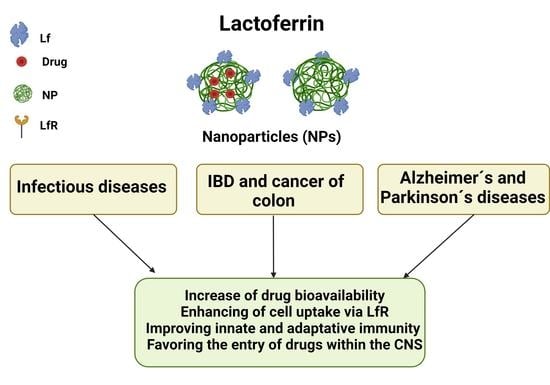
Lactoferrin as a Component of Pharmaceutical Preparations: An Experimental Focus
Abstract
1. Introduction
2. Pharmacologic of Lactoferrin Formulations: General Aspects
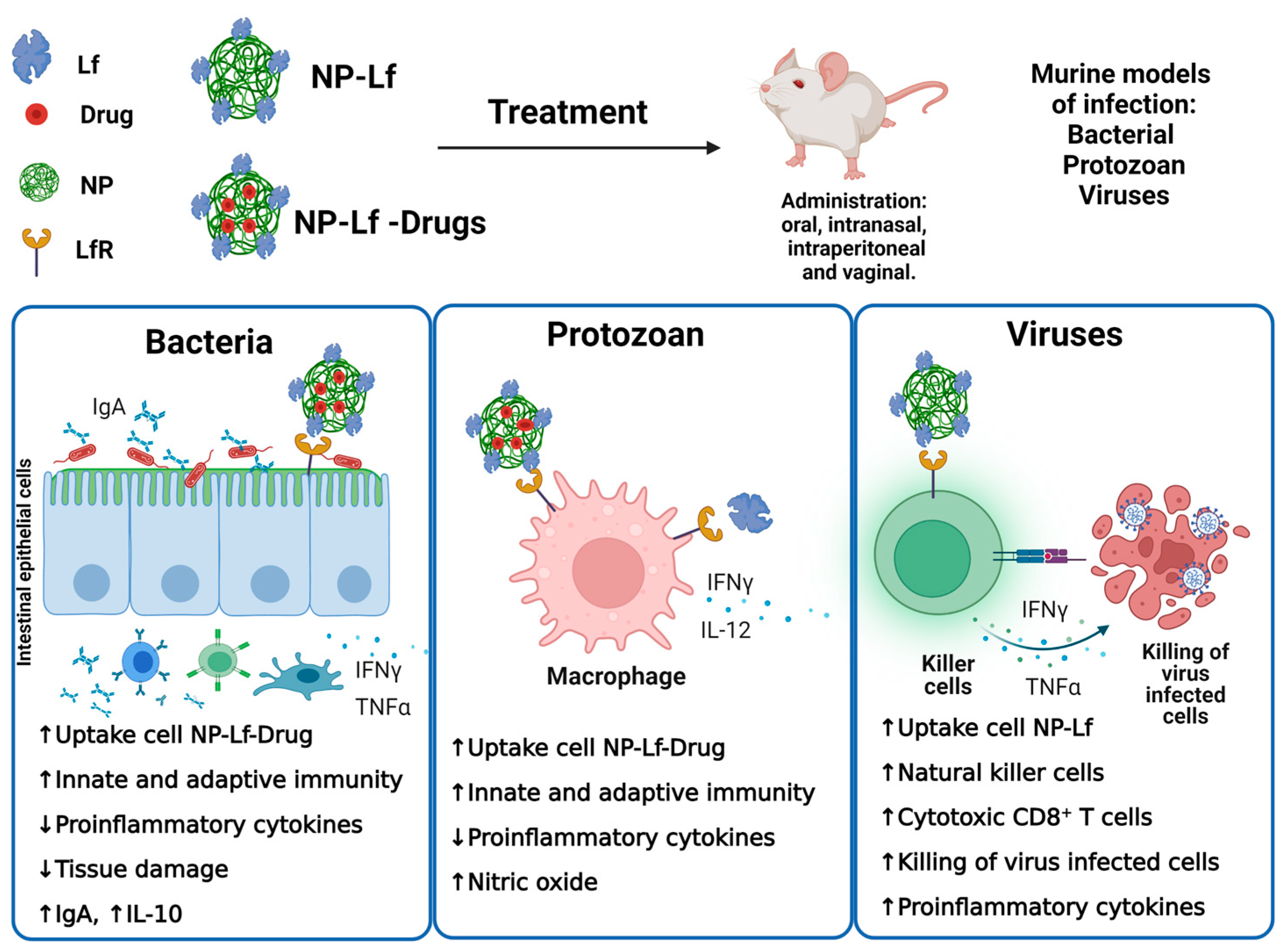
3. Antimicrobial Products
3.1. Antibacterial Products
3.2. Antiparasite Products
3.3. Antiviral Products
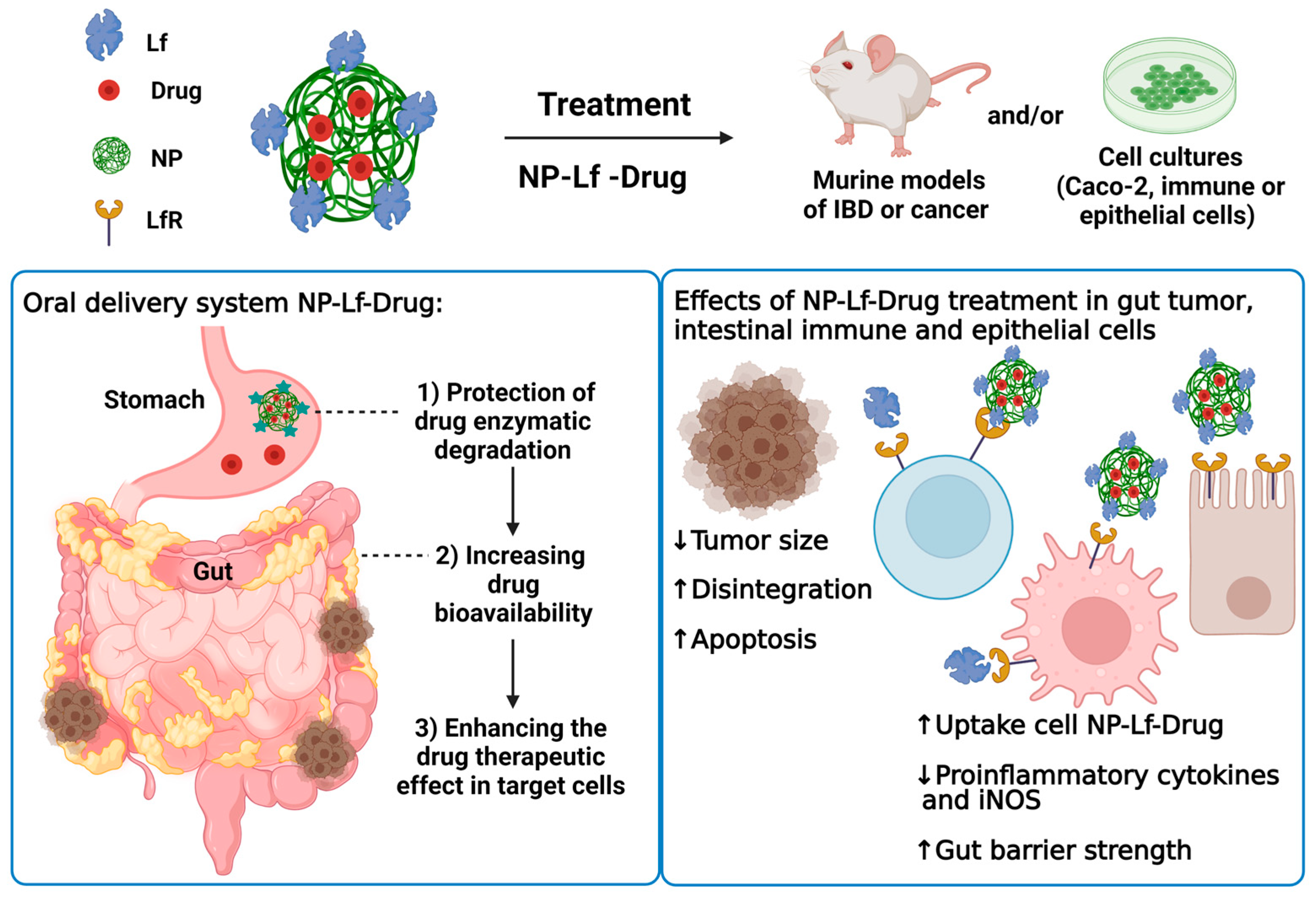
4. Intestinal Dysfunctions
4.1. Inflammatory Bowel Diseases: Ulcerative Colitis and Crohn’s Disease
4.2. Colon Cancer
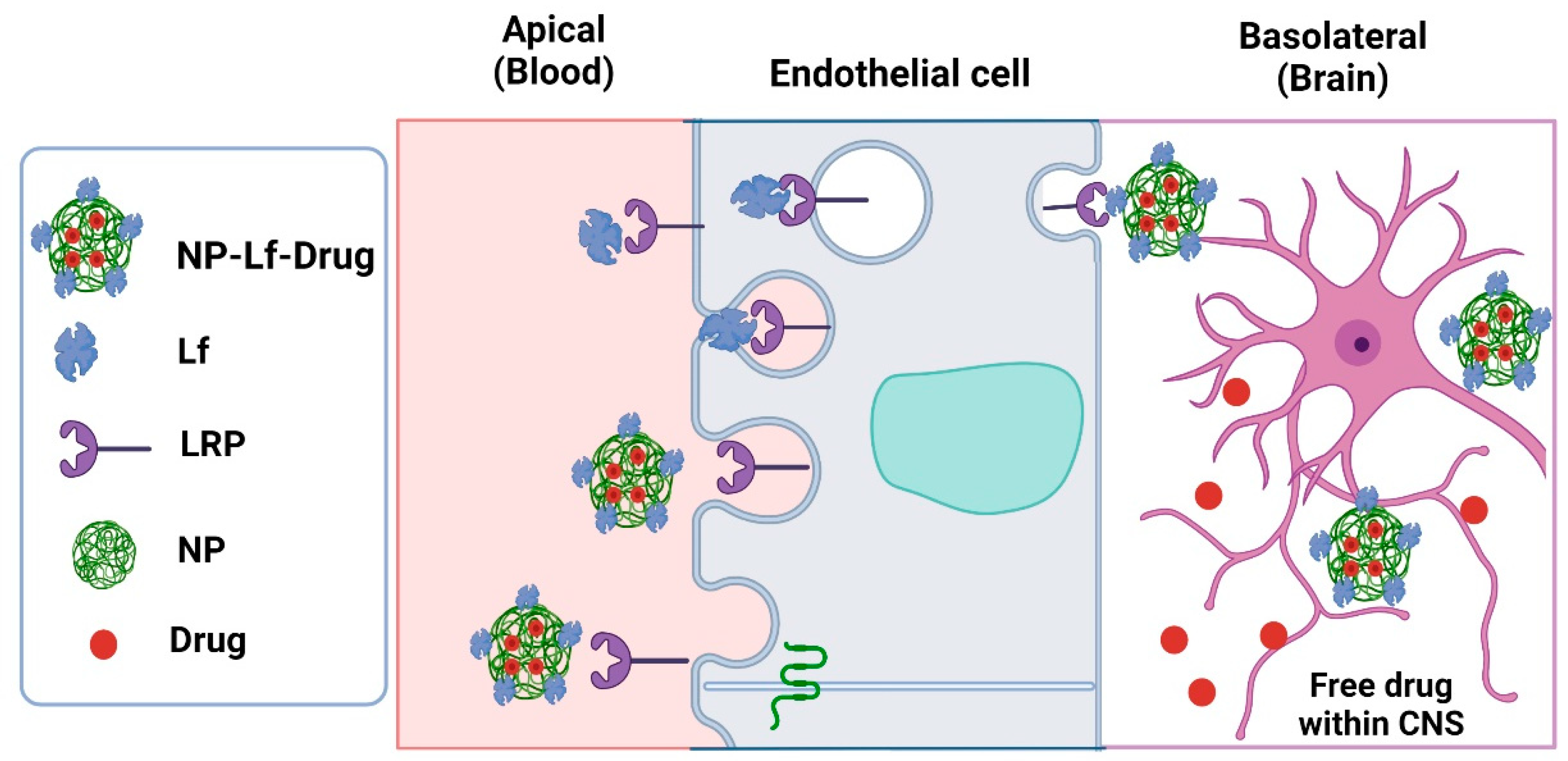
5. Neurodegenerative Diseases
5.1. Alzheimer’s Disease
5.2. Parkinson’s Disease
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baker, E.N.; Anderson, B.F.; Baker, H.M.; Day, C.L.; Haridas, M.; Norris, G.E.; Rumball, S.V.; Smith, C.A.; Thomas, D.H. Three-dimensional structure of lactoferrin in various functional states. Adv. Exp. Med. Biol. 1994, 357, 1–12. [Google Scholar] [PubMed]
- Superti, F. Lactoferrin from Bovine Milk: A Protective Companion for Life. Nutrients 2020, 12, 2562. [Google Scholar] [CrossRef]
- Gruden, Š.; Poklar Ulrih, N. Diverse Mechanisms of Antimicrobial Activities of Lactoferrins, Lactoferricins, and Other Lactoferrin-Derived Peptides. Int. J. Mol. Sci. 2021, 22, 11264. [Google Scholar] [CrossRef] [PubMed]
- Oda, H.; Kolawole, A.O.; Mirabelli, C.; Wakabayashi, H.; Tanaka, M.; Yamauchi, K.; Abe, F.; Wobus, C.E. Antiviral effects of bovine lactoferrin on human norovirus. Biochem. Cell Biol. 2021, 99, 166–172. [Google Scholar] [CrossRef]
- Sinopoli, A.; Isonne, C.; Santoro, M.M.; Baccolini, V. The effects of orally administered lactoferrin in the prevention and management of viral infections: A systematic review. Rev. Med. Virol. 2022, 32, e2261. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Kaczyńska, K.; Kleczkowska, P.; Bukowska-Ośko, I.; Kramkowski, K.; Sulejczak, D. The Lactoferrin Phenomenon-A Miracle Molecule. Molecules 2022, 27, 1–16. [Google Scholar]
- Suzuki, Y.A.; Lopez, V.; Lönnerdal, B. Mammalian lactoferrin receptors: Structure and function. Cell Mol. Life Sci. 2005, 62, 2560–2575. [Google Scholar] [CrossRef] [PubMed]
- Rosa, L.; Lepanto, M.S.; Cutone, A.; Siciliano, R.A.; Paesano, R.; Costi, R.; Musci, G.; Valenti, P. Influence of oral administration mode on the efficacy of commercial bovine Lactoferrin against iron and inflammatory homeostasis disorders. Biometals 2020, 33, 59–168. [Google Scholar] [CrossRef] [PubMed]
- Haddadzadegan, S.; Dorkoosh, F.; Bernkop-Schnürch, A. Oral delivery of therapeutic peptides and proteins: Technology landscape of lipid-based nanocarriers. Adv. Drug Deliv. Rev. 2022, 182, 114097. [Google Scholar] [PubMed]
- Karamanidou, T.; Karidi, K.; Bourganis, V.; Kontonikola, K.; Kammona, O.; Kiparissides, C. Effective incorporation of insulin in mucus permeating self-nanoemulsifying drug delivery systems. Eur. J. Pharm. Biopharm. 2015, 97, 223–229. [Google Scholar]
- Tian, M.; Han, J.; Ye, A.; Liu, W.; Xu, X.; Yao, Y.; Li, K.; Kong, Y.; Wei, F.; Zhou, W. Structural characterization and biological fate of lactoferrin-loaded liposomes during simulated infant digestion. J. Sci. Food Agric. 2019, 99, 2677–2684. [Google Scholar] [CrossRef] [PubMed]
- Ong, R.; Cornish, J.; Wen, J. Nanoparticular and other carriers to deliver lactoferrin for antimicrobial, antibiofilm and bone-regenerating effects: A review. Biometals 2022, 431, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.S.; Feng, K.; Li, S.F.; Hu, T.G.; Linhardt, R.J.; Zong, M.H.; Wu, H. Oral fate and stabilization technologies of lactoferrin: A systematic review. Crit. Rev. Food Sci. Nutr. 2022, 62, 6341–6358. [Google Scholar] [CrossRef] [PubMed]
- El-Fakharany, E.M. Nanoformulation of lactoferrin potentiates its activity and enhances novel biotechnological applications. Int. J. Biol. Macromol. 2020, 165, 970–984. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Timilsena, Y.P.; Blanch, E.; Adhikari, B. Mild thermal treatment and in-vitro digestion of three forms of bovine lactoferrin: Effects on functional properties. Int. Dairy J. 2017, 64, 22–30. [Google Scholar] [CrossRef]
- David-Birman, T.; Mackie, A.; Lesmes, U. Impact of dietary fibers on the properties and proteolytic digestibility of lactoferrin nano-particles. Food Hydrocoll. 2013, 31, 33–41. [Google Scholar] [CrossRef]
- Zhang, Y.; Pu, C.; Tang, W.; Wang, S.; Sun, Q. Gallic acid liposomes decorated with lactoferrin: Characterization, in vitro digestion and antibacterial activity. Food Chem. 2019, 293, 315–322. [Google Scholar] [CrossRef]
- Liu, W.; Ye, A.; Liu, W.; Liu, C.; Singh, H. Stability during in vitro digestion of lactoferrin-loaded liposomes prepared from milk fat globule membrane-derived phospholipids. J. Dairy Sci. 2013, 96, 2061–2070. [Google Scholar] [CrossRef]
- Kilic, E.; Novoselova, M.V.; Lim, S.H.; Pyataev, N.A.; Pinyaev, S.I.; Kulikov, O.A.; Sindeeva, O.A.; Mayorova, O.A.; Murney, R.; Antipina, M.N.; et al. Formulation for Oral Delivery of Lactoferrin Based on Bovine Serum Albumin and Tannic Acid Multilayer Microcapsules. Sci. Rep. 2017, 7, 44159. [Google Scholar] [CrossRef]
- Yao, X.; Bunt, C.; Cornish, J.; Quek, S.Y.; Wen, J. Oral Delivery of Bovine Lactoferrin Using Pectin- and Chitosan-Modified Liposomes and Solid Lipid Particles: Improvement of Stability of Lactoferrin. Chem. Biol. Drug Des. 2015, 86, 466–475. [Google Scholar] [CrossRef]
- Akiyama, Y.; Oshima, K.; Shin, K.; Wakabayashi, H.; Abe, F.; Nadano, D.; Matsuda, T. Intracellular retention and subsequent release of bovine milk lactoferrin taken up by human enterocyte-like cell lines, Caco-2, C2BBe1 and HT-29. Biosci. Biotechnol. Biochem. 2013, 77, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Pu, X.; Ye, N.; Lin, M.; Chen, Q.; Dong, L.; Xu, H.; Luo, R.; Han, X.; Qi, S.; Nie, W. β-1,3-d-Glucan based yeast cell wall system loaded emodin with dual-targeting layers for ulcerative colitis treatment. Carbohydr. Polym. 2021, 273, 118612. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.; Lin, M.; Fu, C.; Zhang, J.; Chen, Q.; Zhang, C.; Shi, J.; Pu, X.; Dong, L.; Xu, H.; et al. Calcium pectinate and hyaluronic acid modified lactoferrin nanoparticles loaded rhein with dual-targeting for ulcerative colitis treatment. Carbohydr. Polym. 2021, 263, 117998. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, T.; Nakamura, M.; Nogita, T.; Sato, A. Cellular Uptake and Release of Intact Lactoferrin and Its Derivatives in an Intestinal Enterocyte Model of Caco-2 Cells. Biol. Pharm. Bull. 2019, 42, 989–995. [Google Scholar] [CrossRef]
- Takeuchi, T.; Kitagawa, H.; Harada, E. Evidence of lactoferrin transportation into blood circulation from intestine via lymphatic pathway in adult rats. Exp. Physiol. 2004, 89, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Ishikado, A.; Imanaka, H.; Takeuchi, T.; Harada, E.; Makino, T. Liposomalization of lactoferrin enhanced it’s anti-inflammatory effects via oral administration. Biol. Pharm. Bull. 2005, 28, 1717–1721. [Google Scholar] [CrossRef] [PubMed]
- Anand, N.; Sehgal, R.; Kanwar, R.K.; Dubey, M.L.; Vasishta, R.K.; Kanwar, J.R. Oral administration of encapsulated bovine lactoferrin protein nanocapsules against intracellular parasite toxoplasma gondii. Int. J. Nanomed. 2015, 10, 6355. [Google Scholar]
- Kanwar, J.R.; Mahidhara, G.; Roy, K.; Sasidharan, S.; Krishnakumar, S.; Prasad, N.; Sehgal, R.; Kanwar, R.K. Fe-bLf nanoformulation targets survivin to kill colon cancer stem cells and maintains absorption of iron, calcium and zinc. Nanomedicine 2015, 10, 35–55. [Google Scholar] [CrossRef]
- Auderset, L.; Cullen, C.L.; Young, K.M. Low Density Lipoprotein-Receptor Related Protein 1 Is Differentially Expressed by Neuronal and Glial Populations in the Developing and Mature Mouse Central Nervous System. PLoS ONE 2016, 11, e0155878. [Google Scholar] [CrossRef]
- Nikolakopoulou, A.M.; Wang, Y.; Ma, Q.; Sagare, A.P.; Montagne, A.; Huuskonen, M.T.; Rege, S.V.; Kisler, K.; Dai, Z.; Körbelin, J.; et al. Endothelial LRP1 protects against neurodegeneration by blocking cyclophilin A. J. Exp. Med. 2021, 218, e20202207. [Google Scholar] [CrossRef]
- Fillebeen, C.; Descamps, L.; Dehouck, M.P.; Fenart, L.; Benaïssa, M.; Spik, G.; Cecchelli, R.; Pierce, A. Receptor-mediated transcytosis of lactoferrin through the blood-brain barrier. J. Biol. Chem. 1999, 274, 7011–7017. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Ke, W.; Han, L.; Liu, Y.; Shao, K.; Jiang, C.; Pei, Y. Lactoferrin-modified nanoparticles could mediate efficient gene delivery to the brain in vivo. Brain Res. Bull. 2010, 81, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Hua, H.; Jiang, Y.; Wang, Y.; Mu, H.; Wu, Z. Intranasal delivery of Huperzine A to the brain using lactoferrin-conjugated N-trimethylated chitosan surface-modified PLGA nanoparticles for treatment of Alzheimer’s disease. Int. J. Nanomed. 2018, 13, 705–718. [Google Scholar] [CrossRef] [PubMed]
- Goldwater, P.N.; Bettelheim, K.A. Treatment of enterohemorrhagic Escherichia coli (EHEC) infection and hemolytic uremic syndrome (HUS). BMC Med. 2012, 10, 12. [Google Scholar] [CrossRef]
- Jung, S.H.; Ryu, C.M.; Kim, J.S. Bacterial persistence: Fundamentals and clinical importance. J. Microbiol. 2019, 57, 829–835. [Google Scholar] [CrossRef]
- Mittal, A.K.; Bhardwaj, R.; Mishra, P.; Rajput, S.K. Antimicrobials Misuse/Overuse: Adverse Effect, Mechanism, Challenges and Strategies to Combat Resistance. Open Biotechnol. J. 2020, 14, 107–112. [Google Scholar] [CrossRef]
- Huttner, A.; Harbarth, S.; Carlet, J.; Cosgrove, S.; Goossens, H.; Holmes, A.; Jarlier, V.; Voss, A.; Pittet, D. Antimicrobial resistance: A global view from the 2013 World Healthcare-Associated Infections Forum. Antimicrob. Resist. Infect. Control 2013, 2, 317–328. [Google Scholar] [CrossRef]
- Gupta, I.; Sehgal, R.; Kanwar, R.K.; Punj, V.; Kanwar, J.R. Nanocapsules loaded with iron-saturated bovine lactoferrin have antimicrobial therapeutic potential and maintain calcium, zinc and iron metabolism. Nanomedicine 2015, 10, 1289–1314. [Google Scholar] [CrossRef]
- Fulgione, A.; Nocerino, N.; Iannaccone, M.; Roperto, S.; Capuano, F.; Roveri, N.; Lelli, M.; Crasto, A.; Calogero, A.; Pilloni, A.P.; et al. Lactoferrin Adsorbed onto Biomimetic Hydroxyapatite Nanocrystals Controlling—In Vivo—the Helicobacter pylori Infection. PLoS ONE 2016, 11, e0158646. [Google Scholar] [CrossRef]
- Ishikado, A.; Uesaki, S.; Suido, H.; Nomura, Y.; Sumikawa, K.; Maeda, M.; Miyauchi, M.; Takata, T.; Makino, T. Human trial of liposomal lactoferrin supplementation for periodontal disease. Biol. Pharm. Bull. 2010, 33, 1758–1762. [Google Scholar] [CrossRef]
- Asthana, S.; Gupta, P.K.; Jaiswal, A.K.; Dube, A.; Chourasia, M.K. Targeted chemotherapy of visceral leishmaniasis by lactoferrin-appended amphotericin B-loaded nanoreservoir: In vitro and in vivo studies. Nanomedicine 2015, 10, 1093–1109. [Google Scholar] [CrossRef] [PubMed]
- Halder, A.; Shukla, D.; Das, S.; Roy, P.; Mukherjee, A.; Saha, B. Cytokine Lactoferrin-modi fi ed Betulinic Acid-loaded PLGA nanoparticles are strong. Cytokine 2018, 110, 412–415. [Google Scholar] [CrossRef] [PubMed]
- Anand, N.; Kanwar, R.K.; Sehgal, R.; Kanwar, J.R. Antiparasitic and immunomodulatory potential of oral nanocapsules encapsulated lactoferrin protein against Plasmodium berghei. Nanomedicine 2016, 11, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Krzyzowska, M.; Chodkowski, M.; Janicka, M.; Dmowska, D.; Grobelny, J. Lactoferrin-Functionalized Noble Metal Nanoparticles as New Antivirals for HSV-2 Infection. Microorganisms 2022, 10, 110. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Lakshmi, Y.S.; Golla, K.; Kondapi, A.K. Improved Safety, Bioavailability and Pharmacokinetics of Zidovudine through Lactoferrin Nanoparticles during Oral Administration in Rats. PLoS ONE 2015, 10, e0140399. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, Y.S.; Kumar, P.; Kishore, G.; Bhaskar, C.; Kondapi, A.K. Triple combination MPT vaginal microbicide using curcumin and efavirenz loaded lactoferrin nanoparticles. Sci. Rep. 2016, 6, 25479. [Google Scholar] [CrossRef]
- Madugulla, L.; Ravula, A.R.; Kondapi, A.K.; Yenugu, S. Evaluation of the reproductive toxicity of antiretroviral drug loaded lactoferrin nanoparticles. Syst. Biol. Reprod. Med. 2019, 65, 205–213. [Google Scholar] [CrossRef]
- Yeruva, S.L.; Kumar, P.; Deepa, S.; Kondapi, A.K. Lactoferrin nanoparticles coencapsulated with curcumin and tenofovir improve vaginal defense against HIV-1 infection. Nanomedicine 2021, 16, 569–586. [Google Scholar] [CrossRef]
- Schultz, B.M.; Melo-Gonzalez, F.; Salazar, G.A.; Porto, B.N.; Riedel, C.A.; Kalergis, A.M.; Bueno, S.M. New Insights on the Early Interaction Between Typhoid and Non-typhoid Salmonella Serovars and the Host Cells. Front. Microbiol. 2021, 12, 647044. [Google Scholar] [CrossRef]
- Wang, X.; Biswas, S.; Paudyal, N.; Pan, H.; Li, X.; Fang, W.; Yue, M. Antibiotic Resistance in Salmonella Typhimurium Isolates Recovered from the Food Chain Through National Antimicrobial Resistance Monitoring System Between 1996 and 2016. Front. Microbiol. 2019, 10, 985. [Google Scholar] [CrossRef]
- Nista, E.C.; Pellegrino, A.; Giuli, L.; Candelli, M.; Schepis, T.; De Lucia, S.S.; Ojetti, V.; Franceschi, F.; Gasbarrini, A. Clinical Implications of Helicobacter pylori Antibiotic Resistance in Italy: A Review of the Literature. Antibiotics 2022, 11, 1452. [Google Scholar] [CrossRef] [PubMed]
- Sao, P.; Vats, S.; Singh, S. Porphyromonas gingivalis resistance and virulence: An integrated functional network analysis. Gene 2022, 839, 146734. [Google Scholar] [CrossRef] [PubMed]
- Kochanowsky, J.A.; Koshy, A.A. Toxoplasma gondii. Curr. Biol. 2018, 28, R770–R771. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Ewald, S.E. The molecular biology and immune control of chronic Toxoplasma gondii infection. J. Clin. Investig. 2020, 130, 3370–3380. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Kumar, V.; Tiwari, R.K.; Ravidas, V.; Pandey, K.; Kumar, A. Amphotericin B: A drug of choice for Visceral Leishmaniasis. Acta Trop. 2022, 235, 106661. [Google Scholar] [CrossRef] [PubMed]
- Menard, D.; Dondorp, A. Antimalarial Drug Resistance: A Threat to Malaria Elimination. Cold Spring Harb. Perspect. Med. 2017, 7, a025619. [Google Scholar] [CrossRef]
- Dehghan, H.; Oshaghi, M.A.; Mosa-Kazemi, S.H.; Abai, M.R.; Rafie, F.; Nateghpour, M.; Mohammadzadeh, H.; Farivar, L.; Bavani, M.M. Experimental Study on Plasmodium berghei, Anopheles Stephensi, and BALB/c Mouse System: Implications for Malaria Transmission Blocking Assays. Iran. J. Parasitol. 2018, 3, 549–559. [Google Scholar]
- Ahmed, J.; Rawre, J.; Dhawan, N.; Dudani, P.; Khanna, N.; Dhawan, B. Genital ulcer disease: A review. J. Fam. Med. Prim. Care 2022, 11, 4255–4262. [Google Scholar]
- Li, G.; Wang, Y.; De Clercq, E. Approved HIV reverse transcriptase inhibitors in the past decade. Acta Pharm. Sin. B 2022, 12, 1567–1590. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, S.; Li, J. Treatment of Inflammatory Bowel Disease: A Comprehensive Review. Front. Med. 2021, 8, 765474. [Google Scholar] [CrossRef]
- Kozuch, P.L.; Hanauer, S.B. Treatment of inflammatory bowel disease: A review of medical therapy. World J. Gastroenterol. 2008, 14, 354–377. [Google Scholar] [CrossRef] [PubMed]
- Majka, G.; Więcek, G.; Śróttek, M.; Śpiewak, K.; Brindell, M.; Koziel, J.; Marcinkiewicz, J.; Strus, M. The impact of lactoferrin with different levels of metal saturation on the intestinal epithelial barrier function and mucosal inflammation. Biometals 2016, 29, 1019–1033. [Google Scholar] [CrossRef] [PubMed]
- MacManus, C.F.; Collins, C.B.; Nguyen, T.T.; Alfano, R.W.; Jedlicka, P.; de Zoeten, E.F. VEN-120, a Recombinant Human Lactoferrin, Promotes a Regulatory T Cell [Treg] Phenotype and Drives Resolution of Inflammation in Distinct Murine Models of Inflammatory Bowel Disease. J. Crohns Colitis 2017, 11, 1101–1112. [Google Scholar] [CrossRef] [PubMed]
- Ho, G.T.; Cartwright, J.A.; Thompson, E.J.; Bain, C.C.; Rossi, A.G. Resolution of Inflammation and Gut Repair in IBD: Translational Steps Towards Complete Mucosal Healing. Inflamm. Bowel Dis. 2020, 26, 1131–1143. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Lv, B.; Jin, H.F.; Zhang, S. A meta-analysis of the therapeutic effects of tumor necrosis factor-α blockers on ulcerative colitis. Eur. J. Clin. Pharmacol. 2011, 67, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Ou, A.T.; Zhang, J.X.; Fang, Y.F.; Wang, R.; Tang, X.P.; Zhao, P.F.; Zhao, Y.G.; Zhang, M.; Huang, Y.Z. Disulfiram-loaded lactoferrin nanoparticles for treating inflammatory diseases. Acta Pharmacol. Sin. 2021, 42, 1913–1920. [Google Scholar] [CrossRef]
- Ahmed, F.; Kumari, S.; Kondapi, A.K. Evaluation of Antiproliferative Activity, Safety and Biodistribution of Oxaliplatin and 5-Fluorouracil Loaded Lactoferrin Nanoparticles for the Management of Colon Adenocarcinoma: An In Vitro and an In Vivo Study. Pharm. Res. 2018, 35, 178. [Google Scholar] [CrossRef]
- Kamalapuram, S.K.; Kanwar, R.K.; Roy, K.; Chaudhary, R.; Sehgal, R.; Kanwar, J.R. Theranostic multimodular potential of zinc-doped ferrite-saturated metal-binding protein-loaded novel nanocapsules in cancers. Int. J. Nanomed. 2016, 11, 1349–1366. [Google Scholar]
- Kanwar, J.R.; Mahidhara, G.; Kanwar, R.K. Novel alginate-enclosed chitosan-calcium phosphate-loaded iron-saturated bovine lactoferrin nanocarriers for oral delivery in colon cancer therapy. Nanomedicine 2012, 7, 1521–1550. [Google Scholar] [CrossRef]
- Saif, M.W. Alternative Treatment Options in Patients with Colorectal Cancer Who Encounter Fluoropyrimidine-Induced Cardiotoxicity. Onco Targets Ther. 2020, 13, 10197–10206. [Google Scholar] [CrossRef]
- Hering, N.A.; Luettig, J.; Krug, S.M.; Wiegand, S.; Gross, G.; Van Tol, E.A.; Schulzke, J.D.; Rosenthal, R. Lactoferrin protects against intestinal inflammation and bacteria-induced barrier dysfunction in vitro. Ann. N. Y. Acad. Sci. 2017, 1405, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Pulgar, V.M. Transcytosis to Cross the Blood Brain Barrier, New Advancements and Challenges. Front. Neurosci. 2019, 12, 1019. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Pang, Z.; Lu, W.; Yin, Q.; Gao, H.; Jiang, X. Self-assembled polymersomes conjugated with lactoferrin as novel drug carrier for brain delivery. Pharm. Res. 2012, 29, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Bi, C.C.; Wang, A.P.; Chu, Y.C.; Liu, S.; Mu, H.J.; Liu, W.H.; Wu, Z.M.; Sun, K.X.; Li, Y.X. Intranasal delivery of rotigotine to the brain with lactoferrin-modified PEG-PLGA nanoparticles for Parkinson’s disease treatment. Int. J. Nanomed. 2016, 11, 6547–6559. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Zhou, J.; Ju, F.; Zhu, H. Intranasal delivery of α-asarone to the brain with lactoferrin-modified mPEG-PLA nanoparticles prepared by premix membrane emulsification. Drug Deliv. Transl. Res. 2018, 8, 83–96. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, B.; Kebebe, D.; Guo, L.; Guo, H.; Li, N.; Pi, J.; Qi, D.; Guo, P.; Liu, Z. Preparation, optimization and cellular uptake study of tanshinone I nanoemulsion modified with lactoferrin for brain drug delivery. Pharm. Dev. Technol. 2019, 24, 982–991. [Google Scholar] [CrossRef]
- Goyal, K.; Konar, A.; Kumar, B.S.H.; Koul, V. Lactoferrin-conjugated pH and redox-sensitive polymersomes based on PEG-S-S-PLA-PCL-OH boost delivery of bacosides to the brain. Nanoscale 2018, 10, 17781–17798. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liu, C.; Zhai, W.; Zhuang, N.; Han, T.; Ding, Z. The Optimization Design of Lactoferrin Loaded HupA Nanoemulsion For Targeted Drug Transport Via Intranasal Route. Int. J. Nanomed. 2019, 14, 9217–9234. [Google Scholar] [CrossRef]
- Gothwal, A.; Kumar, H.; Nakhate, K.T.; Ajazuddin; Dutta, A.; Borah, A.; Gupta, U. Lactoferrin Coupled Lower Generation PAMAM Dendrimers for Brain Targeted Delivery of Memantine in Aluminum-Chloride-Induced Alzheimer’s Disease in Mice. Bioconj. Chem. 2019, 30, 2573–2583. [Google Scholar] [CrossRef]
- Kamalinia, G.; Khodagholi, F.; Atyabi, F.; Amini, M.; Shaerzadeh, F.; Sharifzadeh, M.; Dinarvand, R. Enhanced brain delivery of deferasirox-lactoferrin conjugates for iron chelation therapy in neurodegenerative disorders: In vitro and in vivo studies. Mol. Pharm. 2013, 10, 4418–4431. [Google Scholar] [CrossRef]
- Pereira, P.; Barreira, M.; Cruz, C.; Tomás, J.; Luís, Â.; Pedro, A.Q.; Queiroz, J.A.; Sousa, F. Brain-Targeted Delivery of Pre-miR-29b Using Lactoferrin-Stearic Acid-Modified-Chitosan/Polyethyleneimine Polyplexes. Pharmaceuticals 2020, 13, 314. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Wang, A.; Yan, X.; Chu, L.; Yang, X.; Song, Y.; Sun, K.; Yu, X.; Liu, R.; Wu, Z.; et al. Brain-targeted intranasal delivery of dopamine with borneol and lactoferrin co-modified nanoparticles for treating Parkinson’s disease. Drug Deliv. 2019, 26, 700–707. [Google Scholar] [CrossRef]
- Yan, X.; Xu, L.; Bi, C.; Duan, D.; Chu, L.; Yu, X.; Wu, Z.; Wang, A.; Sun, K. Lactoferrin-modified rotigotine nanoparticles for enhanced nose-to-brain delivery: LESA-MS/MS-based drug biodistribution, pharmacodynamics, and neuroprotective effects. Int. J. Nanomed. 2018, 13, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Singh, T.G.; Singh, S.; Garg, N.; Dhiman, S. Apoptotic Pathways and Alzheimer’s Disease: Probing Therapeutic Potential. Neurochem. Res. 2021, 46, 3103–3122. [Google Scholar] [CrossRef] [PubMed]
- Huat, T.J.; Camats-Perna, J.; Newcombe, E.A.; Valmas, N.; Kitazawa, M.; Medeiros, R. Metal Toxicity Links to Alzheimer’s Disease and Neuroinflammation. J. Mol. Biol. 2019, 431, 1843–1868. [Google Scholar] [CrossRef] [PubMed]
- Chia, S.J.; Tan, E.K.; Chao, Y.X. Historical Perspective: Models of Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 2464. [Google Scholar] [CrossRef]
| Product | Test | Ref |
|---|---|---|
| Antibacterial | ||
| Alginate gel-encapsulated ceramic nanocarriers loaded with 1.2% iron-saturated bLf (EAC-CP-Fe-bLf NCs) | BALB/c orally infected with Salmonella typhimurium and then fed EAC-CP-Fe-bLf NCs in diet | [38] |
| Hydroxyapatite nanoparticles coated with lactoferrin (Lf-HA NPs) | BALB/c orally infected with Helicobacter pylori and then orally treated with Lf-HA NPs alone or plus cell free supertant of Lactobacillus paracasei culture | [39] |
| Multilamellar liposomes prepared with soy phosphatidylcholine containing 180 mg of bLf | Human volunteers with periodontal infection treated with bLf-liposomes (4 tablets per day) during 0, 2, and 4 weeks | [40] |
| Antiprotozoan | ||
| Alginate gel-encapsulated ceramic nanocarriers loaded with 1.2% iron-saturated bLf (EAC-CP-Fe-bLf NCs) | BALB/c infected via intraperitoneal with Toxoplasma gondii tachyzoites and then fed EAC-CP-Fe-bLf NCs in diet | [27] |
| Nanoparticles formulated with bLf adsorbed over core poly-D,L, lactide -co-glicolide loaded with amphotericin B (LcfPGNP-Amb) | Hamsters intraperitoneally (ip) infected with Leishmania donovani amastigotes and then treated intraperitoneally with 1 mg/kg via ip for 5 consecutive days with Amb encapsulated in LcfPGNP | [41] |
| Betulinic acid loaded within poly-D,L,co-glicolide acid nanoparticles coated with bLf (Lf-BANPs) | BALB/c-derived macrophages infected with Leishmania donovani amastigotes treated with Lf-BANPs (2.5 μg/mL) | [42] |
| Alginate-enclosed chitosan-conjugated calcium phosphate nanocapsules with 1.2% w/w iron-saturated buffalo Lf (AEC-CCo-CP-buLf-NCs) | BALB/c mice infected intraperitoneally with parasitized erythrocytes from Swiss mice infected via ip with Plasmodium berghei and fed AEC-CCo-CP-buLf-NCs in diet | [43] |
| Antiviral | ||
| Human recombinant iron-saturated Lf loaded in silver and gold nanoparticles (Lf-Ag-Au NPs) | Female C57BL/6 mice intravaginally infected with herpes simplex virus plaque forming units (PFU) and thereafter washed with 10 μg/mL of Lf-Ag-Au NPs in saline solution | [44] |
| Zidovudine (AZT) loaded in Lf nanoparticles prepared with olive oil (AZT-Lacto-Nano) | Pharmacokinetic and toxicologic assessment in blood, liver, and kidney of Wistar rats orally administered with AZT-lacto-Nano | [45] |
| Efavirenz (anti-HIV) curcumin (antimicrobial spermicide) loaded in Lf nanoparticles (ECLNPs) | Pharmacokinetic, toxicologic, and vaginal Lactobacillus assessment in vaginal lavage of female Wistar rats intravaginally administered with ECLNPs | [46] |
| Efavirenz, curcumin, and Lf nanoparticles (ECLNPs) | Reproductive performance, fertility, and postnatal development in female Wistar rats intravaginally or orally treated with ECLNPs | [47] |
| Tenofovir (anti-HIV) and curcumin loaded in Lf nanoparticles (TCNPs) | Pharmacokinetic and toxicologic assessment of ECLNPs in vaginal epithelium of rats | [48] |
| Product | Test | Ref |
|---|---|---|
| Intestinal Bowel Disease | ||
| Emodin entrapped in Lf nanoparticles and then loaded in yeast wall microparticles (EMON-YPs) | DSS-induced colitis in BALB/c mice orally treated with EMON-YPs | [22] |
| Lf NPs formulated with calcium pectinate and hyaluronic acid as oral carriers of rhein (RH) as anti-inflammatory herbal compound (CP-HA-RH-NPs) | DSS-induced UC in mice orally treated with CP-HA-RH-NPs | [23] |
| Disulfiram-loaded Lf nanoparticles (DSF-Lf-NPs) | DSS-induced colitis in BALB/c mice intravenously injected with DSF-Lf NPs | [66] |
| Colorectal Cancer | ||
| Alginate-enclosed chitosan-conjugated iron-saturated bLf nanocapsules (AEC-CCo-CP-Fe-bLf-NCs) | Athymic nude mice fed AEC-CCo-CP-Fe-bLF-NCs in diet | [28] |
| Lactoferrin nanoparticles loaded with Oxaliplatin (Lacto-Nano-Oxalo) and 5-fluorouracil (Lacto-Nano-5FU) | Adenocarcinoma induced with azoxy-methane in Wistar rats intravenously injected with Lacto-Nano-Oxalo or Lacto-Nano-5FU (40 mg/Kg body weight, four doses in four consecutive weeks) | [67] |
| Zinc-ferrite (Zn-Fe) and iron-saturated bLf (Zn-Fe-bLf) complexes coated with chitosan and alginate nanogel (Zn-Fe-bLf-NCs) | Human xenograft colonic adenocarcinoma model in athymic nu/nu BALB/c mice fed Zn-Fe-bLf-NCs in AIN 93G diet | [68] |
| Alginate-enclosed chitosan calcium phosphate loaded on iron-saturated bLf nanocarriers (AEC-CP-Fe-bLf NCs) | Human xenograft colonic adenocarcinoma model in athymic nu/nu BALB/c mice fed Zn-Fe-bLf-NCs in AIN 93G diet | [69] |
| Product | Test | Ref |
|---|---|---|
| Alzheimer’s | ||
| α-asarone-loaded Lf-NPs | Sprague–Dawley rats intranasally treated with Lf-NPs to assess pharmacokinetics proprieties, distribution in the brain and other tissue, brain targeting, and toxicity | [75] |
| Tanshinone I (TSI) nanoemulsion (TSI-NE) modified with Lf | In vitro assays for evaluating the uptake of TSI-Lf-NE by mouse brain microvascular endothelial cell line (bEnd.3 cells) using Coumarin-6 as a fluorescent probe | [76] |
| Bacoside-loaded-Lf-conjugated polymersomes | In vivo assays of Alzheimer’s model induced by scopolamine in male Swiss albino mice treated via tail vein with bacoside-loaded-Lf-conjugated polymersomes for evaluating uptake of the polymersomes in the brain assayed by LCMS assay | [77] |
| Huperzine A (HupA)-loaded NPs with surface modification by Lf-conjugated nanoemulsions (NEs) | Rats intranasally administred with HupA-NEs, Lf-HupA-NEs, and HupA solution to investigate the brain-targeting effects of these formulations | [78] |
| Huperzine A (HupA)-loaded NPs with surface modification by Lf conjugate | In vivo intranasally administered Hup-A NPs after intranasal administration in KM mice for evaluating the biodistribution | [33] |
| Dendrimers (PAMAM) and Lf conjugate loaded with memantine (MEM). | The in vivo study in AlCl3-induced Alzheimer’s (AD) in Swiss albino mice treated with MEM-PAMAM-Lf intraperitoneally; in vivo biodistribution in the Sprague−Dawley rat model for the brain uptake of MEM-PAMAM-Lf (formulations administrated intraperitoneally) | [79] |
| Deferasirox–Lf conjugated | Rat model of Alzheimer’s disease induced by beta amyloid and treated with the deferasirox–Lf conjugated intraperitoneally for evaluating cognitive disorder | [80] |
| Poly(ethyleneglycol)-poly (D,L-lactic-co-glycolic acid) (PEG-PLGA) polymersomes conjugated with Lf (Lf-POS); S14G-humanin conjugate- Lf-POS | In vivo model of Alzheimer’s disease in Sprague–Dawley rats induced with hippocampal administration of Amyloid-β25–35 and treated with Lf-POS (SHN-Lf-POS) for evaluating the neuroprotective effects | [73] |
| Polymers of chitosan and polyethyleneimine were loaded with recombinant precursor microRNA (pre-miR-29b) and Lf | In vitro assays in N2a695 and RBE4 cell lines incubated with pre-miR-29b-loaded polyplexes to assess expression of BACE-1 | [81] |
| Parkinson’s | ||
| Lactoferrin co-modified nanoparticles (Lf-BNPs) encapsulated dopamine | In vivo model of Parkinson´s disease induced by 6-hydroxydopamine in Sprague–Dawley rats, intranasally administrated with dopamine Lf-BNPs for evaluation of the effects on behavior; in vitro uptake and cytotoxicity studies in SH-SY5Y and 16HBE cells | [82] |
| Lactoferrin-modified rotigotine nanoparticles (Lf-R-NPs) | Intranasal administration of Lf-R-NPs in mice for assay biodistribution; qualitative and quantitative cellular uptake for evaluating the accumulation of Lf-NPs in 16HBE and SH-SY5Y cells | [74] |
| Lactoferrin-modified rotigotine nanoparticles (Lf-R-NPs) | In vivo model of Parkinson’s disease induced by by nigrostriatal administration of 6-hydroxydopamine in Sprague−Dawley rats intranasally treated with Lf-R-NPs for evaluating contralateral rotations and biodistribution | [83] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guzmán-Mejía, F.; Godínez-Victoria, M.; Molotla-Torres, D.E.; Drago-Serrano, M.E. Lactoferrin as a Component of Pharmaceutical Preparations: An Experimental Focus. Pharmaceuticals 2023, 16, 214. https://doi.org/10.3390/ph16020214
Guzmán-Mejía F, Godínez-Victoria M, Molotla-Torres DE, Drago-Serrano ME. Lactoferrin as a Component of Pharmaceutical Preparations: An Experimental Focus. Pharmaceuticals. 2023; 16(2):214. https://doi.org/10.3390/ph16020214
Chicago/Turabian StyleGuzmán-Mejía, Fabiola, Marycarmen Godínez-Victoria, Daniel Efrain Molotla-Torres, and Maria Elisa Drago-Serrano. 2023. "Lactoferrin as a Component of Pharmaceutical Preparations: An Experimental Focus" Pharmaceuticals 16, no. 2: 214. https://doi.org/10.3390/ph16020214
APA StyleGuzmán-Mejía, F., Godínez-Victoria, M., Molotla-Torres, D. E., & Drago-Serrano, M. E. (2023). Lactoferrin as a Component of Pharmaceutical Preparations: An Experimental Focus. Pharmaceuticals, 16(2), 214. https://doi.org/10.3390/ph16020214