Altered Membrane Expression and Function of CD11b Play a Role in the Immunosuppressive Effects of Morphine on Macrophages at the Nanomolar Level
Abstract
:1. Introduction
2. Results
2.1. Nanomolar (nM) Morphine Directly Inhibits Macrophage Phagocytosis of Cancer Cells via MOR but Not by Altering the CD47/SIRPα Axis
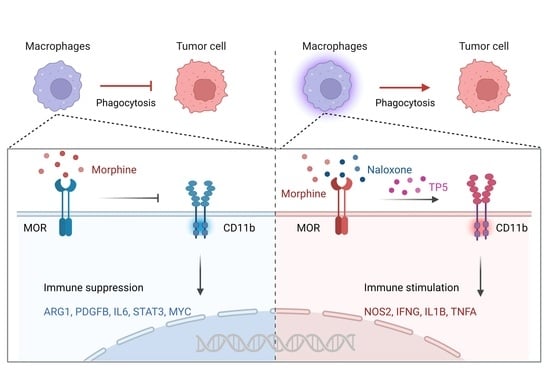
2.2. Morphine Inhibits the Phagocytic Effect of Cancer Cells by Decreasing the Membrane Expression of CD11b on Macrophages
2.3. Leukadherin-1 (LA1) Promotes Macrophage CD11b Activation to Alleviate the Inhibitory Effect of Morphine on Macrophage Phagocytosis
2.4. Nanomolar Morphine Inhibits Phagocytosis of Cancer Cells by Decreasing the Surface Expression of MOR and CD11b on Macrophage Membranes
2.5. The MOR Inhibitor Naloxone Reverses the Inhibitory Effect of Morphine on Macrophage CD11b and Rescues the Phagocytic Function of Macrophages
2.6. Thymopentin (TP5) Reverses the Inhibitory Effect of Morphine on Macrophage Phagocytosis by Promoting the Surface Expression and Function of CD11b
3. Discussion
4. Materials and Methods
4.1. Plasmids and siRNAs
4.2. Chemicals and Cell Culture
4.3. SiRNA Mediated Knockdown
4.4. Other Plasmids Transfection
4.5. Flow Cytometry
4.6. In Vitro Phagocytosis Assays
4.7. RT-qPCR Assay
4.8. Cell Viability Assay
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Hashimi, M.; Scott, S.W.; Thompson, J.P.; Lambert, D.G. Opioids and immune modulation: More questions than answers. Br. J. Anaesth. 2013, 111, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Malafoglia, V.; Ilari, S.; Vitiello, L.; Tenti, M.; Balzani, E.; Muscoli, C.; Raffaeli, W.; Bonci, A. The Interplay between Chronic Pain, Opioids, and the Immune System. Neuroscientist 2022, 28, 613–627. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ma, T.C.; Li, J.L.; Zhou, Y.; Geller, E.B.; Adler, M.W.; Peng, J.S.; Zhou, W.; Zhou, D.J.; Ho, W.Z. Heroin inhibits HIV-restriction miRNAs and enhances HIV infection of macrophages. Front. Microbiol. 2015, 6, 1230. [Google Scholar] [CrossRef] [PubMed]
- Barbaro, J.M.; Jaureguiberry-Bravo, M.; Sidoli, S.; Berman, J.W. Morphine disrupts macrophage functions even during HIV infection. J. Leukoc. Biol. 2022, 112, 1317–1328. [Google Scholar] [CrossRef] [PubMed]
- Labuz, D.; Schmidt, Y.; Schreiter, A.; Rittner, H.L.; Mousa, S.A.; Machelska, H. Immune cell-derived opioids protect against neuropathic pain in mice. J. Clin. Invest. 2009, 119, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Plein, L.M.; Rittner, H.L. Opioids and the immune system—Friend or foe. Br. J. Pharmacol. 2018, 175, 2717–2725. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Blanco, C. The changing opioid crisis: Development, challenges and opportunities. Mol. Psychiatry 2021, 26, 218–233. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Balasubramanian, S.; Sumandeep, S.; Charboneau, R.; Wang, J.; Melnyk, D.; Beilman, G.J.; Vatassery, R.; Barke, R.A. Morphine directs T cells toward T(H2) differentiation. Surgery 2001, 130, 304–309. [Google Scholar] [CrossRef]
- Rojavin, M.; Szabo, I.; Bussiere, J.L.; Rogers, T.J.; Adler, M.W.; Eisenstein, T.K. Morphine treatment in vitro or in vivo decreases phagocytic functions of murine macrophages. Life Sci. 1993, 53, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Eisenstein, T.K. The Role of Opioid Receptors in Immune System Function. Front. Immunol. 2019, 10, 2904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ninkovic, J.; Roy, S. Role of the mu-opioid receptor in opioid modulation of immune function. Amino Acids 2013, 45, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Gaveriaux-Ruff, C.; Matthes, H.W.; Peluso, J.; Kieffer, B.L. Abolition of morphine-immunosuppression in mice lacking the mu-opioid receptor gene. Proc. Natl. Acad. Sci. USA 1998, 95, 6326–6330. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Barke, R.A.; Loh, H.H. MU-opioid receptor-knockout mice: Role of m-opioid receptor in morphine mediated immune functions. Brain Res. Mol. Brain Res. 1998, 61, 190–194. [Google Scholar] [CrossRef]
- Tomassini, N.; Renaud, F.; Roy, S.; Loh, H.H. Morphine inhibits Fc-mediated phagocytosis through mu and delta opioid receptors. J. Neuroimmunol. 2004, 147, 131–133. [Google Scholar] [CrossRef] [PubMed]
- Kosciuczuk, U.; Knapp, P.; Lotowska-Cwiklewska, A.M. Opioid-induced immunosuppression and carcinogenesis promotion theories create the newest trend in acute and chronic pain pharmacotherapy. Clinics 2020, 75, e1554. [Google Scholar] [CrossRef]
- Khan, S.Q.; Khan, I.; Gupta, V. CD11b Activity Modulates Pathogenesis of Lupus Nephritis. Front. Med. 2018, 5, 52. [Google Scholar] [CrossRef]
- Abram, C.L.; Lowell, C.A. The ins and outs of leukocyte integrin signaling. Annu. Rev. Immunol. 2009, 27, 339–362. [Google Scholar] [CrossRef]
- Rosetti, F.; Mayadas, T.N. The many faces of Mac-1 in autoimmune disease. Immunol. Rev. 2016, 269, 175–193. [Google Scholar] [CrossRef]
- Coxon, A.; Rieu, P.; Barkalow, F.J.; Askari, S.; Sharpe, A.H.; von Andrian, U.H.; Arnaout, M.A.; Mayadas, T.N. A novel role for the beta 2 integrin CD11b/CD18 in neutrophil apoptosis: A homeostatic mechanism in inflammation. Immunity 1996, 5, 653–666. [Google Scholar] [CrossRef]
- Zheng, C.; Yang, Q.; Xu, C.; Shou, P.; Cao, J.; Jiang, M.; Chen, Q.; Cao, G.; Han, Y.; Li, F.; et al. CD11b regulates obesity-induced insulin resistance via limiting alternative activation and proliferation of adipose tissue macrophages. Proc. Natl. Acad. Sci. USA 2015, 112, E7239–E7248. [Google Scholar] [CrossRef] [Green Version]
- Ding, C.; Ma, Y.; Chen, X.; Liu, M.; Cai, Y.; Hu, X.; Xiang, D.; Nath, S.; Zhang, H.G.; Ye, H.; et al. Integrin CD11b negatively regulates BCR signalling to maintain autoreactive B cell tolerance. Nat. Commun. 2013, 4, 2813. [Google Scholar] [CrossRef] [PubMed]
- Faridi, M.H.; Khan, S.Q.; Zhao, W.; Lee, H.W.; Altintas, M.M.; Zhang, K.; Kumar, V.; Armstrong, A.R.; Carmona-Rivera, C.; Dorschner, J.M.; et al. CD11b activation suppresses TLR-dependent inflammation and autoimmunity in systemic lupus erythematosus. J. Clin. Invest. 2017, 127, 1271–1283. [Google Scholar] [CrossRef] [PubMed]
- Schmid, M.C.; Khan, S.Q.; Kaneda, M.M.; Pathria, P.; Shepard, R.; Louis, T.L.; Anand, S.; Woo, G.; Leem, C.; Faridi, M.H.; et al. Integrin CD11b activation drives anti-tumor innate immunity. Nat. Commun. 2018, 9, 5379. [Google Scholar] [CrossRef] [PubMed]
- Geraghty, T.; Rajagopalan, A.; Aslam, R.; Pohlman, A.; Venkatesh, I.; Zloza, A.; Cimbaluk, D.; DeNardo, D.G.; Gupta, V. Positive Allosteric Modulation of CD11b as a Novel Therapeutic Strategy Against Lung Cancer. Front. Oncol. 2020, 10, 748. [Google Scholar] [CrossRef]
- Boland, J.W.; Pockley, A.G. Influence of opioids on immune function in patients with cancer pain: From bench to bedside. Br. J. Pharmacol. 2018, 175, 2726–2736. [Google Scholar] [CrossRef]
- Viola, A.; Munari, F.; Sanchez-Rodriguez, R.; Scolaro, T.; Castegna, A. The Metabolic Signature of Macrophage Responses. Front. Immunol. 2019, 10, 1462. [Google Scholar] [CrossRef]
- Fan, Y.Z.; Chang, H.; Yu, Y.; Liu, J.; Zhao, L.; Yang, D.J.; Wang, R. Thymopentin (TP5), an immunomodulatory peptide, suppresses proliferation and induces differentiation in HL-60 cells. Biochim. Biophys. Acta 2006, 1763, 1059–1066. [Google Scholar] [CrossRef]
- Lao, X.; Li, B.; Liu, M.; Chen, J.; Gao, X.; Zheng, H. Increased antitumor activity of tumor-specific peptide modified thymopentin. Biochimie 2014, 107 Pt B, 277–285. [Google Scholar] [CrossRef]
- Wei, X.; Zhang, L.; Zhang, R.; Wu, R.; Petitte, J.N.; Hou, Y.; Si, D.; Ahmad, B.; Guo, H.; Zhang, M.; et al. Targeting the TLR2 Receptor with a Novel Thymopentin-Derived Peptide Modulates Immune Responses. Front. Immunol. 2021, 12, 620494. [Google Scholar] [CrossRef]
- Xiaojing, C.; Yanfang, L.; Yanqing, G.; Fangfang, C. Thymopentin improves cardiac function in older patients with chronic heart failure. Anatol. J. Cardiol. 2017, 17, 24–30. [Google Scholar] [CrossRef]
- Fujioka, N.; Nguyen, J.; Chen, C.; Li, Y.; Pasrija, T.; Niehans, G.; Johnson, K.N.; Gupta, V.; Kratzke, R.A.; Gupta, K. Morphine-induced epidermal growth factor pathway activation in non-small cell lung cancer. Anesth. Analg. 2011, 113, 1353–1364. [Google Scholar] [CrossRef] [PubMed]
- Kuzumaki, N.; Suzuki, A.; Narita, M.; Hosoya, T.; Nagasawa, A.; Imai, S.; Yamamizu, K.; Morita, H.; Nagase, H.; Okada, Y.; et al. Effect of kappa-opioid receptor agonist on the growth of non-small cell lung cancer (NSCLC) cells. Br. J. Cancer 2012, 106, 1148–1152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sun, M.Y.; Zhang, X.; Guo, C.R.; Lei, Y.T.; Wang, W.H.; Fan, Y.Z.; Cao, P.; Li, C.Z.; Wang, R.; et al. Dynamic recognition of naloxone, morphine and endomorphin1 in the same pocket of micro-opioid receptors. Front. Mol. Biosci. 2022, 9, 925404. [Google Scholar] [CrossRef] [PubMed]
- Willingham, S.B.; Volkmer, J.P.; Gentles, A.J.; Sahoo, D.; Dalerba, P.; Mitra, S.S.; Wang, J.; Contreras-Trujillo, H.; Martin, R.; Cohen, J.D.; et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc. Natl. Acad. Sci. USA 2012, 109, 6662–6667. [Google Scholar] [CrossRef] [PubMed]
- Podolnikova, N.P.; Brothwell, J.A.; Ugarova, T.P. The opioid peptide dynorphin A induces leukocyte responses via integrin Mac-1 (alphaMbeta2, CD11b/CD18). Mol. Pain 2015, 11, 33. [Google Scholar] [CrossRef]
- Yao, X.; Dong, G.; Zhu, Y.; Yan, F.; Zhang, H.; Ma, Q.; Fu, X.; Li, X.; Zhang, Q.; Zhang, J.; et al. Leukadherin-1-Mediated Activation of CD11b Inhibits LPS-Induced Pro-inflammatory Response in Macrophages and Protects Mice Against Endotoxic Shock by Blocking LPS-TLR4 Interaction. Front. Immunol. 2019, 10, 215. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Jiang, Y.; Liang, S.; Cheng, Z.; Zhu, X.; Guo, Q. Opioids Regulate the Immune System: Focusing on Macrophages and Their Organelles. Front. Pharmacol. 2021, 12, 814241. [Google Scholar] [CrossRef] [PubMed]
- Tomassini, N.; Renaud, F.L.; Roy, S.; Loh, H.H. Mu and delta receptors mediate morphine effects on phagocytosis by murine peritoneal macrophages. J. Neuroimmunol. 2003, 136, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Finn, A.K.; Whistler, J.L. Endocytosis of the mu opioid receptor reduces tolerance and a cellular hallmark of opiate withdrawal. Neuron 2001, 32, 829–839. [Google Scholar] [CrossRef]
- Yu, P.C.; Liu, D.; Han, Z.X.; Liang, F.; Hao, C.Y.; Lei, Y.T.; Guo, C.R.; Wang, W.H.; Li, X.H.; Yang, X.N.; et al. Thymopentin-Mediated Inhibition of Cancer Stem Cell Stemness Enhances the Cytotoxic Effect of Oxaliplatin on Colon Cancer Cells. Front. Pharmacol. 2022, 13, 779715. [Google Scholar] [CrossRef]
- Reddy, A.; Vidal, M.; Stephen, S.; Baumgartner, K.; Dost, S.; Nguyen, A.; Heung, Y.; Kwan, S.; Wong, A.; Pangemanan, I.; et al. The Conversion Ratio from Intravenous Hydromorphone to Oral Opioids in Cancer Patients. J. Pain Symptom Manag. 2017, 54, 280–288. [Google Scholar] [CrossRef]
- Franken, L.G.; Masman, A.D.; de Winter, B.C.; Koch, B.C.; Baar, F.P.; Tibboel, D.; van Gelder, T.; Mathot, R.A. Pharmacokinetics of Morphine, Morphine-3-Glucuronide and Morphine-6-Glucuronide in Terminally Ill Adult Patients. Clin. Pharmacokinet. 2016, 55, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.L.; Faura, C.C.; Moore, R.A.; McQuay, H.J. Peak plasma concentrations after oral morphine: A systematic review. J. Pain Symptom Manag. 1998, 16, 388–402. [Google Scholar] [CrossRef] [PubMed]
- Lotsch, J.; Skarke, C.; Schmidt, H.; Liefhold, J.; Geisslinger, G. Pharmacokinetic modeling to predict morphine and morphine-6-glucuronide plasma concentrations in healthy young volunteers. Clin. Pharmacol. Ther. 2002, 72, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, D.; Gawali, V.S.; Kallay, L.; Toukam, D.K.; Koehler, A.; Stambrook, P.; Krummel, D.P.; Sengupta, S. Therapeutically leveraging GABA(A) receptors in cancer. Exp. Biol. Med. 2021, 246, 2128–2135. [Google Scholar] [CrossRef]
- Papa, I.; Saliba, D.; Ponzoni, M.; Bustamante, S.; Canete, P.F.; Gonzalez-Figueroa, P.; McNamara, H.A.; Valvo, S.; Grimbaldeston, M.; Sweet, R.A.; et al. T(FH)-derived dopamine accelerates productive synapses in germinal centres. Nature 2017, 547, 318–323. [Google Scholar] [CrossRef]
- Ramirez, V.T.; Godinez, D.R.; Brust-Mascher, I.; Nonnecke, E.B.; Castillo, P.A.; Gardner, M.B.; Tu, D.; Sladek, J.A.; Miller, E.N.; Lebrilla, C.B.; et al. T-cell derived acetylcholine aids host defenses during enteric bacterial infection with Citrobacter rodentium. PLoS Pathog. 2019, 15, e1007719. [Google Scholar] [CrossRef]
- Schloss, M.J.; Hulsmans, M.; Rohde, D.; Lee, I.H.; Severe, N.; Foy, B.H.; Pulous, F.E.; Zhang, S.; Kokkaliaris, K.D.; Frodermann, V.; et al. B lymphocyte-derived acetylcholine limits steady-state and emergency hematopoiesis. Nat. Immunol. 2022, 23, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Iurato, M.P.; Chiarenza, A.; Barbera, N.; Cantarella, G.; Lempereur, L.; Drago, F.; Scapagini, U.; Bernardini, R. Thymic factors influence on behavior in rodents. Pharmacol. Biochem. Behav. 1993, 44, 665–671. [Google Scholar] [CrossRef]
- Wolf, E.; Milazzo, S.; Boehm, K.; Zwahlen, M.; Horneber, M. Thymic peptides for treatment of cancer patients. Cochrane Database Syst. Rev. 2011, 2011, CD003993. [Google Scholar] [CrossRef] [Green Version]
- Cascinelli, N. Margin of resection in the management of primary melanoma. Semin. Surg. Oncol. 1998, 14, 272–275. [Google Scholar] [CrossRef]
- Ren, Z.; He, Y.; Yang, Q.; Guo, J.; Huang, H.; Li, B.; Wang, D.; Yang, Z.; Tian, X. A Comprehensive Analysis of the Glutathione Peroxidase 8 (GPX8) in Human Cancer. Front. Oncol. 2022, 12, 812811. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.Y.; Zhang, X.; Yu, P.C.; Liu, D.; Yang, Y.; Cui, W.W.; Yang, X.N.; Lei, Y.T.; Li, X.H.; Wang, W.H.; et al. Vanilloid agonist-mediated activation of TRPV1 channels requires coordinated movement of the S1–S4 bundle rather than a quiescent state. Sci. Bull. 2022, 67, 1062–1076. [Google Scholar] [CrossRef]
- He, X.; Wan, J.; Yang, X.; Zhang, X.; Huang, D.; Li, X.; Zou, Y.; Chen, C.; Yu, Z.; Xie, L.; et al. Bone marrow niche ATP levels determine leukemia-initiating cell activity via P2X7 in leukemic models. J. Clin. Invest. 2021, 131, JCI140242. [Google Scholar] [CrossRef]
- Barkal, A.A.; Brewer, R.E.; Markovic, M.; Kowarsky, M.; Barkal, S.A.; Zaro, B.W.; Krishnan, V.; Hatakeyama, J.; Dorigo, O.; Barkal, L.J.; et al. CD24 signalling through macrophage Siglec-10 is a target for cancer immunotherapy. Nature 2019, 572, 392–396. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Q.; Hou, B.; Zhang, W.; Yan, M.; Jia, H.; Li, H.; Yan, D.; Zheng, F.; Ding, W.; et al. Concomitant targeting of multiple key transcription factors effectively disrupts cancer stem cells enriched in side population of human pancreatic cancer cells. PLoS ONE 2013, 8, e73942. [Google Scholar] [CrossRef]
- Foldbjerg, R.; Dang, D.A.; Autrup, H. Cytotoxicity and genotoxicity of silver nanoparticles in the human lung cancer cell line, A549. Arch. Toxicol. 2011, 85, 743–750. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, P.-C.; Hao, C.-Y.; Fan, Y.-Z.; Liu, D.; Qiao, Y.-F.; Yao, J.-B.; Li, C.-Z.; Yu, Y. Altered Membrane Expression and Function of CD11b Play a Role in the Immunosuppressive Effects of Morphine on Macrophages at the Nanomolar Level. Pharmaceuticals 2023, 16, 282. https://doi.org/10.3390/ph16020282
Yu P-C, Hao C-Y, Fan Y-Z, Liu D, Qiao Y-F, Yao J-B, Li C-Z, Yu Y. Altered Membrane Expression and Function of CD11b Play a Role in the Immunosuppressive Effects of Morphine on Macrophages at the Nanomolar Level. Pharmaceuticals. 2023; 16(2):282. https://doi.org/10.3390/ph16020282
Chicago/Turabian StyleYu, Peng-Cheng, Cui-Yun Hao, Ying-Zhe Fan, Di Liu, Yi-Fan Qiao, Jia-Bao Yao, Chang-Zhu Li, and Ye Yu. 2023. "Altered Membrane Expression and Function of CD11b Play a Role in the Immunosuppressive Effects of Morphine on Macrophages at the Nanomolar Level" Pharmaceuticals 16, no. 2: 282. https://doi.org/10.3390/ph16020282
APA StyleYu, P.-C., Hao, C.-Y., Fan, Y.-Z., Liu, D., Qiao, Y.-F., Yao, J.-B., Li, C.-Z., & Yu, Y. (2023). Altered Membrane Expression and Function of CD11b Play a Role in the Immunosuppressive Effects of Morphine on Macrophages at the Nanomolar Level. Pharmaceuticals, 16(2), 282. https://doi.org/10.3390/ph16020282