Novel Scaffold Based on Chitosan Hydrogels/Phthalated Cashew Gum for Supporting Human Dental Pulp Stem Cells
Abstract
:1. Introduction
2. Results and Discussion
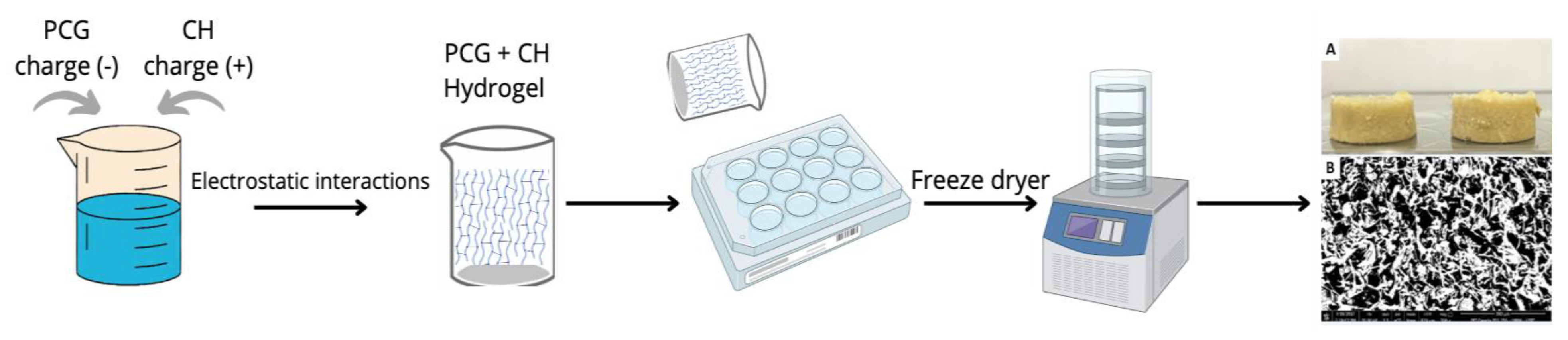
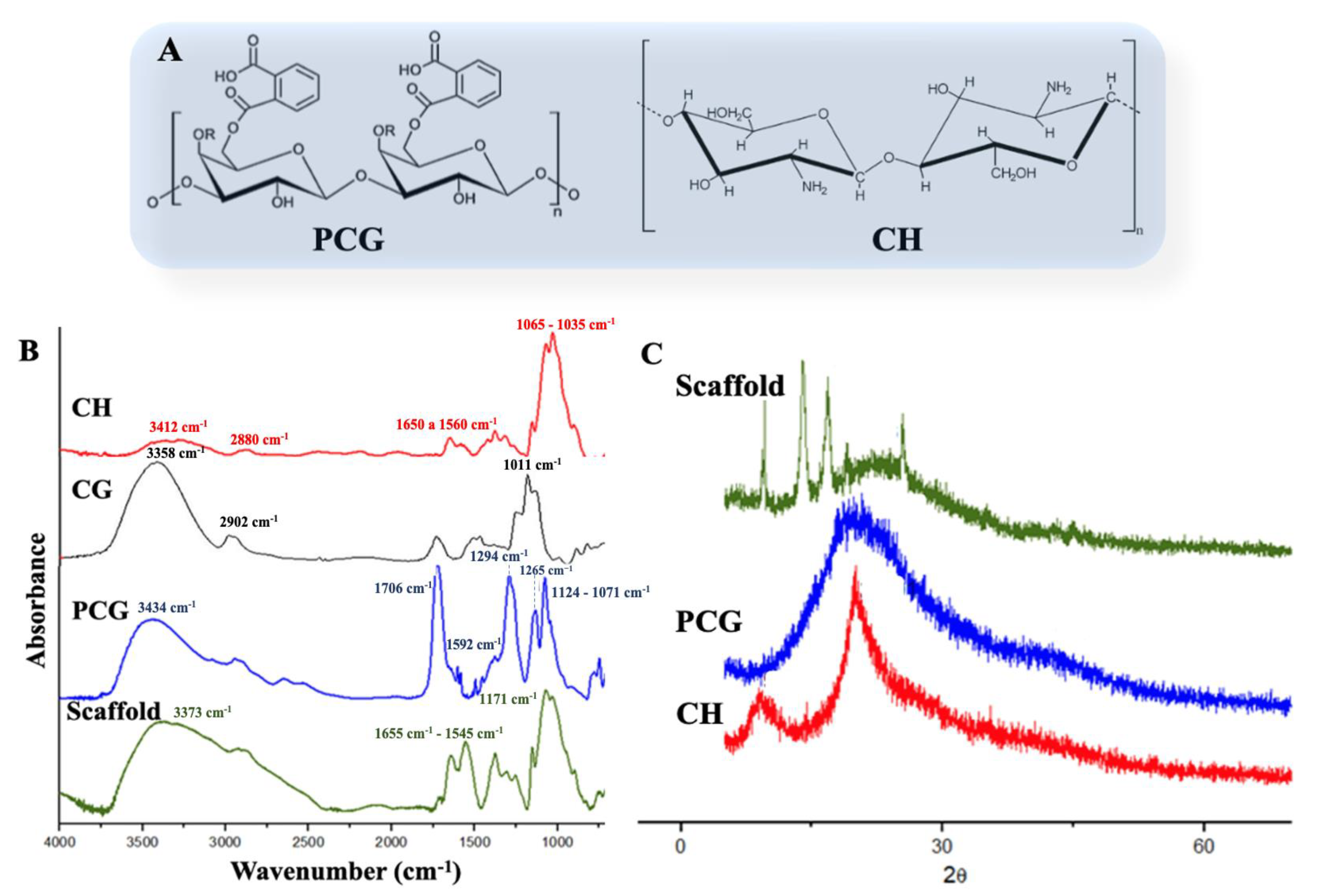
2.1. Scaffolds Preparation and Characterization
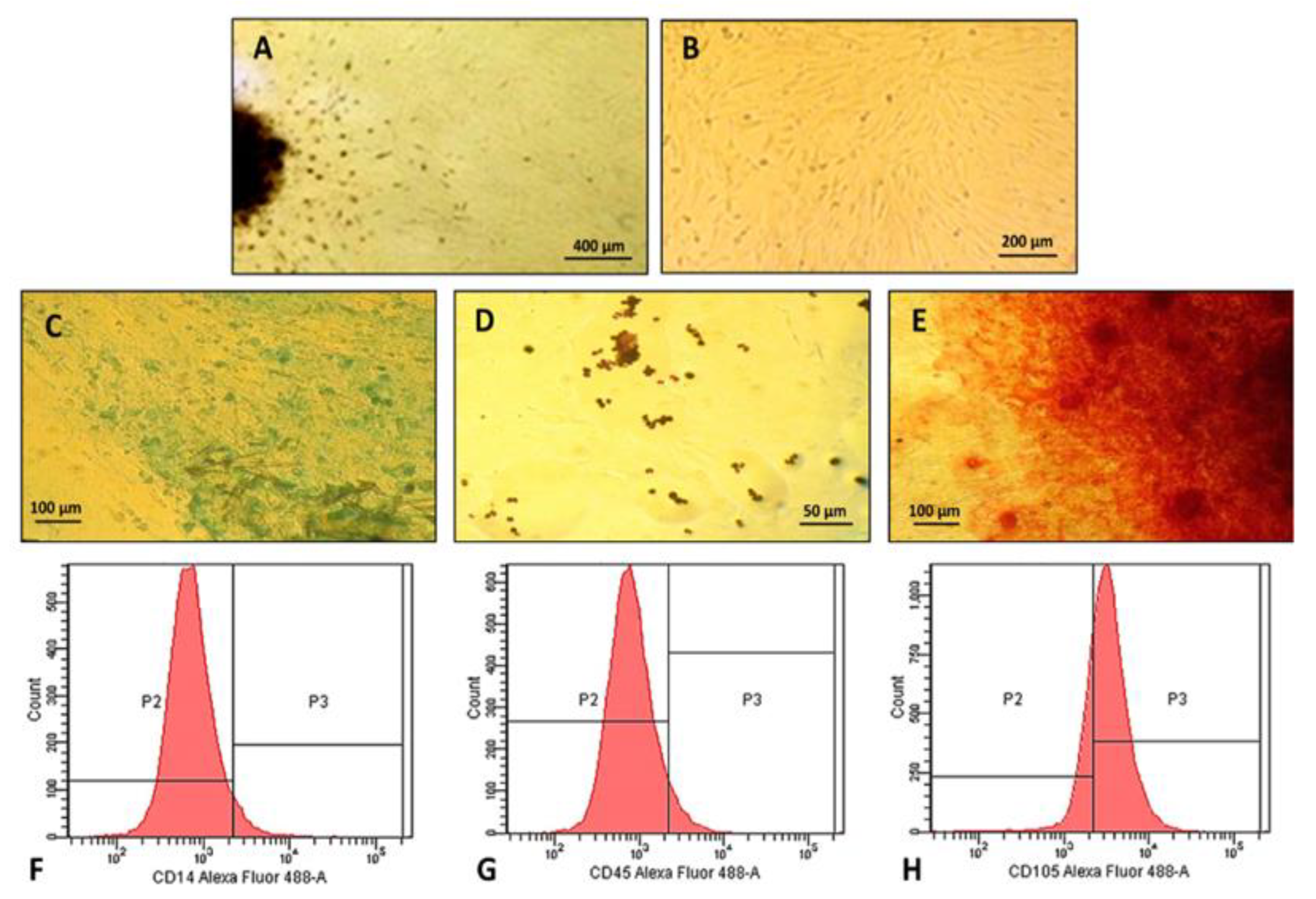
2.2. Characterization of Human Dental Pulp Mesenchymal Stem Cells
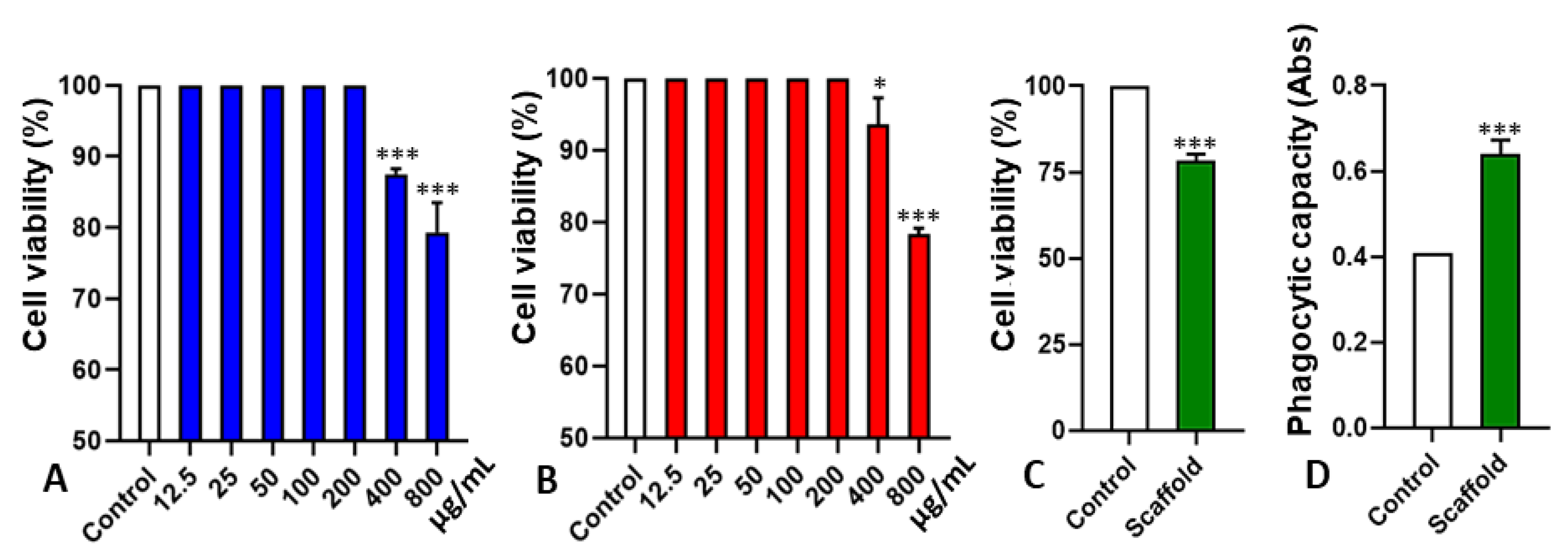
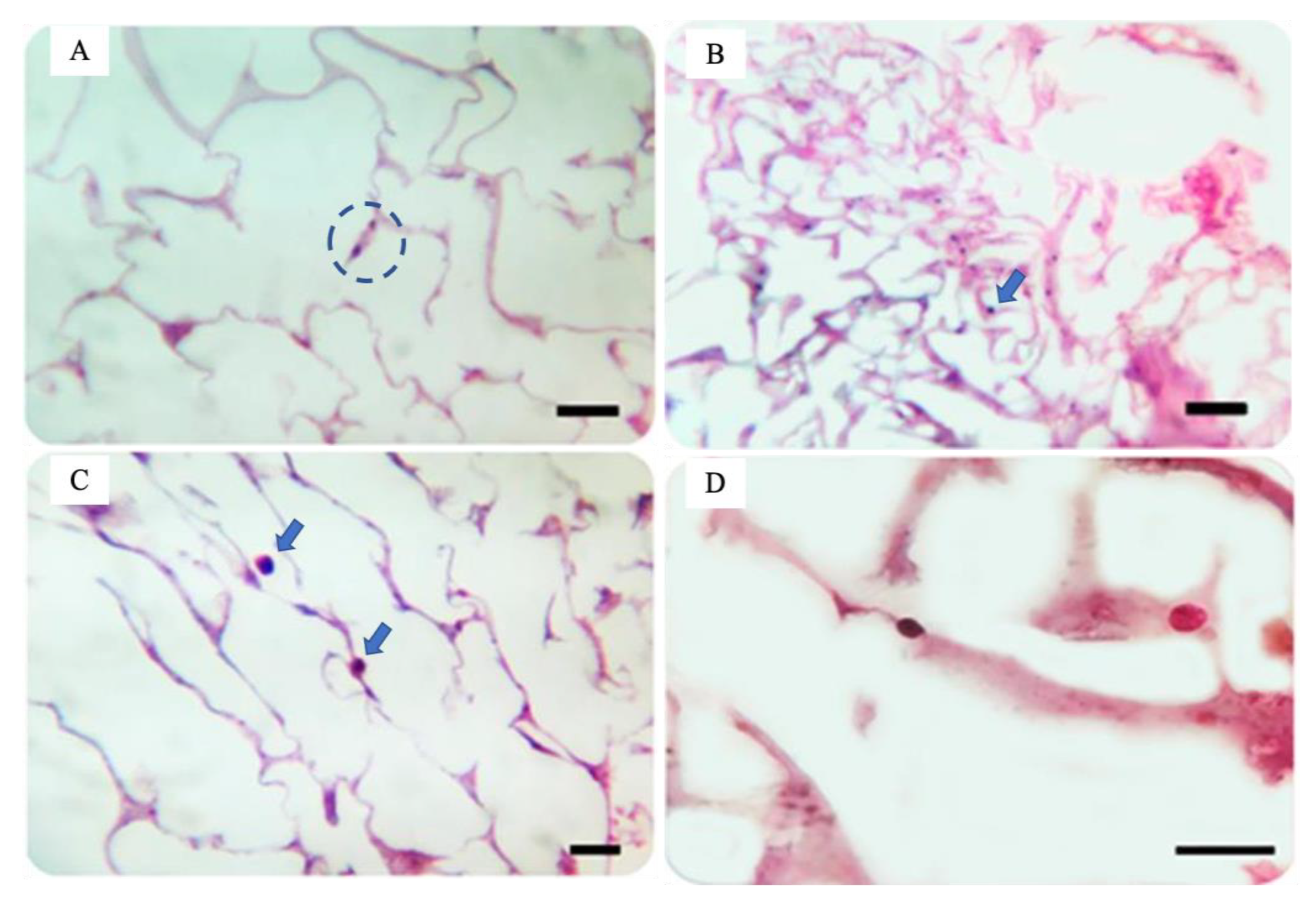
2.3. Cytotoxicity and Interference in Phagocytic Capacity
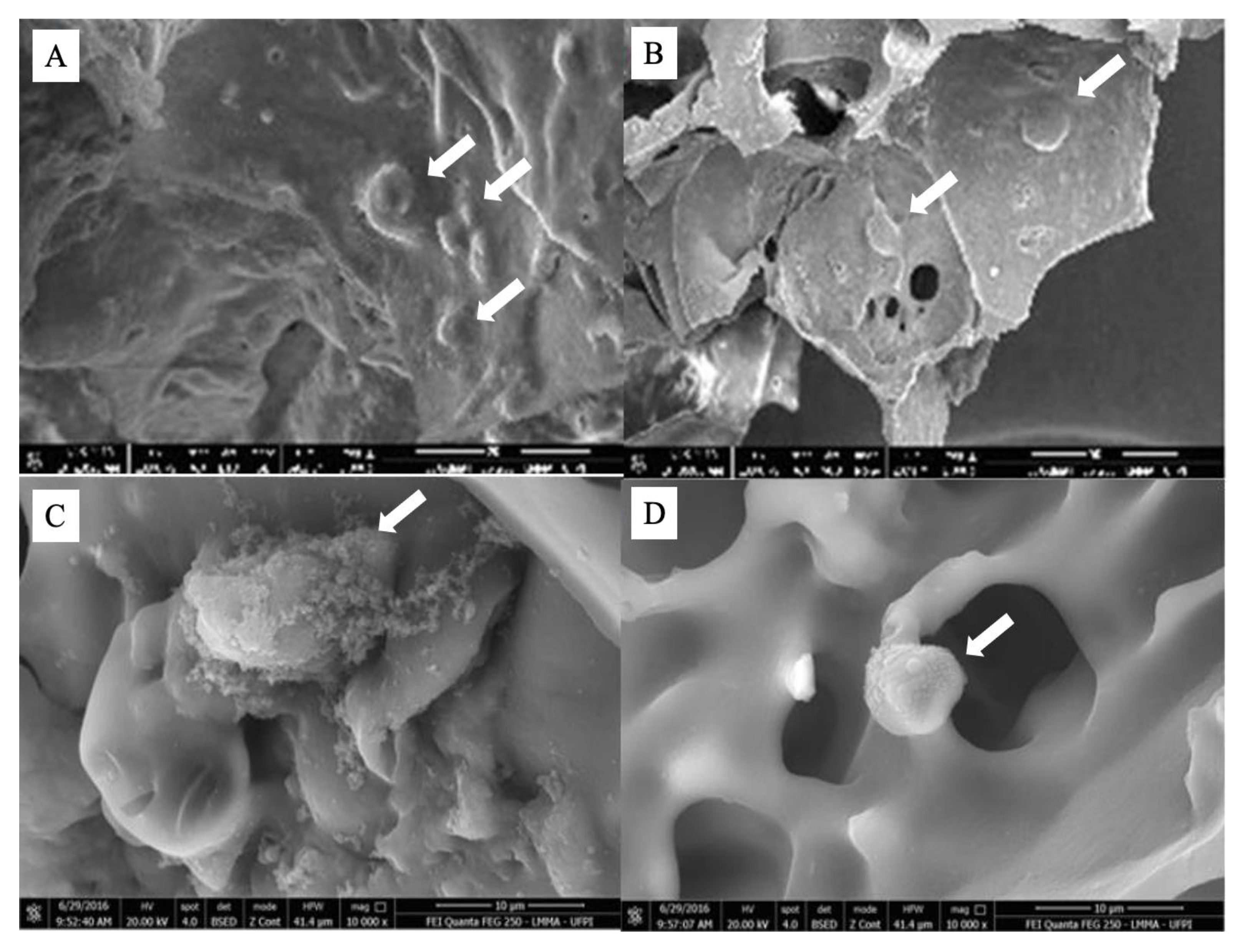
2.4. Stem Cells Adhesion on Scaffold
3. Materials and Methods
3.1. Materials
3.2. Preparation of Phthalate Cashew Gum
3.3. Preparation of Phthalate Cashew Gum/Chitosan Scaffolds (PCG-CH)
3.4. Polymers and Scaffold Characterization
3.4.1. Fourier Transform Infrared Spectroscopy (FTIR)
3.4.2. X-ray Diffraction (XRD)
3.4.3. Thermal Analysis
3.4.4. Swelling Behavior of Scaffolds
3.4.5. Scanning Electron Microscopy (SEM)
3.5. Obtaining and Characterization of Human Dental Pulp Mesenchymal Stem Cells
3.5.1. Human Dental Pulp Stem Cells Isolation and Expansion
3.5.2. Morphological Characteristics, Adhesion Capacity, and Cellular Plasticity
3.5.3. Immunophenotypic Profile of Cultured Cells
3.6. Biocompatibility Assessment of Polymers and Scaffold
3.6.1. CH, PCG, and Scaffold Cytotoxicity on Stem Cells
3.6.2. Macrophages Phagocytic Capacity in the Presence of Scaffold
3.6.3. Evaluation of Stem Cells Adhesion on Scaffold
3.7. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Z.; Feng, Y.; Wang, L.; Liu, D.; Qin, C.; Shi, Y. A review of preparation methods of porous skin tissue engineering scaffolds. Mater. Today Commun. 2022, 32, 104109. [Google Scholar] [CrossRef]
- Khan, S.; Ul-Islam, M.; Ullah, M.W.; Zhu, Y.; Narayanan, K.B.; Han, S.S.; Park, J.K. Fabrication strategies and biomedical applications of three-dimensional bacterial cellulose-based scaffolds: A review. Int. J. Biol. Macromol. 2022, 209, 9–30. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Li, W.; Liu, S.; Li, Y.; Chen, Y.; Dan, N.; Dan, W.; Zhu, M. Fabrication of high-strength, flexible, porous collagen-based scaffolds to promote tissue regeneration. Mater. Today Bio 2022, 16, 100376. [Google Scholar] [CrossRef]
- Singh, P.; Maparu, A.K.; Shah, S.; Rai, B.; Sivakumar, S. Biomimetic algal polysaccharide coated 3D nanofibrous scaffolds promote skin extracellular matrix formation. Mater. Sci. Eng. C 2020, 119, 111580. [Google Scholar] [CrossRef] [PubMed]
- Dinoro, J.; Maher, M.; Talebian, S.; Jafarkhani, M.; Mehrali, M.; Orive, G.; Foroughi, J.; Lord, M.S.; Dolatshahi-Pirouz, A. Sulfated polysaccharide-based scaffolds for orthopaedic tissue engineering. Biomaterials 2019, 214, 119214. [Google Scholar] [CrossRef]
- Nikolova, M.P.; Chavali, M.S. Recent advances in biomaterials for 3D scaffolds: A review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Xu, J.; Pan, X.; Ding, Z.; Xie, H.; Wang, X.; Xie, H. Advances of adipose-derived mesenchymal stem cells-based biomaterial scaffolds for oral and maxillofacial tissue engineering. Bioact. Mater. 2021, 6, 2467–2478. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, H.; Wei, H.; Cheng, H.; Cai, J.; Chen, X.; Xia, L.; Wang, H.; Chai, R. Scaffolds with anisotropic structure for neural tissue engineering. Eng. Regen. 2022, 3, 154–162. [Google Scholar] [CrossRef]
- Porrelli, D.; Gruppuso, M.; Vecchies, F.; Marsich, E.; Turco, G. Alginate bone scaffolds coated with a bioactive lactose modified chitosan for human dental pulp stem cells proliferation and differentiation. Carbohydr. Polym. 2021, 273, 118610. [Google Scholar] [CrossRef]
- Dissanayaka, W.L.; Zhang, C. Scaffold-based and Scaffold-free Strategies in Dental Pulp Regeneration. J. Endod. 2020, 46, S81–S89. [Google Scholar] [CrossRef]
- Ghandforoushan, P.; Hanaee, J.; Aghazadeh, Z.; Samiei, M.; Navali, A.M.; Khatibi, A.; Davaran, S. Novel nanocomposite scaffold based on gelatin/PLGA-PEG-PLGA hydrogels embedded with TGF-β1 for chondrogenic differentiation of human dental pulp stem cells in vitro. Int. J. Biol. Macromol. 2022, 201, 270–287. [Google Scholar] [CrossRef]
- Li, Z.; Ramay, H.R.; Hauch, K.D.; Xiao, D.; Zhang, M. Chitosan–alginate hybrid scaffolds for bone tissue engineering. Biomaterials 2005, 26, 3919–3928. [Google Scholar] [CrossRef]
- Samiei, M.; Abdolahinia, E.D.; Fathi, M.; Barar, J.; Omidi, Y. Chitosan-based bioactive hydrogels for osteogenic differentiation of dental pulp stem cells. J. Drug Deliv. Sci. Technol. 2022, 73, 103478. [Google Scholar] [CrossRef]
- Suh, J.-K.F.; Matthew, H.W.T. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: A review. Biomaterials 2000, 21, 2589–2598. [Google Scholar] [CrossRef] [PubMed]
- Hamedi, H.; Moradi, S.; Hudson, S.M.; Tonelli, A.E.; King, M.W. Chitosan based bioadhesives for biomedical applications: A review. Carbohydr. Polym. 2022, 282, 119100. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Déru, L.; David, G.; Auvergne, R. Chitosan chemistry review for living organisms encapsulation. Carbohydr. Polym. 2022, 295, 119877. [Google Scholar] [CrossRef]
- de Souza, R.F.B.; de Souza, F.C.B.; Thorpe, A.; Mantovani, D.; Popat, K.C.; Moraes, A.M. Phosphorylation of chitosan to improve osteoinduction of chitosan/xanthan-based scaffolds for periosteal tissue engineering. Int. J. Biol. Macromol. 2020, 143, 619–632. [Google Scholar] [CrossRef]
- Sarwar, M.S.; Huang, Q.; Ghaffar, A.; Abid, M.A.; Zafar, M.S.; Khurshid, Z.; Latif, M. A Smart Drug Delivery System Based on Biodegradable Chitosan/Poly(allylamine hydrochloride) Blend Films. Pharmaceutics 2020, 12, 131. [Google Scholar] [CrossRef]
- Park, W.H.; Jeong, L.; Yoo, D.I.; Hudson, S. Effect of chitosan on morphology and conformation of electrospun silk fibroin nanofibers. Polymer 2004, 45, 7151–7157. [Google Scholar] [CrossRef]
- Tan, W.; Krishnaraj, R.; Desai, T.A. Evaluation of Nanostructured Composite Collagen–Chitosan Matrices for Tissue Engineering. Tissue Eng. 2001, 7, 203–210. [Google Scholar] [CrossRef]
- Teng, S.-H.; Wang, P.; Kim, H.-E. Blend fibers of chitosan–agarose by electrospinning. Mater. Lett. 2009, 63, 2510–2512. [Google Scholar] [CrossRef]
- Duda, S.; Dreyer, L.; Behrens, P.; Wienecke, S.; Chakradeo, T.; Glasmacher, B.; Haastert-Talini, K. Outer Electrospun Polycaprolactone Shell Induces Massive Foreign Body Reaction and Impairs Axonal Regeneration through 3D Multichannel Chitosan Nerve Guides. BioMed Res. Int. 2014, 2014, 835269. [Google Scholar] [CrossRef] [PubMed]
- Frohbergh, M.E.; Katsman, A.; Botta, G.P.; Lazarovici, P.; Schauer, C.L.; Wegst, U.G.; Lelkes, P.I. Electrospun hydroxyapatite-containing chitosan nanofibers crosslinked with genipin for bone tissue engineering. Biomaterials 2012, 33, 9167–9178. [Google Scholar] [CrossRef]
- Sivakumar, P.M.; Yetisgin, A.A.; Sahin, S.B.; Demir, E.; Cetinel, S. Bone tissue engineering: Anionic polysaccharides as promising scaffolds. Carbohydr. Polym. 2022, 283, 119142. [Google Scholar] [CrossRef]
- Manzoor, A.; Dar, A.H.; Pandey, V.K.; Shams, R.; Khan, S.; Panesar, P.S.; Kennedy, J.F.; Fayaz, U.; Khan, S.A. Recent insights into polysaccharide-based hydrogels and their potential applications in food sector: A review. Int. J. Biol. Macromol. 2022, 213, 987–1006. [Google Scholar] [CrossRef] [PubMed]
- Nosrati, H.; Khodaei, M.; Alizadeh, Z.; Banitalebi-Dehkordi, M. Cationic, anionic and neutral polysaccharides for skin tissue engineering and wound healing applications. Int. J. Biol. Macromol. 2021, 192, 298–322. [Google Scholar] [CrossRef]
- Bal, T.; Yadav, S.K.; Rai, N.; Swain, S.; Shambhavi; Garg, S.; Sen, G. Invitro evaluations of free radical assisted microwave irradiated polyacrylamide grafted cashew gum (CG) biocompatible graft copolymer (CG-g-PAM) as effective polymeric scaffold. J. Drug Deliv. Sci. Technol. 2020, 56, 101572. [Google Scholar] [CrossRef]
- Ferreira, C.R.D.N.; Ramos, E.L.d.L.; Araujo, L.F.S.; Sousa, L.M.d.S.; Feitosa, J.P.A.; Cunha, A.F.; Oliveira, M.B.; Mano, J.F.; Maciel, J.D.S. Synthesis and characterization of scaffolds produced under mild conditions based on oxidized cashew gums and carboxyethyl chitosan. Int. J. Biol. Macromol. 2021, 176, 26–36. [Google Scholar] [CrossRef] [PubMed]
- de Paula, R.C.; Heatley, F.; Budd, P.M. Characterization of Anacardium occidentale exudate polysaccharide. Polym. Int. 1998, 45, 27–35. [Google Scholar] [CrossRef]
- Gyedu-Akoto, E.; Oduro, I.; Amoah, F.M.; Oldham, J.H.; Ellis, W.O.; Opoku-Ameyaw, K.; Hakeem, R.B. Physico-chemical properties of cashew tree gum. Afr. J. Food Sci. 2008, 2, 60–64. [Google Scholar]
- Abreu, F.O.M.S.; de Oliveira, E.F.; Paula, H.C.B.; de Paula, R.C.M. Chitosan/cashew gum nanogels for essential oil encapsulation. Carbohydr. Polym. 2012, 89, 1277–1282. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Moin, A.; Shruthi, R.; Ahmed, A.; Shivakumar, H.G. Cashew Gum a Versatile Hydrophyllic Polymer: A Review. Curr. Drug Ther. 2012, 7, 2–12. [Google Scholar] [CrossRef]
- Ribeiro, A.J.; de Souza, F.R.L.; Bezerra, J.M.; Oliveira, C.; Nadvorny, D.; Soares, M.F.D.L.R.; Nunes, L.C.; Silva-Filho, E.C.; Veiga, F.; Sobrinho, J.L.S. Gums’ based delivery systems: Review on cashew gum and its derivatives. Carbohydr. Polym. 2016, 147, 188–200. [Google Scholar] [CrossRef]
- Pinto, A.M.; Santos, T.M.; Caceres, C.A.; Lima, J.R.; Ito, E.N.; Azeredo, H.M. Starch-cashew tree gum nanocomposite films and their application for coating cashew nuts. LWT 2015, 62, 549–554. [Google Scholar] [CrossRef]
- Silva, S.M.F.; Ribeiro, H.L.; Mattos, A.L.A.; Borges, M.D.F.; Rosa, M.D.F.; de Azeredo, H.M.C. Films from cashew byproducts: Cashew gum and bacterial cellulose from cashew apple juice. J. Food Sci. Technol. 2020, 58, 1979–1986. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.C.D.J.; de Araújo, A.R.; Quelemes, P.V.; Nadvorny, D.; Soares-Sobrinho, J.L.; Leite, J.R.S.D.A.; da Silva-Filho, E.C.; da Silva, D.A. Solvent-free production of phthalated cashew gum for green synthesis of antimicrobial silver nanoparticles. Carbohydr. Polym. 2019, 213, 176–183. [Google Scholar] [CrossRef]
- de Jesus Oliveira, A.C.; Chaves, L.L.; Ribeiro, F.D.O.S.; de Lima, L.R.M.; Oliveira, T.C.; García-Villén, F.; Viseras, C.; de Paula, R.C.; Rolim-Neto, P.J.; Hallwass, F.; et al. Microwave-initiated rapid synthesis of phthalated cashew gum for drug delivery systems. Carbohydr. Polym. 2020, 254, 117226. [Google Scholar] [CrossRef]
- Lustosa, A.K.M.F.; Oliveira, A.C.D.J.; Quelemes, P.V.; Plácido, A.; Da Silva, F.V.; Oliveira, I.S.; De Almeida, M.P.; Amorim, A.D.G.N.; Delerue-Matos, C.; Oliveira, R.D.C.M.D.; et al. In Situ Synthesis of Silver Nanoparticles in a Hydrogel of Carboxymethyl Cellulose with Phthalated-Cashew Gum as a Promising Antibacterial and Healing Agent. Int. J. Mol. Sci. 2017, 18, 2399. [Google Scholar] [CrossRef]
- de Oliveira, T.C.; Oliveira, A.C.D.J.; Patriota, Y.B.G.; Chaves, L.L.; Ribeiro, F.d.O.S.; de Paula, R.C.; Silva-Filho, E.C.; da Silva, D.A.; Soares, M.F.D.L.R.; Soares-Sobrinho, J.L. Eco-friendly synthesis of phthalate angico gum towards nanoparticles engineering using Quality by Design (QbD) approach. Int. J. Biol. Macromol. 2021, 190, 801–809. [Google Scholar] [CrossRef]
- Yang, Q.; Peng, J.; Xiao, H.; Xu, X.; Qian, Z. Polysaccharide hydrogels: Functionalization, construction and served as scaffold for tissue engineering. Carbohydr. Polym. 2021, 278, 118952. [Google Scholar] [CrossRef]
- Paim, A.; Braghirolli, D.; Cardozo, N.; Pranke, P.; Tessaro, I.C. Human dental pulp stem cell adhesion and detachment in polycaprolactone electrospun scaffolds under direct perfusion. Braz. J. Med. Biol. Res. 2018, 51, e6754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majore, I.; Moretti, P.; Hass, R.; Kasper, C. Identification of subpopulations in mesenchymal stem cell-like cultures from human umbilical cord. Cell Commun. Signal. 2009, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Tien, N.D.; Geng, T.; Heyward, C.A.; Reseland, J.E.; Lyngstadaas, S.P.; Blaker, J.J.; Haugen, H.J. Solution blow spinning of highly deacetylated chitosan nanofiber scaffolds for dermal wound healing. Biomater. Adv. 2022, 137, 212871. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.D.L.V.; Oliveira, A.C.D.J.; Patriota, Y.B.G.; Ribeiro, A.J.; Veiga, F.; Hallwass, F.; Silva-Filho, E.C.; da Silva, D.A.; Soares, M.F.D.L.R.; Wanderley, A.G.; et al. Solvent-free synthesis of acetylated cashew gum for oral delivery system of insulin. Carbohydr. Polym. 2018, 207, 601–608. [Google Scholar] [CrossRef]
- Braz, E.M.D.A.; e Silva, S.C.C.C.; da Silva, D.A.; Carvalho, F.A.D.A.; Barreto, H.M.; Júnior, L.D.S.S.; Filho, E.C.D.S. Modified chitosan-based bioactive material for antimicrobial application: Synthesis and characterization. Int. J. Biol. Macromol. 2018, 117, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Mirmusavi, M.H.; Ahmadian, M.; Karbasi, S. Polycaprolactone-chitosan/multi-walled carbon nanotube: A highly strengthened electrospun nanocomposite scaffold for cartilage tissue engineering. Int. J. Biol. Macromol. 2022, 209, 1801–1814. [Google Scholar] [CrossRef]
- Furuya, D.C.; Costa, S.A.D.; Oliveira, R.C.D.; Ferraz, H.G.; Pessoa, A.; Costa, S.M.D. Fibers obtained from alginate, chitosan and hybrid used in the development of scaffolds. Mater. Res. 2017, 20, 377–386. [Google Scholar] [CrossRef]
- Sadeghianmaryan, A.; Naghieh, S.; Yazdanpanah, Z.; Sardroud, H.A.; Sharma, N.; Wilson, L.D.; Chen, X. Fabrication of chitosan/alginate/hydroxyapatite hybrid scaffolds using 3D printing and impregnating techniques for potential cartilage regeneration. Int. J. Biol. Macromol. 2022, 204, 62–75. [Google Scholar] [CrossRef]
- de Carvalho, Y.K.; Argôlo-Neto, N.M.; Ambrósio, C.E.; Oliveira, L.D.J.D.; Rocha, A.R.D.; Silva, J.B.D.; Carvalho, M.A.M.D.; Alves, F.R. Isolation, expansion and differentiation of cellular progenitors obtained from dental pulp of agouti (Dasyprocta prymnolopha Wagler, 1831). Pesqui. Veterinária Bras. 2015, 35, 590–598. [Google Scholar] [CrossRef]
- Huang, G.T.-J.; Gronthos, S.; Shi, S. Mesenchymal Stem Cells Derived from Dental Tissues vs. Those from Other Sources: Their Biology and Role in Regenerative Medicine. J. Dent. Res. 2009, 88, 792–806. [Google Scholar] [CrossRef] [PubMed]
- Malekfar, A.; Valli, K.S.; Kanafi, M.M.; Bhonde, R.R. Isolation and Characterization of Human Dental Pulp Stem Cells from Cryopreserved Pulp Tissues Obtained from Teeth with Irreversible Pulpitis. J. Endod. 2016, 42, 76–81. [Google Scholar] [CrossRef]
- Lei, M.; Li, K.; Li, B.; Gao, L.-N.; Chen, F.-M.; Jin, Y. Mesenchymal stem cell characteristics of dental pulp and periodontal ligament stem cells after in vivo transplantation. Biomaterials 2014, 35, 6332–6343. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Yamassaki, F.; Lenzi, R.; Campestrini, L.; Bovo, F.; Seyfried, M.; Soldera-Silva, A.; Stevan-Hancke, F.; Zawadzki-Baggio, S.; Pettolino, F.; Bacic, A.; et al. Effect of the native polysaccharide of cashew-nut tree gum exudate on murine peritoneal macrophage modulatory activities. Carbohydr. Polym. 2015, 125, 241–248. [Google Scholar] [CrossRef]
- Galdino, A.G.S.; Oliveira, E.M.; Filippin-Monteiro, F.B.; Zavaglia, C.A.C. Análise de ensaios in vitro do compósito de 50% HA-50% TiO2 fabricados pelo método da esponja polimérica. Cerâmica 2014, 60, 586–593. [Google Scholar] [CrossRef]
- Bolson, J.; Schossler, J.E.; Ornes, R.C.; Mottin, V.; Alberti, T. Clinical, radiological, macroscopical and histological analysis of domestic quail (Coturnix japonica) humerus submitted to implant of polyurethane from castor oil polymer (Ricinnus communis). Ciência Rural 2005, 35, 1123–1130. [Google Scholar] [CrossRef]
- Tamai, N.; Myoui, A.; Tomita, T.; Nakase, T.; Tanaka, J.; Ochi, T.; Yoshikawa, H. Novel hydroxyapatite ceramics with an interconnective porous structure exhibit superior osteoconduction in vivo. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. 2002, 59, 110–117. [Google Scholar] [CrossRef]
- Bispo, V.M.; Mansur, A.; Barbosa-Stancioli, E.; Mansur, H.S. Biocompatibility of Nanostructured Chitosan/Poly(Vinyl Alcohol) Blends Chemically Crosslinked with Genipin for Biomedical Applications. J. Biomed. Nanotechnol. 2010, 6, 166–175. [Google Scholar] [CrossRef]
- Balic, A.; Aguila, H.L.; Caimano, M.J.; Francone, V.P.; Mina, M. Characterization of stem and progenitor cells in the dental pulp of erupted and unerupted murine molars. Bone 2010, 46, 1639–1651. [Google Scholar] [CrossRef]
- Marcomini, R.F.; Souza, D.M.P. Microstructural characterization of ceramic materials using the image digital processing software Image J. Cerâmica 2011, 57, 100–105. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leite, Y.K.d.C.; Oliveira, A.C.d.J.; Quelemes, P.V.; Neto, N.M.A.; Carvalho, C.E.S.d.; Soares Rodrigues, H.W.; Alves, M.M.d.M.; Carvalho, F.A.d.A.; Arcanjo, D.D.R.; Silva-Filho, E.C.d.; et al. Novel Scaffold Based on Chitosan Hydrogels/Phthalated Cashew Gum for Supporting Human Dental Pulp Stem Cells. Pharmaceuticals 2023, 16, 266. https://doi.org/10.3390/ph16020266
Leite YKdC, Oliveira ACdJ, Quelemes PV, Neto NMA, Carvalho CESd, Soares Rodrigues HW, Alves MMdM, Carvalho FAdA, Arcanjo DDR, Silva-Filho ECd, et al. Novel Scaffold Based on Chitosan Hydrogels/Phthalated Cashew Gum for Supporting Human Dental Pulp Stem Cells. Pharmaceuticals. 2023; 16(2):266. https://doi.org/10.3390/ph16020266
Chicago/Turabian StyleLeite, Yulla Klinger de Carvalho, Antônia Carla de Jesus Oliveira, Patrick Veras Quelemes, Napoleão Martins Argolo Neto, Camila Ernanda Sousa de Carvalho, Huanna Waleska Soares Rodrigues, Michel Muálem de Moraes Alves, Fernando Aécio de Amorim Carvalho, Daniel Dias Rufino Arcanjo, Edson Cavalcanti da Silva-Filho, and et al. 2023. "Novel Scaffold Based on Chitosan Hydrogels/Phthalated Cashew Gum for Supporting Human Dental Pulp Stem Cells" Pharmaceuticals 16, no. 2: 266. https://doi.org/10.3390/ph16020266
APA StyleLeite, Y. K. d. C., Oliveira, A. C. d. J., Quelemes, P. V., Neto, N. M. A., Carvalho, C. E. S. d., Soares Rodrigues, H. W., Alves, M. M. d. M., Carvalho, F. A. d. A., Arcanjo, D. D. R., Silva-Filho, E. C. d., Durazzo, A., Lucarini, M., Carvalho, M. A. M. d., Silva, D. A. d., & Leite, J. R. d. S. d. A. (2023). Novel Scaffold Based on Chitosan Hydrogels/Phthalated Cashew Gum for Supporting Human Dental Pulp Stem Cells. Pharmaceuticals, 16(2), 266. https://doi.org/10.3390/ph16020266










