Denosumab Is Superior to Raloxifene in Lowering Risks of Mortality and Ischemic Stroke in Osteoporotic Women
Abstract
:1. Introduction
2. Results
2.1. Patient Characteristics
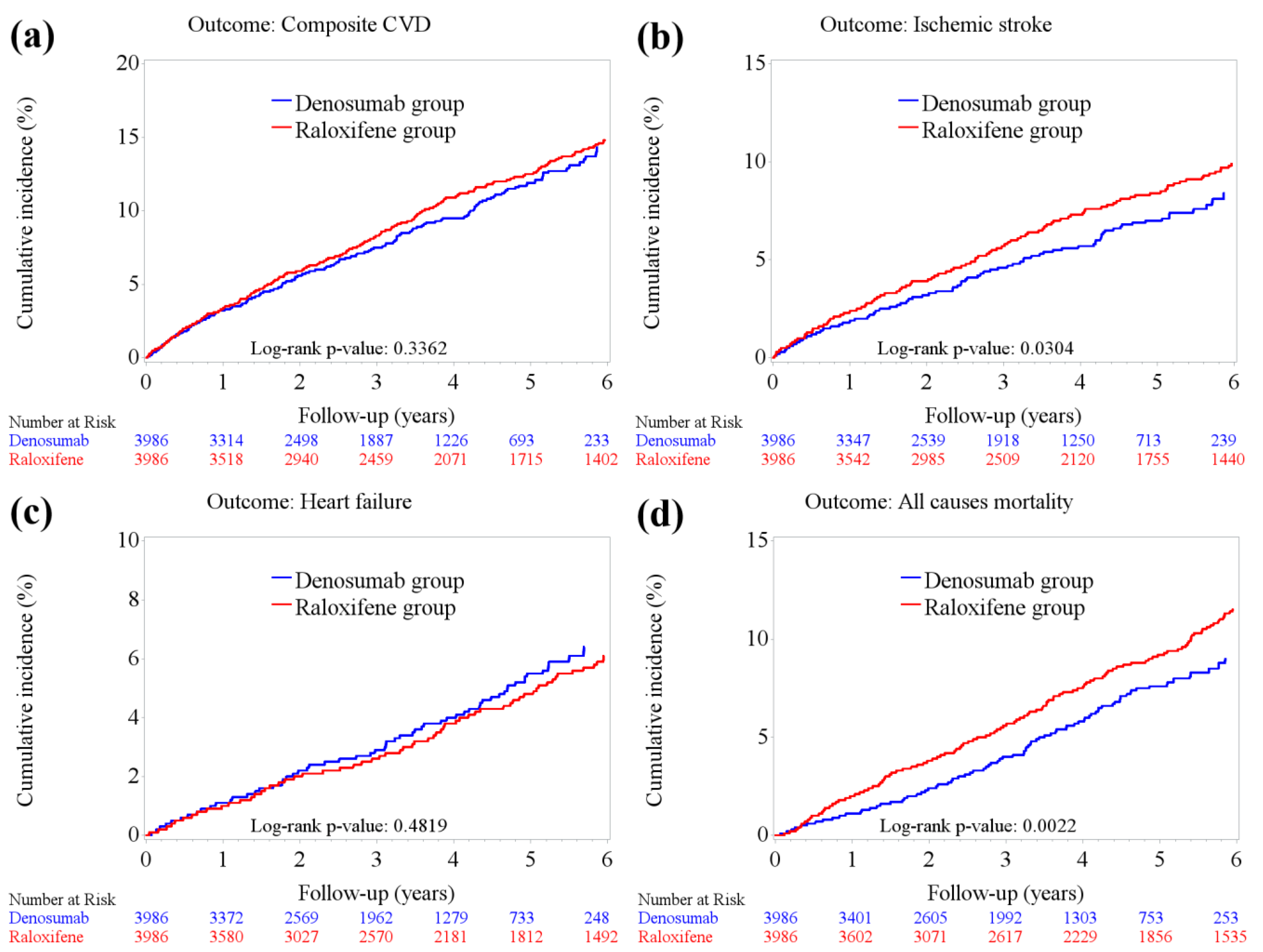
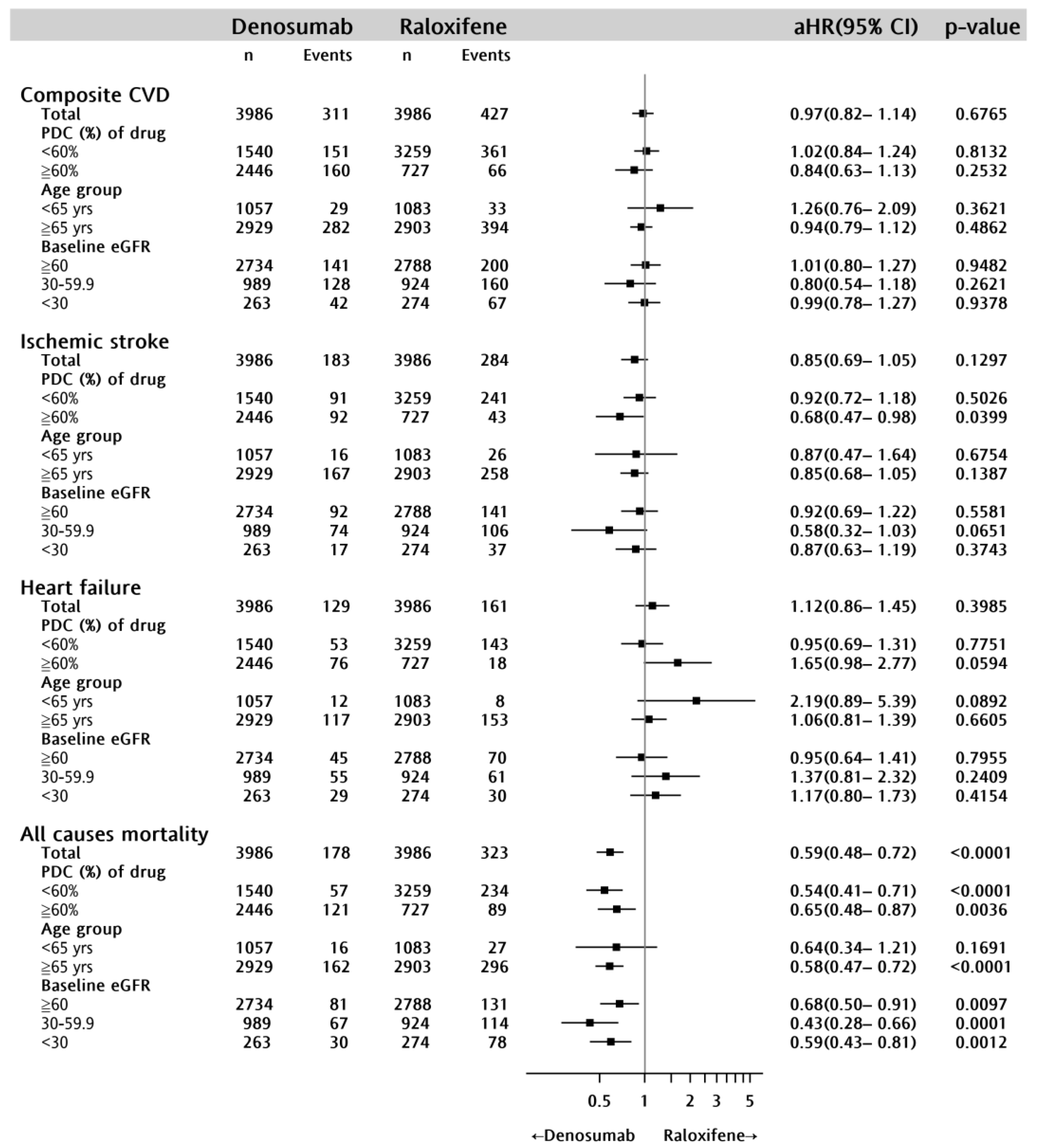
2.2. CVD Incidence
2.3. All-Cause Mortality
3. Discussion
4. Materials and Methods
4.1. Data Source
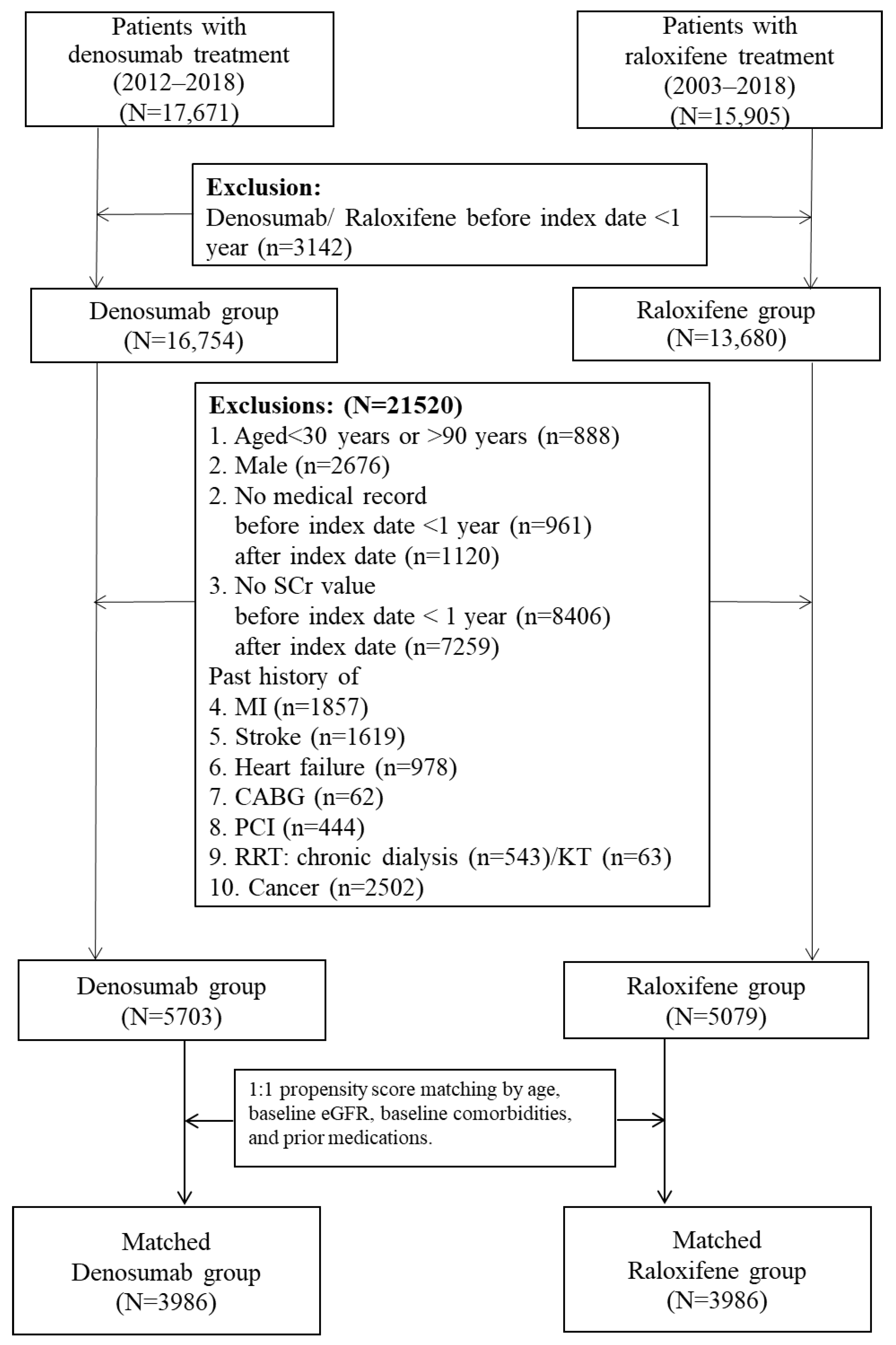
4.2. Study Design and Study Cohort
4.3. Comparison Groups
4.4. Outcomes and Follow-Up
4.5. Study Covariates
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lane, N.E. Epidemiology, etiology, and diagnosis of osteoporosis. Am. J. Obstet. Gynecol. 2006, 194, S3–S11. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Huang, X.; Wu, J.; Lin, X.; Zhou, X.; Zhu, Z.; Pan, X.; Xu, J.; Qiao, J.; Zhang, T.; et al. The Global Burden of Osteoporosis, Low Bone Mass, and Its Related Fracture in 204 Countries and Territories, 1990-2019. Front. Endocrinol. 2022, 13, 882241. [Google Scholar] [CrossRef] [PubMed]
- Lakatta, E.G.; Levy, D. Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises: Part I: Aging arteries: A "set up" for vascular disease. Circulation 2003, 107, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Rajsic, S.; Gothe, H.; Borba, H.H.; Sroczynski, G.; Vujicic, J.; Toell, T.; Siebert, U. Economic burden of stroke: A systematic review on post-stroke care. Eur. J. Health Econ. 2019, 20, 107–134. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, M.; Deal, C. Osteoporosis in elderly: Prevention and treatment. Clin. Geriatr. Med. 2002, 18, 529–555. [Google Scholar] [CrossRef] [PubMed]
- Lello, S.; Capozzi, A.; Scambia, G. Osteoporosis and cardiovascular disease: An update. Gynecol. Endocrinol. 2015, 31, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Nam, S.T.; Mun, S.H.; Lee, S.K.; Kim, H.W.; Park, Y.H.; Kim, B.; Won, K.J.; Kim, H.R.; Park, Y.M.; et al. DJ-1 controls bone homeostasis through the regulation of osteoclast differentiation. Nat. Commun. 2017, 8, 1519. [Google Scholar] [CrossRef]
- Harper, E.; Forde, H.; Davenport, C.; Rochfort, K.D.; Smith, D.; Cummins, P.M. Vascular calcification in type-2 diabetes and cardiovascular disease: Integrative roles for OPG, RANKL and TRAIL. Vascul. Pharmacol. 2016, 82, 30–40. [Google Scholar] [CrossRef]
- Cummings, S.R.; San Martin, J.; McClung, M.R.; Siris, E.S.; Eastell, R.; Reid, I.R.; Delmas, P.; Zoog, H.B.; Austin, M.; Wang, A.; et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N. Engl. J. Med. 2009, 361, 756–765. [Google Scholar] [CrossRef]
- Morony, S.; Tintut, Y.; Zhang, Z.; Cattley, R.C.; Van, G.; Dwyer, D.; Stolina, M.; Kostenuik, P.J.; Demer, L.L. Osteoprotegerin inhibits vascular calcification without affecting atherosclerosis in ldlr(-/-) mice. Circulation 2008, 117, 411–420. [Google Scholar] [CrossRef] [Green Version]
- Helas, S.; Goettsch, C.; Schoppet, M.; Zeitz, U.; Hempel, U.; Morawietz, H.; Kostenuik, P.J.; Erben, R.G.; Hofbauer, L.C. Inhibition of receptor activator of NF-kappaB ligand by denosumab attenuates vascular calcium deposition in mice. Am. J. Pathol. 2009, 175, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Recker, R.; Lappe, J.; Davies, K.; Heaney, R. Characterization of perimenopausal bone loss: A prospective study. J. Bone Miner. Res. 2000, 15, 1965–1973. [Google Scholar] [CrossRef] [PubMed]
- Akhter, M.P.; Lappe, J.M.; Davies, K.M.; Recker, R.R. Transmenopausal changes in the trabecular bone structure. Bone 2007, 41, 111–116. [Google Scholar] [CrossRef] [PubMed]
- D'Amelio, P.; Grimaldi, A.; Di Bella, S.; Brianza, S.Z.M.; Cristofaro, M.A.; Tamone, C.; Giribaldi, G.; Ulliers, D.; Pescarmona, G.P.; Isaia, G. Estrogen deficiency increases osteoclastogenesis up-regulating T cells activity: A key mechanism in osteoporosis. Bone 2008, 43, 92–100. [Google Scholar] [CrossRef] [PubMed]
- D'Amelio, P.; Isaia, G.C. The use of raloxifene in osteoporosis treatment. Expert. Opin. Pharmacother. 2013, 14, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, B.; Black, D.M.; Mitlak, B.H.; Knickerbocker, R.K.; Nickelsen, T.; Genant, H.K.; Christiansen, C.; Delmas, P.D.; Zanchetta, J.R.; Stakkestad, J.; et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: Results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA 1999, 282, 637–645. [Google Scholar] [CrossRef]
- Barrett-Connor, E.; Grady, D.; Sashegyi, A.; Anderson, P.W.; Cox, D.A.; Hoszowski, K.; Rautaharju, P.; Harper, K.D.; Investigators, M. Raloxifene and cardiovascular events in osteoporotic postmenopausal women: Four-year results from the MORE (Multiple Outcomes of Raloxifene Evaluation) randomized trial. JAMA 2002, 287, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Hsu, T.W.; Hsu, C.N.; Wang, S.W.; Huang, C.C.; Li, L.C. Comparison of the Effects of Denosumab and Alendronate on Cardiovascular and Renal Outcomes in Osteoporotic Patients. J. Clin. Med. 2019, 8, 932. [Google Scholar] [CrossRef]
- Chen, C.L.; Chen, N.C.; Wu, F.Z.; Wu, M.T. Impact of denosumab on cardiovascular calcification in patients with secondary hyperparathyroidism undergoing dialysis: A pilot study. Osteoporos. Int. 2020, 31, 1507–1516. [Google Scholar] [CrossRef]
- Saitta, A.; Morabito, N.; Frisina, N.; Cucinotte, D.; Corrado, F.; D'Anna, R.; Altavilla, D.; Squadrito, G.; Minutoli, L.; Arcoraci, V.; et al. Cardiovascular effects of raloxifene hydrochloride. Cardiovasc. Drug. Rev. 2001, 19, 57–74. [Google Scholar] [CrossRef]
- Mijatovic, V.; van der Mooren, M.J.; Kenemans, P.; de Valk-de Roo, G.W.; Netelenbos, C. Raloxifene lowers serum lipoprotein(A) in healthy postmenopausal women: A randomized, double-blind, placebo-controlled comparison with conjugated equine estrogens. Menopause 1999, 6, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Huo, S.; Shi, W.; Ma, H.; Yan, D.; Luo, P.; Guo, J.; Li, C.; Lin, J.; Zhang, C.; Li, S.; et al. Alleviation of Inflammation and Oxidative Stress in Pressure Overload-Induced Cardiac Remodeling and Heart Failure via IL-6/STAT3 Inhibition by Raloxifene. Oxid. Med. Cell. Longev. 2021, 2021, 6699054. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Santos, H.O.; de Souza, I.G.O.; Hoilat, G.J.; Martins, C.E.C.; Varkaneh, H.K.; Alkhwildi, J.A.; Hejji, A.T.; Almuqayyid, F.; Abu-Zaid, A. The Effect of Raloxifene Treatment on Lipid Profile in Elderly Individuals: A Systematic Review and Meta-analysis of Randomized Clinical Trials. Clin. Ther. 2021, 43, 297–317. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Li, N.; Gaman, M.A.; Wang, N. Raloxifene has favorable effects on the lipid profile in women explaining its beneficial effect on cardiovascular risk: A meta-analysis of randomized controlled trials. Pharmacol. Res. 2021, 166, 105512. [Google Scholar] [CrossRef] [PubMed]
- Shoback, D.; Rosen, C.J.; Black, D.M.; Cheung, A.M.; Murad, M.H.; Eastell, R. Pharmacological Management of Osteoporosis in Postmenopausal Women: An Endocrine Society Guideline Update. J. Clin. Endocrinol. Metab. 2020, 105, 587–594. [Google Scholar] [CrossRef]
- Kanis, J.A.; Cooper, C.; Rizzoli, R.; Reginster, J.Y.; Scientific Advisory Board of the European Society for Clinical and Economic Aspects of Osteoporosis (ESCEO); Committees of Scientific Advisors and National Societies of the International Osteoporosis Foundation. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos. Int. 2019, 30, 3–44. [Google Scholar] [CrossRef] [PubMed]
- The North American Menopause Society. Management of Osteoporosis in Postmenopausal Women: The Position Statement of The North American Menopause Society'' Editorial, P. Management of osteoporosis in postmenopausal women: The 2021 position statement of The North American Menopause Society. Menopause 2021, 28, 973–997. [Google Scholar] [CrossRef]
- Arceo-Mendoza, R.M.; Camacho, P.M. Postmenopausal Osteoporosis: Latest Guidelines. Endocrinol. Metab. Clin. North Am. 2021, 50, 167–178. [Google Scholar] [CrossRef]
- Fraser, S.D.; Taal, M.W. Multimorbidity in people with chronic kidney disease: Implications for outcomes and treatment. Curr. Opin. Nephrol. Hypertens. 2016, 25, 465–472. [Google Scholar] [CrossRef]
- Bowling, C.B.; Plantinga, L.; Phillips, L.S.; McClellan, W.; Echt, K.; Chumbler, N.; McGwin, G.; Vandenberg, A.; Allman, R.M.; Johnson, T.M., 2nd. Association of Multimorbidity with Mortality and Healthcare Utilization in Chronic Kidney Disease. J. Am. Geriatr. Soc. 2017, 65, 704–711. [Google Scholar] [CrossRef]
- Lee, W.C.; Lee, Y.T.; Li, L.C.; Ng, H.Y.; Kuo, W.H.; Lin, P.T.; Liao, Y.C.; Chiou, T.T.; Lee, C.T. The Number of Comorbidities Predicts Renal Outcomes in Patients with Stage 3(-)5 Chronic Kidney Disease. J. Clin. Med. 2018, 7, 493. [Google Scholar] [CrossRef] [PubMed]
- Cicek, M.; Buckley, J.; Pearson-Stuttard, J.; Gregg, E.W. Characterizing Multimorbidity from Type 2 Diabetes: Insights from Clustering Approaches. Endocrinol. Metab. Clin. North Am. 2021, 50, 531–558. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.M.; Lu, C.L.; Hung, K.C.; Wu, P.C.; Pan, C.F.; Wu, C.J.; Syu, R.S.; Chen, J.S.; Hsiao, P.J.; Lu, K.C. The Paradoxical Role of Uric Acid in Osteoporosis. Nutrients 2019, 11, 2111. [Google Scholar] [CrossRef] [PubMed]
- Barrett-Connor, E.; Mosca, L.; Collins, P.; Geiger, M.J.; Grady, D.; Kornitzer, M.; McNabb, M.A.; Wenger, N.K.; Raloxifene Use for The Heart Trial, I. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N. Engl. J. Med. 2006, 355, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Dayspring, T.; Qu, Y.; Keech, C. Effects of raloxifene on lipid and lipoprotein levels in postmenopausal osteoporotic women with and without hypertriglyceridemia. Metabolism 2006, 55, 972–979. [Google Scholar] [CrossRef]
- Hofbauer, L.C.; Brueck, C.C.; Shanahan, C.M.; Schoppet, M.; Dobnig, H. Vascular calcification and osteoporosis--from clinical observation towards molecular understanding. Osteoporos. Int. 2007, 18, 251–259. [Google Scholar] [CrossRef]
- Samelson, E.J.; Miller, P.D.; Christiansen, C.; Daizadeh, N.S.; Grazette, L.; Anthony, M.S.; Egbuna, O.; Wang, A.; Siddhanti, S.R.; Cheung, A.M.; et al. RANKL inhibition with denosumab does not influence 3-year progression of aortic calcification or incidence of adverse cardiovascular events in postmenopausal women with osteoporosis and high cardiovascular risk. J. Bone. Miner. Res. 2014, 29, 450–457. [Google Scholar] [CrossRef]
- Suzuki, S.; Suzuki, M.; Hanafusa, N.; Tsuchiya, K.; Nitta, K. Denosumab Recovers Aortic Arch Calcification During Long-Term Hemodialysis. Kidney. Int. Rep. 2021, 6, 605–612. [Google Scholar] [CrossRef]
- Eriksen, E.F.; Lyles, K.W.; Colon-Emeric, C.S.; Pieper, C.F.; Magaziner, J.S.; Adachi, J.D.; Hyldstrup, L.; Recknor, C.; Nordsletten, L.; Lavecchia, C.; et al. Antifracture efficacy and reduction of mortality in relation to timing of the first dose of zoledronic acid after hip fracture. J. Bone Miner. Res. 2009, 24, 1308–1313. [Google Scholar] [CrossRef]
- Wu, P.H.; Lin, M.Y.; Huang, T.H.; Lee, T.C.; Lin, S.Y.; Chen, C.H.; Kuo, M.C.; Chiu, Y.W.; Chang, J.M.; Hwang, S.J. Kidney Function Change and All-Cause Mortality in Denosumab Users with and without Chronic Kidney Disease. J. Pers. Med. 2022, 12, 185. [Google Scholar] [CrossRef]
- Ono, T.; Hayashi, M.; Sasaki, F.; Nakashima, T. RANKL biology: Bone metabolism, the immune system, and beyond. Inflamm. Regen. 2020, 40, 2. [Google Scholar] [CrossRef] [PubMed]
- Bando, J.K.; Gilfillan, S.; Song, C.; McDonald, K.G.; Huang, S.C.; Newberry, R.D.; Kobayashi, Y.; Allan, D.S.J.; Carlyle, J.R.; Cella, M.; et al. The Tumor Necrosis Factor Superfamily Member RANKL Suppresses Effector Cytokine Production in Group 3 Innate Lymphoid Cells. Immunity 2018, 48, 1208–1219.e1204. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, M.M.; Okamoto, K.; Komatsu, N.; Sawa, S.; Danks, L.; Penninger, J.M.; Nakashima, T.; Takayanagi, H. Inhibition of the TNF Family Cytokine RANKL Prevents Autoimmune Inflammation in the Central Nervous System. Immunity 2015, 43, 1174–1185. [Google Scholar] [CrossRef] [PubMed]
- Loser, K.; Mehling, A.; Loeser, S.; Apelt, J.; Kuhn, A.; Grabbe, S.; Schwarz, T.; Penninger, J.M.; Beissert, S. Epidermal RANKL controls regulatory T-cell numbers via activation of dendritic cells. Nat. Med. 2006, 12, 1372–1379. [Google Scholar] [CrossRef]
- Hanada, R.; Leibbrandt, A.; Hanada, T.; Kitaoka, S.; Furuyashiki, T.; Fujihara, H.; Trichereau, J.; Paolino, M.; Qadri, F.; Plehm, R.; et al. Central control of fever and female body temperature by RANKL/RANK. Nature 2009, 462, 505–509. [Google Scholar] [CrossRef]
- Shao, S.C.; Chan, Y.Y.; Kao Yang, Y.H.; Lin, S.J.; Hung, M.J.; Chien, R.N.; Lai, C.C.; Lai, E.C. The Chang Gung Research Database-A multi-institutional electronic medical records database for real-world epidemiological studies in Taiwan. Pharmacoepidemiol. Drug Saf. 2019, 28, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.M. Taiwan's new national health insurance program: Genesis and experience so far. Health Aff. 2003, 22, 61–76. [Google Scholar] [CrossRef]
- Huang, Y.T.; Chen, Y.J.; Chang, S.H.; Kuo, C.F.; Chen, M.H. Discharge status validation of the Chang Gung Research database in Taiwan. Biomed. J. 2021, 45, 907–913. [Google Scholar] [CrossRef]
- Sundararajan, V.; Henderson, T.; Perry, C.; Muggivan, A.; Quan, H.; Ghali, W.A. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J. Clin. Epidemiol. 2004, 57, 1288–1294. [Google Scholar] [CrossRef]
- Austin, P.C. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun. Stat. Simul. Comput. 2009, 38, 1228–1234. [Google Scholar] [CrossRef]
| Before PSM | After PSM | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Denosumab (n = 5703) | Raloxifene (n = 5079) | Denosumab (n = 3986) | Raloxifene (n = 3986) | |||||||||||
| N | n | (%) | n | (%) | SMD | p-Value | N | n | (%) | n | (%) | SMD | p-Value | |
| Age at the index date (years), mean (SD) | 10,782 | 73.18 | ±9.72 | 69.58 | ±10.76 | 0.35 | <0.0001 | 7972 | 71.31 | ± 9.97 | 71.32 | ± 10.00 | 0.00 | 0.9587 |
| Baseline eGFR, mean (SD) | 10,782 | 76.07 | ±30.95 | 73.01 | ±29.44 | 0.10 | <0.0001 | 7972 | 74.11 | ± 29.56 | 74.29 | ± 30.16 | 0.01 | 0.7977 |
| Baseline comorbidities | ||||||||||||||
| Peripheral vascular diseases | 154 | 90 | (1.58) | 64 | (1.26) | 0.03 | 0.1648 | 109 | 54 | (1.35) | 55 | (1.38) | 0.00 | 0.9232 |
| Dementia | 464 | 300 | (5.26) | 164 | (3.23) | 0.10 | <0.0001 | 320 | 165 | (4.14) | 155 | (3.89) | 0.01 | 0.5683 |
| Pulmonary disease | 1084 | 618 | (10.84) | 466 | (9.18) | 0.06 | 0.0042 | 783 | 387 | (9.71) | 396 | (9.93) | 0.01 | 0.7348 |
| Connective tissue disorder | 527 | 301 | (5.28) | 226 | (4.45) | 0.04 | 0.0465 | 393 | 196 | (4.92) | 197 | (4.94) | 0.00 | 0.9587 |
| Peptic ulcer | 2227 | 1160 | (20.34) | 1067 | (21.01) | 0.02 | 0.3925 | 1659 | 841 | (21.10) | 818 | (20.52) | 0.01 | 0.5257 |
| Liver diseases | 1291 | 739 | (12.96) | 552 | (10.87) | 0.06 | 0.0008 | 942 | 464 | (11.64) | 478 | (11.99) | 0.01 | 0.6271 |
| Diabetes | 2520 | 1422 | (24.93) | 1098 | (21.62) | 0.08 | <0.0001 | 1851 | 930 | (23.33) | 921 | (23.11) | 0.01 | 0.8113 |
| Diabetes complications | 756 | 445 | (7.80) | 311 | (6.12) | 0.07 | 0.0007 | 542 | 273 | (6.85) | 269 | (6.75) | 0.00 | 0.8587 |
| Paraplegia | 64 | 28 | (0.49) | 36 | (0.71) | 0.03 | 0.1416 | 43 | 24 | (0.60) | 19 | (0.48) | 0.02 | 0.4445 |
| Renal disease | 1007 | 623 | (10.92) | 384 | (7.56) | 0.12 | <0.0001 | 687 | 345 | (8.66) | 342 | (8.58) | 0.00 | 0.9047 |
| Severe liver diseases | 41 | 22 | (0.39) | 19 | (0.37) | 0.00 | 0.9217 | 31 | 12 | (0.30) | 19 | (0.48) | 0.03 | 0.2078 |
| Metastatic cancer | 4 | 3 | (0.05) | 1 | (0.02) | 0.02 | 0.3757 | 2 | 1 | (0.03) | 1 | (0.03) | 0.00 | 1.0000 |
| Hypertension | 5058 | 2911 | (51.04) | 2147 | (42.27) | 0.18 | <0.0001 | 3626 | 1822 | (45.71) | 1804 | (45.26) | 0.01 | 0.6856 |
| Hyperlipidemia | 2991 | 1760 | (30.86) | 1231 | (24.24) | 0.15 | <0.0001 | 2154 | 1085 | (27.22) | 1069 | (26.82) | 0.01 | 0.6865 |
| Thyroid function abnormal | 288 | 176 | (3.09) | 112 | (2.21) | 0.05 | 0.0046 | 183 | 84 | (2.11) | 99 | (2.48) | 0.03 | 0.2620 |
| Obstructive sleep apnea | 269 | 204 | (3.58) | 65 | (1.28) | 0.15 | <0.0001 | 136 | 72 | (1.81) | 64 | (1.61) | 0.02 | 0.4890 |
| Prior medications | ||||||||||||||
| Oral anticoagulants | 175 | 121 | (2.12) | 54 | (1.06) | 0.08 | <0.0001 | 98 | 48 | (1.20) | 50 | (1.25) | 0.00 | 0.8389 |
| Anti-platelet | 1536 | 858 | (15.04) | 678 | (13.35) | 0.05 | 0.0119 | 1154 | 597 | (14.98) | 557 | (13.97) | 0.03 | 0.2029 |
| Aspirin | 1183 | 667 | (11.70) | 516 | (10.16) | 0.05 | 0.0109 | 886 | 460 | (11.54) | 426 | (10.69) | 0.03 | 0.2257 |
| Statins | 2244 | 1368 | (23.99) | 876 | (17.25) | 0.17 | <0.0001 | 1605 | 818 | (20.52) | 787 | (19.74) | 0.02 | 0.3866 |
| Fibrates | 225 | 147 | (2.58) | 78 | (1.54) | 0.07 | 0.0002 | 143 | 72 | (1.81) | 71 | (1.78) | 0.00 | 0.9328 |
| Anti-diabetics | 2033 | 1143 | (20.04) | 890 | (17.52) | 0.06 | 0.0008 | 1490 | 750 | (18.82) | 740 | (18.56) | 0.01 | 0.7739 |
| ACEI/ARB/Aliskiren | 3046 | 1794 | (31.46) | 1252 | (24.65) | 0.15 | <0.0001 | 2162 | 1096 | (27.50) | 1066 | (26.74) | 0.02 | 0.4498 |
| Diuretics | 518 | 217 | (3.81) | 301 | (5.93) | 0.10 | <0.0001 | 368 | 186 | (4.67) | 182 | (4.57) | 0.00 | 0.8309 |
| Alendronate | 1431 | 770 | (13.50) | 661 | (13.01) | 0.01 | 0.4566 | 1045 | 511 | (12.82) | 534 | (13.40) | 0.02 | 0.4453 |
| Forteo (Teriparatide) | 279 | 160 | (2.81) | 119 | (2.34) | 0.03 | 0.1310 | 226 | 116 | (2.91) | 110 | (2.76) | 0.01 | 0.6856 |
| Calcium | 3192 | 1363 | (23.90) | 1829 | (36.01) | 0.27 | <0.0001 | 2343 | 1157 | (29.03) | 1186 | (29.75) | 0.02 | 0.4759 |
| Vit. D | 2264 | 945 | (16.57) | 1319 | (25.97) | 0.23 | <0.0001 | 1698 | 837 | (21.00) | 861 | (21.60) | 0.01 | 0.5115 |
| N | Denosumab (n = 3986) | Raloxifene (n = 3986) | p-Value | |||
|---|---|---|---|---|---|---|
| n | (%) | n | (%) | |||
| Primary outcomes | ||||||
| Any CVD | 738 | 311 | (7.80) | 427 | (10.71) | <0.0001 |
| Myocardial infarction | 102 | 40 | (1.00) | 62 | (1.56) | 0.0284 |
| Ischemic stroke | 467 | 183 | (4.59) | 284 | (7.12) | <0.0001 |
| Congestive heart failure | 290 | 129 | (3.24) | 161 | (4.04) | 0.0556 |
| In-hospital death from any cause | 501 | 178 | (4.47) | 323 | (8.10) | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.-C.; Hsu, C.-N.; Lee, W.-C.; Wang, S.-W.; Huang, C.-C.; Lee, Y.-T.; Fu, C.-M.; Chen, J.-B.; Li, L.-C. Denosumab Is Superior to Raloxifene in Lowering Risks of Mortality and Ischemic Stroke in Osteoporotic Women. Pharmaceuticals 2023, 16, 222. https://doi.org/10.3390/ph16020222
Liu T-C, Hsu C-N, Lee W-C, Wang S-W, Huang C-C, Lee Y-T, Fu C-M, Chen J-B, Li L-C. Denosumab Is Superior to Raloxifene in Lowering Risks of Mortality and Ischemic Stroke in Osteoporotic Women. Pharmaceuticals. 2023; 16(2):222. https://doi.org/10.3390/ph16020222
Chicago/Turabian StyleLiu, Ting-Chun, Chien-Ning Hsu, Wen-Chin Lee, Shih-Wei Wang, Chiang-Chi Huang, Yueh-Ting Lee, Chung-Ming Fu, Jin-Bor Chen, and Lung-Chih Li. 2023. "Denosumab Is Superior to Raloxifene in Lowering Risks of Mortality and Ischemic Stroke in Osteoporotic Women" Pharmaceuticals 16, no. 2: 222. https://doi.org/10.3390/ph16020222
APA StyleLiu, T.-C., Hsu, C.-N., Lee, W.-C., Wang, S.-W., Huang, C.-C., Lee, Y.-T., Fu, C.-M., Chen, J.-B., & Li, L.-C. (2023). Denosumab Is Superior to Raloxifene in Lowering Risks of Mortality and Ischemic Stroke in Osteoporotic Women. Pharmaceuticals, 16(2), 222. https://doi.org/10.3390/ph16020222








