In Vitro and In Silico Study on the Impact of Chlorogenic Acid in Colorectal Cancer Cells: Proliferation, Apoptosis, and Interaction with β-Catenin and LRP6
Abstract
:1. Introduction
2. Results
2.1. CGA Treatment Affects Cell Viability
2.2. CGA Induces Mitochondrial Reactive Oxygen Species (ROS) Production
2.3. CGA Produces DNA Fragmentation Preferentially in SW480 Cells
2.4. Molecular Docking
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Treatment Outline
4.4. Cell Viability
4.5. Mitochondrial ROS Production
4.6. DNA Content
4.7. Molecular Docking
4.7.1. Ligand Selection and Preparation
4.7.2. Protein Preparation
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization Cancer Today. Available online: https://gco.iarc.fr/ (accessed on 30 November 2022).
- Cheng, X.; Xu, X.; Chen, D.; Zhao, F.; Wang, W. Therapeutic Potential of Targeting the Wnt/β-Catenin Signaling Pathway in Colorectal Cancer. Biomed. Pharmacother. 2019, 110, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Wild, C.P.; Weiderpass, E.S.B. (Eds.) World Cancer Report: Cancer Research for Cancer Prevention; International Agency for Research on Cancer, 2020. Available online: http://publications.iarc.fr/586 (accessed on 23 January 2023).
- Chen, H.J.; Hsu, L.S.; Shia, Y.T.; Lin, M.W.; Lin, C.M. The β-Catenin/TCF Complex as a Novel Target of Resveratrol in the Wnt/β-Catenin Signaling Pathway. Biochem. Pharmacol. 2012, 84, 1143–1153. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, S.; Ohishi, T.; Miyoshi, N.; Oishi, Y. Anti-Cancer Effects of Green Tea Epigallocatchin-3-Gallate and Coffee Chlorogenic Acid. Molecules 2020, 25, 4553. [Google Scholar] [CrossRef]
- Ekbatan, S.S.; Li, X.Q.; Ghorbani, M.; Azadi, B.; Kubow, S. Chlorogenic Acid and Its Microbial Metabolites Exert Anti-Proliferative Effects, S-Phase Cell-Cycle Arrest and Apoptosis in Human Colon Cancer Caco-2 Cells. Int. J. Mol. Sci. 2018, 19, 723. [Google Scholar] [CrossRef]
- Villota, H.; Moreno-Ceballos, M.; Santa-González, G.A.; Uribe, D.; Castañeda, I.C.H.; Preciado, L.M.; Pedroza-Díaz, J. Biological Impact of Phenolic Compounds from Coffee on Colorectal Cancer. Pharmaceuticals 2021, 14, 761. [Google Scholar] [CrossRef]
- Villota, H.; Santa-González, G.A.; Uribe, D.; Henao, I.C.; Arroyave-Ospina, J.C.; Barrera-Causil, C.J.; Pedroza-Díaz, J. Modulatory Effect of Chlorogenic Acid and Coffee Extracts on Wnt/β-Catenin Pathway in Colorectal Cancer Cells. Nutrients 2022, 14, 4880. [Google Scholar] [CrossRef]
- Yan, Y.; Li, J.; Han, J.; Hou, N.; Song, Y.; Dong, L. Chlorogenic Acid Enhances the Effects of 5-Fluorouracil in Human Hepatocellular Carcinoma Cells through the Inhibition of Extracellular Signal-Regulated Kinases. Anticancer. Drugs 2015, 26, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Liu, N.; Hou, N.; Dong, L.; Li, J. Chlorogenic Acid Inhibits Hepatocellular Carcinoma in vitro and in vivo. J. Nutr. Biochem. 2017, 46, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Feng, Y.; Li, Y.; Hu, Y.; Zhang, Q.; Huang, Y.; Shi, K.; Ran, C.; Hou, J.; Zhou, G.; et al. Chlorogenic Acid Decreases Malignant Characteristics of Hepatocellular Carcinoma Cells by Inhibiting DNMT1 Expression. Front. Pharmacol. 2020, 11, 867. [Google Scholar] [CrossRef]
- Zeng, A.; Liang, X.; Zhu, S.; Liu, C.; Wang, S.; Zhang, Q.; Zhao, J.; Song, L. Chlorogenic Acid Induces Apoptosis, Inhibits Metastasis and Improves Antitumor Immunity in Breast Cancer via the NF-ΚB Signaling Pathway. Oncol. Rep. 2021, 45, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Schuster, C.; Wolpert, N.; Moustaid-Moussa, N.; Gollahon, L.S. Combinatorial Effects of the Natural Products Arctigenin, Chlorogenic Acid, and Cinnamaldehyde Commit Oxidation Assassination on Breast Cancer Cells. Antioxidants 2022, 11, 591. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K.; Izawa, Y.; Onodera, D.; Tagami, M. Chlorogenic Acid Regulates Apoptosis and Stem Cell Marker-Related Gene Expression in A549 Human Lung Cancer Cells. Mol. Cell. Biochem. 2018, 441, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Du, H.; Chen, P. Chlorogenic Acid Inhibits the Proliferation of Human Lung Cancer A549 Cell Lines by Targeting Annexin A2 in vitrso and in vivo. Biomed. Pharm. 2020, 131, 110673. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.S.; Liu, C.W.; Ma, Y.S.; Weng, S.W.; Tang, N.Y.; Wu, S.H.; Ji, B.C.; Ma, C.Y.; Ko, Y.C.; Funayama, S.; et al. Chlorogenic Acid Induces Apoptotic Cell Death in U937 Leukemia Cells through Caspase-and Mitochondria-Dependent Pathways. In Vivo 2012, 26, 971–978. [Google Scholar]
- Liu, Y.J.; Zhou, C.Y.; Qiu, C.H.; Lu, X.M.; Wang, Y.T. Chlorogenic Acid Induced Apoptosis and Inhibition of Proliferation in Human Acute Promyelocytic Leukemia HL-60 Cells. Mol. Med. Rep. 2013, 8, 1106–1110. [Google Scholar] [CrossRef]
- Sapio, L.; Salzillo, A.; Illiano, M.; Ragone, A.; Spina, A.; Chiosi, E.; Pacifico, S.; Catauro, M.; Naviglio, S. Chlorogenic Acid Activates ERK1/2 and Inhibits Proliferation of Osteosarcoma Cells. J. Cell. Pharmacol. 2020, 235, 3741–3752. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, J.; Xie, Z.; Rao, J.; Xu, G.; Huang, K.; Li, W.; Yin, Z. Chlorogenic Acid Inhibits Proliferation and Induces Apoptosis in A498 Human Kidney Cancer Cells via Inactivating PI3K/Akt/MTOR Signalling Pathway. J. Pharm. Pharm. 2019, 71, 1100–1109. [Google Scholar] [CrossRef]
- Disoma, C.; Zhou, Y.; Li, S.; Peng, J.; Xia, Z. Wnt/β-Catenin Signaling in Colorectal Cancer: Is Therapeutic Targeting Even Possible? Biochimie 2022, 195, 39–53. [Google Scholar] [CrossRef]
- Pai, S.G.; Carneiro, B.A.; Mota, J.M.; Costa, R.; Leite, C.A.; Barroso-Sousa, R.; Kaplan, J.B.; Chae, Y.K.; Giles, F.J. Wnt/Beta-Catenin Pathway: Modulating Anticancer Immune Response. J. Hematol. Oncol. 2017, 10, 101. [Google Scholar] [CrossRef]
- Garg, A.; Kant, K.; Roy, K.K.; Sahoo, A.; Malakar, C.C.; Gupta, S. Docking-Based Evaluation against Human Tankyrase-1 and Tankyrase-2 Enzyme. Mater. Today Proc. 2022, 57, 300–306. [Google Scholar] [CrossRef]
- Enayatkhani, M.; Salimi, M.; Azadmanesh, K.; Teimoori-Toolabi, L. In-Silico Identification of New Inhibitors for Low-Density Lipoprotein Receptor-Related Protein6 (LRP6). J. Biomol. Struct. Dyn. 2022, 40, 4440–4450. [Google Scholar] [CrossRef] [PubMed]
- Rismani, E.; Rahimi, H.; Arab, S.S.; Azadmanesh, K.; Karimipoor, M.; Teimoori-Toolabi, L. Computationally Design of Inhibitory Peptides against Wnt Signaling Pathway: In Silico Insight on Complex of DKK1 and LRP6. Int. J. Pept. Res. Ther. 2018, 24, 49–60. [Google Scholar] [CrossRef]
- Lu, W.; Lin, C.; Roberts, M.J.; Waud, W.R.; Piazza, G.A.; Li, Y. Niclosamide Suppresses Cancer Cell Growth by Inducing Wnt Co-Receptor LRP6 Degradation and Inhibiting the Wnt/β-Catenin Pathway. PLoS ONE 2011, 6, e29290. [Google Scholar] [CrossRef] [PubMed]
- Suliman, M.A.; Zhang, Z.; Na, H.; Ribeiro, A.L.L.; Zhang, Y.; Niang, B.; Hamid, A.S.; Zhang, H.; Xu, L.; Zuo, Y. Niclosamide Inhibits Colon Cancer Progression through Downregulation of the Notch Pathway and Upregulation of the Tumor Suppressor MiR-200 Family. Int. J. Mol. Med. 2016, 38, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Ye, T.; Liu, Z.; Fang, A.; Luo, Y.; Wei, W.; Li, Y.; Li, Y.; Zeng, A.; Deng, Y.; et al. Niclosamide Induces Colorectal Cancer Apoptosis, Impairs Metastasis and Reduces Immunosuppressive Cells in vivo. RSC Adv. 2016, 6, 106019–106030. [Google Scholar] [CrossRef]
- Low, J.L.; Du, W.; Gocha, T.; Oguz, G.; Zhang, X.; Chen, M.W.; Masirevic, S.; Yim, D.G.R.; Tan, I.B.H.; Ramasamy, A.; et al. Molecular Docking-Aided Identification of Small Molecule Inhibitors Targeting β-Catenin-TCF4 Interaction. iScience 2021, 24, 102544. [Google Scholar] [CrossRef]
- Gonsalves, F.C.; Klein, K.; Carson, B.B.; Katz, S.; Ekas, L.A.; Evans, S.; Nagourney, R.; Cardozo, T.; Brown, A.M.C.; Das Gupta, R. An RNAi-Based Chemical Genetic Screen Identifies Three Small-Molecule Inhibitors of the Wnt/Wingless Signaling Pathway. Proc. Natl. Acad. Sci. USA 2011, 108, 5954–5963. [Google Scholar] [CrossRef]
- Koelman, E.M.R.; Yeste-Vázquez, A.; Grossmann, T.N. Targeting the Interaction of β-Catenin and TCF/LEF Transcription Factors to Inhibit Oncogenic Wnt Signaling. Bioorg. Med. Chem. 2022, 70, 116920. [Google Scholar] [CrossRef]
- Cheltsov, A.; Nomura, N.; Yenugonda, V.M.; Roper, J.; Mukthavaram, R.; Jiang, P.; Her, N.G.; Babic, I.; Kesari, S.; Nurmemmedov, E. Allosteric Inhibitor of β-Catenin Selectively Targets Oncogenic Wnt Signaling in Colon Cancer. Sci. Rep. 2020, 10, 8096. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, W.; Evans, P.M.; Chen, X.; He, X.; Liu, C. Adenomatous Polyposis Coli (APC) Differentially Regulates β-Catenin Phosphorylation and Ubiquitination in Colon Cancer Cells. J. Biol. Chem. 2006, 281, 17751–17757. [Google Scholar] [CrossRef]
- Chandra, S.H.V.; Wacker, I.; Appelt, U.K.; Behrens, J.; Schneikert, J. A Common Role for Various Human Truncated Adenomatous Polyposis Coli Isoforms in the Control of Beta-Catenin Activity and Cell Proliferation. PLoS ONE 2012, 7, e34479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novellasdemunt, L.; Foglizzo, V.; Cuadrado, L.; Antas, P.; Kucharska, A.; Encheva, V.; Snijders, A.P.; Li, V.S.W. USP7 Is a Tumor-Specific WNT Activator for APC-Mutated Colorectal Cancer by Mediating β-Catenin Deubiquitination. Cell Rep. 2017, 21, 612–627. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, S.; Maresca, M.; Yumashev, A.V.; Choopani, R.; Hajimehdipoor, H. The Most Competent Plant-derived Natural Products for Targeting Apoptosis in Cancer Therapy. Biomolecules 2021, 11, 534. [Google Scholar] [CrossRef] [PubMed]
- Lowe, S.W.; Lin, A.W. Apoptosis in Cancer. Carcinogenesis 2000, 21, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wang, L.; He, Y.; Wei, M.; Yan, H.; Zhu, H. Chlorogenic Acid Promotes Osteogenic Differentiation of Human Dental Pulp Stem Cells through Wnt Signaling. Stem. Cells Dev. 2021, 30, 641–650. [Google Scholar] [CrossRef]
- Liu, M.; Qin, J.; Cong, J.; Yang, Y. Chlorogenic Acids Inhibit Adipogenesis: Implications of Wnt/ β-Catenin Signaling Pathway. Int. J. Endocrinol. 2021, 2021, 2215274. [Google Scholar] [CrossRef]
- Rao, S.; Chinkwo, K.; Santhakumar, A.; Johnson, S.; Blanchard, C. Apoptosis Induction Pathway in Human Colorectal Cancer Cell Line SW480 Exposed to Cereal Phenolic Extracts. Molecules 2019, 24, 2465. [Google Scholar] [CrossRef]
- Plesca, D.; Mazumder, S.; Almasan, A. Chapter Six: DNA Damage Response and Apoptosis. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 2008; Volume 446, pp. 107–122. ISBN 9780123744647. [Google Scholar]
- Kajstura, M.; Halicka, H.D.; Pryjma, J.; Darzynkiewicz, Z. Discontinuous Fragmentation of Nuclear DNA during Apoptosis Revealed by Discrete “Sub-G1” Peaks on DNA Content Histograms. Cytom. Part A 2007, 71, 125–131. [Google Scholar] [CrossRef]
- Nicoletti, I.; Migliorati, G.; Pagliacci, M.C.; Grignani, F.; Riccardi, C. A Rapid and Simple Method for Measuring Thymocyte Apoptosis by Propidium Iodide Staining and Flow Cytometry. J. Immunol. Methods 1991, 139, 271–279. [Google Scholar] [CrossRef]
- Moreno-Ceballos, M.; Arroyave, J.C.; Cortes-Mancera, F.M.; Röthlisberger, S. Chemopreventive Effect of Coffee against Colorectal Cancer and Hepatocellular Carcinoma. Int. J. Food Prop. 2019, 22, 536–555. [Google Scholar] [CrossRef]
- Yepes, Y.; Uribe, D.; Röthlisberger, S. A Review of the Chemopreventive Effects of the Main Bioactive Compounds in Coffee in Colorectal Cancer. J. Appl. Pharm. Sci. 2021, 11, 046–054. [Google Scholar] [CrossRef]
- Ardini, M.N.; Irillo, E.C.; Atella, F.N.; Caccini, C.S. Absorption of Phenolic Acids in Humans after Coffee Consumption. J. Agric. Food Chem. 2002, 50, 5735–5741. [Google Scholar] [CrossRef] [PubMed]
- Olthof, M.R.; Hollman, P.C.H.; Katan, M.B. Human Nutrition and Metabolism Chlorogenic Acid and Caffeic Acid Are Absorbed in Humans 1. J. Nutr. 2001, 131, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.Q.; Rajadurai, P.; Abas, F.; Othman, I.; Naidu, R. Proteomic Analysis on Anti-Proliferative and Apoptosis Effects of Curcumin Analog, 1,5-Bis(4-Hydroxy-3-Methyoxyphenyl)-1,4-Pentadiene-3-One-Treated Human Glioblastoma and Neuroblastoma Cells. Front. Mol. Biosci. 2021, 8, 645856. [Google Scholar] [CrossRef]
- Tian, B.; Liu, J. Resveratrol: A Review of Plant Sources, Synthesis, Stability, Modification and Food Application. J. Sci. Food Agric. 2020, 100, 1392–1404. [Google Scholar] [CrossRef]
- ASSOCIAÇÃO BRASILEIRA DE QUÍMICA 35° CLAQ—Congresso Latinoamericano de Química 61° CBQ—Congresso Brasileiro de Química. Available online: https://www.abq.org.br/cbq/2022/trabalhos/11/875-762.html (accessed on 13 December 2022).
- Sena, L.A.; Chandel, N.S. Physiological Roles of Mitochondrial Reactive Oxygen Species. Mol. Cell 2012, 48, 158–167. [Google Scholar] [CrossRef] [PubMed]
- NavaneethaKrishnan, S.; Rosales, J.L.; Lee, K.Y. ROS-Mediated Cancer Cell Killing through Dietary Phytochemicals. Oxid. Med. Cell. Longev. 2019, 2019, 9051542. [Google Scholar] [CrossRef] [PubMed]
- Davalli, P.; Marverti, G.; Lauriola, A.; D’Arca, D. Targeting Oxidatively Induced DNA Damage Response in Cancer: Opportunities for Novel Cancer Therapies. Oxid. Med. Cell. Longev. 2018, 2018, 2389523. [Google Scholar] [CrossRef] [PubMed]
- Van Houten, B.; Santa-Gonzalez, G.A.; Camargo, M. DNA Repair after Oxidative Stress: Current Challenges. Curr. Opin. Toxicol. 2018, 7, 9–16. [Google Scholar] [CrossRef]
- Santa-Gonzalez, G.A.; Gomez-Molina, A.; Arcos-Burgos, M.; Meyer, J.N.; Camargo, M. Distinctive Adaptive Response to Repeated Exposure to Hydrogen Peroxide Associated with Upregulation of DNA Repair Genes and Cell Cycle Arrest. Redox Biol. 2016, 9, 124–133. [Google Scholar] [CrossRef]
- Lauricella, M.; Galbo, V.L.; Cernigliaro, C.; Maggio, A.; Piccionello, A.P.; Calvaruso, G.; Carlisi, D.; Emanuele, S.; Giuliano, M.; D’Anneo, A. The Anti-Cancer Effect of Mangifera indica, L. Peel Extract Is Associated to ΓH2Ax-Mediated Apoptosis in Colon Cancer Cells. Antioxidants 2019, 8, 422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramirez, V.; Arango, S.S.; Maldonado, M.E.; Uribe, D.; Aguillon, J.; Quintero, J.P.; Loango, N. Biological Activity of Passiflora edulis f. Flavicarpa Ethanolic Leaves Extract on Human Colonic Adenocarcinoma Cells. J. Appl. Pharm. Sci. 2019, 9, 64–71. [Google Scholar] [CrossRef]
- Agudelo, C.D.; Arango, S.; Cortés-Mancera, F.; Rojano, B.; Maldonado, M.E. Antiproliferative and Pro-Apoptotic Effects of Andean Berry Juice (Vaccinium Meridionale Swartz) on Human Colon Adenocarcinoma SW480 Cells. J. Med. Plants Res. 2017, 11, 393–402. [Google Scholar] [CrossRef]
- García-Gutiérrez, N.; Maldonado-Celis, M.E.; Rojas-López, M.; Loarca-Piña, G.F.; Campos-Vega, R. The Fermented Non-Digestible Fraction of Spent Coffee Grounds Induces Apoptosis in Human Colon Cancer Cells (SW480). J. Funct. Foods 2017, 30, 237–246. [Google Scholar] [CrossRef]
- Caicedo-Lopez, L.H.; Cuellar-Nuñez, M.L.; Luzardo-Ocampo, I.; Campos-Vega, R.; Lóarca-Piña, G. Colonic Metabolites from Digested Moringa Oleifera Leaves Induced HT-29 Cell Death via Apoptosis, Necrosis, and Autophagy. Int. J. Food Sci. Nutr. 2021, 72, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Han, C.; Guo, D.; Chin, Y.W.; Ding, Y.; Kinghorn, A.D.; D’Ambrosio, S.M. Selective Induction of Apoptosis of Human Oral Cancer Cell Lines by Avocado Extracts via a ROS-Mediated Mechanism. Nutr. Cancer 2009, 61, 348–356. [Google Scholar] [CrossRef]
- Raja, S.B.; Rajendiran, V.; Kasinathan, N.K.; Amrithalakshmi, A.P.; Venkatabalasubramanian, S.; Murali, M.R.; Devaraj, H.; Devaraj, S.N. Differential Cytotoxic Activity of Quercetin on Colonic Cancer Cells Depends on ROS Generation through COX-2 Expression. Food Chem. Toxicol. 2017, 106, 92–106. [Google Scholar] [CrossRef]
- Maldonado-Celis, M.E.; Bousserouel, S.; Gossé, F.; Minker, C.; Lobstein, A.; Raul, F. Differential Induction of Apoptosis by Apple Procyanidins in TRAIL-Sensitive Human Colon Tumor Cells and Derived TRAIL-Resistant Metastatic Cells. J. Cancer Mol. 2009, 5, 21–30. [Google Scholar]
- Zhang, X.; Qin, Y.; Pan, Z.; Li, M.; Liu, X.; Chen, X.; Qu, G.; Zhou, L.; Xu, M.; Zheng, Q.; et al. Cannabidiol Induces Cell Cycle Arrest and Cell Apoptosis in Human Gastric Cancer SGC-7901 Cells. Biomolecules 2019, 9, 302. [Google Scholar] [CrossRef]
- Heo, J.R.; Kim, S.M.; Hwang, K.A.; Kang, J.H.; Choi, K.C. Resveratrol Induced Reactive Oxygen Species and Endoplasmic Reticulum Stress-Mediated Apoptosis, and Cell Cycle Arrest in the A375SM Malignant Melanoma Cell Line. Int. J. Mol. Med. 2018, 42, 1427–1435. [Google Scholar] [CrossRef]
- Sritharan, S.; Sivalingam, N. Curcumin Induced Apoptosis Is Mediated through Oxidative Stress in Mutated P53 and Wild Type P53 Colon Adenocarcinoma Cell Lines. J. Biochem. Mol. Toxicol. 2021, 35, e22616. [Google Scholar] [CrossRef] [PubMed]
- Khiewkamrop, P.; Phunsomboon, P.; Richert, L.; Pekthong, D.; Srisawang, P. Epistructured Catechins, EGCG and EC Facilitate Apoptosis Induction through Targeting de Novo Lipogenesis Pathway in HepG2 Cells. Cancer Cell Int. 2018, 18, 46. [Google Scholar] [CrossRef]
- Jaganathan, S.K.; Supriyanto, E.; Mandal, M. Events Associated with Apoptotic Effect of p-Coumaric Acid in HCT-15 Colon Cancer Cells. World, J. Gastroenterol. 2013, 19, 7726–7734. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas, C.; Quesada, A.R.; Medina, M.Á. Insights on the Antitumor Effects of Kahweol on Human Breast Cancer: Decreased Survival and Increased Production of Reactive Oxygen Species and Cytotoxicity. Biochem. Biophys. Res. Commun. 2014, 447, 452–458. [Google Scholar] [CrossRef]
- Hou, N.; Liu, N.; Han, J.; Yan, Y.; Li, J. Chlorogenic Acid Induces Reactive Oxygen Species Generation and Inhibits the Viability of Human Colon Cancer Cells. Anticancer. Drugs 2017, 28, 59–65. [Google Scholar] [CrossRef]
- Murad, L.D.; Soares, N.D.C.P.; Brand, C.; Monteiro, M.C.; Teodoro, A.J. Effects of Caffeic and 5-Caffeoylquinic Acids on Cell Viability and Cellular Uptake in Human Colon Adenocarcinoma Cells. Nutr. Cancer 2015, 67, 532–542. [Google Scholar] [CrossRef]
- Gibellini, L.; Pinti, M.; Nasi, M.; de Biasi, S.; Roat, E.; Bertoncelli, L.; Cossarizza, A. Interfering with ROS Metabolism in Cancer Cells: The Potential Role of Quercetin. Cancers 2010, 2, 1288–1311. [Google Scholar] [CrossRef] [PubMed]
- Forester, S.C.; Lambert, J.D. The Role of Antioxidant versus Pro-Oxidant Effects of Green Tea Polyphenols in Cancer Prevention. Mol. Nutr. Food Res. 2011, 55, 844–854. [Google Scholar] [CrossRef]
- Sakihama, Y.; Cohen, M.F.; Grace, S.C.; Yamasaki, H. Plant Phenolic Antioxidant and Prooxidant Activities: Phenolics-Induced Oxidative Damage Mediated by Metals in Plants. Toxicology 2002, 177, 67–80. [Google Scholar] [CrossRef]
- Zhu, N.; Huang, T.C.; Yu, Y.; LaVoie, E.J.; Yang, C.S.; Ho, C.T. Identification of Oxidation Products of (-)-Epigallocatechin Gallate and (-)-Epigallocatechin with H2O2. J. Agric. Food Chem. 2000, 48, 979–981. [Google Scholar] [CrossRef]
- Malik, A.; Azam, S.; Hadi, N.; Hadi, S.M. DNA Degradation by Water Extract of Green Tea in the Presence of Copper Ions: Implications for Anticancer Properties. Phyther. Res. 2003, 17, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Catalán, M.; Olmedo, I.; Faúndez, J.; Jara, J.A. Medicinal Chemistry Targeting Mitochondria: From New Vehicles and Pharmacophore Groups to Old Drugs with Mitochondrial Activity. Int. J. Mol. Sci. 2020, 21, 8684. [Google Scholar] [CrossRef]
- del Blanquer-Rosselló, M.M.; Hernández-López, R.; Roca, P.; Oliver, J.; Valle, A. Resveratrol Induces Mitochondrial Respiration and Apoptosis in SW620 Colon Cancer Cells. Biochim. Biophys. Acta-Gen. Subj. 2017, 1861, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, D.; Eide, P.W.; Eilertsen, I.A.; Danielsen, S.A.; Eknæs, M.; Hektoen, M.; Lind, G.E.; Lothe, R.A. Epigenetic and Genetic Features of 24 Colon Cancer Cell Lines. Oncogenesis 2013, 2, e71. [Google Scholar] [CrossRef] [PubMed]
- Berg, K.C.G.; Eide, P.W.; Eilertsen, I.A.; Johannessen, B.; Bruun, J.; Danielsen, S.A.; Bjørnslett, M.; Meza-Zepeda, L.A.; Eknæs, M.; Lind, G.E.; et al. Multi-Omics of 34 Colorectal Cancer Cell Lines—A Resource for Biomedical Studies. Mol. Cancer 2017, 16, 116. [Google Scholar] [CrossRef]
- Singh, M.P.; Rai, S.; Pandey, A.; Singh, N.K.; Srivastava, S. Molecular Subtypes of Colorectal Cancer: An Emerging Therapeutic Opportunity for Personalized Medicine. Genes Dis. 2021, 8, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Fennell, L.J.; Kane, A.; Liu, C.; McKeone, D.; Fernando, W.; Su, C.; Bond, C.; Jamieson, S.; Dumenil, T.; Patch, A.M.; et al. APC Mutation Marks an Aggressive Subtype of BRAF Mutant Colorectal Cancers. Cancers 2020, 12, 1171. [Google Scholar] [CrossRef]
- Luo, F.; Poulogiannis, G.; Ye, H.; Hamoudi, R.; Arends, M.J. Synergism between K-RasVal12 and Mutant Apc Accelerates Murine Large Intestinal Tumourigenesis. Oncol. Rep. 2011, 26, 125–133. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, J.M.; Roh, Y.J.; Kim, I.W.; Hasan, T.; Choi, M.G. Enhanced Efficacy of Photodynamic Therapy by Inhibiting ABCG2 in Colon Cancers. BMC Cancer 2015, 15, 504. [Google Scholar] [CrossRef] [PubMed]
- Aires, V.; Colin, D.J.; Doreau, A.; Pietro, A.D.; Heydel, J.M.; Artur, Y.; Latruffe, N.; Delmas, D. P-Glycoprotein 1 Affects Chemoactivities of Resveratrol against Human Colorectal Cancer Cells. Nutrients 2019, 11, 7–9. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Q.; Liu, F.; Cheng, X.; Wu, X.; Wang, H.; Wu, M.; Ma, Y.; Wang, G.; Hao, H. UDP-Glucuronosyltransferase 1A Compromises Intracellular Accumulation and Anti-Cancer Effect of Tanshinone IIA in Human Colon Cancer Cells. PLoS ONE 2013, 8, e79172. [Google Scholar] [CrossRef] [PubMed]
- Landmann, H.; Proia, D.A.; He, S.; Ogawa, L.S.; Kramer, F.; Beibarth, T.; Grade, M.; Gaedcke, J.; Ghadimi, M.; Moll, U.; et al. UDP Glucuronosyltransferase 1A Expression Levels Determine the Response of Colorectal Cancer Cells to the Heat Shock Protein 90 Inhibitor Ganetespib. Cell Death Dis. 2014, 5, e1411. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Tian, Z.; Cui, Y.; Liu, Z.; Ma, X. Chlorogenic Acid: A Comprehensive Review of the Dietary Sources, Processing Effects, Bioavailability, Beneficial Properties, Mechanisms of Action, and Future Directions. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3130–3158. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Kulkarni, K.; Basu, S.; Zhang, S.; Hu, M. First Pass Metabolism via UDP-Glucuronosyltransferase: A Barrier to Oral Bioavailability of Phenolics. Pharm. Sci. 2011, 100, 3655–3681. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, Z.H.; Xia, J.; Li, X.P.; Li, K.Q.; Xiong, W.; Li, J.; Chen, D.L. 20(S)-Ginsenoside Rh2 Inhibits the Proliferation and Induces the Apoptosis of KG-1a Cells through the Wnt/β-Catenin Signaling Pathway. Oncol. Rep. 2016, 36, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.N.; Yuan, F.; Liu, J.Q.; Peng, X.R.; An, T.; Li, X.; Kong, L.M.; Qiu, M.H.; Li, Y. Physalis Peruviana-Derived 4β-Hydroxywithanolide E, a Novel Antagonist of Wnt Signaling, Inhibits Colorectal Cancer in vitro and in vivo. Molecules 2019, 24, 1146. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, K.; Velmurugan, B.; Gu, M.; Singh, R.P.; Aagarwal, R. Inhibitory Effect of Silibinin against Azoxymethane-Induced Colon Tumorigenesis in A/J Mice. Bone 2010, 16, 4595–4606. [Google Scholar] [CrossRef]
- Li, H.; Huang, J.; Yang, B.; Xiang, T.; Yin, X.; Peng, W.; Cheng, W.; Wan, J.; Luo, F.; Li, H.; et al. Mangiferin Exerts Antitumor Activity in Breast Cancer Cells by Regulating Matrix Metalloproteinases, Epithelial to Mesenchymal Transition, and β-Catenin Signaling Pathway. Toxicol. Appl. Pharmacol. 2013, 272, 180–190. [Google Scholar] [CrossRef]
- Prasad, C.P.; Rath, G.; Mathur, S.; Bhatnagar, D.; Ralhan, R. Potent Growth Suppressive Activity of Curcumin in Human Breast Cancer Cells: Modulation of Wnt/β-Catenin Signaling. Chem. Biol. Interact. 2009, 181, 263–271. [Google Scholar] [CrossRef]
- Sultan, A.S.; Khalil, M.I.M.; Sami, B.M.; Alkhuriji, A.F.; Sadek, O. Quercetin Induces Apoptosis in Triple-Negative Breast Cancer Cells via Inhibiting Fatty Acid Synthase and ß-Catenin. Int. J. Clin. Exp. Pathol. 2017, 10, 156–172. [Google Scholar]
- Mineda, A.; Nishimura, M.; Kagawa, T.; Takiguchi, E.; Kawakita, T.; Abe, A.; Irahara, M. Resveratrol Suppresses Proliferation and Induces Apoptosis of Uterine Sarcoma Cells by Inhibiting the Wnt Signaling Pathway. Exp. Ther. Med. 2019, 17, 2242–2246. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Zhu, H.; Yang, X.; Xie, F.; Peng, J.; Jiang, D.; Xie, J.; Qi, M.; Yu, L. Shizukaol D, a Dimeric Sesquiterpene Isolated from Chloranthus Serratus, Represses the Growth of Human Liver Cancer Cells by Modulating Wnt Signalling Pathway. PLoS ONE 2016, 11, e0152012. [Google Scholar] [CrossRef]
- Kaur, M.; Velmurugan, B.; Tyagi, A.; Agarwal, C.; Singh, R.P.; Agarwal, R. Silibinin Suppresses Growth of Human Colorectal Carcinoma SW480 Cells in Culture and Xenograft through Down-Regulation of β-Catenin-Dependent Signaling. Neoplasia 2010, 12, 415–424. [Google Scholar] [CrossRef] [Green Version]
- Ren, K.; Zhang, W.; Wu, G.; Ren, J.; Lu, H.; Li, Z.; Han, X. Synergistic Anti-Cancer Effects of Galangin and Berberine through Apoptosis Induction and Proliferation Inhibition in Oesophageal Carcinoma Cells. Biomed. Pharmacother. 2016, 84, 1748–1759. [Google Scholar] [CrossRef]
- Sur, S.; Pal, D.; Mandal, S.; Roy, A.; Panda, C.K. Tea Polyphenols Epigallocatechin Gallete and Theaflavin Restrict Mouse Liver Carcinogenesis through Modulation of Self-Renewal Wnt and Hedgehog Pathways. J. Nutr. Biochem. 2016, 27, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Li, X.; Geng, S.; Wu, J.; Zhong, C.; Li, X.; Li, Y.; Chen, Y.; Wang, X.; Meng, Y.; et al. Wnt/β-Catenin Pathway Mediates (−)-Epigallocatechin-3-Gallate (EGCG) Inhibition of Lung Cancer Stem Cells. Biochem. Biophys. Res. Commun. 2017, 482, 15–21. [Google Scholar] [CrossRef]
- Bi, X.; Zhao, Y.; Fang, W.; Yang, W. Anticancer Activity of Panax Notoginseng Extract 20(S)-25-OCH3-PPD: Targetting β-Catenin Signalling. Clin. Exp. Pharmacol. Physiol. 2009, 36, 1074–1078. [Google Scholar] [CrossRef]
- Leow, P.C.; Tian, Q.; Ong, Z.Y.; Yang, Z.; Ee, P.L.R. Antitumor Activity of Natural Compounds, Curcumin and PKF118-310, as Wnt/β-Catenin Antagonists against Human Osteosarcoma Cells. Investig. New Drugs 2010, 28, 766–782. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Chen, L.; Guo, S.; Li, Y. Baicalin Induced Colon Cancer Cells Apoptosis through MiR-217/DKK1-Mediated Inhibition of Wnt Signaling Pathway. Mol. Biol. Rep. 2019, 46, 1693–1700. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.Q.; Zhang, Q.; Zhu, J.Y.; Li, Y.; Xie, C.F.; Li, X.T.; Wu, J.S.; Geng, S.S.; Zhong, C.Y.; et al. (-)-Epigallocatechin-3-Gallate Inhibits Colorectal Cancer Stem Cells by Suppressing Wnt/β-Catenin Pathway. Nutrients 2017, 9, 572. [Google Scholar] [CrossRef]
- Suh, Y.; Afaq, F.; Johnson, J.J.; Mukhtar, H. A Plant Flavonoid Fisetin Induces Apoptosis in Colon Cancer Cells by Inhibition of COX2 and Wnt/EGFR/NF-ΚB-Signaling Pathways. Carcinogenesis 2009, 30, 300–307. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H. Genistein Attenuates WNT Signaling by Up-Regulating SFRP2 in a Human Colon Cancer Cell Line. Exp. Biol. Med. 2011, 236, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Hirata, H.; Ueno, K.; Nakajima, K.; Tabatabai, Z.L.; Hinoda, Y.; Ishii, N.; Dahiya, R. Genistein Downregulates Onco-MiR-1260b and Inhibits Wnt-Signalling in Renal Cancer Cells. Br. J. Cancer 2013, 108, 2070–2078. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Tu, Y.; He, W.; Peng, Y.; Qiu, Z. A Novel Mechanism of Hepatocellular Carcinoma Cell Apoptosis Induced by Lupeol via Brain-Derived Neurotrophic Factor Inhibition and Glycogen Synthase Kinase 3 Beta Reactivation. Eur. J. Pharmacol. 2015, 762, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Bugter, J.M.; Fenderico, N.; Maurice, M.M. Mutations and Mechanisms of WNT Pathway Tumour Suppressors in Cancer. Nat. Rev. Cancer 2021, 21, 5–21. [Google Scholar] [CrossRef] [PubMed]
- van Neerven, S.M.; Vermeulen, L. The Interplay between Intrinsic and Extrinsic Wnt Signaling in Controlling Intestinal Transformation. Differentiation 2019, 108, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.S.; Park, J.I. Wnt Signaling in Cancer: Therapeutic Targeting of Wnt Signaling beyond β-Catenin and the Destruction Complex. Exp. Mol. Med. 2020, 52, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X. Targeting the Wnt/β-Catenin Signaling Pathway in Cancer. J. Hematol. Oncol. 2020, 13, 165. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Lv, J.; Sun, D.; Huang, Y. Therapeutic Strategies Targeting Wnt/β-Catenin Signaling for Colorectal Cancer (Review). Int. J. Mol. Med. 2021, 49, 1–17. [Google Scholar] [CrossRef]
- Sferrazza, G.; Corti, M.; Brusotti, G.; Pierimarchi, P.; Temporini, C.; Serafino, A.; Calleri, E. Nature-Derived Compounds Modulating Wnt/β-Catenin Pathway: A Preventive and Therapeutic Opportunity in Neoplastic Diseases. Acta Pharm. Sin. B 2020, 10, 1814–1834. [Google Scholar] [CrossRef]
- Yu, W.K.; Xu, Z.Y.; Yuan, L.; Mo, S.; Xu, B.; Cheng, X.D.; Qin, J.J. Targeting β-Catenin Signaling by Natural Products for Cancer Prevention and Therapy. Front. Pharmacol. 2020, 11, 984. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, A.; Holder, A. GaussView Version 5.0. Available online: https://gaussian.com/gaussview6/ (accessed on 23 January 2023).
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian Version 09. Available online: https://gaussian.com/glossary/g09/ (accessed on 23 January 2023).
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.; Goddard, T.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—a Visualization System for Exploratory Research and Analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trott, O.; Olson, A.J. Software News and Update AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2009, 31, 455–461. [Google Scholar]
- Salentin, S.; Schreiber, S.; Haupt, V.J.; Adasme, M.F.; Schroeder, M. PLIP: Fully Automated Protein-Ligand Interaction Profiler. Nucleic Acids Res. 2015, 43, W443–W447. [Google Scholar] [CrossRef]

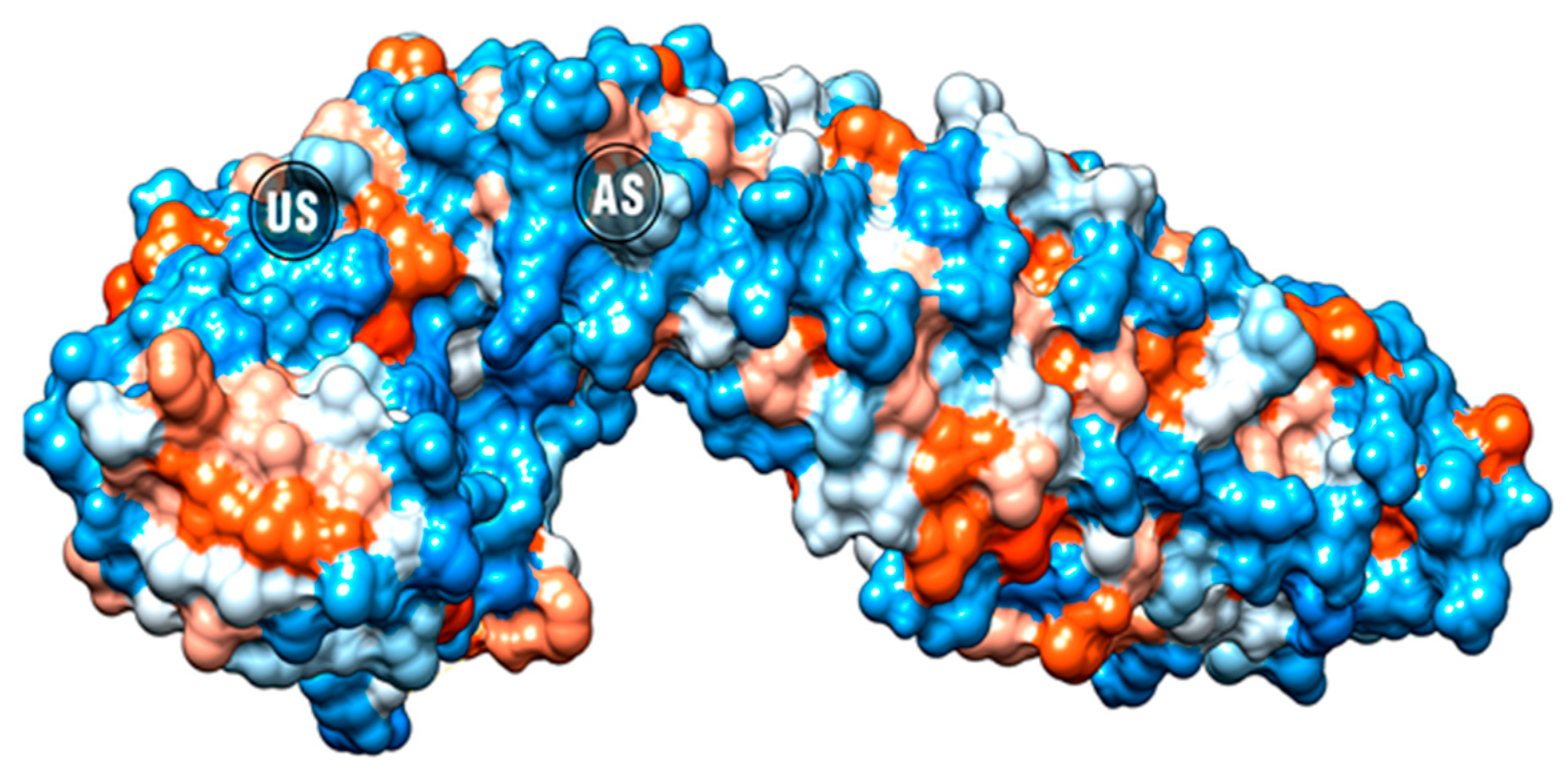





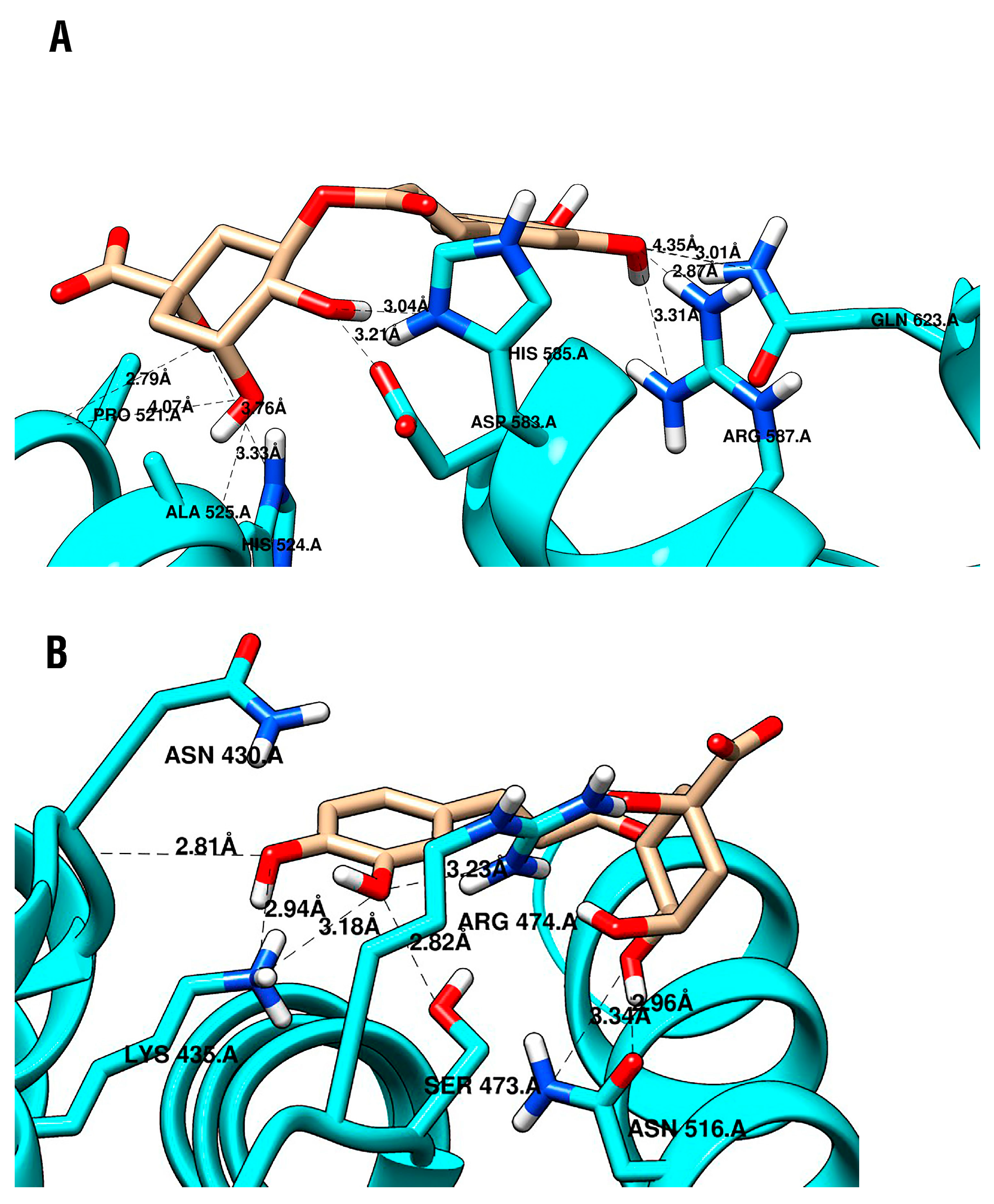

| Protein | Compound | Vina Score (Kcal/mol) | Protein-Ligand Interactions | ||
|---|---|---|---|---|---|
| Hydrogen Bonds | π Interactions | Hydrophobic Interactions | |||
| β-catenin AS | CGA | −5.5 | ARG587 | ||
| ASP583 | |||||
| HIS585 | |||||
| PRO521 | |||||
| HIS524 | |||||
| ALA525 | |||||
| GLN623 | |||||
| C2 | −4.6 | ARG587 | |||
| ASP583 | |||||
| VAL584 | |||||
| β-catenin US | CGA | −6.5 | ASN430 | HIS470 | ARG469 |
| LYS435 | |||||
| ARG474 | |||||
| ASN516 | |||||
| SER473 | |||||
| iCRT14 | −5.3 | ARG469 | ARG474 ARG515 | ||
| HIS470 | |||||
| LYS508 | |||||
| Protein | Compound | Vina Score (Kcal/mol) | Protein–Ligand Interactions | ||
|---|---|---|---|---|---|
| Hydrogen Bonds | π Interactions | Hydrophobic Interactions | |||
| LRP6 | CGA | −6.3 | SER665 GLU708 | GLU708 | |
| ARG751 | |||||
| TRP767 | |||||
| LEU810 | |||||
| PHE836 | |||||
| Niclosamide | −6.3 | ASP811 HIS834 | HIS834 | TRP767 | |
| LEU810 | |||||
| PHE836 | |||||
| Protein Subsite | Center of the Grid | Size | Exhaustiveness |
|---|---|---|---|
| β-catenin US | x = 11.527, y = 22.308, z = 62.347 | 17 Å3 | 20 |
| β-catenin AS | x = 2.805, y = 14.864, z = 79.543 | 17 Å3 | 20 |
| LRP6 E3 | x = 26.038, y = 5.167, z = −15.270 | 24 Å3 | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vélez-Vargas, L.C.; Santa-González, G.A.; Uribe, D.; Henao-Castañeda, I.C.; Pedroza-Díaz, J. In Vitro and In Silico Study on the Impact of Chlorogenic Acid in Colorectal Cancer Cells: Proliferation, Apoptosis, and Interaction with β-Catenin and LRP6. Pharmaceuticals 2023, 16, 276. https://doi.org/10.3390/ph16020276
Vélez-Vargas LC, Santa-González GA, Uribe D, Henao-Castañeda IC, Pedroza-Díaz J. In Vitro and In Silico Study on the Impact of Chlorogenic Acid in Colorectal Cancer Cells: Proliferation, Apoptosis, and Interaction with β-Catenin and LRP6. Pharmaceuticals. 2023; 16(2):276. https://doi.org/10.3390/ph16020276
Chicago/Turabian StyleVélez-Vargas, Laura Catalina, Gloria A. Santa-González, Diego Uribe, Isabel C. Henao-Castañeda, and Johanna Pedroza-Díaz. 2023. "In Vitro and In Silico Study on the Impact of Chlorogenic Acid in Colorectal Cancer Cells: Proliferation, Apoptosis, and Interaction with β-Catenin and LRP6" Pharmaceuticals 16, no. 2: 276. https://doi.org/10.3390/ph16020276
APA StyleVélez-Vargas, L. C., Santa-González, G. A., Uribe, D., Henao-Castañeda, I. C., & Pedroza-Díaz, J. (2023). In Vitro and In Silico Study on the Impact of Chlorogenic Acid in Colorectal Cancer Cells: Proliferation, Apoptosis, and Interaction with β-Catenin and LRP6. Pharmaceuticals, 16(2), 276. https://doi.org/10.3390/ph16020276







