Bátkai, S.; Kunos, G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol. Rev. 2006, 58, 389–462. [Google Scholar] [CrossRef]
- Oddi, S.; Scipioni, L.; Maccarrone, M. Endocannabinoid system and adult neurogenesis: A focused review. Curr. Opin. Pharmacol. 2020, 50, 25–32. [Google Scholar] [CrossRef]
- van Eenige, R.; van der Stelt, M.; Rensen, P.C.; Kooijman, S. Regulation of adipose tissue metabolism by the endocannabinoid system. Trends Endocrinol. Metab. 2018, 29, 326–337. [Google Scholar] [CrossRef]
- Uhelski, M.L.; Khasabova, I.; Simone, D.A. Modulation of Pain by Endocannabinoids in the Periphery. In Recent Advances in Cannabinoid Research; Costain, W.J., Ed.; IntechOpen: London, UK, 2018; Volume 13, pp. 101–118. [Google Scholar]
- Sierra, S.; Luquin, N.; Navarro-Otano, J. The endocannabinoid system in cardiovascular function: Novel insights and clinical implications. Clin. Auton. Res. 2018, 28, 35–52. [Google Scholar] [CrossRef]
- Turcotte, C.; Blanchet, M.-R.; Laviolette, M.; Flamand, N. The CB 2 receptor and its role as a regulator of inflammation. Cell. Mol. Life Sci. 2016, 73, 4449–4470. [Google Scholar] [CrossRef]
- Karsak, M.; Gaffal, E.; Date, R.; Wang-Eckhardt, L.; Rehnelt, J.; Petrosino, S.; Starowicz, K.; Steuder, R.; Schlicker, E.; Cravatt, B. Attenuation of allergic contact dermatitis through the endocannabinoid system. science 2007, 316, 1494–1497. [Google Scholar] [CrossRef]
- Watson, S.; Chambers, D.; Hobbs, C.; Doherty, P.; Graham, A. The endocannabinoid receptor, CB1, is required for normal axonal growth and fasciculation. Mol. Cell. Neurosci. 2008, 38, 89–97. [Google Scholar] [CrossRef]
- Mechoulam, R.; Hanuš, L.O.; Pertwee, R.; Howlett, A.C. Early phytocannabinoid chemistry to endocannabinoids and beyond. Nat. Rev. Neurosci. 2014, 15, 757–764. [Google Scholar] [CrossRef]
- Amenta, P.S.; Jallo, J.I.; Tuma, R.F.; Hooper, D.C.; Elliott, M.B. Cannabinoid receptor type-2 stimulation, blockade, and deletion alter the vascular inflammatory responses to traumatic brain injury. J. Neuroinflamm. 2014, 11, 191. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Rajesh, M.; Horváth, B.; Bátkai, S.; Park, O.; Tanchian, G.; Gao, R.Y.; Patel, V.; Wink, D.A.; Liaudet, L. Cannabidiol protects against hepatic ischemia/reperfusion injury by attenuating inflammatory signaling and response, oxidative/nitrative stress, and cell death. Free. Radic. Biol. Med. 2011, 50, 1368–1381. [Google Scholar] [CrossRef] [PubMed]
- Hegde, V.L.; Hegde, S.; Cravatt, B.F.; Hofseth, L.J.; Nagarkatti, M.; Nagarkatti, P.S. Attenuation of experimental autoimmune hepatitis by exogenous and endogenous cannabinoids: Involvement of regulatory T cells. Mol. Pharmacol. 2008, 74, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V. Endocannabinoids: Synthesis and degradation. In Reviews of Physiology Biochemistry and Pharmacology; Springer: Berlin/Heidelberg, Germany, 2006; pp. 1–24. [Google Scholar]
- Mayo, L.M.; Asratian, A.; Lindé, J.; Morena, M.; Haataja, R.; Hammar, V.; Augier, G.; Hill, M.N.; Heilig, M. Elevated anandamide, enhanced recall of fear extinction, and attenuated stress responses following inhibition of fatty acid amide hydrolase: A randomized, controlled experimental medicine trial. Biol. Psychiatry 2020, 87, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Mayo, L.M.; Asratian, A.; Lindé, J.; Holm, L.; Nätt, D.; Augier, G.; Stensson, N.; Vecchiarelli, H.A.; Balsevich, G.; Aukema, R.J. Protective effects of elevated anandamide on stress and fear-related behaviors: Translational evidence from humans and mice. Mol. Psychiatry 2018, 1–13. [Google Scholar] [CrossRef]
- Habib, A.M.; Okorokov, A.L.; Hill, M.N.; Bras, J.T.; Lee, M.-C.; Li, S.; Gossage, S.J.; van Drimmelen, M.; Morena, M.; Houlden, H. Microdeletion in a FAAH pseudogene identified in a patient with high anandamide concentrations and pain insensitivity. Br. J. Anaesth. 2019, 123, e249–e253. [Google Scholar] [CrossRef]
- Cravatt, B.F.; Demarest, K.; Patricelli, M.P.; Bracey, M.H.; Giang, D.K.; Martin, B.R.; Lichtman, A.H. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc. Natl. Acad. Sci. 2001, 98, 9371–9376. [Google Scholar] [CrossRef]
- Schlosburg, J.E.; Blankman, J.L.; Long, J.Z.; Nomura, D.K.; Pan, B.; Kinsey, S.G.; Nguyen, P.T.; Ramesh, D.; Booker, L.; Burston, J.J. Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nat. Neurosci. 2010, 13, 1113. [Google Scholar] [CrossRef]
- Laun, A.S.; Shrader, S.H.; Brown, K.J.; Song, Z.-H. GPR3, GPR6, and GPR12 as novel molecular targets: Their biological functions and interaction with cannabidiol. Acta Pharmacol. Sin. 2019, 40, 300–308. [Google Scholar] [CrossRef]
- Brown, K.J.; Laun, A.S.; Song, Z.-H. Cannabidiol, a novel inverse agonist for GPR12. Biochem. Biophys. Res. Commun. 2017, 493, 451–454. [Google Scholar] [CrossRef]
- Waldeck-Weiermair, M.; Zoratti, C.; Osibow, K.; Balenga, N.; Goessnitzer, E.; Waldhoer, M.; Malli, R.; Graier, W.F. Integrin clustering enables anandamide-induced Ca2+ signaling in endothelial cells via GPR55 by protection against CB1-receptor-triggered repression. J. Cell Sci. 2008, 121, 1704–1717. [Google Scholar] [CrossRef]
- Qin, N.; Neeper, M.P.; Liu, Y.; Hutchinson, T.L.; Lubin, M.L.; Flores, C.M. TRPV2 is activated by cannabidiol and mediates CGRP release in cultured rat dorsal root ganglion neurons. J. Neurosci. 2008, 28, 6231–6238. [Google Scholar] [CrossRef]
- Fogaça, M.V.; Campos, A.C.; Guimarães, F.S. Cannabidiol and 5-HT1A receptors. In Neuropathology of Drug Addictions and Substance Misuse; Elsevier: Cambridge, MA, USA, 2016; pp. 749–759. [Google Scholar]
- Pumroy, R.A.; Samanta, A.; Liu, Y.; Hughes, T.E.; Zhao, S.; Yudin, Y.; Rohacs, T.; Han, S.; Moiseenkova-Bell, V.Y. Molecular mechanism of TRPV2 channel modulation by cannabidiol. Elife 2019, 8, e48792. [Google Scholar] [CrossRef]
- Pelz, M.C.; Schoolcraft, K.D.; Larson, C.; Spring, M.G.; López, H.H. Assessing the role of serotonergic receptors in cannabidiol′s anticonvulsant efficacy. Epilepsy Behav. 2017, 73, 111–118. [Google Scholar] [CrossRef]
- Laun, A.S.; Song, Z.-H. GPR3 and GPR6, novel molecular targets for cannabidiol. Biochem. Biophys. Res. Commun. 2017, 490, 17–21. [Google Scholar] [CrossRef]
- Huang, Y.; Skwarek-Maruszewska, A.; Horré, K.; Vandewyer, E.; Wolfs, L.; Snellinx, A.; Saito, T.; Radaelli, E.; Corthout, N.; Colombelli, J. Loss of GPR3 reduces the amyloid plaque burden and improves memory in Alzheimer’s disease mouse models. Sci. Transl. Med. 2015, 7, 309ra164-309ra164. [Google Scholar] [CrossRef]
- Massi, P.; Solinas, M.; Cinquina, V.; Parolaro, D. Cannabidiol as potential anticancer drug. Br. J. Clin. Pharmacol. 2013, 75, 303–312. [Google Scholar] [CrossRef]
- O′Sullivan, S.E. An update on PPAR activation by cannabinoids. Br. J. Pharmacol. 2016, 173, 1899–1910. [Google Scholar] [CrossRef]
- Jurkus, R.; Day, H.L.; Guimarães, F.S.; Lee, J.L.; Bertoglio, L.J.; Stevenson, C.W. Cannabidiol regulation of learned fear: Implications for treating anxiety-related disorders. Front. Pharmacol. 2016, 7, 454. [Google Scholar] [CrossRef]
- Silva, R.L.; Silveira, G.T.; Wanderlei, C.W.; Cecilio, N.T.; Maganin, A.G.; Franchin, M.; Marques, L.M.; Lopes, N.P.; Crippa, J.A.; Guimarães, F.S. DMH-CBD, a cannabidiol analog with reduced cytotoxicity, inhibits TNF production by targeting NF-kB activity dependent on A2A receptor. Toxicol. Appl. Pharmacol. 2019, 368, 63–71. [Google Scholar] [CrossRef]
- Hind, W.H.; England, T.J.; O′Sullivan, S.E. Cannabidiol protects an in vitro model of the blood–brain barrier from oxygen-glucose deprivation via PPARγ and 5-HT1A receptors. Br. J. Pharmacol. 2016, 173, 815–825. [Google Scholar] [CrossRef]
- Nichol, K.; Stott, C.; Jones, N.; Gray, R.A.; Bazelot, M.; Whalley, B.J. The proposed multimodal mechanism of action of cannabidiol (CBD) in epilepsy: Modulation of intracellular calcium and adenosine-mediated signaling (P5. 5-007). Neurology 2019, 92, P5.5-007. [Google Scholar]
- Rimmerman, N.; Ben-Hail, D.; Porat, Z.; Juknat, A.; Kozela, E.; Daniels, M.P.; Connelly, P.S.; Leishman, E.; Bradshaw, H.B.; Shoshan-Barmatz, V.; et al. Direct modulation of the outer mitochondrial membrane channel, voltage-dependent anion channel 1 (VDAC1) by cannabidiol: A novel mechanism for cannabinoid-induced cell death. Cell Death Dis. 2013, 4, e949-e949. [Google Scholar] [CrossRef] [PubMed]
- Ryan, D.; Drysdale, A.J.; Lafourcade, C.; Pertwee, R.G.; Platt, B. Cannabidiol targets mitochondria to regulate intracellular Ca2+ levels. J. Neurosci. 2009, 29, 2053–2063. [Google Scholar] [CrossRef]
- Sartim, A.G.; Guimarães, F.S.; Joca, S.R.L. Antidepressant-like effect of cannabidiol injection into the ventral medial prefrontal cortex—Possible involvement of 5-HT1A and CB1 receptors. Behav. Brain Res. 2016, 303, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Laprairie, R.; Bagher, A.; Kelly, M.; Denovan-Wright, E. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br. J. Pharmacol. 2015, 172, 4790–4805. [Google Scholar] [CrossRef]
- Howlett, A.C.; Abood, M.E. CB1 and CB2 receptor pharmacology. In Advances in Pharmacology; Elsevier: Cambridge, MA, USA, 2017; Volume 80, pp. 169–206. [Google Scholar]
- De Petrocellis, L.; Orlando, P.; Moriello, A.S.; Aviello, G.; Stott, C.; Izzo, A.; Di Marzo, V. Cannabinoid actions at TRPV channels: Effects on TRPV3 and TRPV4 and their potential relevance to gastrointestinal inflammation. Acta Physiol. 2012, 204, 255–266. [Google Scholar] [CrossRef]
- De Petrocellis, L.; Ligresti, A.; Moriello, A.S.; Allarà, M.; Bisogno, T.; Petrosino, S.; Stott, C.G.; Di Marzo, V. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br. J. Pharmacol. 2011, 163, 1479–1494. [Google Scholar] [CrossRef]
- De Petrocellis, L.; Vellani, V.; Schiano-Moriello, A.; Marini, P.; Magherini, P.C.; Orlando, P.; Di Marzo, V. Plant-derived cannabinoids modulate the activity of transient receptor potential channels of ankyrin type-1 and melastatin type-8. J. Pharmacol. Exp. Ther. 2008, 325, 1007–1015. [Google Scholar] [CrossRef]
- Cascio, M.G.; Gauson, L.A.; Stevenson, L.A.; Ross, R.A.; Pertwee, R.G. Evidence that the plant cannabinoid cannabigerol is a highly potent α2-adrenoceptor agonist and moderately potent 5HT1A receptor antagonist. Br. J. Pharmacol. 2010, 159, 129–141. [Google Scholar] [CrossRef]
- Linge, R.; Jiménez-Sánchez, L.; Campa, L.; Pilar-Cuéllar, F.; Vidal, R.; Pazos, A.; Adell, A.; Díaz, A. Cannabidiol enhancement of serotonergic and glutamatergic signaling in a mouse model of depression induces fast and maintained antidepressant actions: Implication of 5-HT1A receptors. 2016. Available online: https://digital.csic.es/bitstream/10261/164285/1/cannabidiolreceptor.pdf (accessed on 1 December 2022).
- Linge, R.; Jiménez-Sánchez, L.; Campa, L.; Pilar-Cuéllar, F.; Vidal, R.; Pazos, A.; Adell, A.; Díaz, Á. Cannabidiol induces rapid-acting antidepressant-like effects and enhances cortical 5-HT/glutamate neurotransmission: Role of 5-HT1A receptors. Neuropharmacology 2016, 103, 16–26. [Google Scholar] [CrossRef]
- Chiurchiù, V.; Lanuti, M.; De Bardi, M.; Battistini, L.; Maccarrone, M. The differential characterization of GPR55 receptor in human peripheral blood reveals a distinctive expression in monocytes and NK cells and a proinflammatory role in these innate cells. Int. Immunol. 2015, 27, 153–160. [Google Scholar] [CrossRef]
- Lin, X.H.; Yuece, B.; Li, Y.Y.; Feng, Y.J.; Feng, J.Y.; Yu, L.Y.; Li, K.; Li, Y.N.; Storr, M. A novel CB receptor GPR55 and its ligands are involved in regulation of gut movement in rodents. Neurogastroenterol. Motil. 2011, 23, 862-e342. [Google Scholar] [CrossRef]
- Sonego, A.B.; Prado, D.S.; Vale, G.T.; Sepulveda-Diaz, J.E.; Cunha, T.M.; Tirapelli, C.R.; Del Bel, E.A.; Raisman-Vozari, R.; Guimarães, F.S. Cannabidiol prevents haloperidol-induced vacuos chewing movements and inflammatory changes in mice via PPARγ receptors. Brain Behav. Immun. 2018, 74, 241–251. [Google Scholar] [CrossRef]
- Rajan, T.S.; Giacoppo, S.; Iori, R.; De Nicola, G.R.; Grassi, G.; Pollastro, F.; Bramanti, P.; Mazzon, E. Anti-inflammatory and antioxidant effects of a combination of cannabidiol and moringin in LPS-stimulated macrophages. Fitoterapia 2016, 112, 104–115. [Google Scholar] [CrossRef]
- Petrosino, S.; Verde, R.; Vaia, M.; Allarà, M.; Iuvone, T.; Di Marzo, V. Anti-inflammatory properties of cannabidiol, a nonpsychotropic cannabinoid, in experimental allergic contact dermatitis. J. Pharmacol. Exp. Ther. 2018, 365, 652–663. [Google Scholar] [CrossRef]
- Pellati, F.; Borgonetti, V.; Brighenti, V.; Biagi, M.; Benvenuti, S.; Corsi, L. Cannabis sativa L. and nonpsychoactive cannabinoids: Their chemistry and role against oxidative stress, inflammation, and cancer. BioMed Res. Int. 2018, 2018, 1691428. [Google Scholar] [CrossRef]
- Weiss, L.; Zeira, M.; Reich, S.; Har-Noy, M.; Mechoulam, R.; Slavin, S.; Gallily, R. Cannabidiol lowers incidence of diabetes in non-obese diabetic mice. Autoimmunity 2006, 39, 143–151. [Google Scholar] [CrossRef]
- Sacerdote, P.; Martucci, C.; Vaccani, A.; Bariselli, F.; Panerai, A.; Colombo, A.; Parolaro, D.; Massi, P. The nonpsychoactive component of marijuana cannabidiol modulates chemotaxis and IL-10 and IL-12 production of murine macrophages both in vivo and in vitro. J. Neuroimmunol. 2005, 159, 97–105. [Google Scholar] [CrossRef]
- Hamelink, C.; Hampson, A.; Wink, D.A.; Eiden, L.E.; Eskay, R.L. Comparison of cannabidiol, antioxidants, and diuretics in reversing binge ethanol-induced neurotoxicity. J. Pharmacol. Exp. Ther. 2005, 314, 780–788. [Google Scholar] [CrossRef]
- dos-Santos-Pereira, M.; Guimarães, F.S.; Del-Bel, E.; Raisman-Vozari, R.; Michel, P.P. Cannabidiol prevents LPS-induced microglial inflammation by inhibiting ROS/NF-κB-dependent signaling and glucose consumption. Glia 2020, 68, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Jastrząb, A.; Gęgotek, A.; Skrzydlewska, E. Cannabidiol regulates the expression of keratinocyte proteins involved in the inflammation process through transcriptional regulation. Cells 2019, 8, 827. [Google Scholar] [CrossRef] [PubMed]
- Castillo, A.; Tolón, M.; Fernández-Ruiz, J.; Romero, J.; Martinez-Orgado, J. The neuroprotective effect of cannabidiol in an in vitro model of newborn hypoxic–ischemic brain damage in mice is mediated by CB2 and adenosine receptors. Neurobiol. Dis. 2010, 37, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Hroudová, J.; Fišar, Z. Cannabinoid-induced changes in the activity of electron transport chain complexes of brain mitochondria. J. Mol. Neurosci. 2015, 56, 926–931. [Google Scholar] [CrossRef]
- Ross, H.R.; Napier, I.; Connor, M. Inhibition of recombinant human T-type calcium channels by Δ9-tetrahydrocannabinol and cannabidiol. J. Biol. Chem. 2008, 283, 16124–16134. [Google Scholar] [CrossRef]
- Hill, A.J.; Jones, N.A.; Smith, I.; Hill, C.L.; Williams, C.M.; Stephens, G.J.; Whalley, B.J. Voltage-gated sodium (NaV) channel blockade by plant cannabinoids does not confer anticonvulsant effects per se. Neurosci. Lett. 2014, 566, 269–274. [Google Scholar] [CrossRef]
- Pedrazzi, J.F.C.; Issy, A.; Gomes, F.; Guimarães, F.; Del-Bel, E. Cannabidiol effects in the prepulse inhibition disruption induced by amphetamine. Psychopharmacology 2015, 232, 3057–3065. [Google Scholar] [CrossRef]
- Bisogno, T. Endogenous cannabinoids: Structure and metabolism. J. Neuroendocrinol. 2008, 20, 1–9. [Google Scholar] [CrossRef]
- Klotz, K.A.; Grob, D.; Hirsch, M.; Metternich, B.; Schulze-Bonhage, A.; Jacobs, J. Efficacy and tolerance of synthetic cannabidiol for treatment of intractable epilepsy. Front. Neurol. 2019, 10, 1313. [Google Scholar] [CrossRef]
- Wilson, J.T.; Fief, C.A.; Jackson, K.D.; Mercer, S.L.; Deweese, J.E. HU-331 and oxidized cannabidiol act as inhibitors of human topoisomerase IIα and β. Chem. Res. Toxicol. 2018, 31, 137–144. [Google Scholar] [CrossRef]
- Sumariwalla, P.F.; Gallily, R.; Tchilibon, S.; Fride, E.; Mechoulam, R.; Feldmann, M. A novel synthetic, nonpsychoactive cannabinoid acid (HU-320) with antiinflammatory properties in murine collagen-induced arthritis. Arthritis Rheum. 2004, 50, 985–998. [Google Scholar] [CrossRef]
- Çakır, M.; Tekin, S.; Doğanyiğit, Z.; Erden, Y.; Soytürk, M.; Çiğremiş, Y.; Sandal, S. Cannabinoid type 2 receptor agonist JWH-133, attenuates Okadaic acid induced spatial memory impairment and neurodegeneration in rats. Life Sci. 2019, 217, 25–33. [Google Scholar] [CrossRef]
- Xu, H.; Cheng, C.L.; Chen, M.; Manivannan, A.; Cabay, L.; Pertwee, R.G.; Coutts, A.; Forrester, J.V. Anti-inflammatory property of the cannabinoid receptor-2-selective agonist JWH-133 in a rodent model of autoimmune uveoretinitis. J. Leukoc. Biol. 2007, 82, 532–541. [Google Scholar] [CrossRef]
- Aso, E.; Juvés, S.; Maldonado, R.; Ferrer, I. CB 2 cannabinoid receptor agonist ameliorates Alzheimer-like phenotype in AβPP/PS1 mice. J. Alzheimer′s Dis. 2013, 35, 847–858. [Google Scholar] [CrossRef]
- Qamri, Z.; Preet, A.; Nasser, M.W.; Bass, C.E.; Leone, G.; Barsky, S.H.; Ganju, R.K. Synthetic cannabinoid receptor agonists inhibit tumor growth and metastasis of breast cancer. Mol. Cancer Ther. 2009, 8, 3117–3129. [Google Scholar] [CrossRef]
- Bisogno, T.; Oddi, S.; Piccoli, A.; Fazio, D.; Maccarrone, M. Type-2 cannabinoid receptors in neurodegeneration. Pharmacol. Res. 2016, 111, 721–730. [Google Scholar] [CrossRef]
- Kruk-Slomka, M.; Banaszkiewicz, I.; Biala, G. The impact of CB2 receptor ligands on the MK-801-induced hyperactivity in mice. Neurotox. Res. 2017, 31, 410–420. [Google Scholar] [CrossRef]
- Bolognini, D.; Cascio, M.G.; Parolaro, D.; Pertwee, R.G. AM630 behaves as a protean ligand at the human cannabinoid CB2 receptor. Br. J. Pharmacol. 2012, 165, 2561–2574. [Google Scholar] [CrossRef]
- Ottani, A.; Giuliani, D. HU 210: A potent tool for investigations of the cannabinoid system. CNS Drug Rev. 2001, 7, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Skrabek, R.Q.; Galimova, L.; Ethans, K.; Perry, D. Nabilone for the treatment of pain in fibromyalgia. J. Pain 2008, 9, 164–173. [Google Scholar] [CrossRef]
- Maas, A.I.; Murray, G.; Henney III, H.; Kassem, N.; Legrand, V.; Mangelus, M.; Muizelaar, J.-P.; Stocchetti, N.; Knoller, N. Efficacy and safety of dexanabinol in severe traumatic brain injury: Results of a phase III randomised, placebo-controlled, clinical trial. Lancet Neurol. 2006, 5, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Wyss-Coray, T. Ageing, neurodegeneration and brain rejuvenation. Nature 2016, 539, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Ransohoff, R.M. How neuroinflammation contributes to neurodegeneration. Science 2016, 353, 777–783. [Google Scholar] [CrossRef]
- Allan, S.M.; Rothwell, N.J. Cytokines and acute neurodegeneration. Nat. Rev. Neurosci. 2001, 2, 734–744. [Google Scholar] [CrossRef]
- Mattson, M.P. Calcium and neurodegeneration. Aging Cell 2007, 6, 337–350. [Google Scholar] [CrossRef]
- Knott, A.B.; Perkins, G.; Schwarzenbacher, R.; Bossy-Wetzel, E. Mitochondrial fragmentation in neurodegeneration. Nat. Rev. Neurosci. 2008, 9, 505–518. [Google Scholar] [CrossRef]
- Esposito, G.; Scuderi, C.; Savani, C.; Steardo Jr, L.; De Filippis, D.; Cottone, P.; Iuvone, T.; Cuomo, V.; Steardo, L. Cannabidiol in vivo blunts β-amyloid induced neuroinflammation by suppressing IL-1β and iNOS expression. Br. J. Pharmacol. 2007, 151, 1272–1279. [Google Scholar] [CrossRef]
- Mori, M.A.; Meyer, E.; Soares, L.M.; Milani, H.; Guimarães, F.S.; de Oliveira, R.M.W. Cannabidiol reduces neuroinflammation and promotes neuroplasticity and functional recovery after brain ischemia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 75, 94–105. [Google Scholar] [CrossRef]
- Vallée, A.; Lecarpentier, Y.; Guillevin, R.; Vallée, J.-N. Effects of cannabidiol interactions with Wnt/β-catenin pathway and PPARγ on oxidative stress and neuroinflammation in Alzheimer′s disease. Acta Biochim. Et Biophys. Sin. 2017, 49, 853–866. [Google Scholar] [CrossRef]
- Lambert, A.J.; Brand, M.D. Reactive oxygen species production by mitochondria. In Mitochondrial DNA; Stuart, J.A., Ed.; Springer: Boston, MA, USA, 2009; Volume 554, pp. 165–181. [Google Scholar]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, T.; Ziegler, A.C.; Dimitrion, P.; Zuo, L. Oxidative stress in neurodegenerative diseases: From molecular mechanisms to clinical applications. Oxidative Med. Cell. Longev. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Rego, A.C.; Oliveira, C.R. Mitochondrial Dysfunction and Reactive Oxygen Species in Excitotoxicity and Apoptosis: Implications for the Pathogenesis of Neurodegenerative Diseases. Neurochem. Res. 2003, 28, 1563–1574. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Michaelis, E.K. Selective neuronal vulnerability to oxidative stress in the brain. Front. Aging Neurosci. 2010, 2, 12. [Google Scholar] [CrossRef]
- Bih, C.I.; Chen, T.; Nunn, A.V.; Bazelot, M.; Dallas, M.; Whalley, B.J. Molecular targets of cannabidiol in neurological disorders. Neurotherapeutics 2015, 12, 699–730. [Google Scholar]
- Campos, A.C.; Fogaça, M.V.; Sonego, A.B.; Guimarães, F.S. Cannabidiol, neuroprotection and neuropsychiatric disorders. Pharmacol. Res. 2016, 112, 119–127. [Google Scholar] [CrossRef]
- Rosenberg, E.C.; Tsien, R.W.; Whalley, B.J.; Devinsky, O. Cannabinoids and epilepsy. Neurotherapeutics 2015, 12, 747–768. [Google Scholar] [CrossRef]
- Pamplona, F.A.; da Silva, L.R.; Coan, A.C. Potential clinical benefits of CBD-rich Cannabis extracts over purified CBD in treatment-resistant epilepsy: Observational data meta-analysis. Front. Neurol. 2018, 9, 759. [Google Scholar] [CrossRef]
- Hussain, S.A.; Zhou, R.; Jacobson, C.; Weng, J.; Cheng, E.; Lay, J.; Hung, P.; Lerner, J.T.; Sankar, R. Perceived efficacy of cannabidiol-enriched cannabis extracts for treatment of pediatric epilepsy: A potential role for infantile spasms and Lennox–Gastaut syndrome. Epilepsy Behav. 2015, 47, 138–141. [Google Scholar] [CrossRef]
- Silvestro, S.; Mammana, S.; Cavalli, E.; Bramanti, P.; Mazzon, E. Use of cannabidiol in the treatment of epilepsy: Efficacy and security in clinical trials. Molecules 2019, 24, 1459. [Google Scholar] [CrossRef]
- Pazos, M.R.; Mohammed, N.; Lafuente, H.; Santos, M.; Martínez-Pinilla, E.; Moreno, E.; Valdizan, E.; Romero, J.; Pazos, A.; Franco, R. Mechanisms of cannabidiol neuroprotection in hypoxic–ischemic newborn pigs: Role of 5HT1A and CB2 receptors. Neuropharmacology 2013, 71, 282–291. [Google Scholar] [CrossRef]
- Chen, J.W.; Borgelt, L.M.; Blackmer, A.B. Cannabidiol: A new hope for patients with Dravet or Lennox-Gastaut syndromes. Ann. Pharmacother. 2019, 53, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Maggio, N.; Shavit Stein, E.; Segal, M. Cannabidiol regulates long term potentiation following status epilepticus: Mediation by calcium stores and serotonin. Front. Mol. Neurosci. 2018, 11, 32. [Google Scholar] [CrossRef]
- Fernández-Ruiz, J.; Romero, J.; Ramos, J.A. Endocannabinoids and neurodegenerative disorders: Parkinson’s disease, Huntington’s chorea, Alzheimer’s disease, and others. In Endocannabinoids; Springer: New York, NY, USA, 2015; pp. 233–259. [Google Scholar]
- Nazıroğlu, M.; Övey, I. Involvement of apoptosis and calcium accumulation through TRPV1 channels in neurobiology of epilepsy. Neuroscience 2015, 293, 55–66. [Google Scholar] [CrossRef]
- Lattanzi, S.; Brigo, F.; Trinka, E.; Zaccara, G.; Cagnetti, C.; Del Giovane, C.; Silvestrini, M. Efficacy and safety of cannabidiol in epilepsy: A systematic review and meta-analysis. Drugs 2018, 78, 1791–1804. [Google Scholar] [CrossRef]
- Sveinbjornsdottir, S. The clinical symptoms of Parkinson′s disease. J. Neurochem. 2016, 139, 318–324. [Google Scholar] [CrossRef]
- Sonego, A.B.; Gomes, F.V.; Del Bel, E.A.; Guimaraes, F.S. Cannabidiol attenuates haloperidol-induced catalepsy and c-Fos protein expression in the dorsolateral striatum via 5-HT1A receptors in mice. Behav. Brain Res. 2016, 309, 22–28. [Google Scholar] [CrossRef]
- Peres, F.F.; Levin, R.; Suiama, M.A.; Diana, M.C.; Gouvêa, D.A.; Almeida, V.; Santos, C.M.; Lungato, L.; Zuardi, A.W.; Hallak, J.E. Cannabidiol prevents motor and cognitive impairments induced by reserpine in rats. Front. Pharmacol. 2016, 7, 343. [Google Scholar] [CrossRef]
- Peres, F.F.; Lima, A.C.; Hallak, J.E.; Crippa, J.A.; Silva, R.H.; Abílio, V.C. Cannabidiol as a promising strategy to treat and prevent movement disorders? Front. Pharmacol. 2018, 9, 482. [Google Scholar] [CrossRef]
- Zuardi, A.W.; Crippa, J.; Hallak, J.E.C.; Pinto, J.; Chagas, M.H.N.; Rodrigues, G.; Dursun, S.; Tumas, V. Cannabidiol for the treatment of psychosis in Parkinson’s disease. J. Psychopharmacol. 2009, 23, 979–983. [Google Scholar] [CrossRef]
- Gomes, F.V.; Del Bel, E.A.; Guimarães, F.S. Cannabidiol attenuates catalepsy induced by distinct pharmacological mechanisms via 5-HT1A receptor activation in mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 46, 43–47. [Google Scholar] [CrossRef]
- Oeckl, P.; Hengerer, B.; Ferger, B. G-protein coupled receptor 6 deficiency alters striatal dopamine and cAMP concentrations and reduces dyskinesia in a mouse model of Parkinson′s disease. Exp. Neurol. 2014, 257, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Congdon, E.E.; Sigurdsson, E.M. Tau-targeting therapies for Alzheimer disease. Nat. Rev. Neurol. 2018, 14, 399–415. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; De Filippis, D.; Carnuccio, R.; Izzo, A.A.; Iuvone, T. The marijuana component cannabidiol inhibits β-amyloid-induced tau protein hyperphosphorylation through Wnt/β-catenin pathway rescue in PC12 cells. J. Mol. Med. 2006, 84, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Casarejos, M.J.; Perucho, J.; Gomez, A.; Munoz, M.P.; Fernandez-Estevez, M.; Sagredo, O.; Fernandez Ruiz, J.; Guzman, M.; de Yebenes, J.G.; Mena, M.A. Natural cannabinoids improve dopamine neurotransmission and tau and amyloid pathology in a mouse model of tauopathy. J. Alzheimer′s Dis. 2013, 35, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Martín-Moreno, A.M.; Reigada, D.; Ramírez, B.G.; Mechoulam, R.; Innamorato, N.; Cuadrado, A.; de Ceballos, M.L. Cannabidiol and other cannabinoids reduce microglial activation in vitro and in vivo: Relevance to Alzheimer′s disease. Mol. Pharmacol. 2011, 79, 964–973. [Google Scholar] [CrossRef]
- Janefjord, E.; Mååg, J.L.; Harvey, B.S.; Smid, S.D. Cannabinoid effects on β amyloid fibril and aggregate formation, neuronal and microglial-activated neurotoxicity in vitro. Cell. Mol. Neurobiol. 2014, 34, 31–42. [Google Scholar] [CrossRef]
- Watt, G.; Karl, T. In vivo evidence for therapeutic properties of cannabidiol (CBD) for Alzheimer′s disease. Front. Pharmacol. 2017, 8, 20. [Google Scholar] [CrossRef]
- Thameem Dheen, S.; Kaur, C.; Ling, E.-A. Microglial activation and its implications in the brain diseases. Curr. Med. Chem. 2007, 14, 1189–1197. [Google Scholar] [CrossRef]
- Maresz, K.; Carrier, E.J.; Ponomarev, E.D.; Hillard, C.J.; Dittel, B.N. Modulation of the cannabinoid CB2 receptor in microglial cells in response to inflammatory stimuli. J. Neurochem. 2005, 95, 437–445. [Google Scholar] [CrossRef]
- Hassan, S.; Eldeeb, K.; Millns, P.J.; Bennett, A.J.; Alexander, S.P.; Kendall, D.A. Cannabidiol enhances microglial phagocytosis via transient receptor potential (TRP) channel activation. Br. J. Pharmacol. 2014, 171, 2426–2439. [Google Scholar] [CrossRef]
- Manczak, M.; Sheiko, T.; Craigen, W.J.; Reddy, P.H. Reduced VDAC1 protects against Alzheimer′s disease, mitochondria, and synaptic deficiencies. J. Alzheimer′s Dis. 2013, 37, 679–690. [Google Scholar] [CrossRef]
- Leehey, M.A.; Liu, Y.; Hart, F.; Epstein, C.; Cook, M.; Sillau, S.; Klawitter, J.; Newman, H.; Sempio, C.; Forman, L. Safety and tolerability of cannabidiol in Parkinson disease: An open label, dose-escalation study. Cannabis Cannabinoid Res. 2020, 5, 326–336. [Google Scholar] [CrossRef]
- Patel, A.D.; Mazurkiewicz-Bełdzińska, M.; Chin, R.F.; Gil-Nagel, A.; Gunning, B.; Halford, J.J.; Mitchell, W.; Perry, M.S.; Thiele, E.A.; Weinstock, A. Long-term safety and efficacy of add-on cannabidiol in patients with Lennox–Gastaut syndrome: Results of a long-term open-label extension trial. Epilepsia 2021, 62, 2228–2239. [Google Scholar] [CrossRef]
- Xu, C.; Chang, T.; Du, Y.; Yu, C.; Tan, X.; Li, X. Pharmacokinetics of oral and intravenous cannabidiol and its antidepressant-like effects in chronic mild stress mouse model. Environ. Toxicol. Pharmacol. 2019, 70, 103202. [Google Scholar] [CrossRef]
- Shbiro, L.; Hen-Shoval, D.; Hazut, N.; Rapps, K.; Dar, S.; Zalsman, G.; Mechoulam, R.; Weller, A.; Shoval, G. Effects of cannabidiol in males and females in two different rat models of depression. Physiol. Behav. 2019, 201, 59–63. [Google Scholar] [CrossRef]
- Masataka, N. Anxiolytic effects of repeated cannabidiol treatment in teenagers with social anxiety disorders. Front. Psychol. 2019, 10, 2466. [Google Scholar] [CrossRef]
- Giorgi, V.; Marotto, D.; Batticciotto, A.; Atzeni, F.; Bongiovanni, S.; Sarzi-Puttini, P. Cannabis and autoimmunity: Possible mechanisms of action. ImmunoTargets Ther. 2021, 10, 261. [Google Scholar] [CrossRef]
- Peball, M.; Seppi, K.; Krismer, F.; Knaus, H.G.; Spielberger, S.; Heim, B.; Ellmerer, P.; Werkmann, M.; Poewe, W.; Djamshidian, A. Effects of Nabilone on Sleep Outcomes in Patients with Parkinson′s Disease: A Post-hoc Analysis of NMS-Nab Study. Mov. Disord. Clin. Pract. 2022, 9, 751–758. [Google Scholar] [CrossRef]
- Wheless, J.W.; Dlugos, D.; Miller, I.; Oh, D.A.; Parikh, N.; Phillips, S.; Renfroe, J.B.; Roberts, C.M.; Saeed, I.; Sparagana, S.P. Pharmacokinetics and tolerability of multiple doses of pharmaceutical-grade synthetic cannabidiol in pediatric patients with treatment-resistant epilepsy. CNS Drugs 2019, 33, 593–604. [Google Scholar] [CrossRef]
- Szaflarski, J.P.; Hernando, K.; Bebin, E.M.; Gaston, T.E.; Grayson, L.E.; Ampah, S.B.; Moreadith, R. Higher cannabidiol plasma levels are associated with better seizure response following treatment with a pharmaceutical grade cannabidiol. Epilepsy Behav. 2019, 95, 131–136. [Google Scholar] [CrossRef]
- Katchan, V.; David, P.; Shoenfeld, Y. Cannabinoids and autoimmune diseases: A systematic review. Autoimmun. Rev. 2016, 15, 513–528. [Google Scholar] [CrossRef] [PubMed]
- Furgiuele, A.; Cosentino, M.; Ferrari, M.; Marino, F. Immunomodulatory potential of cannabidiol in multiple sclerosis: A systematic review. J. Neuroimmune Pharmacol. 2021, 16, 251–269. [Google Scholar] [CrossRef] [PubMed]
- Keating, G.M. Delta-9-tetrahydrocannabinol/cannabidiol oromucosal spray (Sativex®): A review in multiple sclerosis-related spasticity. Drugs 2017, 77, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Mecha, M.; Feliú, A.; Iñigo, P.; Mestre, L.; Carrillo-Salinas, F.; Guaza, C. Cannabidiol provides long-lasting protection against the deleterious effects of inflammation in a viral model of multiple sclerosis: A role for A2A receptors. Neurobiol. Dis. 2013, 59, 141–150. [Google Scholar] [CrossRef]
- Kozela, E.; Lev, N.; Kaushansky, N.; Eilam, R.; Rimmerman, N.; Levy, R.; Ben-Nun, A.; Juknat, A.; Vogel, Z. Cannabidiol inhibits pathogenic T cells, decreases spinal microglial activation and ameliorates multiple sclerosis-like disease in C57BL/6 mice. Br. J. Pharmacol. 2011, 163, 1507–1519. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Hu, C.-A.A. Therapeutic potential of amino acids in inflammatory bowel disease. Nutrients 2017, 9, 920. [Google Scholar] [CrossRef]
- Ambrose, T.; Simmons, A. Cannabis, cannabinoids, and the endocannabinoid system—is there therapeutic potential for inflammatory bowel disease? J. Crohn′s Colitis 2019, 13, 525–535. [Google Scholar] [CrossRef]
- Hasenoehrl, C.; Storr, M.; Schicho, R. Cannabinoids for treating inflammatory bowel diseases: Where are we and where do we go? Expert Rev. Gastroenterol. Hepatol. 2017, 11, 329–337. [Google Scholar] [CrossRef]
- Kienzl, M.; Storr, M.; Schicho, R. Cannabinoids and opioids in the treatment of inflammatory bowel diseases. Clin. Transl. Gastroenterol. 2020, 11. [Google Scholar] [CrossRef]
- Martínez, V.; Iriondo De-Hond, A.; Borrelli, F.; Capasso, R.; Del Castillo, M.D.; Abalo, R. Cannabidiol and other non-psychoactive cannabinoids for prevention and treatment of gastrointestinal disorders: Useful nutraceuticals? Int. J. Mol. Sci. 2020, 21, 3067. [Google Scholar] [CrossRef]
- Vermeulen, W.; De Man, J.G.; Pelckmans, P.A.; De Winter, B.Y. Neuroanatomy of lower gastrointestinal pain disorders. World J. Gastroenterol. WJG 2014, 20, 1005. [Google Scholar] [CrossRef]
- Krohn, R.M.; Parsons, S.A.; Fichna, J.; Patel, K.D.; Yates, R.M.; Sharkey, K.A.; Storr, M.A. Abnormal cannabidiol attenuates experimental colitis in mice, promotes wound healing and inhibits neutrophil recruitment. J. Inflamm. 2016, 13, 1–11. [Google Scholar] [CrossRef]
- Couch, D.G.; Cook, H.; Ortori, C.; Barrett, D.; Lund, J.N.; O’Sullivan, S.E. Palmitoylethanolamide and cannabidiol prevent inflammation-induced hyperpermeability of the human gut in vitro and in vivo—A randomized, placebo-controlled, double-blind controlled trial. Inflamm. Bowel Dis. 2019, 25, 1006–1018. [Google Scholar] [CrossRef]
- Couch, D.G.; Tasker, C.; Theophilidou, E.; Lund, J.N.; O’Sullivan, S.E. Cannabidiol and palmitoylethanolamide are anti-inflammatory in the acutely inflamed human colon. Clin. Sci. 2017, 131, 2611–2626. [Google Scholar] [CrossRef]
- Pagano, E.; Capasso, R.; Piscitelli, F.; Romano, B.; Parisi, O.A.; Finizio, S.; Lauritano, A.; Marzo, V.D.; Izzo, A.A.; Borrelli, F. An orally active cannabis extract with high content in cannabidiol attenuates chemically-induced intestinal inflammation and hypermotility in the mouse. Front. Pharmacol. 2016, 7, 341. [Google Scholar] [CrossRef]
- Jamontt, J.; Molleman, A.; Pertwee, R.G.; Parsons, M. The effects of Δ9-tetrahydrocannabinol and cannabidiol alone and in combination on damage, inflammation and in vitro motility disturbances in rat colitis. Br. J. Pharmacol. 2010, 160, 712–723. [Google Scholar] [CrossRef]
- Borrelli, F.; Aviello, G.; Romano, B.; Orlando, P.; Capasso, R.; Maiello, F.; Guadagno, F.; Petrosino, S.; Capasso, F.; Di Marzo, V. Cannabidiol, a safe and non-psychotropic ingredient of the marijuana plant Cannabis sativa, is protective in a murine model of colitis. J. Mol. Med. 2009, 87, 1111–1121. [Google Scholar] [CrossRef]
- Khoury, M.; Cohen, I.; Bar-Sela, G. “The Two Sides of the Same Coin”—Medical Cannabis, Cannabinoids and Immunity: Pros and Cons Explained. Pharmaceutics 2022, 14, 389. [Google Scholar] [CrossRef]
- Dörner, T.; Furie, R. Novel paradigms in systemic lupus erythematosus. Lancet 2019, 393, 2344–2358. [Google Scholar] [CrossRef]
- Fava, A.; Petri, M. Systemic lupus erythematosus: Diagnosis and clinical management. J. Autoimmun. 2019, 96, 1–13. [Google Scholar] [CrossRef]
- Naftali, T.; Mechulam, R.; Marii, A.; Gabay, G.; Stein, A.; Bronshtain, M.; Laish, I.; Benjaminov, F.; Konikoff, F.M. Low-dose cannabidiol is safe but not effective in the treatment for Crohn’s disease, a randomized controlled trial. Dig. Dis. Sci. 2017, 62, 1615–1620. [Google Scholar] [CrossRef] [PubMed]
- Naftali, T.; Bar-Lev Schleider, L.; Almog, S.; Meiri, D.; Konikoff, F.M. Oral CBD-rich cannabis induces clinical but not endoscopic response in patients with Crohn’s disease, a randomised controlled trial. J. Crohn′s Colitis 2021, 15, 1799–1806. [Google Scholar] [CrossRef] [PubMed]
- Irving, P.M.; Iqbal, T.; Nwokolo, C.; Subramanian, S.; Bloom, S.; Prasad, N.; Hart, A.; Murray, C.; Lindsay, J.O.; Taylor, A. A randomized, double-blind, placebo-controlled, parallel-group, pilot study of cannabidiol-rich botanical extract in the symptomatic treatment of ulcerative colitis. Inflamm. Bowel Dis. 2018, 24, 714–724. [Google Scholar] [CrossRef] [PubMed]
- Blake, D.R.; Robson, P.; Ho, M.; Jubb, R.W.; McCabe, C.S. Preliminary assessment of the efficacy, tolerability and safety of a cannabis-based medicine (Sativex) in the treatment of pain caused by rheumatoid arthritis. Rheumatology 2006, 45, 50–52. [Google Scholar] [CrossRef]
- Hendricks, O.; Andersen, T.E.; Christiansen, A.A.; Primdahl, J.; Hauge, E.M.; Ellingsen, T.; Horsted, T.I.; Bachmann, A.G.; Loft, A.G.; Bojesen, A.B. Efficacy and safety of cannabidiol followed by an open label add-on of tetrahydrocannabinol for the treatment of chronic pain in patients with rheumatoid arthritis or ankylosing spondylitis: Protocol for a multicentre, randomised, placebo-controlled study. BMJ Open 2019, 9, e028197. [Google Scholar] [CrossRef]
- Kiriakidou, M.; Ching, C.L. Systemic Lupus Erythematosus. Ann. Intern. Med. 2020, 172, Itc81–Itc96. [Google Scholar] [CrossRef]
- Navarini, L.; Bisogno, T.; Mozetic, P.; Piscitelli, F.; Margiotta, D.P.E.; Basta, F.; Afeltra, A.; Maccarrone, M. Endocannabinoid system in systemic lupus erythematosus: First evidence for a deranged 2-arachidonoylglycerol metabolism. Int. J. Biochem. Cell Biol. 2018, 99, 161–168. [Google Scholar] [CrossRef]
- Rahaman, O.; Bhattacharya, R.; Liu, C.S.C.; Raychaudhuri, D.; Ghosh, A.R.; Bandopadhyay, P.; Pal, S.; Goswami, R.P.; Sircar, G.; Ghosh, P. Cutting edge: Dysregulated endocannabinoid-rheostat for plasmacytoid dendritic cell activation in a systemic lupus endophenotype. J. Immunol. 2019, 202, 1674–1679. [Google Scholar] [CrossRef]
- Henriquez, J.E.; Crawford, R.B.; Kaminski, N.E. Suppression of CpG-ODN-mediated IFNα and TNFα response in human plasmacytoid dendritic cells (pDC) by cannabinoid receptor 2 (CB2)-specific agonists. Toxicol. Appl. Pharmacol. 2019, 369, 82–89. [Google Scholar] [CrossRef]
- Maddukuri, S.; Patel, J.; Diaz, D.A.; Chen, K.L.; Wysocka, M.; Bax, C.; Li, Y.; Ravishankar, A.; Grinnell, M.; Zeidi, M. Cannabinoid type 2 receptor (CB2R) distribution in dermatomyositis skin and peripheral blood mononuclear cells (PBMCs) and in vivo effects of LenabasumTM. Arthritis Res. Ther. 2022, 24, 1–11. [Google Scholar] [CrossRef]
- Aletaha, D.; Smolen, J.S. Diagnosis and management of rheumatoid arthritis: A review. Jama 2018, 320, 1360–1372. [Google Scholar] [CrossRef]
- Burmester, G.R.; Pope, J.E. Novel treatment strategies in rheumatoid arthritis. Lancet 2017, 389, 2338–2348. [Google Scholar] [CrossRef]
- Lowin, T.; Schneider, M.; Pongratz, G. Joints for joints: Cannabinoids in the treatment of rheumatoid arthritis. Curr. Opin. Rheumatol. 2019, 31, 271–278. [Google Scholar] [CrossRef]
- Gui, H.; Liu, X.; Wang, Z.-W.; He, D.-Y.; Su, D.-F.; Dai, S.-M. Expression of cannabinoid receptor 2 and its inhibitory effects on synovial fibroblasts in rheumatoid arthritis. Rheumatology 2014, 53, 802–809. [Google Scholar] [CrossRef]
- Gui, H.; Liu, X.; Liu, L.-R.; Su, D.-F.; Dai, S.-M. Activation of cannabinoid receptor 2 attenuates synovitis and joint distruction in collagen-induced arthritis. Immunobiology 2015, 220, 817–822. [Google Scholar] [CrossRef]
- Lowin, T.; Tingting, R.; Zurmahr, J.; Classen, T.; Schneider, M.; Pongratz, G. Cannabidiol (CBD): A killer for inflammatory rheumatoid arthritis synovial fibroblasts. Cell Death Dis. 2020, 11, 1–11. [Google Scholar] [CrossRef]
- Campanati, A.; Marani, A.; Martina, E.; Diotallevi, F.; Radi, G.; Offidani, A. Psoriasis as an immune-mediated and inflammatory systemic disease: From pathophysiology to novel therapeutic approaches. Biomedicines 2021, 9, 1511. [Google Scholar] [CrossRef]
- Dopytalska, K.; Ciechanowicz, P.; Wiszniewski, K.; Szymańska, E.; Walecka, I. The role of epigenetic factors in psoriasis. Int. J. Mol. Sci. 2021, 22, 9294. [Google Scholar] [CrossRef]
- Carvalho, A.L.; Hedrich, C.M. The molecular pathophysiology of psoriatic arthritis—the complex interplay between genetic predisposition, epigenetics factors, and the microbiome. Front. Mol. Biosci. 2021, 8, 662047. [Google Scholar] [CrossRef]
- Sheriff, T.; Lin, M.J.; Dubin, D.; Khorasani, H. The potential role of cannabinoids in dermatology. J. Dermatol. Treat. 2020, 31, 839–845. [Google Scholar] [CrossRef]
- Ramot, Y.; Sugawara, K.; Zákány, N.; Toth, B.I.; Bíró, T.; Paus, R. A novel control of human keratin expression: Cannabinoid receptor 1-mediated signaling down-regulates the expression of keratins K6 and K16 in human keratinocytes in vitro and in situ. PeerJ 2013, 1, e40. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, J.D.; Williamson, E.M. Cannabinoids inhibit human keratinocyte proliferation through a non-CB1/CB2 mechanism and have a potential therapeutic value in the treatment of psoriasis. J. Dermatol. Sci. 2007, 45, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Norooznezhad, A.H.; Norooznezhad, F. Cannabinoids: Possible agents for treatment of psoriasis via suppression of angiogenesis and inflammation. Med. Hypotheses 2017, 99, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Scheau, C.; Badarau, I.A.; Mihai, L.-G.; Scheau, A.-E.; Costache, D.O.; Constantin, C.; Calina, D.; Caruntu, C.; Costache, R.S.; Caruntu, A. Cannabinoids in the pathophysiology of skin inflammation. Molecules 2020, 25, 652. [Google Scholar] [CrossRef]
- Stella, A.; Palmieri, B.; Laurino, C.; Vadalà, M. A therapeutic effect of cbd-enriched ointment in inflammatory skin diseases and cutaneous scars. La Clin. Ter. 2019, 170, e93–e99. [Google Scholar]
- Sinai, A.; Turner, Z.; Baruch, Y. Cannabis-based extracts and topical formulations for use in skin disorders. U.S. Patent 10,603,301, 31 March 2020. [Google Scholar]
- Guzmán, M. Cannabinoids: Potential anticancer agents. Nat. Rev. Cancer 2003, 3, 745–755. [Google Scholar] [CrossRef]
- Aggarwal, S.K.; Carter, G.T.; Sullivan, M.D.; ZumBrunnen, C.; Morrill, R.; Mayer, J.D. Medicinal use of cannabis in the United States: Historical perspectives, current trends, and future directions. J. Opioid Manag. 2009, 5, 153–168. [Google Scholar] [CrossRef]
- Reuter, S.E.; Martin, J.H. Pharmacokinetics of cannabis in cancer cachexia-anorexia syndrome. Clin. Pharmacokinet. 2016, 55, 807–812. [Google Scholar] [CrossRef]
- Borgelt, L.M.; Franson, K.L.; Nussbaum, A.M.; Wang, G.S. The P harmacologic and C linical E ffects of M edical C annabis. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2013, 33, 195–209. [Google Scholar] [CrossRef]
- Barnes, M.P. Sativex®: Clinical efficacy and tolerability in the treatment of symptoms of multiple sclerosis and neuropathic pain. Expert Opin. Pharmacother. 2006, 7, 607–615. [Google Scholar] [CrossRef]
- Blake, A.; Wan, B.A.; Malek, L.; DeAngelis, C.; Diaz, P.; Lao, N.; O’Hearn, S. A selective review of medical cannabis in cancer pain management. Ann. Palliat. Med. 2017, 6, s215–s222. [Google Scholar] [CrossRef]
- Velasco, G.; Sánchez, C.; Guzmán, M. Towards the use of cannabinoids as antitumour agents. Nat. Rev. Cancer 2012, 12, 436–444. [Google Scholar] [CrossRef]
- Chakravarti, B.; Ravi, J.; Ganju, R.K. Cannabinoids as therapeutic agents in cancer: Current status and future implications. Oncotarget 2014, 5, 5852. [Google Scholar] [CrossRef]
- Velasco, G.; Sánchez, C.; Guzmán, M. Anticancer mechanisms of cannabinoids. Curr. Oncol. 2016, 23, S23. [Google Scholar] [CrossRef]
- Pisanti, S.; Malfitano, A.M.; Ciaglia, E.; Lamberti, A.; Ranieri, R.; Cuomo, G.; Abate, M.; Faggiana, G.; Proto, M.C.; Fiore, D. Cannabidiol: State of the art and new challenges for therapeutic applications. Pharmacol. Ther. 2017, 175, 133–150. [Google Scholar] [CrossRef]
- Kim, J.L.; Kim, B.R.; Kim, D.Y.; Jeong, Y.A.; Jeong, S.; Na, Y.J.; Park, S.H.; Yun, H.K.; Jo, M.J.; Kim, B.G. Cannabidiol Enhances the Therapeutic Effects of TRAIL by Upregulating DR5 in Colorectal Cancer. Cancers 2019, 11, 642. [Google Scholar] [CrossRef]
- Lukhele, S.T.; Motadi, L.R. Cannabidiol rather than Cannabis sativa extracts inhibit cell growth and induce apoptosis in cervical cancer cells. BMC Complement. Altern. Med. 2016, 16, 335. [Google Scholar] [CrossRef]
- Elbaz, M.; Nasser, M.W.; Ravi, J.; Wani, N.A.; Ahirwar, D.K.; Zhao, H.; Oghumu, S.; Satoskar, A.R.; Shilo, K.; Carson III, W.E. Modulation of the tumor microenvironment and inhibition of EGF/EGFR pathway: Novel anti-tumor mechanisms of Cannabidiol in breast cancer. Mol. Oncol. 2015, 9, 906–919. [Google Scholar] [CrossRef]
- Jeong, S.; Jo, M.J.; Yun, H.K.; Kim, D.Y.; Kim, B.R.; Kim, J.L.; Park, S.H.; Na, Y.J.; Jeong, Y.A.; Kim, B.G. Cannabidiol promotes apoptosis via regulation of XIAP/Smac in gastric cancer. Cell Death Dis. 2019, 10, 1–13. [Google Scholar] [CrossRef]
- Huang, T.; Xu, T.; Wang, Y.; Zhou, Y.; Yu, D.; Wang, Z.; He, L.; Chen, Z.; Zhang, Y.; Davidson, D. Cannabidiol inhibits human glioma by induction of lethal mitophagy through activating TRPV4. Autophagy 2021, 17, 3592–3606. [Google Scholar] [CrossRef]
- Kosgodage, U.S.; Mould, R.; Henley, A.B.; Nunn, A.V.; Guy, G.W.; Thomas, E.L.; Inal, J.M.; Bell, J.D.; Lange, S. Cannabidiol (CBD) is a novel inhibitor for exosome and microvesicle (EMV) release in cancer. Front. Pharmacol. 2018, 9, 889. [Google Scholar] [CrossRef] [PubMed]
- Schoeman, R.; Beukes, N.; Frost, C. Cannabinoid combination induces cytoplasmic vacuolation in MCF-7 breast cancer cells. Molecules 2020, 25, 4682. [Google Scholar] [CrossRef] [PubMed]
- Milian, L.; Mata, M.; Alcacer, J.; Oliver, M.; Sancho-Tello, M.; Martín de Llano, J.J.; Camps, C.; Galbis, J.; Carretero, J.; Carda, C. Cannabinoid receptor expression in non-small cell lung cancer. Effectiveness of tetrahydrocannabinol and cannabidiol inhibiting cell proliferation and epithelial-mesenchymal transition in vitro. PLoS ONE 2020, 15, e0228909. [Google Scholar] [CrossRef]
- Nabissi, M.; Morelli, M.B.; Offidani, M.; Amantini, C.; Gentili, S.; Soriani, A.; Cardinali, C.; Leoni, P.; Santoni, G. Cannabinoids synergize with carfilzomib, reducing multiple myeloma cells viability and migration. Oncotarget 2016, 7, 77543. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Kim, B.G.; Kim, D.Y.; Kim, B.R.; Kim, J.L.; Park, S.H.; Na, Y.J.; Jo, M.J.; Yun, H.K.; Jeong, Y.A. Cannabidiol overcomes oxaliplatin resistance by enhancing NOS3-and SOD2-Induced autophagy in human colorectal cancer cells. Cancers 2019, 11, 781. [Google Scholar] [CrossRef]
- Deng, L.; Ng, L.; Ozawa, T.; Stella, N. Quantitative analyses of synergistic responses between cannabidiol and DNA-damaging agents on the proliferation and viability of glioblastoma and neural progenitor cells in culture. J. Pharmacol. Exp. Ther. 2017, 360, 215–224. [Google Scholar] [CrossRef]
- López-Valero, I.; Saiz-Ladera, C.; Torres, S.; Hernández-Tiedra, S.; García-Taboada, E.; Rodríguez-Fornés, F.; Barba, M.; Dávila, D.; Salvador-Tormo, N.; Guzmán, M. Targeting glioma initiating cells with a combined therapy of cannabinoids and temozolomide. Biochem. Pharmacol. 2018, 157, 266–274. [Google Scholar] [CrossRef]
- Fraguas-Sánchez, A.; Fernández-Carballido, A.; Simancas-Herbada, R.; Martin-Sabroso, C.; Torres-Suárez, A. CBD loaded microparticles as a potential formulation to improve paclitaxel and doxorubicin-based chemotherapy in breast cancer. Int. J. Pharm. 2020, 574, 118916. [Google Scholar] [CrossRef]
- Twelves, C.; Sabel, M.; Checketts, D.; Miller, S.; Tayo, B.; Jove, M.; Brazil, L.; Short, S.C. A phase 1b randomised, placebo-controlled trial of nabiximols cannabinoid oromucosal spray with temozolomide in patients with recurrent glioblastoma. Br. J. Cancer 2021, 124, 1379–1387. [Google Scholar] [CrossRef]
- Bar-Sela, G.; Cohen, I.; Campisi-Pinto, S.; Lewitus, G.M.; Oz-Ari, L.; Jehassi, A.; Peer, A.; Turgeman, I.; Vernicova, O.; Berman, P. Cannabis consumption used by cancer patients during immunotherapy correlates with poor clinical outcome. Cancers 2020, 12, 2447. [Google Scholar] [CrossRef]
- Chesney, E.; Oliver, D.; Green, A.; Sovi, S.; Wilson, J.; Englund, A.; Freeman, T.P.; McGuire, P. Adverse effects of cannabidiol: A systematic review and meta-analysis of randomized clinical trials. Neuropsychopharmacology 2020, 45, 1799–1806. [Google Scholar] [CrossRef] [PubMed]
| Receptor/Target | Action | Effect | Dose | Reference |
|---|---|---|---|---|
| CB1 | Inverse agonist/negative allosteric modulator | Antidepressant-like effect. | 10–60 nmol (in vivo); 0.01–5 µM | [67,68] |
| CB2 | Inverse agonist/negative allosteric modulator | Anti-inflammatory effect. | <1 µM | [25,69] |
| TRPV1-4 | Agonist | Nociceptor desensitization effect. | 1–10 µM | [70,71] |
| TRPM8 | Antagonist | Inhibition of [Ca2+] elevation induced by menthol and icilin. | <0.1 µM | [71,72] |
| TRPA1 | Agonist | Regulation of TRPV1 function. | EC50 12 µM | [72] |
| 5-HT1a receptors | Activation through direct, allosteric, or indirect effects | Associated with an antidepressant and anxiolytic effect. | 50 mg/Kg (in vivo) | [73,74,75] |
| GPR55 | Antagonist | Antagonization of proinflammatory effects. | 10 mg/Kg (in vivo) | [76,77] |
| GPR3, GPR6 and GPR12 | Inverse agonist | GPR3 is suggested as a biomarker for the prognosis of multiple sclerosis. GPR6 has been implicated in both HD and PD. GPR12 has been implicated in cell survival and neurite outgrow. | 0.1–10 µM | [50,57] |
| PPAR-γ | Agonist/Up-regulator | Associated with anti-inflammatory and antioxidant properties through interaction with different transcription factors. | 10 µM | [60,63,78] |
| TNF-α, IFN-β, IFN-γ, IL-1β, IL-17, IL-6 | Modulator, decreases levels | Decrease in inflammation levels by targeting different pathways’ activity. | <20 µM | [79,80,81] |
| IL-4 and IL-10 | Increases levels | Anti-inflammatory cytokines. | 5 mg/Kg (in vivo) | [82,83] |
| ROS | Inhibitor | CBD inhibits a mechanism related to NADPH oxidase-mediated ROS production and NF-κB-dependent signaling events | <10 µM | [84,85] |
| iNOS and COX2 | Inhibition of expression | Inhibition of the transcription of pro-inflammatory genes (i.e., iNOS, COX-2) contributing to CBD anti-inflammatory effect. | 100 µM | [86,87] |
| Mitochondrial complexes I-IV | Inhibition | Decreases the activity of mitochondrial complexes (I, II, II-III, and IV). | 50 µM | [88] |
| CaV3 | Antagonist | Inhibition of the CaV3 channels might be involved in CBD analgesic effect. | 1 µM | [89] |
| NaV | Inhibitor | Linked to antiseizure effects. | 10 µM | [90] |
| VDAC1 | Modulator | Associated to anticancer and immunosuppressive properties. | 10 µM | [65] |
| AEA | Inhibitor | CBD acts in part by interfering with AEA inactivation and enhancing its inhibitory action on inflammation. | 60 mg/Kg (in vivo) | [91,92] |
| FAAH | Inhibitor | Linked to CBD’s antipsychotic effect. | IC50 15 µM | [2,71] |
| Compound | Dosage and Treatment | Condition | Phase | Status and Results | Clinical Trial Code |
|---|---|---|---|---|---|
| High CBD and low THC | Hemp derived solution to be administered sublingually twice daily | AD and anxiety | Early 1 | Still recruiting | NCT04075435 |
| THC-free CBD oil | Starting at a dosage of 15 mg twice per day with up titration to 45 mg twice per day; oral solution | AD and dementia | 2 | Still recruiting | NCT04436081 |
| GWP42003 (purified CBD) | Started at 5 mg/kg/day and is increased by 2.5–5 mg/kg at 3–5-day intervals to a target dose of 20 mg/kg/day; oral solution | Tremor in PD | 2 | 3 participants dropped out due to study drug intolerance. The remaining participants demonstrated improvements in cognition, depression, and emotional issues associated with PD. | NCT02818777 |
| Medical cannabis | Inhaled dried buds or sublingual oil extract | Non-motor symptoms of PD | 2 | Still recruiting | NCT05106504 |
| Nabilone | 0.25–2 mg per day. Capsules, taken orally daily | Non-motor symptoms of PD | 2 | Positive effects on anxious mood and night-time sleep problems, with no serious adverse effects registered [154]. | NCT03769896 |
| GWP42003 (purified CBD) | 150 mg/day, increased by 150 mg/day each week for the remaining three weeks. Maximum dose of 600 mg/day during the fourth week; oral solution | Psychosis in PD | 2 | No adverse effect was observed during the treatment. Psychosis symptoms decreased [148]. | NCT02818777 |
| Epidiolex | 100 mg/mL oral solution titrated to a target maintenance dose of 20 mg/kg/day over 2 weeks | Epilepsy | 3 | The study demonstrated long-term benefits with a 43.9% reduction in monthly drop and total seizures frequency in the CBD group, observed through 156 weeks [149]. | NCT02224573 |
| CBD (nonplant-based) | 3 multiple ascending doses: 10 mg/kg/day, 20 mg/kg/day and 40 mg/kg/day; oral solution | Epilepsy | 1/2 | Short-term administration was generally safe and well tolerated. Inter-individual variability decreased with multiple doses [155]. | NCT02324673 |
| Epidiolex | Various doses between 5 mg/kg/day and 50 mg/kg/day; oral solution | Epilepsy | - | Seizure control is proportional to CBD plasma level, with a linear relationship between dosage and level of CBD [156]. | NCT02695537 |
| CBD (in corn oil) | 5–25 mg/kg/day; oral solution | Epilepsy | 3 | On-going | NCT02783092 |
| CBD (supplement with a bio-terpene complex) | 1 capsule/day (Hemp Extract 35 mg; Bio-Terpene Complex 52 mg) | Anxiety and stress | - | Not shared | NCT05518019 |
| Cannabis oil | Single dose of CBD (300 mg) | Social anxiety disorder | - | CBD significantly decreased anxiety measured by two different scales [152]. | JCT0018004564 |
| Compound | Dosage and Treatment | Condition | Phase | Status and Results | Clinical Trial Code |
|---|---|---|---|---|---|
| CBD | 10 mg daily. Sublingual | IBD (CD and UC) | 1/2 | Safe but not effective as treatment [177]. | NCT01037322 |
| CBD and THC oil | Initially 16 mg CBD and 4 mg THC/day. Maximum: 320 mg CBD and 80 mg THC/day. Sublingual | CD | 1/2 | Significant clinical and QOL improvement without significant changes in inflammatory parameters or endoscopic scores [178]. | NCT01826188 |
| CBD (rich botanical extract) | 50 mg up to 250 mg daily. Ingestion | UC | 2 | No remission after treatment, yet promoted QOL improvement [179]. | NCT01562314 |
| Medical cannabis | Inhalation | IBD (CD and UC) | 2 | On-going | NCT05578313 |
| Medical cannabis | Inhalation | IBD (CD and UC) | 2 | On-going | NCT03944447 |
| Sativex (CBD and THC) | 2.7 mg THC and 2.5 mg CBD. Oromucosal spray | RA | 2 | A significant analgesic effect was observed, and disease activity was significantly suppressed following Sativex treatment [180]. | - |
| Medical cannabis | Inhalation | RA and PSA | 2 | On-going. | NCT04269993 |
| CBD | 400 or 800 mg daily. Ingestion | RA | 1/2 | On-going. | NCT04911127 |
| CBD | Initial: 10 mg CBD/day. Maximum: 30 mg CBD/day. Ingestion | RA | 2 | On-going [181]. | EudraCT 2017-004226-15 |
| CBD and THC | 3% CBD + 3% THC; topical | Psoriasis | 1 | Not shared. | NCT02976779 |
| CBD | Initial: 10 mg CBD/day. Maximum: 30 mg CBD/day. Ingestion | PSA | 2 | Not shared. | NCT03693833 |
| Compound | Other Drugs/Treatment | Type of Cancer | Phase | Status and Results | Clinical Trial Code |
|---|---|---|---|---|---|
| Epidiolex | - | Prostate cancer | 1 | Recruitment completed No results shared | NCT04428203 |
| Epidiolex | Mifepristone (antiprogesterone) | Breast cancer | 3 | Not yet recruiting | NCT05016349 |
| Tamoxifen Retinoic acid | data | ||||
| BRCX014 (CBD 200 mg) | Bortezomib Leucovorin 5-FU Oxaliplatin Bevacizumab Irinotecan Gemcitabine Temozolomide | Multiple myeloma Glioblastoma multiforme GI malignancies | 1/2 | Unknown | NCT03607643 |
| Sativex (27 mg/mL THC and 25 mg/mL CBD) | Advanced cancer | 1 | Withdrawn | NCT02432612 | |
| Sativex | Temozolomide | Recurrent Glioblastoma | 1/2 | Completed [226] | NCT01812603 NCT01812616 |
| Sativex | Head and neck squamous cell carcinoma | 1 | Terminated (slow recruitment) | NCT01975688 | |
| TN-TC11G (5 mg of CBD, 5 mg of THC) | Temozolomide Radiation therapy | Newly diagnosed Glioblastoma | 1/2 | Estimated completion in 2024 | NCT03529448 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luz-Veiga, M.; Azevedo-Silva, J.; Fernandes, J.C. Beyond Pain Relief: A Review on Cannabidiol Potential in Medical Therapies. Pharmaceuticals 2023, 16, 155. https://doi.org/10.3390/ph16020155
Luz-Veiga M, Azevedo-Silva J, Fernandes JC. Beyond Pain Relief: A Review on Cannabidiol Potential in Medical Therapies. Pharmaceuticals. 2023; 16(2):155. https://doi.org/10.3390/ph16020155
Chicago/Turabian StyleLuz-Veiga, Mariana, João Azevedo-Silva, and João C. Fernandes. 2023. "Beyond Pain Relief: A Review on Cannabidiol Potential in Medical Therapies" Pharmaceuticals 16, no. 2: 155. https://doi.org/10.3390/ph16020155
APA StyleLuz-Veiga, M., Azevedo-Silva, J., & Fernandes, J. C. (2023). Beyond Pain Relief: A Review on Cannabidiol Potential in Medical Therapies. Pharmaceuticals, 16(2), 155. https://doi.org/10.3390/ph16020155







