Autophagy Induction by Scutellaria Flavones in Cancer: Recent Advances
Abstract
:1. Introduction
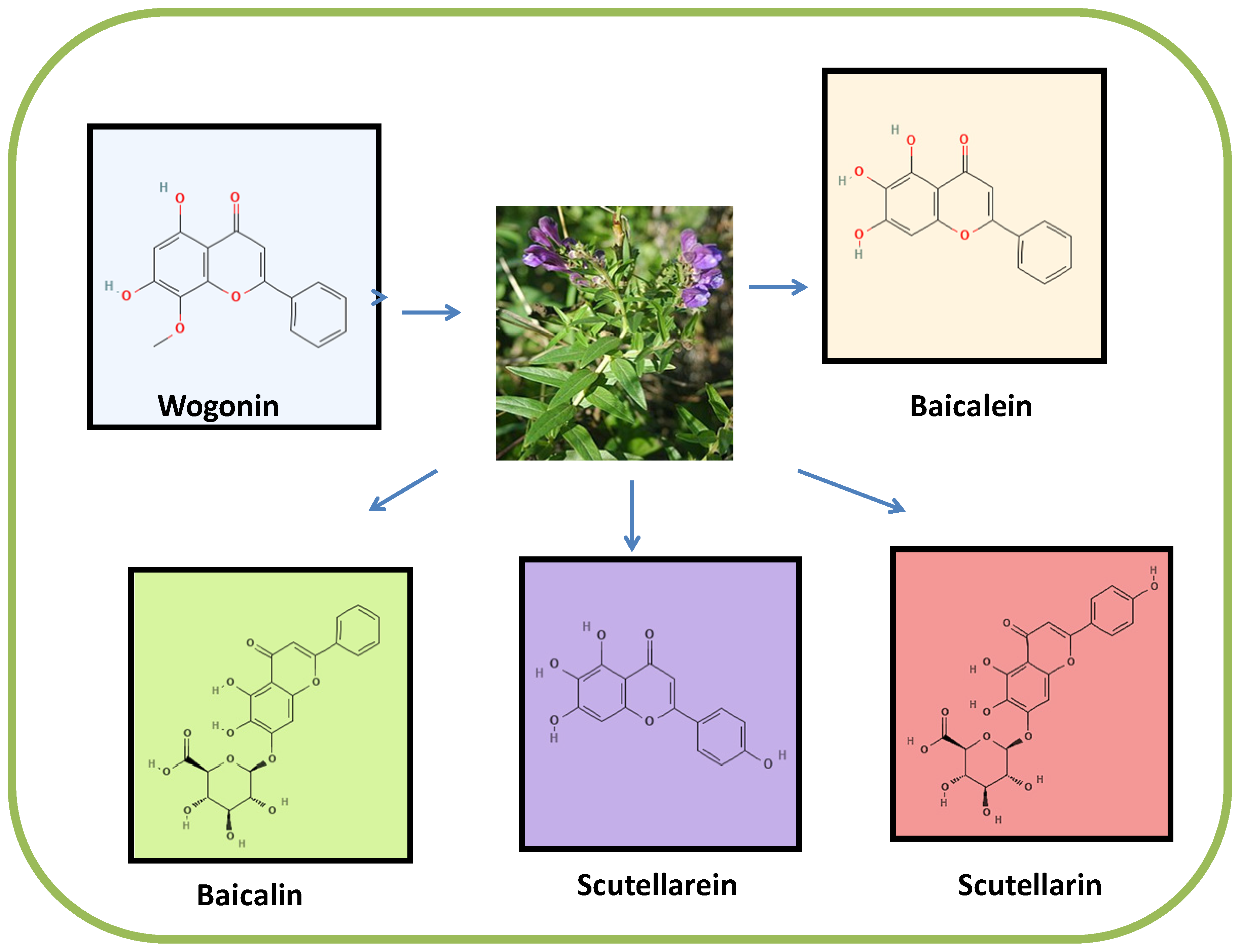
2. Sources and Chemistry of Scutellaria Flavones (Wogonin, Baicalein, Baicalin, Scutellarein and Scutellarin)
2.1. Wogonin
2.2. Baicalein
2.3. Baicalin
2.4. Scutellarein
2.5. Scutellarin
3. Absorption and Metabolism of Scutellaria Flavones
4. Mechanistic Role of Autophagic Death in Cancer
4.1. Antitumorigenic
4.2. Protumorigenic
5. Regulation of Autophagy by Scutellaria Flavones
5.1. Wogonin and Autophagy Induction
5.2. Baicalein and Baicalin Induce Autophagy
5.3. Scutellarein and Scutellarin Induce Autophagy
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petrovska, B.B. Historical Review of Medicinal Plants’ Usage. Pharmacogn. Rev. 2012, 6, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ekor, M. The Growing Use of Herbal Medicines: Issues Relating to Adverse Reactions and Challenges in Monitoring Safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sak, K. Anticancer Action of Plant Products: Changing Stereotyped Attitudes. Explor. Target Antitumor. Ther. 2022, 3, 423–427. [Google Scholar] [CrossRef]
- Hsieh, Y.-S.; Yang, S.-F.; Sethi, G.; Hu, D.-N. Natural Bioactives in Cancer Treatment and Prevention. Biomed. Res. Int. 2015, 2015, 182835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salehi, B.; Quispe, C.; Imran, M.; Ul-Haq, I.; Živković, J.; Abu-Reidah, I.M.; Sen, S.; Taheri, Y.; Acharya, K.; Azadi, H.; et al. Nigella Plants—Traditional Uses, Bioactive Phytoconstituents, Preclinical and Clinical Studies. Front. Pharmacol. 2021, 12, 625386. [Google Scholar] [CrossRef] [PubMed]
- Li-Weber, M. New Therapeutic Aspects of Flavones: The Anticancer Properties of Scutellaria and Its Main Active Constituents Wogonin, Baicalein and Baicalin. Cancer Treat. Rev. 2009, 35, 57–68. [Google Scholar] [CrossRef]
- Shah, M.; Mubin, S.; Hassan, S.S.U.; Tagde, P.; Ullah, O.; Rahman, M.H.; Al-Harrasi, A.; Rehman, N.U.; Murad, W. Phytochemical Profiling and Bio-Potentiality of Genus Scutellaria: Biomedical Approach. Biomolecules 2022, 12, 936. [Google Scholar] [CrossRef] [PubMed]
- EghbaliFeriz, S.; Taleghani, A.; Tayarani-Najaran, Z. Scutellaria: Debates on the Anticancer Property. Biomed. Pharmacother. 2018, 105, 1299–1310. [Google Scholar] [CrossRef]
- Singh, S.; Meena, A.; Luqman, S. Baicalin Mediated Regulation of Key Signaling Pathways in Cancer. Pharmacol. Res. 2021, 164, 105387. [Google Scholar] [CrossRef]
- Liu, H.; Dong, Y.; Gao, Y.; Du, Z.; Wang, Y.; Cheng, P.; Chen, A.; Huang, H. The Fascinating Effects of Baicalein on Cancer: A Review. Int. J. Mol. Sci. 2016, 17, 1681. [Google Scholar] [CrossRef]
- Banik, K.; Khatoon, E.; Harsha, C.; Rana, V.; Parama, D.; Thakur, K.K.; Bishayee, A.; Kunnumakkara, A.B. Wogonin and Its Analogs for the Prevention and Treatment of Cancer: A Systematic Review. Phytother. Res. 2022, 36, 1854–1883. [Google Scholar] [CrossRef] [PubMed]
- Huynh, D.L.; Sharma, N.; Kumar Singh, A.; Singh Sodhi, S.; Zhang, J.J.; Mongre, R.K.; Ghosh, M.; Kim, N.; Ho Park, Y.; Kee Jeong, D. Anti-tumor activity of wogonin, an extract from Scutellaria baicalensis, through regulating different signaling pathways. Chin. J. Nat. Med. 2017, 15, 15–40. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, T.; Tao, W.; Liao, N.; Yan, Q.; Li, L.; Tan, J.; Shen, W.; Cheng, H.; Sun, D. Flavonoids from Scutellaria Barbata D. Don Exert Antitumor Activity in Colorectal Cancer through Inhibited Autophagy and Promoted Apoptosis via ATF4/Sestrin2 Pathway. Phytomedicine 2022, 99, 154007. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.; Nguyen, V.H.; A’lincourt Salazar, M.; Wong, P.; Diamond, D.J.; Yim, J.H.; Melstrom, L.G. Inhibition of Autophagy Amplifies Baicalein-Induced Apoptosis in Human Colorectal Cancer. Mol. Ther. Oncolytics 2020, 19, 1–7. [Google Scholar] [CrossRef]
- Kim, H.I.; Hong, S.H.; Ku, J.M.; Lim, Y.S.; Lee, S.J.; Song, J.; Kim, T.Y.; Cheon, C.; Ko, S.-G. Scutellaria Radix Promotes Apoptosis in Non-Small Cell Lung Cancer Cells via Induction of AMPK-Dependent Autophagy. Am. J. Chin. Med. 2019, 47, 691–705. [Google Scholar] [CrossRef]
- Bisol, Â.; Campos, P.S.; Lamers, M.L. Flavonoids as Anticancer Therapies: A Systematic Review of Clinical Trials. Phytother. Res. 2020, 34, 568–582. [Google Scholar] [CrossRef]
- Elkin, Y.N.; Kulesh, N.I.; Stepanova, A.Y.; Solovieva, A.I.; Kargin, V.M.; Manyakhin, A.Y. Methylated Flavones of the Hairy Root Culture Scutellaria Baicalensis. J. Plant Physiol. 2018, 231, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Ye, J.; Gao, L.; Liu, Y. The Main Bioactive Compounds of Scutellaria Baicalensis Georgi. for Alleviation of Inflammatory Cytokines: A Comprehensive Review. Biomed. Pharmacother. 2021, 133, 110917. [Google Scholar] [CrossRef]
- Timberlake, C.F.; Henry, B.S. Plant Pigments as Natural Food Colours. Endeavour 1986, 10, 31–36. [Google Scholar] [CrossRef]
- Shang, X.; He, X.; He, X.; Li, M.; Zhang, R.; Fan, P.; Zhang, Q.; Jia, Z. The Genus Scutellaria an Ethnopharmacological and Phytochemical Review. J. Ethnopharmacol. 2010, 128, 279–313. [Google Scholar] [CrossRef]
- Hui, K.M.; Huen, M.S.Y.; Wang, H.Y.; Zheng, H.; Sigel, E.; Baur, R.; Ren, H.; Li, Z.W.; Wong, J.T.-F.; Xue, H. Anxiolytic Effect of Wogonin, a Benzodiazepine Receptor Ligand Isolated from Scutellaria Baicalensis Georgi. Biochem. Pharmacol. 2002, 64, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- INAGAKI, I.; HISADA, S.; SHIMA, K. Studies on the Constituents of Anodendron Affine DURCE. I.: Isolation of Wogonin, Dambonitol, Sucrose and Some Other Components from Stems. Yakugaku Zasshi 1971, 91, 1133–1136. [Google Scholar] [CrossRef] [Green Version]
- Sukmawati, R.; Guntoro, D.; Junaedi, A. Flavone Content Analysis of Wogonin (5,7- Dihydroxy-8-Methoxy) from Tetracera Indica L. MERR. Rasayan J. Chem. 2020, 13, 1124–1128. [Google Scholar] [CrossRef]
- Wessely, F.; Moser, G.H. Synthese und Konstitution des Skutellareins. Monatsh. Chem. 1930, 56, 97–105. [Google Scholar] [CrossRef]
- Xia, Y.; Wu, C.; Liu, W.; Feng, F.; Li, Z. A Novel Process Related Impurity and Forced Degradation Study of Synthetic Wogonin: Development of a Liquid Chromatographic Method for Purity Control. J. Pharm. Biomed. Anal. 2012, 71, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Huang, K.; Yang, X.; Xu, H. Free Radical Scavenging and Antioxidant Activities of Flavonoids Extracted from the Radix of Scutellaria Baicalensis Georgi. Biochim. Biophys. Acta Gen. Subj. 1999, 1472, 643–650. [Google Scholar] [CrossRef]
- Park, B.K.; Heo, M.Y.; Park, H.; Kim, H.P. Inhibition of TPA-Induced Cyclooxygenase-2 Expression and Skin Inflammation in Mice by Wogonin, a Plant Flavone from Scutellaria Radix. Eur. J. Pharmacol. 2001, 425, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Enomoto, R.; Koshiba, C.; Hirano, H. Inhibition of P-Glycoprotein by Wogonin Is Involved with the Potentiation of Etoposide-Induced Apoptosis in Cancer Cells. Ann. N. Y. Acad. Sci. 2009, 1171, 132–136. [Google Scholar] [CrossRef]
- Zhao, Z.; Nian, M.; Qiao, H.; Yang, X.; Wu, S.; Zheng, X. Review of Bioactivity and Structure-Activity Relationship on Baicalein (5,6,7-Trihydroxyflavone) and Wogonin (5,7-Dihydroxy-8-Methoxyflavone) Derivatives: Structural Modifications Inspired from Flavonoids in Scutellaria Baicalensis. Eur. J. Med. Chem. 2022, 243, 114733. [Google Scholar] [CrossRef]
- Moghaddam, E.; Teoh, B.-T.; Sam, S.-S.; Lani, R.; Hassandarvish, P.; Chik, Z.; Yueh, A.; Abubakar, S.; Zandi, K. Baicalin, a Metabolite of Baicalein with Antiviral Activity against Dengue Virus. Sci. Rep. 2015, 4, 5452. [Google Scholar] [CrossRef]
- Cathcart, M.-C.; Useckaite, Z.; Drakeford, C.; Semik, V.; Lysaght, J.; Gately, K.; O’Byrne, K.J.; Pidgeon, G.P. Anti-Cancer Effects of Baicalein in Non-Small Cell Lung Cancer in-Vitro and in-Vivo. BMC Cancer 2016, 16, 707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, K.; Li, J.; Xu, J.; Zhang, L.; Liu, Q.-Y.; Huang, Y.-K.; Zhang, Z.-G.; Kang, Y.; Xu, C.-J. Baicalein Ameliorates Chronic Stress-Mediated Ovarian Dysfunction by Upregulating the Expression of Gamma-Aminobutyric Acid B2 Receptor. Reprod. Dev. Med. 2018, 2, 21–29. [Google Scholar] [CrossRef]
- Takahashi, H.; Chen, M.C.; Pham, H.; Angst, E.; King, J.C.; Park, J.; Brovman, E.Y.; Ishiguro, H.; Harris, D.M.; Reber, H.A.; et al. Baicalein, a Component of Scutellaria Baicalensis, Induces Apoptosis by Mcl-1 down-Regulation in Human Pancreatic Cancer Cells. Biochim. Biophys. Acta Mol. Cell Res. 2011, 1813, 1465–1474. [Google Scholar] [CrossRef] [Green Version]
- Ruan, C.; Xiao, X.; Li, G. Microwave-Assisted Extraction Coupled with Countercurrent Chromatography for the Rapid Preparation of Flavonoids from Scutellaria Barbata D. Don. J. Sep. Sci. 2014, 37, 1364–1369. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Han, X.; Chen, P.; Wu, Q.; Feng, J.; Duan, T.; Chen, X.; Pan, T.; Yan, L.; Jin, T.; et al. Baicalin Induces Apoptosis and Suppresses the Cell Cycle Progression of Lung Cancer Cells Through Downregulating Akt/MTOR Signaling Pathway. Front. Mol. Biosci. 2021, 7, 602282. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Zhan, S.; Wang, Y.; Zhou, G.; Liang, H.; Chen, X.; Shen, H. Baicalin, the Major Component of Traditional Chinese Medicine Scutellaria Baicalensis Induces Colon Cancer Cell Apoptosis through Inhibition of OncomiRNAs. Sci. Rep. 2018, 8, 14477. [Google Scholar] [CrossRef] [Green Version]
- Lu, Q.-Y.; Zhang, L.; Moro, A.; Chen, M.C.; Harris, D.M.; Eibl, G.; Go, V.-L.W. Detection of Baicalin Metabolites Baicalein and Oroxylin-A in Mouse Pancreas and Pancreatic Xenografts. Pancreas 2012, 41, 571–576. [Google Scholar] [CrossRef] [Green Version]
- Li, H.-B.; Chen, F. Isolation and Purification of Baicalein, Wogonin and Oroxylin A from the Medicinal Plant Scutellaria Baicalensis by High-Speed Counter-Current Chromatography. J. Chromatogr. A 2005, 1074, 107–110. [Google Scholar] [CrossRef]
- Lin, M.-Y.; Cheng, W.-T.; Cheng, H.-C.; Chou, W.-C.; Chen, H.-I.; Ou, H.-C.; Tsai, K.-L. Baicalin Enhances Chemosensitivity to Doxorubicin in Breast Cancer Cells via Upregulation of Oxidative Stress-Mediated Mitochondria-Dependent Apoptosis. Antioxidants 2021, 10, 1506. [Google Scholar] [CrossRef]
- Wang, S.; Fu, J.L.; Hao, H.F.; Jiao, Y.N.; Li, P.P.; Han, S.Y. Metabolic reprogramming by traditional Chinese medicine and its role in effective cancer therapy. Pharmacol. Res. 2021, 170, 105728. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Li, Q.; Zhou, X.; Pang, Q.; Xu, Y. A UHPLC–MS/MS Method for Simultaneous Determination of Twelve Constituents from Erigeron Breviscapus Extract in Rat Plasma: Application to a Pharmacokinetic Study. J. Chromatogr. B 2017, 1046, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tan, L.; Jiao, K.; Xue, C.; Tang, Q.; Jiang, S.; Ren, Y.; Chen, H.; El-Aziz, T.M.A.; Abdelazeem, K.N.M.; et al. Scutellarein Attenuates Atopic Dermatitis by Selectively Inhibiting Transient Receptor Potential Vanilloid 3 Channels. Br. J. Pharmacol. 2022, 179, 4792–4808. [Google Scholar] [CrossRef] [PubMed]
- Gu, T.; Zhong, Y.; Lu, Y.-T.; Sun, Y.; Dong, Z.-X.; Wu, W.-Y.; Shi, Z.-H.; Li, N.-G.; Xue, X.; Fang, F.; et al. Synthesis and Bioactivity Characterization of Scutellarein Sulfonated Derivative. Molecules 2017, 22, 1028. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Firrman, J.; Liu, L.; Yam, K. A Review on Flavonoid Apigenin: Dietary Intake, ADME, Antimicrobial Effects, and Interactions with Human Gut Microbiota. Biomed. Res. Int. 2019, 2019, 7010467. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Meng, Z.; Chen, Y.; Yu, L.; Gao, B.; Zheng, Y.; Guan, S. Apigenin Induced Autophagy and Stimulated Autophagic Lipid Degradation. Food Funct. 2020, 11, 9208–9215. [Google Scholar] [CrossRef] [PubMed]
- Ni, G.; Tang, Y.; Li, M.; He, Y.; Rao, G. Synthesis of Scutellarein Derivatives with a Long Aliphatic Chain and Their Biological Evaluation against Human Cancer Cells. Molecules 2018, 23, 310. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.; Tian, T.; Zheng, Y.; Zhou, L.; Dai, C.; Wang, M.; Lin, S.; Deng, Y.; Hao, Q.; Zhai, Z.; et al. Scutellarin Inhibits Proliferation and Invasion of Hepatocellular Carcinoma Cells via Down-regulation of JAK2/STAT3 Pathway. J. Cell. Mol. Med. 2019, 23, 3040–3044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, Y.; Fang, M.; Wu, C.-Y.; Ling, E.-A. Scutellarin as a Potential Therapeutic Agent for Microglia-Mediated Neuroinflammation in Cerebral Ischemia. Neuromolecular Med. 2016, 18, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Zhou, Y.; Jiang, Z.; Zhao, Y.; Zhang, D.; Cong, X.; Cao, R.; Li, H.; Tian, W. Cytotoxic and Chemosensitization Effects of Scutellarin from Traditional Chinese Herb Scutellaria Altissima L. in Human Prostate Cancer Cells. Oncol. Rep. 2017, 38, 1491–1499. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.; Zhao, Y.-L.; Yang, X.; Liao, X.-L.; Yang, J.; Zhang, J.-H.; Gao, C.-Z. Scutellarin-Cyclodextrin Conjugates: Synthesis, Characterization and Anticancer Activity. Carbohydr. Polym. 2013, 92, 1308–1314. [Google Scholar] [CrossRef]
- Liu, Y.; Jing, Y.-Y.; Zeng, C.-Y.; Li, C.-G.; Xu, L.-H.; Yan, L.; Bai, W.-J.; Zha, Q.-B.; Ouyang, D.-Y.; He, X.-H. Scutellarin Suppresses NLRP3 Inflammasome Activation in Macrophages and Protects Mice against Bacterial Sepsis. Front. Pharmacol. 2018, 8, 975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, E.W.C.; Lim, C.S.Q.; Lim, W.Y.; Loong, Z.J.; Wong, C.W. Role of Scutellarin in Human Cancer—A Review. J. Appl. Pharm. Sci. 2019, 9, 143–146. [Google Scholar]
- Hollman, P.C.H.; Bijsman, M.N.C.P.; van Gameren, Y.; Cnossen, E.P.J.; de Vries, J.H.M.; Katan, M.B. The Sugar Moiety Is a Major Determinant of the Absorption of Dietary Flavonoid Glycosides in Man. Free Radic. Res. 1999, 31, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J. Dietary Flavonoid Aglycones and Their Glycosides: Which Show Better Biological Significance? Crit. Rev. Food Sci. Nutr. 2015, 57, 1874–1905. [Google Scholar] [CrossRef]
- Naeem, A.; Ming, Y.; Pengyi, H.; Jie, K.Y.; Yali, L.; Haiyan, Z.; Shuai, X.; Wenjing, L.; Ling, W.; Xia, Z.M.; et al. The Fate of Flavonoids after Oral Administration: A Comprehensive Overview of Its Bioavailability. Crit. Rev. Food Sci. Nutr. 2022, 62, 6169–6186. [Google Scholar] [CrossRef]
- Day, A.J.; Cañada, F.J.; Dñaz, J.C.; Kroon, P.A.; Mclauchlan, R.; Faulds, C.B.; Plumb, G.W.; Morgan, M.R.A.; Williamson, G. Dietary Flavonoid and Isoflavone Glycosides Are Hydrolysed by the Lactase Site of Lactase Phlorizin Hydrolase. FEBS Lett. 2000, 468, 166–170. [Google Scholar] [CrossRef] [Green Version]
- Akhlaghi, M.; Foshati, S. Bioavailability and Metabolism of Flavonoids: A Review. Int. J. Nutr. Sci. 2017, 2, 180–184. [Google Scholar]
- Xu, J.; Zhang, B.; Chu, Z.; Jiang, F.; Han, J. Wogonin Alleviates Cisplatin-Induced Cardiotoxicity in Mice Via Inhibiting Gasdermin D-Mediated Pyroptosis. J. Cardiovasc. Pharmacol. 2021, 78, 597–603. [Google Scholar] [CrossRef]
- Wang, T.; Long, F.; Jiang, G.; Cai, H.; Jiang, Q.; Cheng, K.; Hu, Z.; Wang, Y. Pharmacokinetic Properties of Wogonin and Its Herb-Drug Interactions with Docetaxel in Rats with Mammary Tumors. Biomed. Chromatogr. 2018, 32, e4264. [Google Scholar] [CrossRef]
- Fong, S.Y.K.; Wong, Y.C.; Zuo, Z. Development of a SPE-LC/MS/MS Method for Simultaneous Quantification of Baicalein, Wogonin, Oroxylin A and Their Glucuronides Baicalin, Wogonoside and Oroxyloside in Rats and Its Application to Brain Uptake and Plasma Pharmacokinetic Studies. J. Pharm. Biomed. Anal. 2014, 97, 9–23. [Google Scholar] [CrossRef]
- Wang, Q.; Shi, R.; Dai, Y.; Li, Y.; Wang, T.; Ma, Y.; Cheng, N. Mechanism in the Existent Difference in Form of Wogonin/Wogonoside between Plasma and Intestine/Liver in Rats. RSC Adv. 2018, 8, 3364–3373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talbi, A.; Zhao, D.; Liu, Q.; Li, J.; Fan, A.; Yang, W.; Han, X.; Chen, X. Pharmacokinetics, Tissue Distribution, Excretion and Plasma Protein Binding Studies of Wogonin in Rats. Molecules 2014, 19, 5538–5549. [Google Scholar] [CrossRef]
- Zhang, J.; Lv, H.; Jiang, K.; Gao, Y. Enhanced Bioavailability after Oral and Pulmonary Administration of Baicalein Nanocrystal. Int. J. Pharm. 2011, 420, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.-L.; Liang, X.-L.; Zhao, L.-J.; Liao, Z.-G.; Zhao, G.-W.; Cao, Y.-C.; Zhang, J.; Luo, Y. Elucidation of the Transport Mechanism of Baicalin and the Influence of a Radix Angelicae Dahuricae Extract on the Absorption of Baicalin in a Caco-2 Cell Monolayer Model. J. Ethnopharmacol. 2013, 150, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, X.; Li, J.; Liu, R.; Shu, L.; Jin, J. Effects of Labrasol on the Corneal Drug Delivery of Baicalin. Drug Deliv. 2009, 16, 399–404. [Google Scholar] [CrossRef]
- Taiming, L.; Xuehua, J. Investigation of the Absorption Mechanisms of Baicalin and Baicalein in Rats. J. Pharm. Sci. 2006, 95, 1326–1333. [Google Scholar] [CrossRef]
- Xing, J.; Chen, X.; Sun, Y.; Luan, Y.; Zhong, D. Interaction of Baicalin and Baicalein with Antibiotics in the Gastrointestinal Tract. J. Pharm. Pharmacol. 2010, 57, 743–750. [Google Scholar] [CrossRef]
- Lai, M.-Y.; Hsiu, S.-L.; Tsai, S.-Y.; Hou, Y.-C.; Chao, P.-D.L. Comparison of Metabolic Pharmacokinetics of Baicalin and Baicalein in Rats. J. Pharm. Pharmacol. 2010, 55, 205–209. [Google Scholar] [CrossRef]
- Wei, J.; Liu, R.; Zhang, J.; Liu, S.; Yan, D.; Wen, X.; Tian, X. Baicalin Enhanced Oral Bioavailability of Sorafenib in Rats by Inducing Intestine Absorption. Front. Pharmacol. 2021, 12. [Google Scholar] [CrossRef]
- Abe, K.; Inoue, O.; Yumioka, E. Biliary Excretion of Metabolites of Baicalin, and Baicalein in Rats. Chem. Pharm. Bull. 1990, 38, 208–211. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Lin, G.; Chang, Q.; Zuo, Z. Role of Intestinal First-Pass Metabolism of Baicalein in Its Absorption Process. Pharm. Res. 2005, 22, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-L.; Che, Q.-M.; Li, S.-Z.; Zhou, T.-H. Study on Metabolism of Scutellarin in Rats by HPLC-MS and HPLC-NMR. J. Asian Nat. Prod. Res. 2003, 5, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; Xia, H.; Zhang, T.; Di, X.; Qu, G.; Yao, X. Two Major Urinary Metabolites of Scutellarin in Rats. Planta Med. 2007, 73, 363–365. [Google Scholar] [CrossRef]
- Gao, H.-M.; Wang, Z.-M.; Tian, J. Pharmacokinetics and Metabolites of Scutellarin in Normal and Model Rats. Acta Pharm. Sin. 2005, 40, 1024–1027. [Google Scholar]
- Gao, C.; Zhang, H.; Guo, Z.; You, T.; Chen, X.; Zhong, D. Mechanistic Studies on the Absorption and Disposition of Scutellarin in Humans: Selective OATP2B1-Mediated Hepatic Uptake Is a Likely Key Determinant for Its Unique Pharmacokinetic Characteristics. Drug Metab. Dispos. 2012, 40, 2009–2020. [Google Scholar] [CrossRef] [Green Version]
- Gao, C.; Chen, X.; Zhong, D. Absorption and Disposition of Scutellarin in Rats: A Pharmacokinetic Explanation for the High Exposure of Its Isomeric Metabolite. Drug Metab. Dispos. 2011, 39, 2034–2044. [Google Scholar] [CrossRef]
- Patra, S.; Mishra, S.R.; Behera, B.P.; Mahapatra, K.K.; Panigrahi, D.P.; Bhol, C.S.; Praharaj, P.P.; Sethi, G.; Patra, S.K.; Bhutia, S.K. Autophagy-Modulating Phytochemicals in Cancer Therapeutics: Current Evidences and Future Perspectives. Semin. Cancer Biol. 2022, 80, 205–217. [Google Scholar] [CrossRef]
- Bhutia, S.K.; Behera, B.; Nandini Das, D.; Mukhopadhyay, S.; Sinha, N.; Panda, P.K.; Naik, P.P.; Patra, S.K.; Mandal, M.; Sarkar, S.; et al. Abrus Agglutinin Is a Potent Anti-Proliferative and Anti-Angiogenic Agent in Human Breast Cancer. Int. J. Cancer 2016, 139, 457–466. [Google Scholar] [CrossRef] [Green Version]
- Mathew, L.K.; Sengupta, S.; Kawakami, A.; Andreasen, E.A.; Löhr, C.V.; Loynes, C.A.; Renshaw, S.A.; Peterson, R.T.; Tanguay, R.L. Unraveling Tissue Regeneration Pathways Using Chemical Genetics. J. Biol. Chem. 2007, 282, 35202–35210. [Google Scholar] [CrossRef] [Green Version]
- Kanzawa, T.; Bedwell, J.; Kondo, Y.; Kondo, S.; Germano, I.M. Inhibition of DNA Repair for Sensitizing Resistant Glioma Cells to Temozolomide. J. Neurosurg. 2003, 99, 1047–1052. [Google Scholar] [CrossRef] [Green Version]
- Ostenfeld, M.S.; Fehrenbacher, N.; Høyer-Hansen, M.; Thomsen, C.; Farkas, T.; Jäättelä, M. Effective Tumor Cell Death by σ-2 Receptor Ligand Siramesine Involves Lysosomal Leakage and Oxidative Stress. Cancer Res. 2005, 65, 8975–8983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fazi, B.; Bursch, W.; Fimia, G.M.; Nardacci, R.; Piacentini, M.; di Sano, F.; Piredda, L. Fenretinide Induces Autophagic Cell Death in Caspase-Defective Breast Cancer Cells. Autophagy 2008, 4, 435–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grandér, D.; Kharaziha, P.; Laane, E.; Pokrovskaja, K.; Panaretakis, T. Autophagy as the Main Means of Cytotoxicity by Glucocorticoids in Hematological Malignancies. Autophagy 2009, 5, 1198–1200. [Google Scholar] [CrossRef] [PubMed]
- Laane, E.; Tamm, K.P.; Buentke, E.; Ito, K.; Khahariza, P.; Oscarsson, J.; Corcoran, M.; Björklund, A.-C.; Hultenby, K.; Lundin, J.; et al. Cell Death Induced by Dexamethasone in Lymphoid Leukemia Is Mediated through Initiation of Autophagy. Cell Death Differ. 2009, 16, 1018–1029. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.-H.; Sarbassov, D.D.; Ali, S.M.; King, J.E.; Latek, R.R.; Erdjument-Bromage, H.; Tempst, P.; Sabatini, D.M. MTOR Interacts with Raptor to Form a Nutrient-Sensitive Complex That Signals to the Cell Growth Machinery. Cell 2002, 110, 163–175. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.; Zhang, L.; Zhang, C.; Hao, Y.; Zhao, X. Rapamycin May Inhibit Murine S180 Sarcoma Growth by Regulating the Pathways Associated with Autophagy and Cancer Stem Cells. J. Cancer Res. Ther. 2019, 15, 398–403. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Yang, P.-M.; Shun, C.-T.; Wu, M.-S.; Weng, J.-R.; Chen, C.-C. Autophagy Potentiates the Anti-Cancer Effects of the Histone Deacetylase Inhibitors in Hepatocellular Carcinoma. Autophagy 2010, 6, 1057–1065. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.; Han, L.; Weng, J.; Wang, K.; Chen, T. Rapamycin Inhibits Proliferation and Induces Autophagy in Human Neuroblastoma Cells. Biosci. Rep. 2018, 38, BSR20181822. [Google Scholar] [CrossRef] [Green Version]
- Metibemu, D.S.; Akinloye, O.A.; Akamo, A.J.; Ojo, D.A.; Okeowo, O.T.; Omotuyi, I.O. Exploring Receptor Tyrosine Kinases-Inhibitors in Cancer Treatments. Egypt. J. Med. Hum. Genet. 2019, 20, 35. [Google Scholar] [CrossRef] [Green Version]
- Kondo, Y.; Kondo, S. Autophagy and Cancer Therapy. Autophagy 2006, 2, 85–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, R.; Chen, Y.; Liu, G.; Li, C.; Song, Y.; Cao, Z.; Li, W.; Hu, J.; Lu, C.; Liu, Y. PI3K/AKT Pathway as a Key Link Modulates the Multidrug Resistance of Cancers. Cell Death Dis. 2020, 11, 797. [Google Scholar] [CrossRef] [PubMed]
- Filomeni, G.; de Zio, D.; Cecconi, F. Oxidative Stress and Autophagy: The Clash between Damage and Metabolic Needs. Cell Death Differ. 2015, 22, 377–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poillet-Perez, L.; White, E. Role of Tumor and Host Autophagy in Cancer Metabolism. Genes Dev. 2019, 33, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Chouaib, S.; Noman, M.Z.; Kosmatopoulos, K.; Curran, M.A. Hypoxic Stress: Obstacles and Opportunities for Innovative Immunotherapy of Cancer. Oncogene 2017, 36, 439–445. [Google Scholar] [CrossRef] [Green Version]
- Paglin, S.; Hollister, T.; Delohery, T.; Hackett, N.; McMahill, M.; Sphicas, E.; Domingo, D.; Yahalom, J. A Novel Response of Cancer Cells to Radiation Involves Autophagy and Formation of Acidic Vesicles. Cancer Res. 2001, 61, 439–444. [Google Scholar]
- Liang, C.; Feng, P.; Ku, B.; Dotan, I.; Canaani, D.; Oh, B.-H.; Jung, J.U. Autophagic and Tumour Suppressor Activity of a Novel Beclin1-Binding Protein UVRAG. Nat. Cell Biol. 2006, 8, 688–698. [Google Scholar] [CrossRef]
- Hoare, M.; Young, A.R.J.; Narita, M. Autophagy in Cancer: Having Your Cake and Eating It. Semin. Cancer Biol. 2011, 21, 397–404. [Google Scholar] [CrossRef]
- White, E.; DiPaola, R.S. The Double-Edged Sword of Autophagy Modulation in Cancer. Clin. Cancer Res. 2009, 15, 5308–5316. [Google Scholar] [CrossRef] [Green Version]
- Chen, N.; Karantza-Wadsworth, V. Role and Regulation of Autophagy in Cancer. Biochim. Biophys. Acta Mol. Cell Res. 2009, 1793, 1516–1523. [Google Scholar] [CrossRef] [Green Version]
- Sikder, S.; Kumari, S.; Mustafi, P.; Ramdas, N.; Padhi, S.; Saha, A.; Bhaduri, U.; Banerjee, B.; Manjithaya, R.; Kundu, T.K. Nonhistone Human Chromatin Protein PC4 Is Critical for Genomic Integrity and Negatively Regulates Autophagy. FEBS J. 2019, 286, 4422–4442. [Google Scholar] [CrossRef]
- Maclean, K.H.; Dorsey, F.C.; Cleveland, J.L.; Kastan, M.B. Targeting Lysosomal Degradation Induces P53-Dependent Cell Death and Prevents Cancer in Mouse Models of Lymphomagenesis. J. Clin. Investig. 2008, 118, 79–88. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.; Tang, J.; Liang, Y.; Jin, R.; Cai, X. Suppression of Autophagy by Chloroquine Sensitizes 5-Fluorouracil-Mediated Cell Death in Gallbladder Carcinoma Cells. Cell Biosci. 2014, 4, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boya, P.; González-Polo, R.-A.; Casares, N.; Perfettini, J.-L.; Dessen, P.; Larochette, N.; Métivier, D.; Meley, D.; Souquere, S.; Yoshimori, T.; et al. Inhibition of Macroautophagy Triggers Apoptosis. Mol. Cell. Biol. 2005, 25, 1025–1040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amaravadi, R.K.; Yu, D.; Lum, J.J.; Bui, T.; Christophorou, M.A.; Evan, G.I.; Thomas-Tikhonenko, A.; Thompson, C.B. Autophagy Inhibition Enhances Therapy-Induced Apoptosis in a Myc-Induced Model of Lymphoma. J. Clin. Investig. 2007, 117, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Rehman, S.K.; Zhang, W.; Wen, A.; Yao, L.; Zhang, J. Autophagy Is a Therapeutic Target in Anticancer Drug Resistance. Biochim. Biophys. Acta Rev. Cancer 2010, 1806, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Deldar Abad Paskeh, M.; Mirzaei, S.; Ashrafizadeh, M.; Zarrabi, A.; Sethi, G. Wnt/β-Catenin Signaling as a Driver of Hepatocellular Carcinoma Progression: An Emphasis on Molecular Pathways. J. Hepatocell. Carcinoma 2021, 8, 1415–1444. [Google Scholar] [CrossRef]
- Ong, P.S.; Wang, L.Z.; Dai, X.; Tseng, S.H.; Loo, S.J.; Sethi, G. Judicious Toggling of MTOR Activity to Combat Insulin Resistance and Cancer: Current Evidence and Perspectives. Front. Pharmacol. 2016, 7, 395. [Google Scholar] [CrossRef] [Green Version]
- Lampada, A.; O’Prey, J.; Szabadkai, G.; Ryan, K.M.; Hochhauser, D.; Salomoni, P. MTORC1-Independent Autophagy Regulates Receptor Tyrosine Kinase Phosphorylation in Colorectal Cancer Cells via an MTORC2-Mediated Mechanism. Cell Death Differ. 2017, 24, 1045–1062. [Google Scholar] [CrossRef] [Green Version]
- Alsagaby, S.A.; Iqbal, D.; Ahmad, I.; Patel, H.; Mir, S.A.; Madkhali, Y.A.; Oyouni, A.A.A.; Hawsawi, Y.M.; Alhumaydhi, F.A.; Alshehri, B.; et al. In Silico Investigations Identified Butyl Xanalterate to Competently Target CK2α (CSNK2A1) for Therapy of Chronic Lymphocytic Leukemia. Sci. Rep. 2022, 12, 17648. [Google Scholar] [CrossRef]
- Pottier, C.; Fresnais, M.; Gilon, M.; Jérusalem, G.; Longuespée, R.; Sounni, N.E. Tyrosine Kinase Inhibitors in Cancer: Breakthrough and Challenges of Targeted Therapy. Cancers 2020, 12, 731. [Google Scholar] [CrossRef] [Green Version]
- Arora, A.; Scholar, E.M. Role of Tyrosine Kinase Inhibitors in Cancer Therapy. J. Pharmacol. Exp. Ther. 2005, 315, 971–979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, R.; Harilal, S.; Parambi, D.G.T.; Narayanan, S.E.; Uddin, M.S.; Marathakam, A.; Jose, J.; Mathew, G.E.; Mathew, B. Fascinating Chemopreventive Story of Wogonin: A Chance to Hit on the Head in Cancer Treatment. Curr. Pharm. Des. 2021, 27, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Herrera-Bravo, J.; Salazar, L.A.; Shaheen, S.; Abdulmajid Ayatollahi, S.; Kobarfard, F.; Imran, M.; Imran, A.; Custódio, L.; Dolores López, M.; et al. The Therapeutic Potential of Wogonin Observed in Preclinical Studies. Evid. Based Complement. Altern. Med. 2021, 2021, 9935451. [Google Scholar] [CrossRef] [PubMed]
- Law, B.Y.K.; Chan, W.K.; Xu, S.W.; Wang, J.R.; Bai, L.P.; Liu, L.; Wong, V.K.W. Natural Small-Molecule Enhancers of Autophagy Induce Autophagic Cell Death in Apoptosis-Defective Cells. Sci. Rep. 2014, 4, 5510. [Google Scholar] [CrossRef]
- Wu, K.; Teng, M.; Zhou, W.; Lu, F.; Zhou, Y.; Zeng, J.; Yang, J.; Liu, X.; Zhang, Y.; Ding, Y.; et al. Wogonin Induces Cell Cycle Arrest and Apoptosis of Hepatocellular Carcinoma Cells by Activating Hippo Signaling. Anticancer Agents Med. Chem. 2022, 22, 1551–1560. [Google Scholar] [CrossRef]
- Chen, M.; Wu, H.L.; Wong, T.S.; Chen, B.; Gong, R.-H.; Wong, H.L.X.; Xiao, H.; Bian, Z.; Kwan, H.Y. Combination of Wogonin and Artesunate Exhibits Synergistic Anti-Hepatocellular Carcinoma Effect by Increasing DNA-Damage-Inducible Alpha, Tumor Necrosis Factor α and Tumor Necrosis Factor Receptor-Associated Factor 3-Mediated Apoptosis. Front. Pharmacol. 2021, 12. [Google Scholar] [CrossRef]
- Cui, B.; Yu, J.-M. Autophagy: A New Pathway for Traditional Chinese Medicine. J. Asian Nat. Prod. Res. 2018, 20, 14–26. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, M.; Yao, J.; Guo, Q.; Wei, L. The Synthetic Flavonoid Derivative GL-V9 Induces Apoptosis and Autophagy in Cutaneous Squamous Cell Carcinoma via Suppressing AKT-Regulated HK2 and MTOR Signals. Molecules 2020, 25, 5033. [Google Scholar] [CrossRef]
- Li, S.-J.; Sun, S.-J.; Gao, J.; Sun, F.-B. Wogonin Induces Beclin-1/PI3K and Reactive Oxygen Species-Mediated Autophagy in Human Pancreatic Cancer Cells. Oncol. Lett. 2016, 12, 5059–5067. [Google Scholar] [CrossRef] [Green Version]
- Hong, Z.-P.; Wang, L.-G.; Wang, H.-J.; Ye, W.-F.; Wang, X.-Z. Wogonin Exacerbates the Cytotoxic Effect of Oxaliplatin by Inducing Nitrosative Stress and Autophagy in Human Gastric Cancer Cells. Phytomedicine 2018, 39, 168–175. [Google Scholar] [CrossRef]
- Zhang, H.; Ge, C.; Lin, C.; Yi, L.; Ran, J.; Shi, X.; Tang, C.; Wu, Y.; Nian, W. Combination of Icotinib and Wogonin Induces Apoptosis and Autophagy to Overcome Acquired Resistance in Lung Cancer Harbouring EGFR T790M Mutation. Int. J. Clin. Exp. Med. 2017, 10, 7553–7562. [Google Scholar]
- Huang, S.T.; Wang, C.Y.; Yang, R.C.; Chu, C.J.; Wu, H.T.; Pang, J.H. Wogonin, an active compound in Scutellaria baicalensis, induces apoptosis and reduces telomerase activity in the HL-60 leukemia cells. Phytomedicine 2010, 17, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Wu, F.; Shao, Q.; Chen, G.; Xu, L.; Lu, F. Baicalin Alleviates Oxidative Stress and Inflammation in Diabetic Nephropathy via Nrf2 and MAPK Signaling Pathway. Drug Des. Devel. Ther. 2021, 15, 3207–3221. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Cho, K.-S.; Yi, I.; To, C.-H.; Chen, D.F.; Do, C.-W. Baicalein, Baicalin, and Wogonin: Protective Effects against Ischemia-Induced Neurodegeneration in the Brain and Retina. Oxid. Med. Cell. Longev. 2021, 2021, 8377362. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Fang, J.; Wang, H.; Fei, M.; Tang, T.; Liu, K.; Niu, W.; Zhou, Y. Baicalin Suppresses Proliferation, Migration, and Invasion in Human Glioblastoma Cells via Ca2+-Dependent Pathway. Drug Des. Devel. Ther. 2018, 12, 3247–3261. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Zhao, Z.; Liu, B.; Lu, L.; Dong, J. Exploring the Chemopreventive Properties and Perspectives of Baicalin and Its Aglycone Baicalein in Solid Tumors. Eur. J. Med. Chem. 2017, 126, 844–852. [Google Scholar] [CrossRef]
- Wang, B.; Huang, T.; Fang, Q.; Zhang, X.; Yuan, J.; Li, M.; Ge, H. Bone-Protective and Anti-Tumor Effect of Baicalin in Osteotropic Breast Cancer via Induction of Apoptosis. Breast Cancer Res. Treat. 2020, 184, 711–721. [Google Scholar] [CrossRef]
- Lan, H.; Wang, H.; Gao, M.; Luo, G.; Zhang, J.; Yi, E.; Liang, C.; Xiong, X.; Chen, X.; Wu, Q.; et al. Analysis and Construction of a Competitive Endogenous RNA Regulatory Network of Baicalin-Induced Apoptosis in Human Osteosarcoma Cells. Biomed. Res. Int. 2021, 2021, 9984112. [Google Scholar] [CrossRef]
- Liu, Y.; Hong, Z.; Chen, P.; Wang, J.; Zhou, Y.; Huang, J. Baicalin Inhibits Growth and Induces Apoptosis of Human Osteosarcoma Cells by Suppressing the AKT Pathway. Oncol. Lett. 2019, 18, 3188–3194. [Google Scholar] [CrossRef] [Green Version]
- Wan, D.; Ouyang, H. Baicalin Induces Apoptosis in Human Osteosarcoma Cell through ROS-Mediated Mitochondrial Pathway. Nat. Prod. Res. 2018, 32, 1996–2000. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Zhou, R.; Zhong, W.; Lu, S.; Ma, Z.; Chai, Y. Baicalin Inhibits Human Osteosarcoma Cells Invasion, Metastasis, and Anoikis Resistance by Suppressing the Transforming Growth Factor-Β1-Induced Epithelial-to-Mesenchymal Transition. Anticancer Drugs 2017, 28, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Aryal, P.; Kim, K.; Park, P.-H.; Ham, S.; Cho, J.; Song, K. Baicalein Induces Autophagic Cell Death through AMPK/ULK1 Activation and Downregulation of MTORC1 Complex Components in Human Cancer Cells. FEBS J. 2014, 281, 4644–4658. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-F.; Xu, Y.-L.; Tang, Z.-H.; Li, T.; Zhang, L.-L.; Chen, X.; Lu, J.-H.; Leung, C.-H.; Ma, D.-L.; Qiang, W.-A.; et al. Baicalein Induces Beclin 1- and Extracellular Signal-Regulated Kinase-Dependent Autophagy in Ovarian Cancer Cells. Am. J. Chin. Med. 2017, 45, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Jiang, C.; Chen, W.; Zhang, G.; Luo, D.; Cao, Y.; Wu, J.; Ding, Y.; Liu, B. Baicalein Induces Apoptosis and Autophagy via Endoplasmic Reticulum Stress in Hepatocellular Carcinoma Cells. Biomed. Res. Int. 2014, 2014, 732516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, X.; Liu, J.; Liu, L.; Sun, X.; Huang, J.; Dong, J. Drp1-Mediated Mitochondrial Fission Contributes to Baicalein-Induced Apoptosis and Autophagy in Lung Cancer via Activation of AMPK Signaling Pathway. Int. J. Biol. Sci. 2020, 16, 1403–1416. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Qiu, S.; Qin, J. Baicalein Induced Apoptosis and Autophagy of Undifferentiated Thyroid Cancer Cells by the ERK/PI3K/Akt Pathway. Am. J. Transl. Res. 2019, 11, 3341–3352. [Google Scholar]
- Hu, J.; Wang, R.; Liu, Y.; Zhou, J.; Shen, K.; Dai, Y. Baicalein Represses Cervical Cancer Cell Growth, Cell Cycle Progression and Promotes Apoptosis via Blocking AKT/mTOR Pathway by the Regulation of circHIAT1/miR-19a-3p Axis. Onco. Targets Ther. 2021, 14, 905–916. [Google Scholar] [CrossRef]
- Fattahi, S.; Amjadi-Moheb, F.; Tabaripour, R. PI3K/AKT/mTOR signaling in gastric cancer: Epigenetics and beyond. Life Sci. 2020, 262, 118513. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Hu, J.; Shi, B.; Tie, J. Baicalein Enhanced Cisplatin Sensitivity of Gastric Cancer Cells by Inducing Cell Apoptosis and Autophagy via Akt/MTOR and Nrf2/Keap 1 Pathway. Biochem. Biophys. Res. Commun. 2020, 531, 320–327. [Google Scholar] [CrossRef]
- Pang, H.; Wu, T.; Peng, Z.; Tan, Q.; Peng, X.; Zhan, Z.; Song, L.; Wei, B. Baicalin Induces Apoptosis and Autophagy in Human Osteosarcoma Cells by Increasing ROS to Inhibit PI3K/Akt/MTOR, ERK1/2 and β-Catenin Signaling Pathways. J. Bone Oncol. 2022, 33, 100415. [Google Scholar] [CrossRef]
- Wei, Z.; Chen, J.; Zuo, F.; Guo, J.; Sun, X.; Liu, D.; Liu, C. Traditional Chinese Medicine Has Great Potential as Candidate Drugs for Lung Cancer: A Review. J. Ethnopharmacol. 2023, 300, 115748. [Google Scholar] [CrossRef]
- Wang, L.; Ma, Q. Clinical Benefits and Pharmacology of Scutellarin: A Comprehensive Review. Pharmacol. Ther. 2018, 190, 105–127. [Google Scholar] [CrossRef] [PubMed]
- Birundadevi, M.; Sivashankar, R.; Mathukumar, S. Scutellarein Apoptosis Mediated by Mitochondria in Oral Squamous Cell Carcinomas. Biomed. Biotechnol. Res. J. 2022, 6, 60. [Google Scholar] [CrossRef]
- Tan, H.; Li, X.; Yang, W.H.; Kang, Y. A Flavone, Wogonin from Scutellaria Baicalensis Inhibits the Proliferation of Human Colorectal Cancer Cells by Inducing of Autophagy, Apoptosis and G2/M Cell Cycle Arrest via Modulating the PI3K/AKT and STAT3 Signalling Pathways. J. BUON 2019, 24, 1143–1149. [Google Scholar] [PubMed]
- Zeng, S.; Tan, L.; Sun, Q.; Chen, L.; Zhao, H.; Liu, M.; Yang, H.; Ren, S.; Ming, T.; Tang, S.; et al. Suppression of Colitis-Associated Colorectal Cancer by Scutellarin through Inhibiting Hedgehog Signaling Pathway Activity. Phytomedicine 2022, 98, 153972. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Li, C.; Li, X.; Zhu, Y.; Su, Z.; Wang, X.; He, Q.; Zheng, G.; Feng, B. Scutellarin Induces Apoptosis and Autophagy in NSCLC Cells through ERK1/2 and AKT Signaling Pathways in Vitro and in Vivo. J. Cancer 2018, 9, 3247–3256. [Google Scholar] [CrossRef]
- Sun, C.-Y.; Nie, J.; Zheng, Z.-L.; Zhao, J.; Wu, L.-M.; Zhu, Y.; Su, Z.-Q.; Zheng, G.-J.; Feng, B. Renoprotective Effect of Scutellarin on Cisplatin-Induced Renal Injury in Mice: Impact on Inflammation, Apoptosis, and Autophagy. Biomed. Pharmacother. 2019, 112, 108647. [Google Scholar] [CrossRef]
- Shi, X.; Chen, G.; Liu, X.; Qiu, Y.; Yang, S.; Zhang, Y.; Fang, X.; Zhang, C.; Liu, X. Scutellarein Inhibits Cancer Cell Metastasis in Vitro and Attenuates the Development of Fibrosarcoma in Vivo. Int. J. Mol. Med. 2015, 35, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Ha, S.E.; Kim, S.M.; Vetrivel, P.; Kim, H.H.; Bhosale, P.B.; Heo, J.D.; Lee, H.J.; Kim, G.S. Inhibition of Cell Proliferation and Metastasis by Scutellarein Regulating PI3K/Akt/NF-ΚB Signaling through PTEN Activation in Hepatocellular Carcinoma. Int. J. Mol. Sci. 2021, 22, 8841. [Google Scholar] [CrossRef]
- Nie, J.; Yang, H.-M.; Sun, C.-Y.; Liu, Y.-L.; Zhuo, J.-Y.; Zhang, Z.-B.; Lai, X.-P.; Su, Z.-R.; Li, Y.-C. Scutellarin Enhances Antitumor Effects and Attenuates the Toxicity of Bleomycin in H22 Ascites Tumor-Bearing Mice. Front. Pharmacol. 2018, 9. [Google Scholar] [CrossRef]
- Hou, L.; Chen, L.; Fang, L. Scutellarin Inhibits Proliferation, Invasion, and Tumorigenicity in Human Breast Cancer Cells by Regulating HIPPO-YAP Signaling Pathway. Med. Sci. Monit. 2017, 23, 5130–5138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Xu, Y.; Sui, X.; Lin, H.; Xu, J.; Han, D.; Ye, D.; Lv, G.; Liu, Y.; Qu, X.; et al. Scutellarein Suppresses Aβ-induced Memory Impairment via Inhibition of the NF-κB Pathway in Vivo and in Vitro. Oncol. Lett. 2019, 17, 5581–5589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Yang, B.; Zhao, Y.; Liao, X.; Gao, C.; Jiang, R.; Han, B.; Yang, J.; Liu, M.; Zhou, R. Host-Guest Inclusion System of Scutellarein with 2-Hydroxypropyl-Beta-Cyclodextrin: Preparation, Characterization, and Anticancer Activity. J. Biomater. Sci. Polym. Ed. 2014, 25, 594–607. [Google Scholar] [CrossRef] [PubMed]
- Saralamma, V.V.; Vetrivel, P.; Lee, H.; Kim, S.; Ha, S.; Murugesan, R.; Kim, E.; Heo, J.; Kim, G. Comparative Proteomic Analysis Uncovers Potential Biomarkers Involved in the Anticancer Effect of Scutellarein in Human Gastric Cancer Cells. Oncol. Rep. 2020, 44, 939–958. [Google Scholar] [CrossRef]
- Lang, X.; Chen, Z.; Yang, X.; Yan, Q.; Xu, M.; Liu, W.; He, Q.; Zhang, Y.; Cheng, W.; Zhao, W. Scutellarein Induces Apoptosis and Inhibits Proliferation, Migration, and Invasion in Ovarian Cancer via Inhibition of EZH2/FOXO1 Signaling. J. Biochem. Mol. Toxicol. 2021, 35, e22870. [Google Scholar] [CrossRef]
- Sang Eun, H.; Seong Min, K.; Ho Jeong, L.; Vetrivel, P.; Venkatarame Gowda Saralamma, V.; Jeong Doo, H.; Eun Hee, K.; Sang Joon, L.; Gon Sup, K. Scutellarein Induces Fas-Mediated Extrinsic Apoptosis and G2/M Cell Cycle Arrest in Hep3B Hepatocellular Carcinoma Cells. Nutrients 2019, 11, 263. [Google Scholar] [CrossRef]
- Yang, N.; Zhao, Y.; Wang, Z.; Liu, Y.; Zhang, Y. Scutellarin Suppresses Growth and Causes Apoptosis of Human Colorectal Cancer Cells by Regulating the P53 Pathway. Mol. Med. Rep. 2017, 15, 929–935. [Google Scholar] [CrossRef] [Green Version]
- Chow, S.-E.; Chen, Y.-W.; Liang, C.-A.; Huang, Y.-K.; Wang, J.-S. Wogonin Induces Cross-Regulation between Autophagy and Apoptosis via a Variety of Akt Pathway in Human Nasopharyngeal Carcinoma Cells. J. Cell. Biochem. 2012, 113, 3476–3485. [Google Scholar] [CrossRef]
- Yan, W.; Ma, X.; Zhao, X.; Zhang, S. Baicalein Induces Apoptosis and Autophagy of Breast Cancer Cells via Inhibiting PI3K/AKT Pathway in Vivo and Vitro. Drug Des. Devel. Ther. 2018, 12, 3961–3972. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Ding, L.; Zhang, L.; Wang, S.; Wang, Y.; Wang, B.; Li, L. Baicalein Induces Autophagy and Apoptosis through AMPK Pathway in Human Glioma Cells. Am. J. Chin. Med. 2019, 47, 1405–1418. [Google Scholar] [CrossRef]
- Qiao, D.; Li, Y.; Xing, J.; Sun, P.; Wang, Y.; Zhang, Y.; Chen, L.; Ren, X.; Lin, Z.; Jin, J.; et al. Baicalein Inhibits PI3K/AKT Signaling Pathway and Induces Autophagy of MGC-803 Cells. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2019, 35, 613–618. [Google Scholar] [PubMed]
- Yi, S.; Liu, G.; Wu, Y.; Liang, Q.; Li, L. Baicalein Suppresses the Growth of the Human Thyroid Cancer Cells by Inducing Mitotic Catastrophe, Apoptosis and Autophagy via NF-KB Signalling Pathway. J. BUON 2020, 25, 389–394. [Google Scholar] [PubMed]
- Lin, C.; Tsai, S.-C.; Tseng, M.T.; Peng, S.-F.; Kuo, S.-C.; Lin, M.-W.; Hsu, Y.-M.; Lee, M.-R.; Amagaya, S.; Huang, W.-W.; et al. AKT Serine/Threonine Protein Kinase Modulates Baicalin-Triggered Autophagy in Human Bladder Cancer T24 Cells. Int. J. Oncol. 2013, 42, 993–1000. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Tang, X.; Liu, H.; Li, L.; Hou, Q.; Gao, J. Autophagy Induced by Baicalin Involves Downregulation of CD147 in SMMC-7721 Cells in Vitro. Oncol. Rep. 2012, 27, 1128–1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, C.-Y.; Zhu, Y.; Li, X.-F.; Wang, X.-Q.; Tang, L.-P.; Su, Z.-Q.; Li, C.-Y.; Zheng, G.-J.; Feng, B. Scutellarin Increases Cisplatin-Induced Apoptosis and Autophagy to Overcome Cisplatin Resistance in Non-Small Cell Lung Cancer via ERK/P53 and c-Met/AKT Signaling Pathways. Front. Pharmacol. 2018, 9, 92. [Google Scholar] [CrossRef]

| Compound | Source Plant/Plants | Bioactivities | Reference |
|---|---|---|---|
| Wogonin | Scutellaria baicalensis Georgi radix, Andrographis paniculata Burm.f, Anodendron affine (Hook. & Arn.) Druce, Tetracera indica L. | Antioxidant, antiviral, anti-inflammatory, antiproliferative | [26,27,28] |
| Baicalein | S baicalensis, S lateriflora, Oroxylum indicum | Positive allosteric modulator of GABAA receptor, anxiolytic activity | [29,30,31,32] |
| Baicalin | S. lateriflora, S. galericulata, Thalictrum baicalense, Radix scutellariae | Neuroprotective, cardioprotective, antioxidant, antiatherosclerotic, antibacterial, anticoronaviral | [37,38] |
| Scutellarein | S lateriflora, Scoparia dulcis, Artemisia douglasiana, Asplenium belangeri (Fern) | Anticancer, antiproliferative, antioxidant | [41,42,43,52] |
| Scutellarin | Scutellaria spp., Erigeron spp. | Liver diseases, cerebrovascular diseases, hyperlipidemia | [46,51] |
| Compound | Administration of Compound | Pharmacokinetic Analysis | Reference |
|---|---|---|---|
| Wogonin (W.O.) | Oral administration in C57BL/6 mice H9c2 cells | Modulated Gasdermin D protein in H9c2 cells; Attenuated CDDP-induced cardiotoxicity and showed antipyroptotic effects | [58] |
| Oral administration in rats at 10, 20 and 40 mg/kg | Modulate the activities of CYPs, P-gp and Cmax AUC0-t of W.O. were proportionally increased | [59] | |
| Oral administration of R.S. extract (300 mg/kg) to Sprague–Dawley rats | W.O. showed the ability to cross the blood–brain barrier | [60] | |
| I.G. administration of W.O. in rats | Metabolized/detected in the small intestine and liver | [61] | |
| I.V. WO dose i.e., 10, 20 and 40 mg/kg I.G. 100 mg/kg dose I.V. WO (20 mg/kg) in Sprague–Dawley rats | W.O. was detected in all examined tissues; the highest levels were found in the kidney and liver, and 21% was excreted as an unchanged drug | [62] | |
| Baicalein | Oral administration (121 mg/kg bw) and Pulmonary administration (10 mg/kg) in Male Sprague–Dawley (S.D.) rats | Oral baicalein nanocrystals: Bioavailability of baicalein is 1.67-fold, showing rapid and extensive absorption | [63] |
| Oral administration Male SD rats 30 mg/kg baicalein | Distributed rapidly within 0.25 h and accumulated in the lung and liver Quickly absorbed in plasma lung > kidney >liver having T1/2z 8.08 | [64] | |
| Oral administration in normal rats (65 mg/kg) | Baicalein was significantly higher in the stomach > liver > intestine | [65] | |
| In situ perfusion in Male Wistar rats | Baicalein was moderately absorbed as per the stomach > small intestine >colon | [66] | |
| Baicalin | I.V. administration (230–250 g) of 37 µmol/kg to Male Wistar rats Oral administration of 227 µmol/kg | T ½ = 0.12 ± 0.02 in I.V. administered rats in plasma. Plasma concentration of baicalin displayed a second peak over the 8 –12 h (i.v.) | [67] |
| I.V and oral administration in Male Sprague–Dawley rats | Rapid absorption and simultaneous glucuronidation/sulfation. The absorption rate was slower and the Cmax was lower for oral baicalin compared with I.V baicalein | [68] | |
| I.G. and oral administration in rats at a dose of 160 mg/kg | Coadministration significantly upregulated the Cmax, AUC0-t, and AUC0-∞ of oral dose by 2.02, 1.65, 1.66-fold in male rats | [69] | |
| Oral Administration to Male rats | Baicalein is significantly hydrolyzed in the gastrointestinal tract. The total cumulative amounts of baicalin were 54% of the doses | [70] | |
| Intestinal perfusion model: In rat in situ single-pass | In the rat’s intestinal regions, baicalin underwent considerable metabolism via conjugative processes | [71] | |
| Scutellarin/ Scutellarein | Oral administration 200 mg kg/bw in Male Wistar rats | Scutellarein is obtained after metabolizing scutellarin in the blood by glucuronic acid and methylating enzymes in the liver | [72] |
| Oral administration of 80 mg kg/bw to Male Wistar rats | Two metabolites of Scutellarin, viz., Scutellarein 6,7-di-o-β-D-glucuronide and Scutellarein observed in urine | [73] | |
| I.V administration of 36 mg kg/bw to rats | Total of four metabolites were observed in the plasma. Scutellarin was metabolized via dehydroxylation and methylation | [74] | |
| Oral administration in human subject | Glucuronidation of scutellarin is mediated by uridine 5’-diphosphoglucuronosyltransferase (UGT) in rats and humans | [75] | |
| Scutellarin (S-7-G) at a dose of 75 mg/kg orally to Male Sprague–Dawley rats | S-7-G and S-6-G were spotted in the systemic circulation S-7-G absorbed as aglycone after hydrolyzed in the intestinal Glucuronidation of S-7-G occurs in liver microsomes of rat | [76] |
| Scutellaria Flavones | Type of Cancer | Cell Line | Mechanisms of Action | Ref. |
|---|---|---|---|---|
| Wogonin | Pancreatic cancer | Panc-1 and Colo-357 | ↑ROS, Beclin-1/PI3K | [119] |
| Wogonin and oxaliplatin | Gastric cancer | BGC-823 cells | ↑phospho-JNK (Thr183/Tyr185), phospho-ULK1 (Ser555), ↑LC3II | [120] |
| Wogonin | Nasopharyngeal cancer | NPC-TW076 and NPCTW039 | ↑LC3 I/II cleavage, autophagosome/autolysosome, ↓Raf/ERK | [158] |
| Wogonin | Colorectal cancer | SW1417, SW48, DLD-1, HCT-15, LS-180 and CCD-18Co | ↓AKT and STAT3, ↑Beclin-1 caspases 3/8/9 and Bax expressions | [12] |
| Wogonin derivative GL-V9 | Cutaneous squamous cancer | A431 cells | ↓Akt/mTOR pathway | [118] |
| Baicalein | Breast cancer | MCF-7 and MDA-MB-231 | ↓PI3K/AKT, NF-κB | [159] |
| Baicalein | Glioma cells | U251 cells | ↑LC3, ↑caspase-3, ↑phosphorylation of AMPK | [160] |
| Baicalein | Lung cancer | A549 and H1299 | ↑activated A.M.P.K., ↑Drp1-mediated mitochondrial fission | [135] |
| Baicalein | Ovarian cancer | HEY and A2780 | ↑LC3-II, ↑PARP, ↑phosphorylation of ERK, ↑Beclin-1 | [133] |
| Baicalein | Hepatocellular cancer | SMMC-7721 and Bel-7402 | ↑endoplasmic reticulum (E.R.) stress, ↓Bcl-2, ↑J.N.K. | [153] |
| Baicalein | Thyroid cancer | F.R.O. | ↓Bcl-2/Bax, ↑Caspase-3, ↑Caspase-8, ↑Beclin-1, Atg5, ↓ERK, ↓PI3K/Akt | [136] |
| Baicalein | Gastric cancer | MGC-803 | ↑LC3, ↓PI3K and ↓AKT, ↑P62 | [161] |
| Baicalein | Prostate and breast cancer cell | PC-3, MDA-MB-231 and DU145 | ↓mTOR, ↑ activated AMPK/ULK1 | [132] |
| Baicalein and cisplatin | Gastric cancer | SGC-7901 and SGC-7901/DDP | ↓Akt/mTOR and Nrf2/Keap 1 | [139] |
| Baicalein | Thyroid cancer | MDA-T68 | ↑Bax, ↓NF-kB, ↓Cyclin B1 | [162] |
| Baicalin | Bladder cancer | T24 cells | ↑Atg 5, ↑Atg 7, ↑Atg 12, ↑Beclin-1, ↑LC3-II | [163] |
| Baicalin | Osteosarcoma | HOS, MG63 U2OS and 143B | ↓PI3K/Akt/mTOR, ↓ERK1/2, ↓β-catenin, ↑Bax, ↑caspase-3, ↑cleaved PARP | [140] |
| Baicalin | Hepatocellular cancer | SMMC-7721 | ↓CD147, ↑Beclin-1 | [164] |
| Scutellarin | Breast cancer | MCF-7 | ↑p-YAP, ↓Y.A.P., ↑autophagy | [151] |
| Scutellarin | Lung cancer | PC-9 and H1975, HepG2, Hela | ↑LC3-II, ↓p-AKT | [146] |
| Scutellarin and cisplatin | Lung adenocarcinoma | A549, PC-9, H1975, and A549/DDP | ↑p53 and ↓c-met/AKT | [165] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuli, H.S.; Bhushan, S.; Kumar, A.; Aggarwal, P.; Sak, K.; Ramniwas, S.; Vashishth, K.; Behl, T.; Rana, R.; Haque, S.; et al. Autophagy Induction by Scutellaria Flavones in Cancer: Recent Advances. Pharmaceuticals 2023, 16, 302. https://doi.org/10.3390/ph16020302
Tuli HS, Bhushan S, Kumar A, Aggarwal P, Sak K, Ramniwas S, Vashishth K, Behl T, Rana R, Haque S, et al. Autophagy Induction by Scutellaria Flavones in Cancer: Recent Advances. Pharmaceuticals. 2023; 16(2):302. https://doi.org/10.3390/ph16020302
Chicago/Turabian StyleTuli, Hardeep Singh, Sakshi Bhushan, Ajay Kumar, Poonam Aggarwal, Katrin Sak, Seema Ramniwas, Kanupriya Vashishth, Tapan Behl, Rashmi Rana, Shafiul Haque, and et al. 2023. "Autophagy Induction by Scutellaria Flavones in Cancer: Recent Advances" Pharmaceuticals 16, no. 2: 302. https://doi.org/10.3390/ph16020302
APA StyleTuli, H. S., Bhushan, S., Kumar, A., Aggarwal, P., Sak, K., Ramniwas, S., Vashishth, K., Behl, T., Rana, R., Haque, S., & Prieto, M. A. (2023). Autophagy Induction by Scutellaria Flavones in Cancer: Recent Advances. Pharmaceuticals, 16(2), 302. https://doi.org/10.3390/ph16020302










