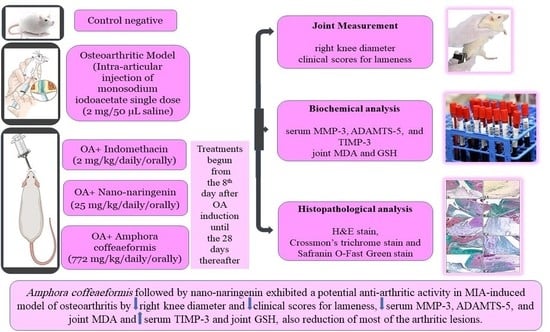
Targeting Some Key Metalloproteinases by Nano-Naringenin and Amphora coffeaeformis as a Novel Strategy for Treatment of Osteoarthritis in Rats
Abstract
:1. Introduction
2. Results
2.1. Effect of Nano-Naringenin and Amphora Coffeaeformis on Body Weight
2.2. Effect of Nano-Naringenin and Amphora Coffeaeformis on Knee Swelling
2.3. Effect of Nano-Naringenin and Amphora Coffeaeformis on Clinical Score for Lameness
2.4. Effect of Nano-Naringenin and Amphora Coffeaeformis on Joint Redox Markers
2.5. Effect of Nano-Naringenin and Amphora Coffeaeformis on Serum Pro-Inflammatory (ADAM TS-5, MMP-3) and Anti-Inflammatory Joint Markers (TIMP-3)
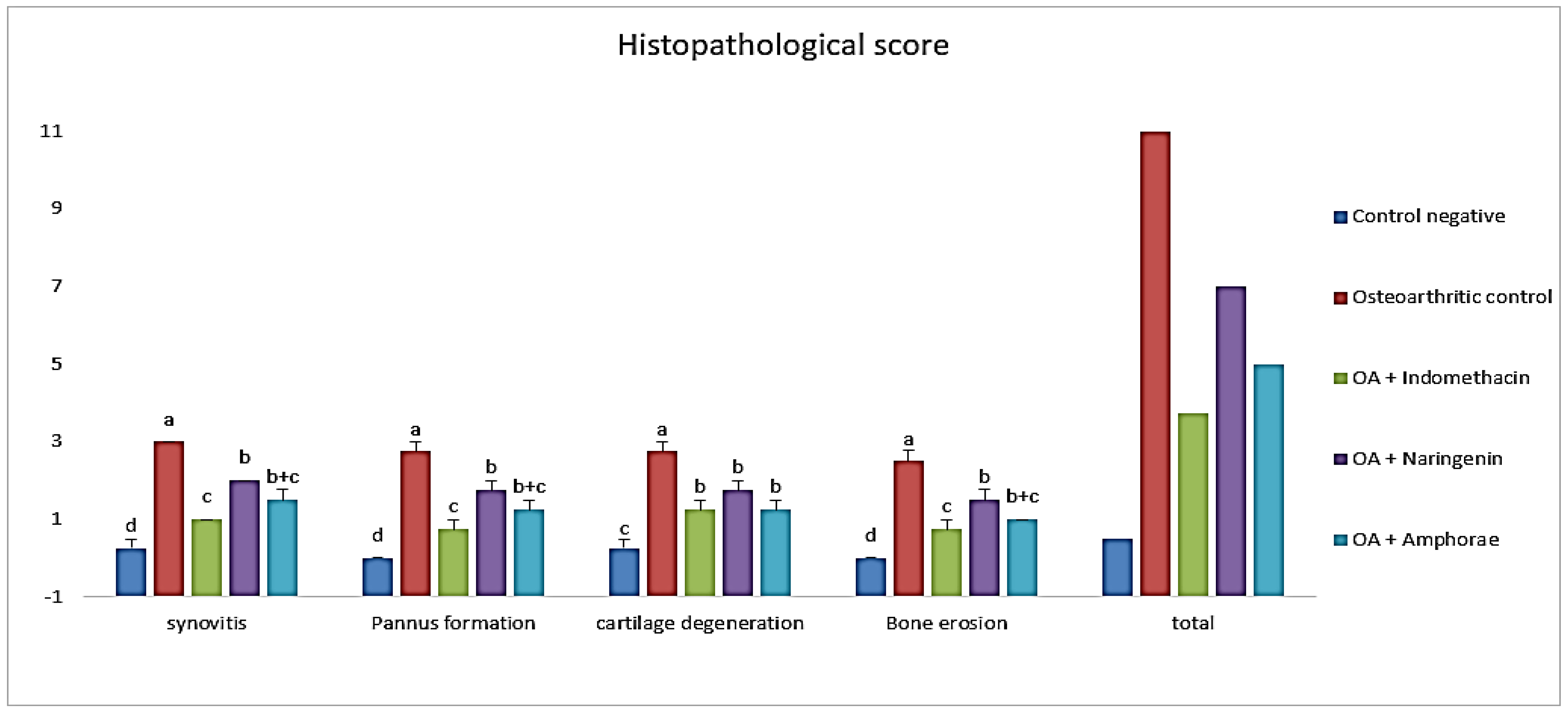
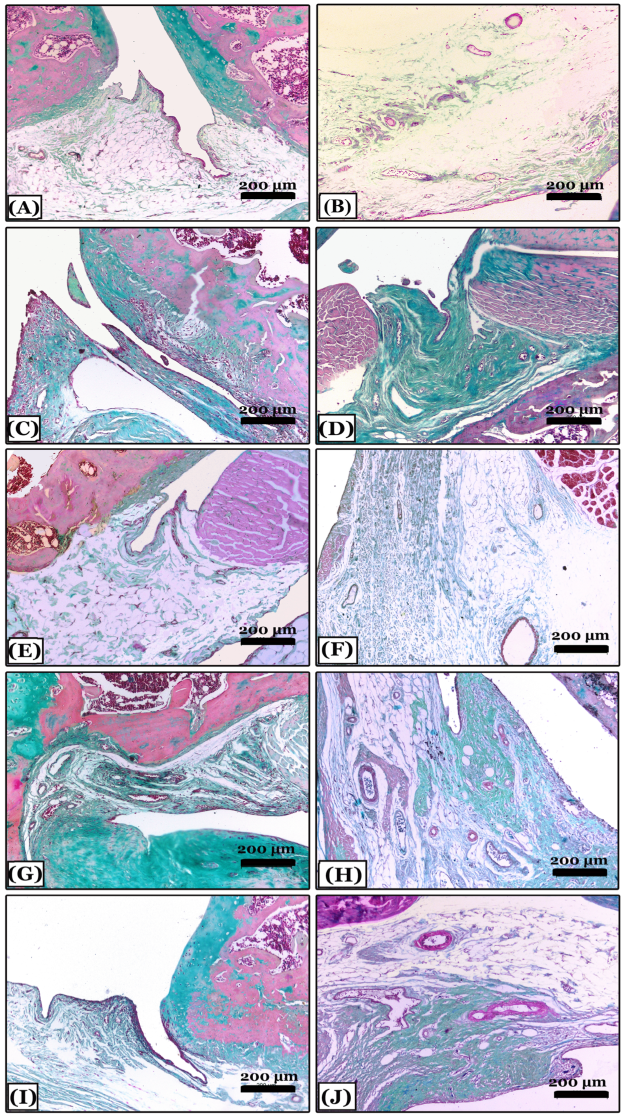
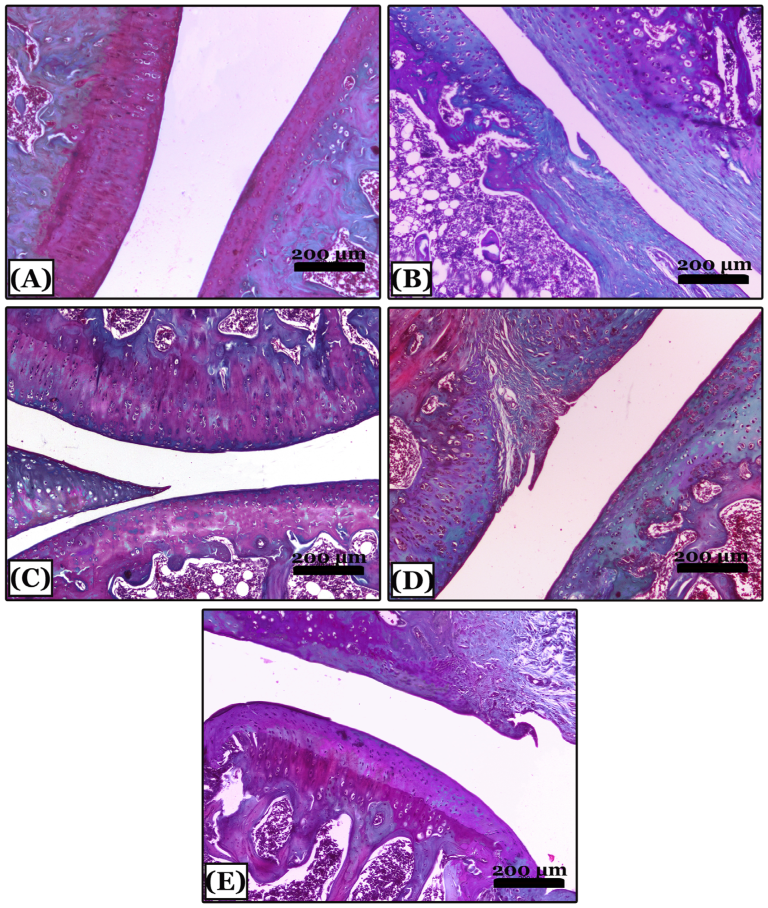
2.6. Histopathological Results
2.6.1. Effect on Histopathological Score
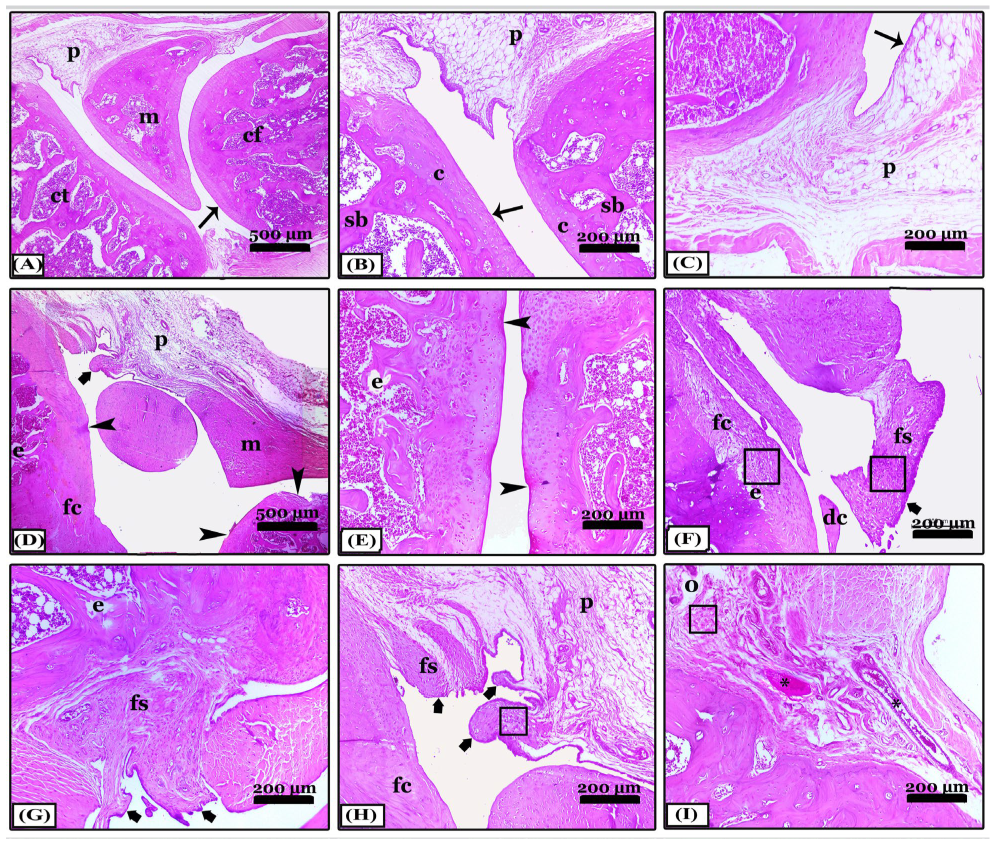
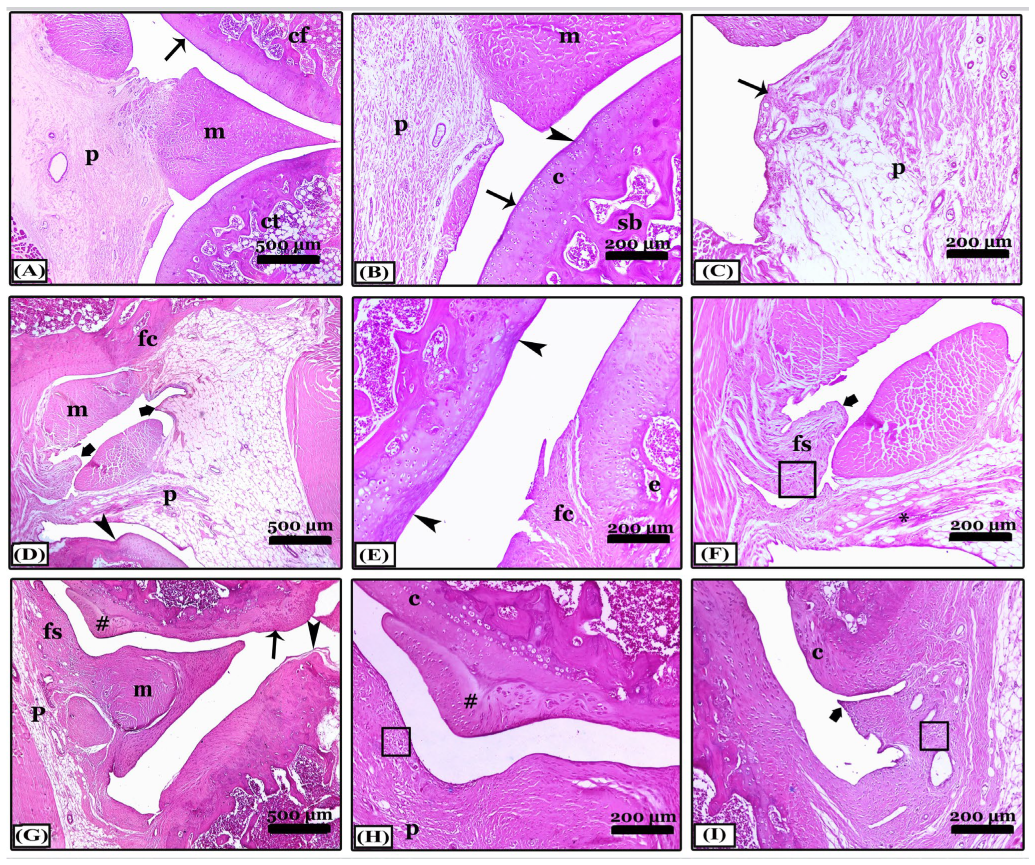
2.6.2. Histopathological Changes in Right Knee Joint
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Nano-Naringenin Preparation and Characterization
4.3. Animals
4.4. Experimental Procedure
4.5. Joint Measurement (Evaluation of Knee Swelling and Degree of Lameness)
4.6. Sampling and Tissue Preparations
4.7. Biochemical Analysis
4.8. Histopathological Analysis
- (a)
- Hematoxylin and eosin stain (H&E) for the general screening. The degree scoring of the OA severity was assessed in joint sections stained with H&E X40 by OARSI system (Osteoarthritis Research Society International) as explained by [69,70,71] for pannus formation, synovitis, cartilage destruction, and bone erosion. The grades; are 0 (no change), 1 (minimal change), 2 (mild change), 3 (moderate change), and 4 (severe inflammation).
- (b)
- Crossman’s Trichrome (CT) stain for collagen fibers identification and arthritis evaluation.
- (c)
- Safranin O-Fast Green (SOFG) stain for proteoglycans demonstration and evaluation of the knee joints.
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nguyen, U.S.D.; Zhang, Y.; Zhu, Y.; Niu, J.; Zhang, B.; Felson, D.T. Increasing prevalence of knee pain and symptomatic knee osteoarthritis: Survey and cohort data. Ann. Intern. Med. 2011, 155, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Juhakoski, R.; Tenhonen, S.; Anttonen, T.; Kauppinen, T.; Arokoski, J.P. Factors affecting self-reported pain and physical function in patients with hip osteoarthritis. Arch. Phys. Med. Rehabil. 2008, 89, 1066–1073. [Google Scholar] [CrossRef] [PubMed]
- Murphy, G.; Nagase, H. Reappraising metalloproteinases in rheumatoid arthritis and osteoarthritis: Destruction or repair? Nat. Clin. Pract. Rheumatol. 2008, 4, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Fraser, A.; Fearon, U.; Billinghurst, R.C.; Ionescu, M.; Reece, R.; Barwick, T.; Veale, D.J. Turnover of type II collagen and aggrecan in cartilage matrix at the onset of inflammatory arthritis in humans: Relationship to mediators of systemic and local inflammation. Arthritis Rheum. 2003, 48, 3085–3095. [Google Scholar] [CrossRef]
- Heinegård, D.; Saxne, T. The role of the cartilage matrix in osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 50–56. [Google Scholar] [CrossRef]
- Mohammed, F.F.; Smookler, D.S.; Khokha, R. Metalloproteinases, inflammation, and rheumatoid arthritis. Ann. Rheum. Dis. 2003, 62, ii43–ii47. [Google Scholar] [CrossRef]
- Blanco, F.J.; Rego, I.; Ruiz-Romero, C. The role of mitochondria in osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 161–169. [Google Scholar] [CrossRef]
- Wehling, M. Non-steroidal anti-inflammatory drug use in chronic pain conditions with special emphasis on the elderly and patients with relevant comorbidities: Management and mitigation of risks and adverse effects. Eur. J. Clin. Pharmacol. 2014, 70, 1159–1172. [Google Scholar] [CrossRef]
- Badal, S.; Turfus, S.; Rajnarayanan, R.; Wilson-Clarke, C.; Sandiford, S.L. Analysis of natural product regulation of opioid receptors in the treatment of human disease. Pharmacol. Ther. 2018, 184, 51–80. [Google Scholar] [CrossRef]
- Mulvihill, E.E.; Allister, E.M.; Sutherland, B.G.; Telford, D.E.; Sawyez, C.G.; Edwards, J.Y.; Markle, J.M.; Hegele, R.A.; Huff, M.W. Naringenin prevents dyslipidemia, apolipoprotein B overproduction, and hyperinsulinemia in LDL receptor–null mice with diet-induced insulin resistance. Diabetes 2009, 58, 2198–2210. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Wang, J.; Wei, T.; Gao, J.; He, H.; Chang, X.; Yan, T. Effects of Naringenin on inflammation in complete Freund’s adjuvant-induced arthritis by regulating Bax/Bcl-2 balance. Inflammation 2015, 38, 245–251. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Ding, J.; Xu, H.; Dai, X.; Hou, Z.; Sun, W. Delivery of ursolic acid (UA) in polymeric nanoparticles effectively promotes the apoptosis of gastric cancer cells through enhanced inhibition of cyclooxygenase 2 (COX-2). Int. J. Pharm. 2013, 441, 261–268. [Google Scholar] [CrossRef]
- Barros, M.P.; Pinto, E.; Sigaud-Kutner, T.C.; Cardozo, K.H.; Colepicolo, P. Rhythmicity and oxidative/nitrosative stress in algae. Biol. Rhythm Res. 2005, 36, 67–82. [Google Scholar] [CrossRef]
- El-Sayed, A.E.K.B.; Aboulthana, W.M.; El-Feky, A.M.; Ibrahim, N.E.; Seif, M.M. Bio and phyto-chemical effect of Amphora coffeaeformis extract against hepatic injury induced by paracetamol in rats. Mol. Biol. Rep. 2018, 45, 2007–2023. [Google Scholar] [CrossRef]
- Goiris, K.; Muylaert, K.; Fraeye, I.; Foubert, I.; De Brabanter, J.; De Cooman, L. Antioxidant potential of microalgae in relation to their phenolic and carotenoid content. J. Appl. Phycol. 2012, 24, 1477–1486. [Google Scholar] [CrossRef]
- Abdel-Wahab, A.M. In-vitro studies on antiviral effects of Galaxaura elongata marine algae on white spot syndrome virus. Benha Vet. Med. J. 2018, 34, 162–171. [Google Scholar] [CrossRef]
- Lee, S.H.; Karawita, R.; Affan, A.; Lee, J.B.; Lee, K.W.; Lee, B.J.; Jeon, Y.J. Potential of benthic diatoms Achnanthes longipes, Amphora coffeaeformis and Navicula sp (Bacillariophyceae) as antioxidant sources. Algae 2009, 24, 47–55. [Google Scholar] [CrossRef]
- Jimbo, S.; Terashima, Y.; Takebayashi, T.; Teramoto, A.; Ogon, I. A novel rat model of ankle osteoarthritis induced by the application of monoiodoacetate. Br. J. Med. Med. Res. 2017, 6, 1000260. [Google Scholar] [CrossRef]
- Wang, Y.; Prentice, L.F.; Vitetta, L.; Wluka, A.E.; Cicuttini, F.M. The effect of nutritional supplements on osteoarthritis. Altern. Med. Rev. 2004, 9, 275–296. [Google Scholar]
- Li, Z.; Gao, M.; Yang, B.; Zhang, H.; Wang, K.; Liu, Z.; Yang, M. Naringin attenuates MLC phosphorylation and NF-κB activation to protect sepsis-induced intestinal injury via RhoA/ROCK pathway. Biomed. Pharmacother. 2018, 103, 50–58. [Google Scholar] [CrossRef]
- Tripathi, A.; Awasthi, H.; Rokaya, D.B.; Srivastava, D.; Srivastava, V. Antimicrobial and wound healing potential of dietary flavonoid naringenin. Nat. Prod. J. 2019, 9, 61–68. [Google Scholar] [CrossRef]
- Ayoub, A.; Pulijala, Y. The application of virtual reality and augmented reality in Oral & Maxillofacial Surgery. BMC Oral Health 2019, 19, 238. [Google Scholar] [CrossRef]
- El-Bahr, S.; Shousha, S.; Shehab, A.; Khattab, W.; Ahmed-Farid, O.; Sabike, I.; Albosadah, K. Effect of dietary microalgae on growth performance, profiles of amino and fatty acids, antioxidant status, and meat quality of broiler chickens. Animals 2020, 10, 761. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Guo, L.; Tian, F.D.; An, N.; Luo, L.; Hao, R.H.; Wang, B.; Zhou, Z.H. Naringenin regulates production of matrix metalloproteinases in the knee-joint and primary cultured articular chondrocytes and alleviates pain in rat osteoarthritis model. Braz. J. Med. Biol. Res. 2017, 50, e5714. [Google Scholar] [CrossRef] [PubMed]
- Bussmann, A.J.; Borghi, S.M.; Zaninelli, T.H.; Dos Santos, T.S.; Guazelli, C.F.; Fattori, V.; Verri, W.A. The citrus flavanone naringenin attenuates zymosan-induced mouse joint inflammation: Induction of Nrf2 expression in recruited CD45+ hematopoietic cells. Inflammopharmacology 2019, 27, 1229–1242. [Google Scholar] [CrossRef]
- Khavari, F.; Saidijam, M.; Taheri, M.; Nouri, F. Microalgae: Therapeutic potentials and applications. Mol. Biol. Rep. 2021, 48, 4757–4765. [Google Scholar] [CrossRef]
- Salvemini, D.; Little, J.W.; Doyle, T.; Neumann, W.L. Roles of reactive oxygen and nitrogen species in pain. Free Radic. Biol. Med. 2011, 51, 951–966. [Google Scholar] [CrossRef]
- Manchope, M.F.; Casagrande, R.; Verri, W.A., Jr. Naringenin: An analgesic and anti-inflammatory citrus flavanone. Oncotarget 2017, 8, 3766. [Google Scholar] [CrossRef]
- Quiñonez-Flores, C.M.; González-Chávez, S.A.; Del Rio Najera, D.; Pacheco-Tena, C. Oxidative stress relevance in the pathogenesis of the rheumatoid arthritis: A systematic review. BioMed Res. Int. 2016, 2016, 6097417. [Google Scholar] [CrossRef]
- Filippin, L.I.; Vercelino, R.; Marroni, N.P.; Xavier, R.M. Redox signalling and the inflammatory response in rheumatoid arthritis. Clin. Exp. Immunol. 2008, 152, 415–422. [Google Scholar] [CrossRef]
- Sharma, H.; Chauhan, P.; Singh, S. Evaluation of the anti-arthritic activity of Cinnamomum cassia bark extract in experimental models. Integr. Med. Res. 2018, 7, 366–373. [Google Scholar] [CrossRef]
- Fan, R.; Pan, T.; Zhu, A.L.; Zhang, M.H. Anti-inflammatory and anti-arthritic properties of naringenin via attenuation of NF-κB and activation of the heme oxygenase (HO)-1/related factor 2 pathway. Pharmacol. Rep. 2017, 69, 1021–1029. [Google Scholar] [CrossRef]
- Renugadevi, J.; Prabu, S.M. Naringenin protects against cadmium-induced oxidative renal dysfunction in rats. Toxicology 2009, 256, 128–134. [Google Scholar] [CrossRef]
- Manchope, M.F.; Calixto-Campos, C.; Coelho-Silva, L.; Zarpelon, A.C.; Pinho-Ribeiro, F.A.; Georgetti, S.R.; Verri, W.A., Jr. Naringenin inhibits superoxide anion-induced inflammatory pain: Role of oxidative stress, cytokines, Nrf-2 and the NO−cGMP−PKG−KATP Channel signaling pathway. PLoS ONE 2016, 11, e0153015. [Google Scholar] [CrossRef]
- Salim, I.H.; Abdel-Aal, M.; Awad, D.O.; El-Sayed, A.B. Productive performance, physiological and antioxidant status of growing v-line rabbits drinking water supplemented with Amphora coffeaeformis diatoms alga extract during hot conditions. Egypt. J. Nutr. Feed. 2019, 22, 577–588. [Google Scholar] [CrossRef]
- Mekkawy, I.A.; Mahmoud, U.M.; Moneeb, R.H.; Sayed, A.E.D.H. Significance assessment of Amphora coffeaeformis in arsenic-induced hemato-biochemical alterations of African catfish (Clarias gariepinus). Front. Mar. Sci. 2020, 7, 191. [Google Scholar] [CrossRef]
- Mazumder, K.; Nabila, A.; Aktar, A.; Farahnaky, A. Bioactive variability and in-vitro and in-vivo antioxidant activity of unprocessed and processed flour of nine cultivars of Australian lupin species: A comprehensive substantiation. Antioxidants 2020, 9, 282. [Google Scholar] [CrossRef]
- Hadjadj, N.; Hazzit, M. Analysis and antioxidant activity of essential oils and methanol extracts of Origanum floribundum Munby. J. Essent. Oil Bear. Plants 2020, 23, 85–96. [Google Scholar] [CrossRef]
- Kevorkian, L.; Young, D.A.; Darrah, C.; Donell, S.T.; Shepstone, L.; Porter, S.; Clark, I.M. Expression profiling of metalloproteinases and their inhibitors in cartilage. Arthritis Rheum. 2004, 50, 131–141. [Google Scholar] [CrossRef]
- Adamcova, M.; Šimko, F. Multiplex biomarker approach to cardiovascular diseases. Acta Pharmacol. Sin. 2018, 39, 1068–1072. [Google Scholar] [CrossRef]
- Fitzgerald, J.B.; Jin, M.; Dean, D.; Wood, D.J.; Zheng, M.H.; Grodzinsky, A.J. Mechanical compression of cartilage explants induces multiple time-dependent gene expression patterns and involves intracellular calcium and cyclic AMP. J. Biol. Chem. 2004, 279, 19502–19511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pengas, I.; Eldridge, S.; Assiotis, A.; McNicholas, M.; Mendes, J.E.; Laver, L. MMP-3 in the peripheral serum as a biomarker of knee osteoarthritis, 40 years after open total knee meniscectomy. J. Exp. Orthop. 2018, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- Patwari, P.; Gao, G.; Lee, J.H.; Grodzinsky, A.J.; Sandy, J.D. Analysis of ADAMTS4 and MT4-MMP indicates that both are involved in aggrecanolysis in interleukin-1-treated bovine cartilage. Osteoarthr. Cartil. 2005, 13, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Echtermeyer, F.; Bertrand, J.; Dreier, R.; Meinecke, I.; Neugebauer, K.; Fuerst, M.; Pap, T. Syndecan-4 regulates ADAMTS-5 activation and cartilage breakdown in osteoarthritis. Nat. Med. 2009, 15, 1072–1076. [Google Scholar] [CrossRef]
- Glasson, S.S.; Askew, R.; Sheppard, B.; Carito, B.; Blanchet, T.; Ma, H.L.; Morris, E.A. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature 2005, 434, 644–648. [Google Scholar] [CrossRef]
- Baker, A.H.; Kritz, A.; Work, L.M.; Nicklin, S.A. Cell-selective viral gene delivery vectors for the vasculature. Exp. Physiol. 2005, 90, 27–31. [Google Scholar] [CrossRef]
- Kashiwagi, M.; Tortorella, M.; Nagase, H.; Brew, K. TIMP-3 is a potent inhibitor of aggrecanase 1 (ADAM-TS4) and aggrecanase 2 (ADAM-TS5). J. Biol. Chem. 2001, 276, 12501–12504. [Google Scholar] [CrossRef]
- Lee, J.H.; Fitzgerald, J.B.; DiMicco, M.A.; Grodzinsky, A.J. Mechanical injury of cartilage explants causes specific time-dependent changes in chondrocyte gene expression. Arthritis Rheum. 2005, 52, 2386–2395. [Google Scholar] [CrossRef]
- Ahmed, S.; Wang, N.; Lalonde, M.; Goldberg, V.M.; Haqqi, T.M. Green tea polyphenol epigallocatechin-3-gallate (EGCG) differentially inhibits interleukin-1β-induced expression of matrix metalloproteinase-1 and-13 in human chondrocytes. J. Pharmacol. Exp. Ther. 2004, 308, 767–773. [Google Scholar] [CrossRef]
- Mix, K.S.; Mengshol, J.A.; Benbow, U.; Vincenti, M.P.; Sporn, M.B.; Brinckerhoff, C.E. A synthetic triterpenoid selectively inhibits the induction of matrix metalloproteinases 1 and 13 by inflammatory cytokines. Arthritis Rheum. 2001, 44, 1096–1104. [Google Scholar] [CrossRef]
- Lin, N.; Sato, T.; Ito, A. Triptolide, a novel diterpenoid triepoxide from Tripterygium wilfordii Hook. f., suppresses the production and gene expression of pro–matrix metalloproteinases 1 and 3 and augments those of tissue inhibitors of metalloproteinases 1 and 2 in human synovial fibroblasts. Arthritis Rheum. 2001, 44, 2193–2200. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, S.; Su, H.; Cheng, J. Isoliquiritigenin inhibits IL-1β-induced production of matrix metalloproteinase in articular chondrocytes. Mol. Ther. Methods Clin. Dev. 2018, 9, 153–159. [Google Scholar] [CrossRef]
- Van der Laan, W.H.; Quax, P.H.A.; Seemayer, C.A.; Huisman, L.G.M.; Pieterman, E.J.; Grimbergen, J.M.; Verheijen, J.H.; Breedveld, F.C.; Gay, R.E.; Gay, S.; et al. Cartilage degradation and invasion by rheumatoid synovial fibroblasts is inhibited by gene transfer of TIMP-1 and TIMP-3. Gene Ther. 2003, 10, 234–242. [Google Scholar] [CrossRef]
- El-Moghazy, M.; Zedan, N.S.; El-Atrsh, A.M.; El-Gogary, M.; Tousson, E. The possible effect of diets containing fish oil (omega-3) on hematological, biochemical and histopathogical alterations of rabbit liver and kidney. Biomed. Prev. Nutr. 2014, 4, 371–377. [Google Scholar] [CrossRef]
- Riccio, G.; Lauritano, C. Microalgae with immunomodulatory activities. Mar. Drugs 2020, 18, 2. [Google Scholar] [CrossRef]
- Knowles, H.J.; Moskovsky, L.; Thompson, M.S.; Grunhen, J.; Cheng, X.; Kashima, T.G.; Athanasou, N.A. Chondroclasts are mature osteoclasts which are capable of cartilage matrix resorption. Virchows Arch. 2012, 461, 205–210. [Google Scholar] [CrossRef]
- Romas, E.; Sims, N.A.; Hards, D.K.; Lindsay, M.; Quinn, J.W.; Ryan, P.F.; Gillespie, M.T. Osteoprotegerin reduces osteoclast numbers and prevents bone erosion in collagen-induced arthritis. Am. J. Pathol. 2002, 161, 1419–1427. [Google Scholar] [CrossRef]
- Kamarudin, T.A.; Othman, F.; Ramli, E.S.M.; Isa, N.M.; Das, S. Protective effect of curcumin on experimentally induced arthritic rats: Detailed histopathological study of the joints and white blood cell count. EXCLI J. 2012, 11, 226–236. [Google Scholar]
- Funk, J.L.; Oyarzo, J.N.; Frye, J.B.; Chen, G.; Lantz, R.C.; Jolad, S.D.; Solyom, A.M.; Timmermann, B.N. Turmeric extracts containing curcuminoids prevent experimental rheumatoid arthritis. J. Nat. Prod. 2006, 69, 351–355. [Google Scholar] [CrossRef]
- Gusev, A.I.; Kurlov, A.S. Production of nanocrystalline powders by high-energy ball milling: Model and experiment. Nanotechnology 2008, 19, 265302. [Google Scholar] [CrossRef]
- Möller, K.Ä.; Klein, S.; Seeliger, F.; Finn, A.; Stenfors, C.; Svensson, C.I. Monosodium iodoacetate-induced monoarthritis develops differently in knee versus ankle joint in rats. Neurobiol. Pain 2019, 6, 100036. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Zhang, Z.F.; Sun, W.X. Effect of naringin on monosodium iodoacetate-induced osteoarthritis pain in rats. Med. Sci. Monit. 2017, 23, e939357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, J.; Yu, T.; Liu, Y.; Jiang, J.; Xu, J.; Zhao, Y.; Wu, C. Naringenin-loaded solid lipid nanoparticles: Preparation, controlled delivery, cellular uptake, and pulmonary pharmacokinetics. Drug Des. Dev. Ther. 2016, 10, 911–925. [Google Scholar] [CrossRef]
- Khan, H.M.; Ashraf, M.; Hashmi, A.S.; Ahmad, M.U.D.; Anjum, A.A. Clinical assessment of experimentally induced osteoarthritis rat model in relation to time. J. Anim. Plant Sci. 2012, 22, 960–965. Available online: https://www.thejaps.org.pk/Volume/2012/22-4/abstracts/23.php (accessed on 21 January 2023).
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar] [CrossRef]
- Beutler, E. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar]
- Tietz, N.W. Clinical Guide to Laboratory Tests; Saunders: Philadelphia, PA, USA, 1995; 1096p. [Google Scholar]
- Suvarna, K.S.; Layton, C.; Bancroft, J.D. (Eds.) Bancroft’s Theory and Practice of Histological Techniques (E-Book); Elsevier Health Sciences: Amsterdam, The Netherlands, 2018; ISBN 978-0-7020-4226-3. [Google Scholar]
- Sancho, D.; Gómez, M.; Viedma, F.; Esplugues, E.; Gordón-Alonso, M.; García-López, M.A.; Sánchez-Madrid, F. CD69 downregulates autoimmune reactivity through active transforming growth factor-β production in collagen-induced arthritis. J. Clin. Investig. 2003, 112, 872–882. [Google Scholar] [CrossRef]
- Pritzker, K.P.; Gay, S.; Jimenez, S.A.; Ostergaard, K.; Pelletier, J.P.; Revell, P.A.; Salter, D.; Van den Berg, W.B. Osteoarthritis cartilage histopathology: Grading and staging. Osteoarthr. Cartil. 2006, 14, 13–29. [Google Scholar] [CrossRef]
- Allam, G.; Mahdi, E.A.; Alzahrani, A.M.; Abuelsaad, A.S. Ellagic acid alleviates adjuvant induced arthritis by modulation of pro- and anti-inflammatory cytokines. Cent. Eur. J. Immunol. 2016, 41, 339–349. [Google Scholar] [CrossRef] [Green Version]

| Groups | Zero Day | 1st Week | 2nd Week | 3rd Week | 4th Week |
|---|---|---|---|---|---|
| Control negative | 200.5 ± 5.45 a | 203.5 ± 2.93 a | 192.13 ± 2.63 ab | 210.88 ± 3.19 ab | 216.13 ± 6.02 a |
| Osteoarthritic (OA) | 193.25 ± 3.37 a | 189 ± 3.28 b | 185.63 ± 4.98 b | 192.5 ± 5.20 c | 197.75 ± 2.81 b |
| OA + indomethacin | 189.25 ± 2.17 a | 189.5 ± 3.74 b | 194.63 ± 3.6 ab | 196.63 ± 2.47 bc | 207.63 ± 4.35 ab |
| OA + nano-naringenin | 190.25 ± 2.12 a | 193.25 ± 3.56 b | 202.25 ± 4.99 a | 208.38 ± 4.70 ab | 220.25 ± 6.14 a |
| OA + Amphora coffeaeformis | 190.13 ± 5.81 a | 197.63 ± 2.22 ab | 203.38 ± 5.73 a | 213.88 ± 6.95 a | 220.63 ± 8.39 a |
| Groups | Zero Day | 1st Week | 2nd Week | 3rd Week | 4th Week |
|---|---|---|---|---|---|
| Control negative | 7.5 ± 0.19 a | 7.88 ± 0.30 b | 7.5 ± 0.19 b | 7.63 ± 0.18 b | 7.88 ± 0.30 c |
| Osteoarthritic (OA) | 7.5 ± 0.19 a | 18.13 ± 0.93 a | 15.5 ± 0.78 a | 11.63 ± 0.71 a | 10.5 ± 0.46 a |
| OA + indomethacin | 7.38 ± 0.18 a | 17.25 ± 0.70 a | 12.38 ± 0.50 ab | 9 ± 0.33 ab | 8.63 ± 0.26 b |
| OA + nano-naringenin | 7.63 ± 0.18 a | 17.38 ± 0.65 a | 13.63 ± 0.70 ab | 9.38 ± 0.42 ab | 9.13 ± 0.30 bc |
| OA + Amphora coffeaeformis | 7.5 ± 0.19 a | 17.13 ± 0.72 a | 13.37 ± 0.60 ab | 8.88 ± 0.30 ab | 8.63 ± 0.50 bc |
| Groups | Zero Day | 1st Week | 2nd Week | 3rd Week | 4th Week |
|---|---|---|---|---|---|
| Control negative | 0 | 0.38 ± 0.18 b | 0.38 ± 0.18 c | 0.25 ± 0.16 c | 0.13 ± 0.13 c |
| Osteoarthritic (OA) | 0 | 3.75 ± 0.16 a | 3.63 ± 0.18 a | 3.13 ± 0.30 a | 2.63 ± 0.18 a |
| OA + indomethacin | 0 | 3.63 ± 0.18 a | 2.88 ± 0.30 b | 2.13 ± 0.30 b | 0.75 ± 0.25 bc |
| OA + nano-naringenin | 0 | 3.63 ± 0.18 a | 3.25 ± 0.25 ab | 2.38 ± 0.18 b | 1.25 ± 0.25 b |
| OA + Amphora coffeaeformis | 0 | 3.63 ± 0.18 a | 3.00 ± 0.27 ab | 2.13 ± 0.30 b | 0.63 ± 0.26 bc |
| Groups | MDA (nmol/mg Tissue) | GSH (µg/mg Protein) |
|---|---|---|
| Control negative | 0.61 ± 0.09 c | 1.61 ± 0.10 a |
| Osteoarthritic (OA) | 2.48 ± 0.05 a | 0.65 ± 0.06 c |
| OA + indomethacin | 0.68 ± 0.09 c | 1.69 ± 0.10 a |
| OA + nano-naringenin | 1.75 ± 0.05 b | 1.17 ± 0.04 b |
| OA + Amphora coffeaeformis | 0.73 ± 0.07 c | 1.65 ± 0.08 a |
| Groups | ADAM TS-5 (ng mL−1) | MMP-3 (ng mL−1) | TIMP-3 (ng mL−1) |
|---|---|---|---|
| Control negative | 2.30 ± 0.12 d | 1.05 ± 0.1 c | 5.70 ± 0.32 a |
| Osteoarthritic (OA) | 10.70 ± 0.82 a | 3.17 ± 0.09 a | 1.97 ± 0.68 b |
| OA + indomethacin | 4.93 ± 0.30 c | 1.13 ± 0.09 c | 5.47 ± 1.08 a |
| OA + nano-naringenin | 8.93 ± 0.28 b | 1.63 ± 0.12 b | 3.43 ± 0.70 ab |
| OA + Amphora coffeaeformis | 6.07 ± 0.67 c | 1.13 ± 0.18 c | 5.50.64 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaban, N.S.; Radi, A.M.; Abdelgawad, M.A.; Ghoneim, M.M.; Al-Serwi, R.H.; Hassan, R.M.; Mohammed, E.T.; Radi, R.A.; Halfaya, F.M. Targeting Some Key Metalloproteinases by Nano-Naringenin and Amphora coffeaeformis as a Novel Strategy for Treatment of Osteoarthritis in Rats. Pharmaceuticals 2023, 16, 260. https://doi.org/10.3390/ph16020260
Shaban NS, Radi AM, Abdelgawad MA, Ghoneim MM, Al-Serwi RH, Hassan RM, Mohammed ET, Radi RA, Halfaya FM. Targeting Some Key Metalloproteinases by Nano-Naringenin and Amphora coffeaeformis as a Novel Strategy for Treatment of Osteoarthritis in Rats. Pharmaceuticals. 2023; 16(2):260. https://doi.org/10.3390/ph16020260
Chicago/Turabian StyleShaban, Nema S., Abeer M. Radi, Mohamed A. Abdelgawad, Mohammed M. Ghoneim, Rasha Hamed Al-Serwi, Randa M. Hassan, Eman T. Mohammed, Rania A. Radi, and Fatma M. Halfaya. 2023. "Targeting Some Key Metalloproteinases by Nano-Naringenin and Amphora coffeaeformis as a Novel Strategy for Treatment of Osteoarthritis in Rats" Pharmaceuticals 16, no. 2: 260. https://doi.org/10.3390/ph16020260
APA StyleShaban, N. S., Radi, A. M., Abdelgawad, M. A., Ghoneim, M. M., Al-Serwi, R. H., Hassan, R. M., Mohammed, E. T., Radi, R. A., & Halfaya, F. M. (2023). Targeting Some Key Metalloproteinases by Nano-Naringenin and Amphora coffeaeformis as a Novel Strategy for Treatment of Osteoarthritis in Rats. Pharmaceuticals, 16(2), 260. https://doi.org/10.3390/ph16020260