In Silico and In Vitro Screening of Serine Racemase Agonist and In Vivo Efficacy on Alzheimer’s Disease Drosophila melanogaster
Abstract
:1. Introduction
2. Results
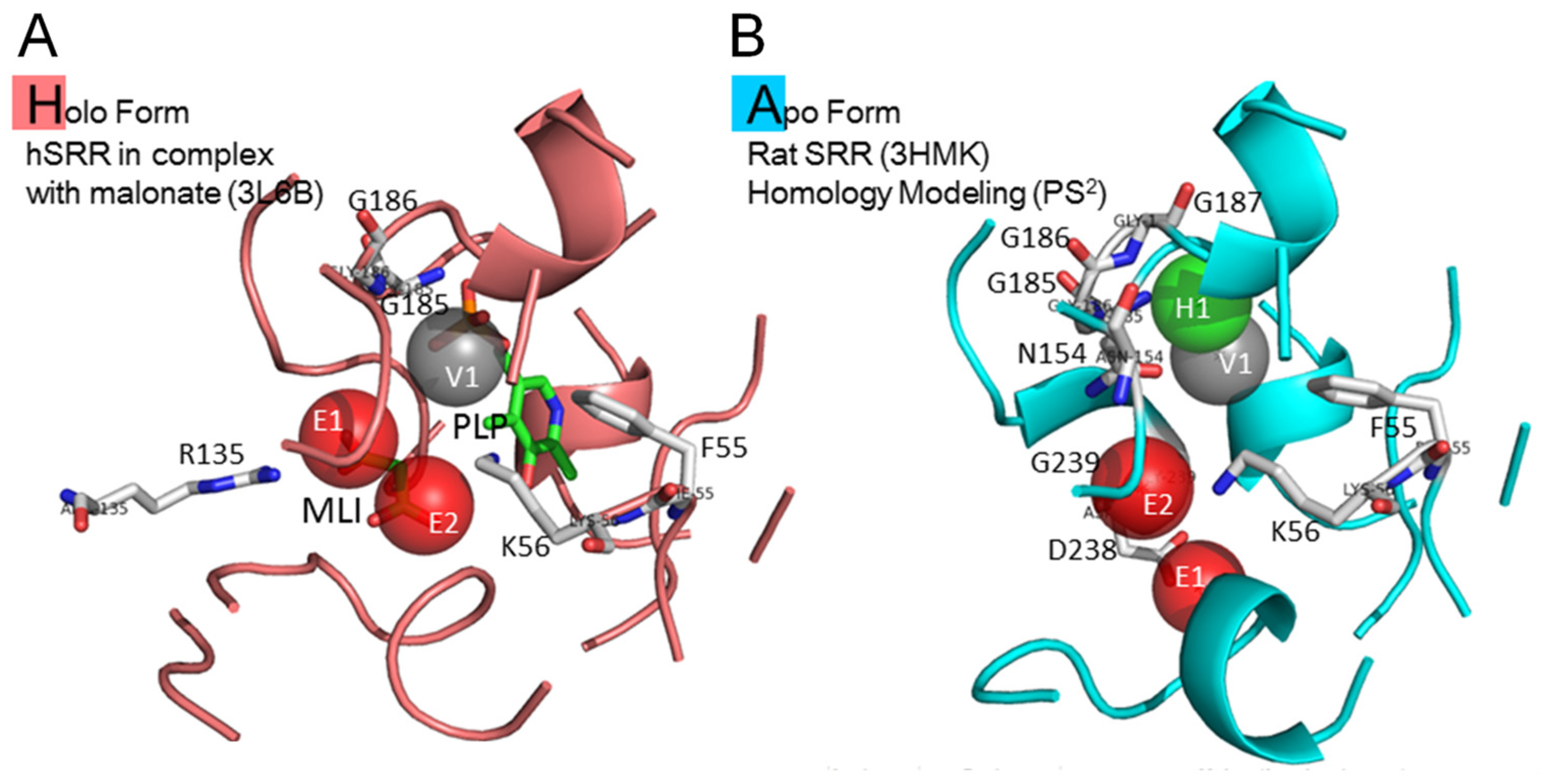
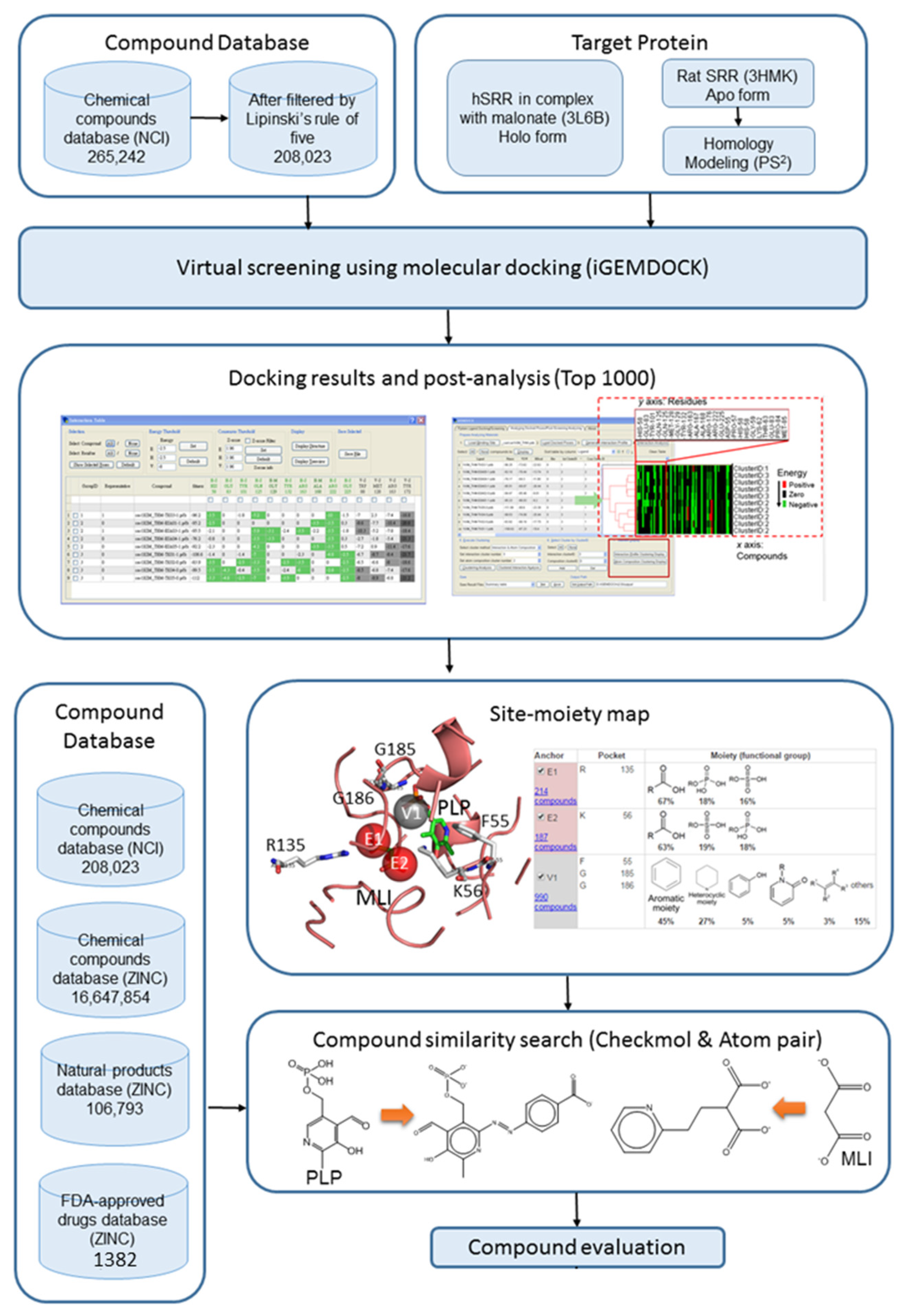
2.1. Site Moiety Map of Binding Pocket
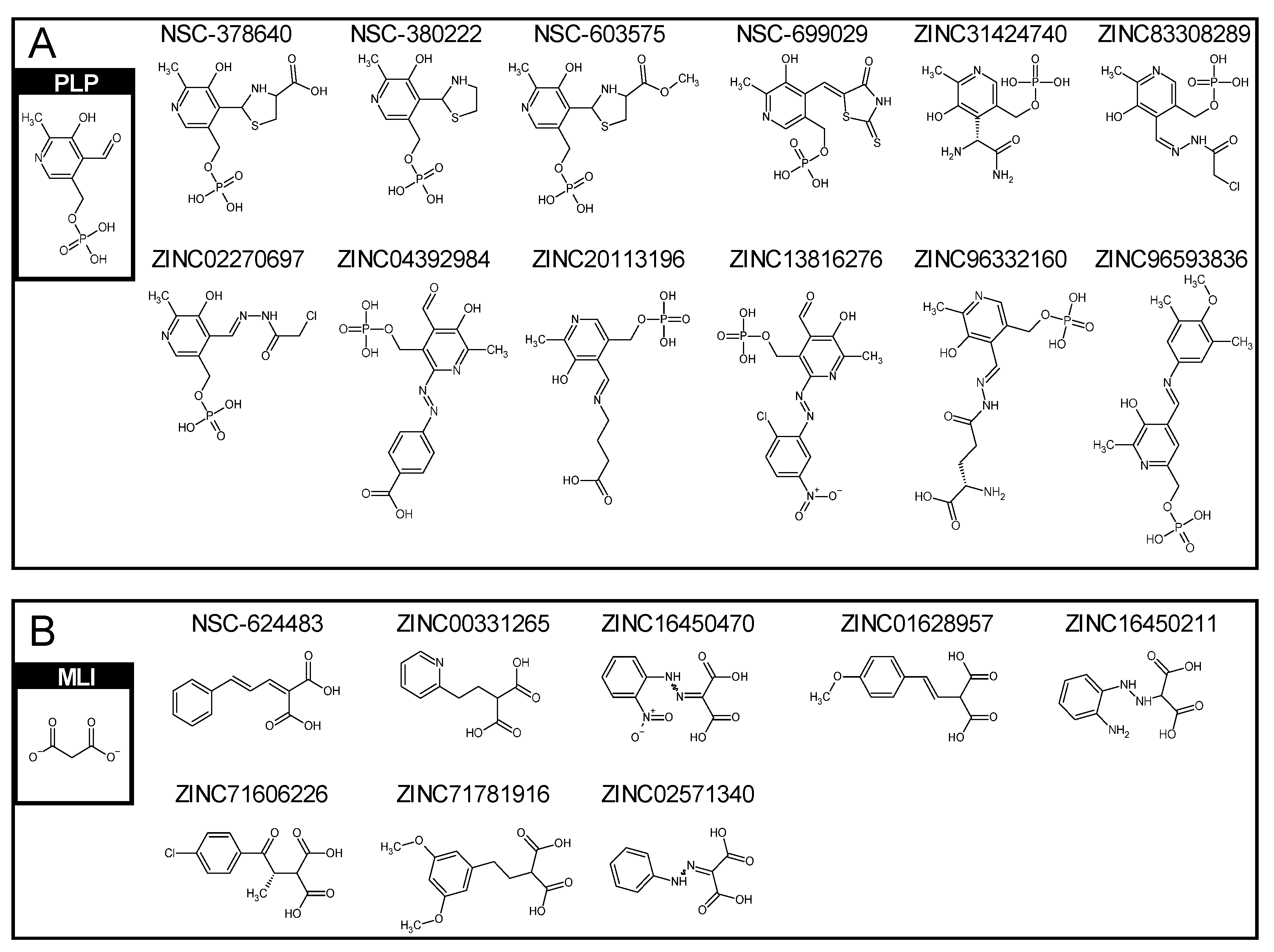
2.2. Similar Compounds to Vitamin B6 Phosphate and Malonate
2.3. Similar Compounds to Vitamin B6 Phosphate and Malonate
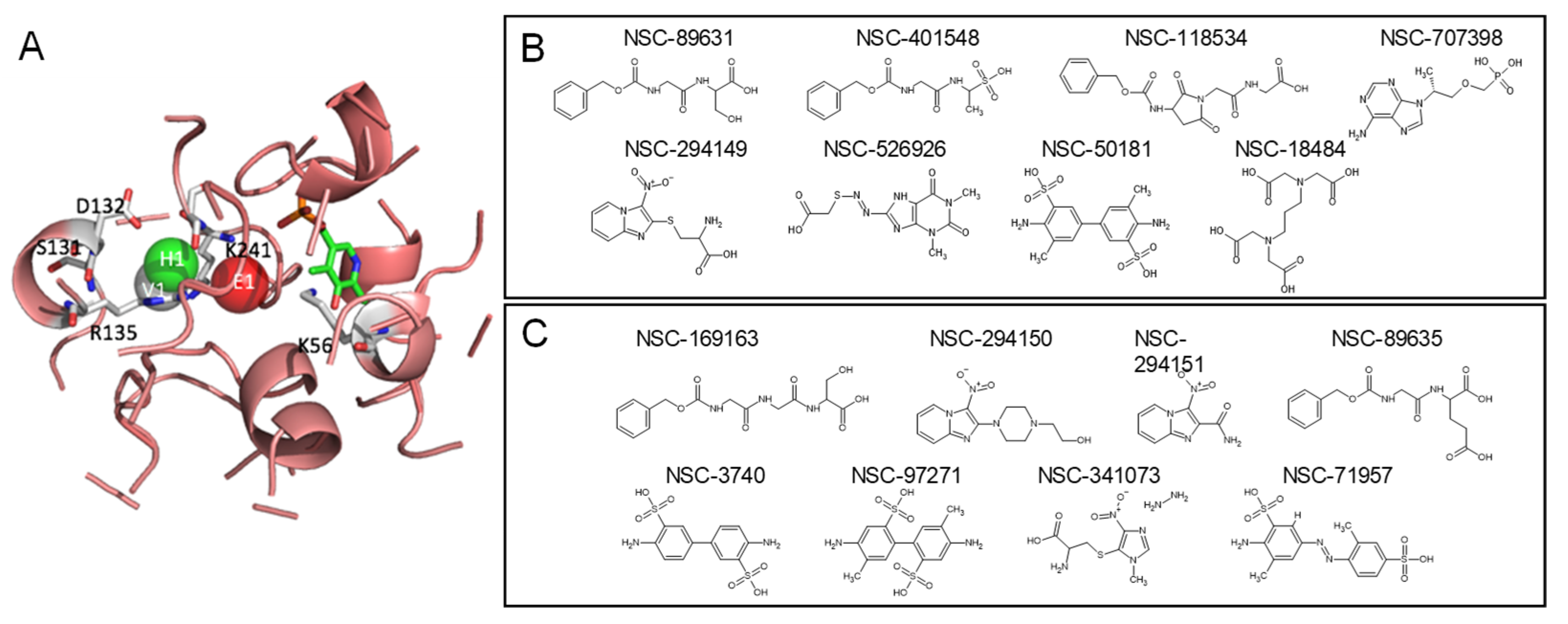
2.4. Top Candidates of Potential SRR Modulators
2.5. Keeping PLP as a Cofactor Ligand
2.6. In Vitro Screening the SRR Modulators
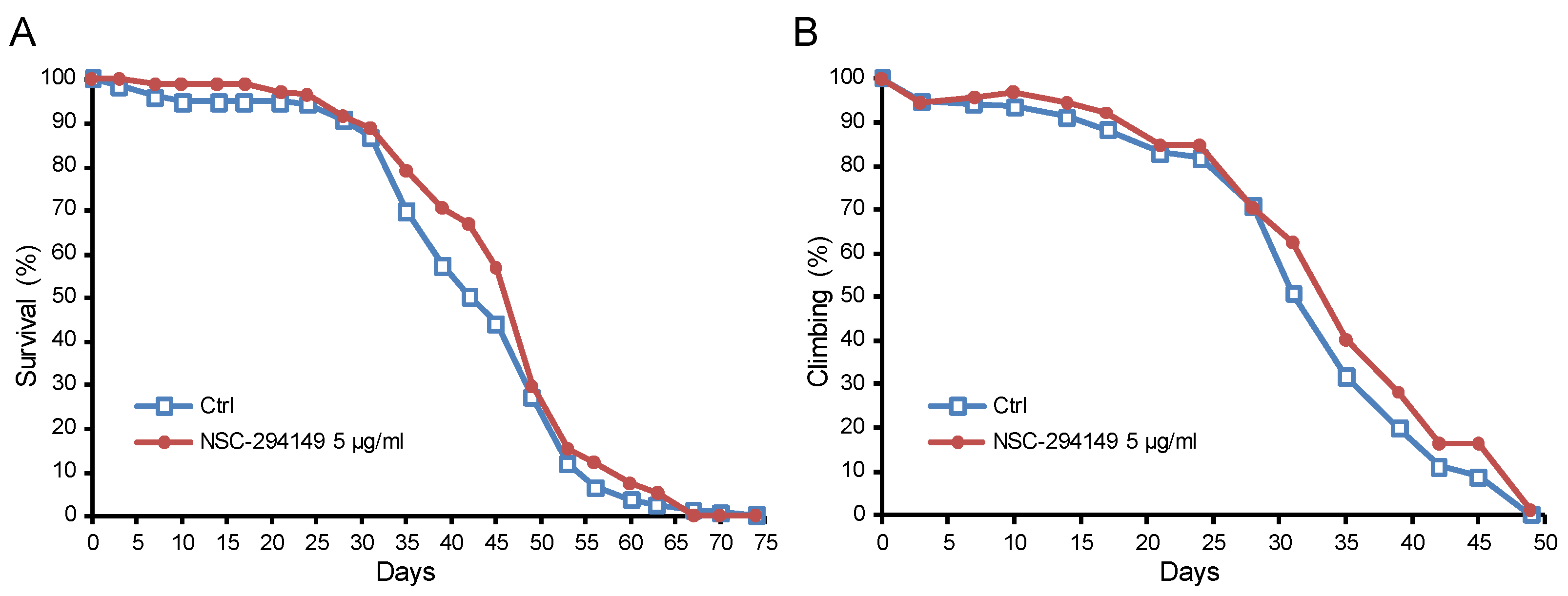
2.7. The SRR Agonist NSC294149 Ameliorated Survival of AD Drosophila
3. Discussion
4. Materials and Methods
4.1. Target Protein Structures
4.2. Compound Databases
4.3. Molecular Docking and Post-Analysis
4.4. Compound Similarity Search
4.5. The SRR Enzymatic Activity Assay
4.6. Fruit Fly Model of Alzheimer’s Disease
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Panatier, A.; Theodosis, D.T.; Mothet, J.-P.; Touquet, B.; Pollegioni, L.; Poulain, D.A.; Oliet, S.H. Glia-derived D-serine controls NMDA receptor activity and synaptic memory. Cell 2006, 125, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Wolosker, H.; Sheth, K.N.; Takahashi, M.; Mothet, J.-P.; Brady, R.O.; Ferris, C.D.; Snyder, S.H. Purification of serine racemase: Biosynthesis of the neuromodulator D-serine. Proc. Natl. Acad. Sci. USA 1999, 96, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Suzuki, M.; Sasabe, J.; Takahashi, S.; Unekawa, M.; Mashima, K.; Iizumi, T.; Hamase, K.; Konno, R.; Aiso, S. Cellular origin and regulation of D-and L-serine in in vitro and in vivo models of cerebral ischemia. J. Cereb. Blood Flow Metab. 2014, 34, 1928–1935. [Google Scholar] [CrossRef] [PubMed]
- Olney, J.W.; Newcomer, J.W.; Farber, N.B. NMDA receptor hypofunction model of schizophrenia. J. Psychiatr. Res. 1999, 33, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Farber, N.B. The NMDA receptor hypofunction model of psychosis. Ann. N. Y. Acad. Sci. 2003, 1003, 119–130. [Google Scholar] [CrossRef]
- Newcomer, J.W.; Farber, N.B.; Jevtovic-Todorovic, V.; Selke, G.; Melson, A.K.; Hershey, T.; Craft, S.; Olney, J.W. Ketamine-induced NMDA receptor hypofunction as a model of memory impairment and psychosis. Neuropsychopharmacology 1999, 20, 106–118. [Google Scholar] [CrossRef]
- Foster, T.; Kyritsopoulos, C.; Kumar, A. Central role for NMDA receptors in redox mediated impairment of synaptic function during aging and Alzheimer’s disease. Behav. Brain Res. 2017, 322, 223–232. [Google Scholar] [CrossRef]
- Farber, N.B.; Newcomer, J.W.; Olney, J.W. The glutamate synapse in neuropsychiatric disorders: Focus on schizophrenia and Alzheimer’s disease. Prog. Brain Res. 1998, 116, 421–437. [Google Scholar]
- Hirsch, L.; Yang, J.; Bresee, L.; Jette, N.; Patten, S.; Pringsheim, T. Second-generation antipsychotics and metabolic side effects: A systematic review of population-based studies. Drug Saf. 2017, 40, 771–781. [Google Scholar] [CrossRef]
- Mothet, J.; Rouaud, E.; Sinet, P.M.; Potier, B.; Jouvenceau, A.; Dutar, P.; Videau, C.; Epelbaum, J.; Billard, J.M. A critical role for the glial-derived neuromodulator D-serine in the age-related deficits of cellular mechanisms of learning and memory. Aging Cell 2006, 5, 267–274. [Google Scholar] [CrossRef]
- Potier, B.; Turpin, F.R.; Sinet, P.-M.; Rouaud, E.; Mothet, J.-P.; Videau, C.; Epelbaum, J.; Dutar, P.; Billard, J.-M. Contribution of the d-serine-dependent pathway to the cellular mechanisms underlying cognitive aging. Front. Aging Neurosci. 2010, 2, 1. [Google Scholar] [CrossRef] [PubMed]
- Turpin, F.; Potier, B.; Dulong, J.; Sinet, P.-M.; Alliot, J.; Oliet, S.; Dutar, P.; Epelbaum, J.; Mothet, J.-P.; Billard, J.-M. Reduced serine racemase expression contributes to age-related deficits in hippocampal cognitive function. Neurobiol. Aging 2011, 32, 1495–1504. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Qiao, H.; Wen, L.; Zhou, W.; Zhang, Y. D-serine enhances impaired long-term potentiation in CA1 subfield of hippocampal slices from aged senescence-accelerated mouse prone/8. Neurosci. Lett. 2005, 379, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Calcia, M.A.; Madeira, C.; Alheira, F.V.; Silva, T.C.; Tannos, F.M.; Vargas-Lopes, C.; Goldenstein, N.; Brasil, M.A.; Ferreira, S.T.; Panizzutti, R. Plasma levels of D-serine in Brazilian individuals with schizophrenia. Schizophr. Res. 2012, 142, 83–87. [Google Scholar] [CrossRef]
- Rosenberg, D.; Artoul, S.; Segal, A.C.; Kolodney, G.; Radzishevsky, I.; Dikopoltsev, E.; Foltyn, V.N.; Inoue, R.; Mori, H.; Billard, J.-M. Neuronal D-serine and glycine release via the Asc-1 transporter regulates NMDA receptor-dependent synaptic activity. J. Neurosci. 2013, 33, 3533–3544. [Google Scholar] [CrossRef]
- Sason, H.; Billard, J.M.; Smith, G.P.; Safory, H.; Neame, S.; Kaplan, E.; Rosenberg, D.; Zubedat, S.; Foltyn, V.N.; Christoffersen, C.T. Asc-1 transporter regulation of synaptic activity via the tonic release of d-serine in the forebrain. Cerebral cortex 2017, 27, 1573–1587. [Google Scholar] [CrossRef]
- Billard, J.M.; Freret, T. Asc-1 transporter activation: An alternative to rescue age-related alterations in functional plasticity at rat hippocampal CA 3/CA 1 synapses. J. Neurochem. 2018, 147, 514–525. [Google Scholar] [CrossRef]
- Ju, Y.; Tam, K.Y. Pathological mechanisms and therapeutic strategies for Alzheimer’s disease. Neural Regen. Res. 2022, 17, 543. [Google Scholar]
- Sengoku, R. Aging and Alzheimer’s disease pathology. Neuropathology 2020, 40, 22–29. [Google Scholar] [CrossRef]
- Dhillon, S. Aducanumab: First approval. Drugs 2021, 81, 1437–1443. [Google Scholar] [CrossRef]
- Knopman, D.S.; Jones, D.T.; Greicius, M.D. Failure to demonstrate efficacy of aducanumab: An analysis of the EMERGE and ENGAGE trials as reported by Biogen, December 2019. Alzheimer’s Dement. 2021, 17, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Mesulam, M.-M. Neuroplasticity failure in Alzheimer’s disease: Bridging the gap between plaques and tangles. Neuron 1999, 24, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Teter, B.; Ashford, J.W. Neuroplasticity in Alzheimer’s disease. J. Neurosci. Res. 2002, 70, 402–437. [Google Scholar] [CrossRef] [PubMed]
- Koch, G.; Spampinato, D. Alzheimer disease and neuroplasticity. Handb. Clin. Neurol. 2022, 184, 473–479. [Google Scholar] [PubMed]
- Brown, T.H.; Chapman, P.F.; Kairiss, E.W.; Keenan, C.L. Long-term synaptic potentiation. Science 1988, 242, 724–728. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, J.K.; Bachman, P.; Mathalon, D.H.; Roach, B.J.; Asarnow, R.F. Augmenting NMDA receptor signaling boosts experience-dependent neuroplasticity in the adult human brain. Proc. Natl. Acad. Sci. USA 2015, 112, 15331–15336. [Google Scholar] [CrossRef] [PubMed]
- Kessels, H.W.; Nabavi, S.; Malinow, R. Metabotropic NMDA receptor function is required for β-amyloid–induced synaptic depression. Proc. Natl. Acad. Sci. USA 2013, 110, 4033–4038. [Google Scholar] [CrossRef]
- Malinow, R. New developments on the role of NMDA receptors in Alzheimer’s disease. Curr. Opin. Neurobiol. 2012, 22, 559–563. [Google Scholar] [CrossRef]
- Mota, S.I.; Ferreira, I.L.; Rego, A.C. Dysfunctional synapse in Alzheimer’s disease–A focus on NMDA receptors. Neuropharmacology 2014, 76, 16–26. [Google Scholar] [CrossRef]
- Brito-Moreira, J.; Paula-Lima, A.C.; Bomfim, T.R.; Oliveira, F.F.; Sepulveda, F.J.; De Mello, F.G.; Aguayo, L.G.; Panizzutti, R.; Ferreira, S.T. Aβ oligomers induce glutamate release from hippocampal neurons. Curr. Alzheimer Res. 2011, 8, 552–562. [Google Scholar] [CrossRef]
- Talantova, M.; Sanz-Blasco, S.; Zhang, X.; Xia, P.; Akhtar, M.W.; Okamoto, S.-I.; Dziewczapolski, G.; Nakamura, T.; Cao, G.; Pratt, A.E. Aβ induces astrocytic glutamate release, extrasynaptic NMDA receptor activation, and synaptic loss. Proc. Natl. Acad. Sci. USA 2013, 110, E2518–E2527. [Google Scholar] [CrossRef]
- Biemans, E.A.; Verhoeven-Duif, N.M.; Gerrits, J.; Claassen, J.A.; Kuiperij, H.B.; Verbeek, M.M. CSF d-serine concentrations are similar in Alzheimer’s disease, other dementias, and elderly controls. Neurobiol. Aging 2016, 42, 213–216. [Google Scholar] [CrossRef]
- Chouinard, M.L.; Gaitan, D.; Wood, P.L. Presence of the N-Methyl-D-Aspartate-Associated Glycine Receptor Agonist, D-Seine, in Human Temporal Cortex: Comparison of Normal, Parkinson, and Alzheimer Tissues. J. Neurochem. 1993, 61, 1561–1564. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Fukushima, T.; Shimizu, E.; Okada, S.-i.; Komatsu, N.; Okamura, N.; Koike, K.; Koizumi, H.; Kumakiri, C.; Imai, K. Possible role of D-serine in the pathophysiology of Alzheimer’s disease. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2004, 28, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Kumashiro, S.; Hashimoto, A.; Nishikawa, T. Free D-serine in post-mortem brains and spinal cords of individuals with and without neuropsychiatric diseases. Brain Res. 1995, 681, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Chiu, C.-C.; Huang, C.-H.; Yang, H.-T.; Lane, H.-Y. pLG72 levels increase in early phase of Alzheimer’s disease but decrease in late phase. Sci. Rep. 2019, 9, 7843. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Yang, H.-T.; Chiu, C.-C.; Lane, H.-Y. Blood levels of D-amino acid oxidase vs. D-amino acids in reflecting cognitive aging. Sci. Rep. 2017, 7, 14849. [Google Scholar] [CrossRef]
- Madeira, C.; Lourenco, M.V.; Vargas-Lopes, C.; Suemoto, C.K.; Brandão, C.O.; Reis, T.; Leite, R.E.; Laks, J.; Jacob-Filho, W.; Pasqualucci, C.A. d-serine levels in Alzheimer’s disease: Implications for novel biomarker development. Transl. Psychiatry 2015, 5, e561. [Google Scholar] [CrossRef]
- Nagata, Y.; Borghi, M.; Fisher, G.H.; D’Aniello, A. Free D-serine concentration in normal and Alzheimer human brain. Brain Res. Bull. 1995, 38, 181–183. [Google Scholar] [CrossRef]
- Fisher, G.; Lorenzo, N.; Abe, H.; Fujita, E.; Frey, W.; Emory, C.; Di Fiore, M.; D’Aniello, A. Free D-and L-amino acids in ventricular cerebrospinal fluid from Alzheimer and normal subjects. Amino Acids 1998, 15, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Le Douce, J.; Maugard, M.; Veran, J.; Matos, M.; Jégo, P.; Vigneron, P.-A.; Faivre, E.; Toussay, X.; Vandenberghe, M.; Balbastre, Y. Impairment of glycolysis-derived L-serine production in astrocytes contributes to cognitive deficits in Alzheimer’s disease. Cell. Metab. 2020, 31, 503–517.e508. [Google Scholar] [CrossRef] [PubMed]
- Kantrowitz, J.T.; Malhotra, A.K.; Cornblatt, B.; Silipo, G.; Balla, A.; Suckow, R.F.; D’Souza, C.; Saksa, J.; Woods, S.W.; Javitt, D.C. High dose D-serine in the treatment of schizophrenia. Schizophr. Res. 2010, 121, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Krug, A.W.; Volker, K.; Dantzler, W.H.; Silbernagl, S. Why is d-serine nephrotoxic and α-aminoisobutyric acid protective? Am. J. Physiol. Ren. Physiol. 2007, 293, F382–F390. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.; Lock, E. D-serine-induced nephrotoxicity: Possible interaction with tyrosine metabolism. Toxicology 2004, 201, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Finelli, A.; Kelkar, A.; Song, H.-J.; Yang, H.; Konsolaki, M. A model for studying Alzheimer’s Aβ42-induced toxicity in Drosophila melanogaster. Mol. Cell. Neurosci. 2004, 26, 365–375. [Google Scholar] [CrossRef]
- Sterling, T.; Irwin, J.J. ZINC 15–ligand discovery for everyone. J. Chem. Inf. Model. 2015, 55, 2324–2337. [Google Scholar] [CrossRef]
- Hsu, K.-C.; Chen, Y.-F.; Lin, S.-R.; Yang, J.-M. iGEMDOCK: A graphical environment of enhancing GEMDOCK using pharmacological interactions and post-screening analysis. BMC Bioinform. 2011, 12, S33. [Google Scholar] [CrossRef]
- Chen, Y.-F.; Hsu, K.-C.; Lin, S.-R.; Wang, W.-C.; Huang, Y.-C.; Yang, J.-M. SiMMap: A web server for inferring site-moiety map to recognize interaction preferences between protein pockets and compound moieties. Nucleic Acids Res. 2010, 38, W424–W430. [Google Scholar] [CrossRef]
- Henneberger, C.; Papouin, T.; Oliet, S.H.; Rusakov, D.A. Long-term potentiation depends on release of D-serine from astrocytes. Nature 2010, 463, 232–236. [Google Scholar] [CrossRef]
- Yang, Y.; Ge, W.; Chen, Y.; Zhang, Z.; Shen, W.; Wu, C.; Poo, M.; Duan, S. Contribution of astrocytes to hippocampal long-term potentiation through release of D-serine. Proc. Natl. Acad. Sci. USA 2003, 100, 15194–15199. [Google Scholar] [CrossRef]
- Basu, A.C.; Tsai, G.E.; Ma, C.-L.; Ehmsen, J.T.; Mustafa, A.K.; Han, L.; Jiang, Z.I.; Benneyworth, M.A.; Froimowitz, M.P.; Lange, N. Targeted disruption of serine racemase affects glutamatergic neurotransmission and behavior. Mol. Psychiatry 2009, 14, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Labrie, V.; Fukumura, R.; Rastogi, A.; Fick, L.J.; Wang, W.; Boutros, P.C.; Kennedy, J.L.; Semeralul, M.O.; Lee, F.H.; Baker, G.B. Serine racemase is associated with schizophrenia susceptibility in humans and in a mouse model. Hum. Mol. Genet. 2009, 18, 3227–3243. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, M.; Rezania, F.; Lewin, J.; Moore, K.P.; Mani, A.R. d-Serine modulates neurogenic relaxation in rat corpus cavernosum. Biochem. Pharmacol. 2010, 79, 1791–1796. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Lee, S.-A.; Lam, M.; Rapisarda, A.; Kraus, M.; Keefe, R.; Lee, J. The relationship between negative symptom subdomains and cognition. Psychol. Med. 2016, 46, 2169–2177. [Google Scholar] [CrossRef]
- Kristiansen, L.; Beneyto, M.; Haroutunian, V.; Meador-Woodruff, J. Altered NMDA receptor expression in schizophrenia. Mol. Psychiatry 2006, 11, 705. [Google Scholar] [CrossRef] [PubMed]
- Geddes, A.E.; Huang, X.-F.; Newell, K.A. Reciprocal signalling between NR2 subunits of the NMDA receptor and neuregulin1 and their role in schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Weickert, C.S.; Fung, S.; Catts, V.; Schofield, P.; Allen, K.; Moore, L.; Newell, K.A.; Pellen, D.; Huang, X.-F.; Catts, S. Molecular evidence of N-methyl-D-aspartate receptor hypofunction in schizophrenia. Mol. Psychiatry 2013, 18, 1185–1192. [Google Scholar] [CrossRef]
- Mohn, A.R.; Gainetdinov, R.R.; Caron, M.G.; Koller, B.H. Mice with reduced NMDA receptor expression display behaviors related to schizophrenia. Cell 1999, 98, 427–436. [Google Scholar] [CrossRef]
- Morris, B.J.; Cochran, S.M.; Pratt, J.A. PCP: From pharmacology to modelling schizophrenia. Curr. Opin. Pharmacol. 2005, 5, 101–106. [Google Scholar] [CrossRef]
- Snyder, M.A.; Gao, W.-J. NMDA hypofunction as a convergence point for progression and symptoms of schizophrenia. Front. Cell. Neurosci. 2013, 7, 31. [Google Scholar] [CrossRef]
- Smith, M.A.; Mack, V.; Ebneth, A.; Moraes, I.; Felicetti, B.; Wood, M.; Schonfeld, D.; Mather, O.; Cesura, A.; Barker, J. The structure of mammalian serine racemase: Evidence for conformational changes upon inhibitor binding. J. Biol. Chem. 2010, 285, 12873–12881. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The protein data bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-C.; Hwang, J.-K.; Yang, J.-M. 2: Protein structure prediction server. Nucleic Acids Res. 2006, 34, W152–W157. [Google Scholar] [CrossRef] [PubMed]
- Guda, C.; Lu, S.; Scheeff, E.D.; Bourne, P.E.; Shindyalov, I.N. CE-MC: A multiple protein structure alignment server. Nucleic Acids Res. 2004, 32, W100–W103. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A. Lead-and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef]
- Sheridan, R.P.; Miller, M.D.; Underwood, D.J.; Kearsley, S.K. Chemical similarity using geometric atom pair descriptors. J. Chem. Inf. Comput. Sci. 1996, 36, 128–136. [Google Scholar] [CrossRef]
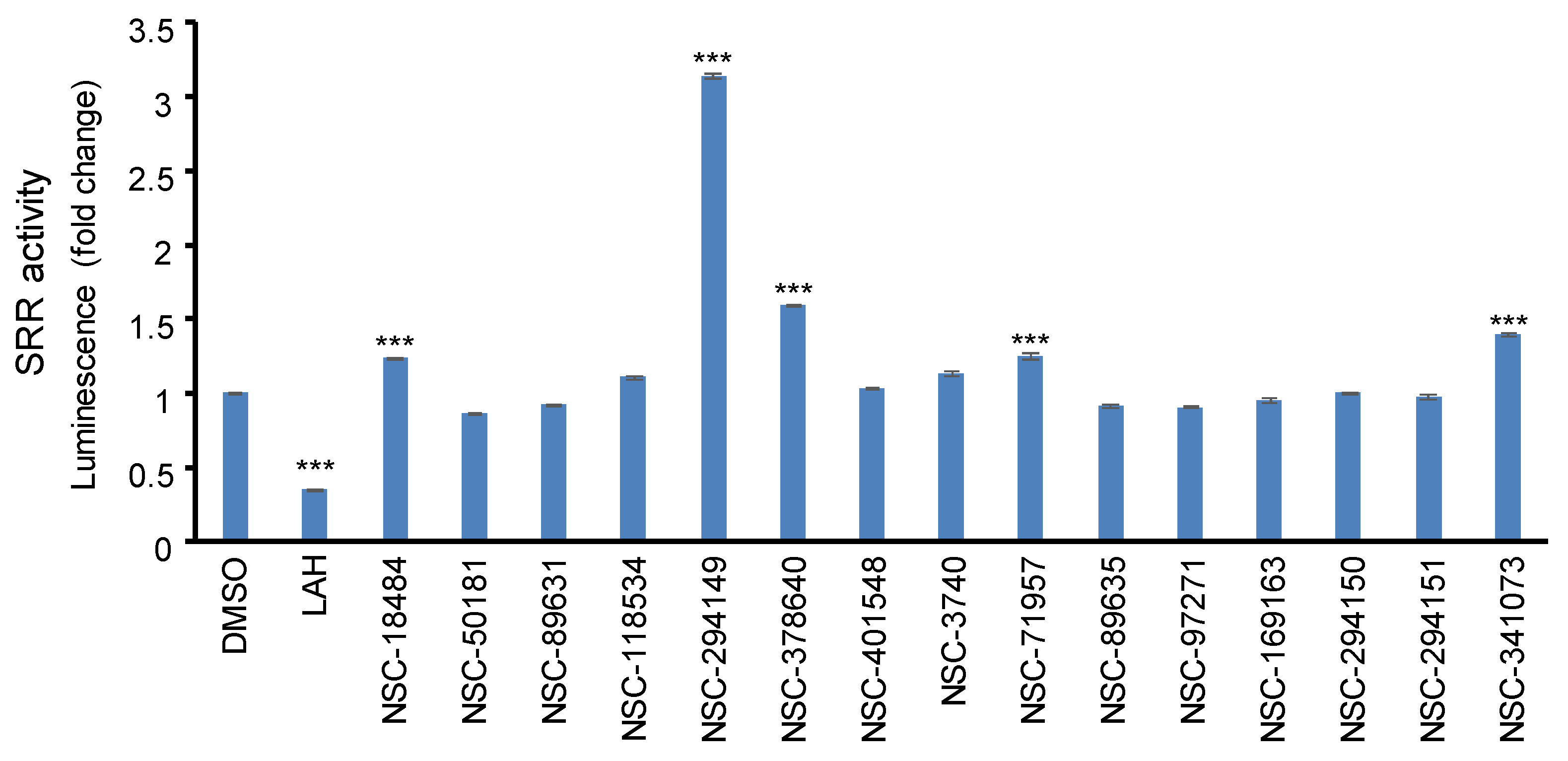
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, C.-H.; Chang, H.-T.; Hsu, L.-F.; Lee, M.-H.; Cheng, J.; Wu, D.C.; Lin, W.-Y. In Silico and In Vitro Screening of Serine Racemase Agonist and In Vivo Efficacy on Alzheimer’s Disease Drosophila melanogaster. Pharmaceuticals 2023, 16, 280. https://doi.org/10.3390/ph16020280
Lu C-H, Chang H-T, Hsu L-F, Lee M-H, Cheng J, Wu DC, Lin W-Y. In Silico and In Vitro Screening of Serine Racemase Agonist and In Vivo Efficacy on Alzheimer’s Disease Drosophila melanogaster. Pharmaceuticals. 2023; 16(2):280. https://doi.org/10.3390/ph16020280
Chicago/Turabian StyleLu, Chih-Hao, Hao-Teng Chang, Lee-Fen Hsu, Ming-Hsueh Lee, Jack Cheng, Dong Chuan Wu, and Wei-Yong Lin. 2023. "In Silico and In Vitro Screening of Serine Racemase Agonist and In Vivo Efficacy on Alzheimer’s Disease Drosophila melanogaster" Pharmaceuticals 16, no. 2: 280. https://doi.org/10.3390/ph16020280
APA StyleLu, C.-H., Chang, H.-T., Hsu, L.-F., Lee, M.-H., Cheng, J., Wu, D. C., & Lin, W.-Y. (2023). In Silico and In Vitro Screening of Serine Racemase Agonist and In Vivo Efficacy on Alzheimer’s Disease Drosophila melanogaster. Pharmaceuticals, 16(2), 280. https://doi.org/10.3390/ph16020280





