A Cross Talk between the Endocannabinoid System and Different Systems Involved in the Pathogenesis of Hypertensive Retinopathy
Abstract
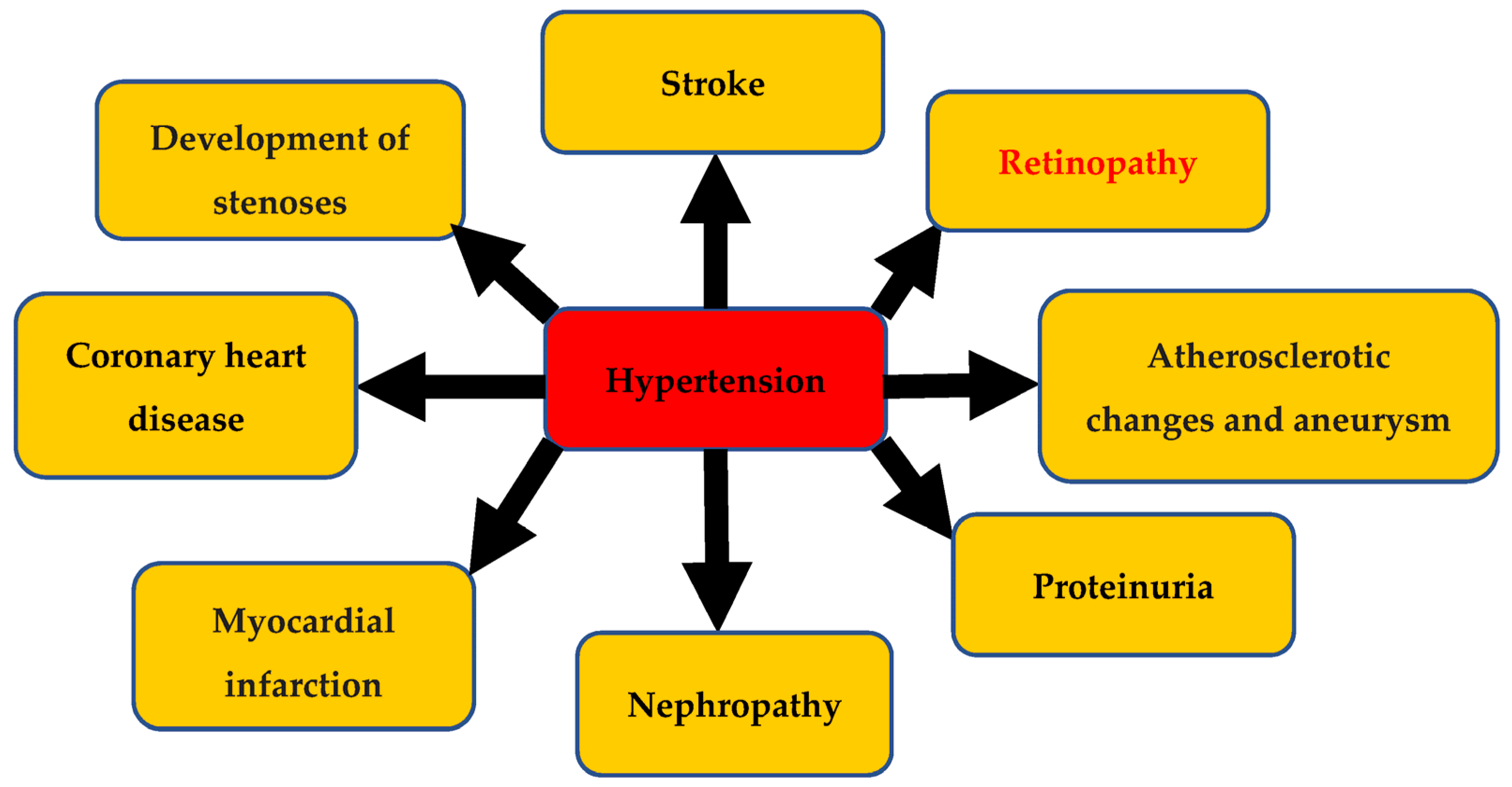
:1. Hypertension and Its Complications
2. Endocannabinoid System and Its Agonists
Biosynthesis of Endocannabinoids and Their Hydrolysis
3. Hypertensive Retinopathy
3.1. Pathophysiology
3.1.1. Vasoconstrictive Phase
3.1.2. Sclerotic Phase
3.1.3. Exudative Phase
3.2. Role of Vasospasm, Oxidative Stress, Inflammation, and Nitric-Oxide-Deficient Endothelium in the Pathogenesis of Hypertensive Retinopathy
3.3. Role of the ECS in the Pathogenesis of Hypertensive Retinopathy
3.4. Role of SNS and RAS in the Pathogenesis of Hypertensive Retinopathy
4. Interaction of Endocannabinoid System with Sympathetic Nervous System and Renin–Angiotensin System
5. Conclusions and Future Directions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stanaway, J.D.; Afshin, A.; Gakidou, E.; Lim, S.S.; Abate, D.; Abate, K.H.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1923–1994. [Google Scholar]
- Mills, K.T.; Bundy, J.D.; Kelly, T.N.; Reed, J.E.; Kearney, P.M.; Reynolds, K.; Chen, J.; He, J. Global disparities of hypertension prevalence and control: A systematic analysis of population-based studies from 90 countries. Circulation 2016, 134, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Schmieder, R.E. End organ damage in hypertension. Dtsch. Ärzteblatt Int. 2010, 107, 866. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.; Coca, A.; De Simone, G.; Dominiczak, A. Authors/Task Force Members. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J. Hypertens. 2018, 36, 1953–2041. [Google Scholar]
- Naser, N.; Dzubur, A.; Durak, A.; Kulic, M.; Naser, N. Blood pressure control in hypertensive patients, cardiovascular risk profile and the prevalence of masked uncontrolled hypertension (MUCH). Med. Arch. 2016, 70, 274. [Google Scholar] [CrossRef] [Green Version]
- Beevers, G.; Lip, G.Y.H.; O’Brien, E. The pathophysiology of hypertension. BMJ 2001, 322, 912–916. [Google Scholar] [CrossRef]
- Dibona, G.F. Sympathetic nervous system and hypertension. Hypertension 2013, 61, 556–560. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Zhang, S.; Gong, Y.; Dai, K.; Sui, M.; Yu, Y.; Ning, G. The role of the autonomic nervous system in hypertension: A bond graph model study. Physiol. Meas. 2008, 29, 473. [Google Scholar] [CrossRef]
- Crowley, S.D.; Gurley, S.B.; Herrera, M.J.; Ruiz, P.; Griffiths, R.; Kumar, A.P.; Kim, H.-S.; Smithies, O.; Le, T.H.; Coffman, T.M. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc. Natl. Acad. Sci. USA 2006, 103, 17985–17990. [Google Scholar] [CrossRef] [Green Version]
- Madhur, M.S.; Lob, H.E.; McCann, L.A.; Iwakura, Y.; Blinder, Y.; Guzik, T.J.; Harrison, D.G. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension 2010, 55, 500–507. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, A.; Dempsey, S.K.; Daneva, Z.; Azam, M.; Li, N.; Li, P.-L.; Ritter, J.K. Role of Nitric Oxide in the Cardiovascular and Renal Systems. Int. J. Mol. Sci. 2018, 19, 2605. [Google Scholar] [CrossRef] [Green Version]
- Oliveira-Paula, G.H.; Lacchini, R.; Tanus-Santos, J.E. Endothelial nitric oxide synthase: From biochemistry and gene structure to clinical implications of NOS3 polymorphisms. Gene 2016, 575, 584–599. [Google Scholar] [CrossRef]
- Node, K.; Kitakaze, M.; Yoshikawa, H.; Kosaka, H.; Hori, M. Reduced plasma concentrations of nitrogen oxide in individuals with essential hypertension. Hypertension 1997, 30, 405–408. [Google Scholar] [CrossRef]
- Panza, J.A.; Quyyumi, A.A.; Brush, J.E., Jr.; Epstein, S.E. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N. Engl. J. Med. 1990, 323, 22–27. [Google Scholar] [CrossRef]
- Basavarajappa, B.S.; Shivakumar, M.; Joshi, V.; Subbanna, S. Endocannabinoid system in neurodegenerative disorders. J. Neurochem. 2017, 142, 624–648. [Google Scholar] [CrossRef] [Green Version]
- Coutts, A.A.; Izzo, A.A. The gastrointestinal pharmacology of cannabinoids: An update. Curr. Opin. Pharmacol. 2004, 4, 572–579. [Google Scholar] [CrossRef]
- Park, F.; Potukuchi, P.K.; Moradi, H.; Kovesdy, C.P. Cannabinoids and the kidney: Effects in health and disease. Am. J. Physiol. Renal. Physiol. 2017, 313, F1124–F1132. [Google Scholar] [CrossRef] [Green Version]
- Cristino, L.; Bisogno, T.; Di Marzo, V. Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat. Rev. Neurol. 2020, 16, 9–29. [Google Scholar] [CrossRef]
- Puhl, S.L. Cannabinoid-sensitive receptors in cardiac physiology and ischaemia. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118462. [Google Scholar] [CrossRef]
- Fraguas-Sánchez, A.I.; Torres-Suárez, A.I. Medical Use of Cannabinoids. Drugs 2018, 78, 1665–1703. [Google Scholar] [CrossRef]
- Maida, V.; Daeninck, P.J. A user’s guide to cannabinoid therapies in oncology. Curr. Oncol. 2016, 23, 398–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DiPatrizio, N.V.; Piomelli, D. The thrifty lipids: Endocannabinoids and the neural control of energy conservation. Trends Neurosci. 2012, 35, 403–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, J.Z.; Li, W.; Booker, L.; Burston, J.J.; Kinsey, S.G.; Schlosburg, J.E.; Pavón, F.J.; Serrano, A.M.; Selley, D.E.; Parsons, L.H.; et al. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat. Chem. Biol. 2009, 5, 37–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kathuria, S.; Gaetani, S.; Fegley, D.; Valiño, F.; Duranti, A.; Tontini, A.; Mor, M.; Tarzia, G.; La Rana, G.; Calignano, A.; et al. Modulation of anxiety through blockade of anandamide hydrolysis. Nat. Med. 2003, 9, 76–81. [Google Scholar] [CrossRef]
- Matsuda, L.A.; Lolait, S.J.; Brownstein, M.J.; Young, A.C.; Bonner, T.I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 1990, 346, 561–564. [Google Scholar] [CrossRef]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993, 365, 61–65. [Google Scholar] [CrossRef]
- Fowler, C.J. Transport of endocannabinoids across the plasma membrane and within the cell. FEBS J. 2013, 280, 1895–1904. [Google Scholar] [CrossRef]
- Di Marzo, V. The endocannabinoid system: Its general strategy of action, tools for its pharmacological manipulation and potential therapeutic exploitation. Pharmacol. Res. 2009, 60, 77–84. [Google Scholar] [CrossRef]
- Devane, W.A.; Hanuš, L.; Breuer, A.; Pertwee, R.G.; Stevenson, L.A.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992, 258, 1946–1949. [Google Scholar] [CrossRef]
- Mechoulam, R.; Ben-Shabat, S.; Hanus, L.; Ligumsky, M.; Kaminski, N.E.; Schatz, A.R.; Gopher, A.; Almog, S.; Martin, B.R.; Compton, D.R. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem. Pharmacol. 1995, 50, 83–90. [Google Scholar] [CrossRef]
- Hanuš, L.; Abu-Lafi, S.; Fride, E.; Breuer, A.; Vogel, Z.; Shalev, D.E.; Kustanovich, I.; Mechoulam, R. 2-arachidonyl glyceryl ether, an endogenous agonist of the cannabinoid CB1 receptor. Proc. Natl. Acad. Sci. USA 2001, 98, 3662–3665. [Google Scholar] [CrossRef] [Green Version]
- Di Marzo, V.; Stella, N.; Zimmer, A. Endocannabinoid signalling and the deteriorating brain. Nat. Rev. Neurosci. 2015, 16, 30–42. [Google Scholar] [CrossRef] [Green Version]
- Di Marzo, V.; Piscitelli, F. The endocannabinoid system and its modulation by phytocannabinoids. Neurotherapeutics 2015, 12, 692–698. [Google Scholar] [CrossRef] [Green Version]
- Freund, T.F.; Katona, I.; Piomelli, D. Role of endogenous cannabinoids in synaptic signaling. Physiol. Rev. 2003, 83, 1017–1066. [Google Scholar] [CrossRef] [Green Version]
- Miranda, A.; Nordstrom, E.; Mannem, A.; Smith, C.; Banerjee, B.; Sengupta, J.N. The role of transient receptor potential vanilloid 1 in mechanical and chemical visceral hyperalgesia following experimental colitis. Neuroscience 2007, 148, 1021–1032. [Google Scholar] [CrossRef] [Green Version]
- Koch, M. Cannabinoid receptor signaling in central regulation of feeding behavior: A mini-review. Front. Neurosci. 2017, 11, 293. [Google Scholar] [CrossRef] [Green Version]
- Eid, B.G.; Neamatallah, T.; Hanafy, A.; El-Bassossy, H.M.; Binmahfouz, L.; Aldawsari, H.M.; Hasan, A.; El-Aziz, G.A.; Vemuri, K.; Makriyannis, A. Interference with TGFβ1-Mediated Inflammation and Fibrosis Underlies Reno-Protective Effects of the CB1 Receptor Neutral Antagonists AM6545 and AM4113 in a Rat Model of Metabolic Syndrome. Molecules 2021, 26, 866. [Google Scholar] [CrossRef]
- Griffin, G.; Wray, E.J.; Tao, Q.; McAllister, S.D.; Rorrer, W.K.; Aung, M.; Martin, B.R.; Abood, M.E. Evaluation of the cannabinoid CB2 receptor-selective antagonist, SR144528: Further evidence for cannabinoid CB2 receptor absence in the rat central nervous system. Eur. J. Pharmacol. 1999, 377, 117–125. [Google Scholar] [CrossRef]
- Ahmad, A.; Dempsey, S.K.; Daneva, Z.; Li, N.; Poklis, J.L.; Li, P.L.; Ritter, J.K. Modulation of mean arterial pressure and diuresis by renomedullary infusion of a selective inhibitor of fatty acid amide hydrolase. Am. J. Physiol.-Ren. Physiol. 2018, 315, F967–F976. [Google Scholar] [CrossRef]
- Daneva, Z.; Dempsey, S.K.; Ahmad, A.; Li, N.; Li, P.-L.; Ritter, J.K. Diuretic, natriuretic, and vasodepressor activity of a lipid fraction enhanced in medium of cultured mouse medullary interstitial cells by a selective fatty acid amide hydrolase inhibitor. J. Pharmacol. Exp. Ther. 2019, 368, 187–198. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, R.K.P. A perspective review on fatty acid amide hydrolase (FAAH) inhibitors as potential therapeutic agents. Eur. J. Med. Chem. 2020, 188, 111953. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Daneva, Z.; Li, G.; Dempsey, S.K.; Li, N.; Poklis, J.L.; Lichtman, A.; Li, P.-L.; Ritter, J.K. Stimulation of diuresis and natriuresis by renomedullary infusion of a dual inhibitor of fatty acid amide hydrolase and monoacylglycerol lipase. Am. J. Physiol.-Ren. Physiol. 2017, 313, F1068–F1076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granchi, C.; Caligiuri, I.; Minutolo, F.; Rizzolio, F.; Tuccinardi, T. A patent review of Monoacylglycerol Lipase (MAGL) inhibitors (2013–2017). Expert Opin. Ther. Pat. 2017, 27, 1341–1351. [Google Scholar] [CrossRef] [PubMed]
- Henderson, A.D.; Bruce, B.B.; Newman, N.J.; Biousse, V.R. Hypertension-related eye abnormalities and the risk of stroke. Rev. Neurol. Dis. 2011, 8, 1–9. [Google Scholar]
- Kabedi, N.N.; Mwanza, J.-C.; Lepira, F.o.B.; Kayembe, T.K.; Kayembe, D.L. Hypertensive retinopathy and its association with cardiovascular, renal and cerebrovascular morbidity in Congolese patients. Cardiovasc. J. Afr. 2014, 25, 228–232. [Google Scholar] [CrossRef] [Green Version]
- Del Brutto, O.H.; Mera, R.M.; Viteri, E.M.; Pólit, J.; Ledesma, E.A.; Cano, J.A.; Plaza, K.J.; Zambrano, M.; Costa, A.F. Hypertensive retinopathy and cerebral small vessel disease in Amerindians living in rural Ecuador: The Atahualpa Project. Int. J. Cardiol. 2016, 218, 65–68. [Google Scholar] [CrossRef]
- Chaine, G.; Kohner, E.M. Hypertensive retinopathy. J. Fr. D’ophtalmologie 1983, 6, 995–1005. [Google Scholar]
- Duncan, B.B.; Wong, T.Y.; Tyroler, H.A.; Davis, C.E.; Fuchs, F.D. Hypertensive retinopathy and incident coronary heart disease in high risk men. Br. J. Ophthalmol. 2002, 86, 1002–1006. [Google Scholar] [CrossRef] [Green Version]
- Ong, Y.T.; Wong, T.Y.; Klein, R.; Klein, B.E.; Mitchell, P.; Sharrett, A.R.; Couper, D.J.; Ikram, M.K. Hypertensive retinopathy and risk of stroke. Hypertension 2013, 62, 706–711. [Google Scholar] [CrossRef]
- Wong, T.Y.; Coresh, J.; Klein, R.; Muntner, P.; Couper, D.J.; Sharrett, A.R.; Klein, B.E.; Heiss, G.; Hubbard, L.D.; Duncan, B.B. Retinal microvascular abnormalities and renal dysfunction: The atherosclerosis risk in communities study. J. Am. Soc. Nephrol. JASN 2004, 15, 2469–2476. [Google Scholar] [CrossRef] [Green Version]
- Wong, T.Y.; Mitchell, P. Hypertensive retinopathy. N. Engl. J. Med. 2004, 351, 2310–2317. [Google Scholar] [CrossRef]
- Bhargava, M.; Ikram, M.; Wong, T.Y. How does hypertension affect your eyes? J. Hum. Hypertens. 2012, 26, 71–83. [Google Scholar] [CrossRef] [Green Version]
- Keith, N.M.; Wagener, H.P.; Barker, N.W. Some different types of essential hypertension: Their course and prognosis. Am. J. Med. Sci. 1974, 268, 336–345. [Google Scholar] [CrossRef]
- Campbell, J.; Zhang, M.; Hwang, T.; Bailey, S.; Wilson, D.; Jia, Y.; Huang, D. Detailed vascular anatomy of the human retina by projection-resolved optical coherence tomography angiography. Sci. Rep. 2017, 7, 42201. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Liu, C.-H.; Sapieha, P. Retinal vascular development. In Anti-Angiogenic Therapy in Ophthalmology; Springer: Cham, Switzerland, 2016; pp. 1–19. [Google Scholar]
- Cunha-Vaz, J.; Bernardes, R.; Lobo, C. Blood-retinal barrier. Eur. J. Ophthalmol. 2011, 21, 3–9. [Google Scholar] [CrossRef]
- Triantafyllou, A.; Anyfanti, P.; Gavriilaki, E.; Zabulis, X.; Gkaliagkousi, E.; Petidis, K.; Triantafyllou, G.; Gkolias, V.; Pyrpasopoulou, A.; Douma, S. Association between retinal vessel caliber and arterial stiffness in a population comprised of normotensive to early-stage hypertensive individuals. Am. J. Hypertens. 2014, 27, 1472–1478. [Google Scholar] [CrossRef] [Green Version]
- Kawasaki, R.; Cheung, N.; Wang, J.J.; Klein, R.; Klein, B.E.; Cotch, M.F.; Sharrett, A.R.; Shea, S.; Islam, F.A.; Wong, T.Y. Retinal vessel diameters and risk of hypertension: The Multiethnic Study of Atherosclerosis. J. Hypertens. 2009, 27, 2386–2393. [Google Scholar] [CrossRef] [Green Version]
- Wong, T.; Mitchell, P. The eye in hypertension. Lancet 2007, 369, 425–435. [Google Scholar] [CrossRef]
- Hayreh, S.S. Pathogenesis of optic disc edema in raised intracranial pressure. Prog. Retin. Eye Res. 2016, 50, 108–144. [Google Scholar] [CrossRef] [Green Version]
- Coban, E.; Alkan, E.; Altuntas, S.; Akar, Y. Serum ferritin levels correlate with hypertensive retinopathy. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2010, 16, CR92–CR95. [Google Scholar]
- Coban, E.; Nizam, I.; Topal, C.; Akar, Y. The association of low-grade systemic inflammation with hypertensive retinopathy. Clin. Exp. Hypertens. 2010, 32, 528–531. [Google Scholar] [CrossRef] [PubMed]
- Delles, C.; Michelson, G.; Harazny, J.; Oehmer, S.; Hilgers, K.F.; Schmieder, R.E. Impaired endothelial function of the retinal vasculature in hypertensive patients. Stroke 2004, 35, 1289–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goncharov, N.V.; Nadeev, A.D.; Jenkins, R.O.; Avdonin, P.V. Markers and biomarkers of endothelium: When something is rotten in the state. Oxidative Med. Cell. Longev. 2017, 2017, 9759735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yazulla, S. Endocannabinoids in the retina: From marijuana to neuroprotection. Prog. Retin. Eye Res. 2008, 27, 501–526. [Google Scholar] [CrossRef] [Green Version]
- Sugiura, T.; Kondo, S.; Sukagawa, A.; Nakane, S.; Shinoda, A.; Itoh, K.; Yamashita, A.; Waku, K. 2-Arachidonoylgylcerol: A possible endogenous cannabinoid receptor ligand in brain. Biochem. Biophys. Res. Commun. 1995, 215, 89–97. [Google Scholar] [CrossRef]
- Shu-Jung Hu, S.; Arnold, A.; Hutchens, J.M.; Radicke, J.; Cravatt, B.F.; Wager-Miller, J.; Mackie, K.; Straiker, A. Architecture of cannabinoid signaling in mouse retina. J. Comp. Neurol. 2010, 518, 3848–3866. [Google Scholar] [CrossRef] [Green Version]
- Zabouri, N.; Bouchard, J.F.; Casanova, C. Cannabinoid receptor type 1 expression during postnatal development of the rat retina. J. Comp. Neurol. 2011, 519, 1258–1280. [Google Scholar] [CrossRef]
- Gilliam, J.C.; Wensel, T.G. TRP channel gene expression in the mouse retina. Vis. Res. 2011, 51, 2440–2452. [Google Scholar] [CrossRef] [Green Version]
- Maccarone, R.; Rapino, C.; Zerti, D.; di Tommaso, M.; Battista, N.; Di Marco, S.; Bisti, S.; Maccarrone, M. Modulation of Type-1 and Type-2 Cannabinoid Receptors by Saffron in a Rat Model of Retinal Neurodegeneration. PLoS ONE 2016, 11, e0166827. [Google Scholar] [CrossRef] [Green Version]
- Araújo, D.S.M.; Miya-Coreixas, V.S.; Pandolfo, P.; Calaza, K.C. Cannabinoid receptors and TRPA1 on neuroprotection in a model of retinal ischemia. Exp. Eye Res. 2017, 154, 116–125. [Google Scholar] [CrossRef]
- Kokona, D.; Thermos, K. Synthetic and endogenous cannabinoids protect retinal neurons from AMPA excitotoxicity in vivo, via activation of CB1 receptors: Involvement of PI3K/Akt and MEK/ERK signaling pathways. Exp. Eye Res. 2015, 136, 45–58. [Google Scholar] [CrossRef]
- Ryskamp, D.A.; Redmon, S.; Jo, A.O.; Križaj, D. TRPV1 and Endocannabinoids: Emerging Molecular Signals that Modulate Mammalian Vision. Cells 2014, 3, 914–938. [Google Scholar] [CrossRef] [Green Version]
- Ramírez-López, M.T.; Vázquez, M.; Lomazzo, E.; Hofmann, C.; Blanco, R.N.; Alén, F.; Antón, M.; Decara, J.; Arco, R.; Orio, L.; et al. A moderate diet restriction during pregnancy alters the levels of endocannabinoids and endocannabinoid-related lipids in the hypothalamus, hippocampus and olfactory bulb of rat offspring in a sex-specific manner. PLoS ONE 2017, 12, e0174307. [Google Scholar] [CrossRef] [Green Version]
- Paloczi, J.; Varga, Z.V.; Hasko, G.; Pacher, P. Neuroprotection in Oxidative Stress-Related Neurodegenerative Diseases: Role of Endocannabinoid System Modulation. Antioxid. Redox Signal 2018, 29, 75–108. [Google Scholar] [CrossRef]
- Hossain, M.Z.; Ando, H.; Unno, S.; Kitagawa, J. Targeting Peripherally Restricted Cannabinoid Receptor 1, Cannabinoid Receptor 2, and Endocannabinoid-Degrading Enzymes for the Treatment of Neuropathic Pain Including Neuropathic Orofacial Pain. Int. J. Mol. Sci. 2020, 21, 1423. [Google Scholar] [CrossRef] [Green Version]
- Reis, R.A.; Ventura, A.L.; Kubrusly, R.C.; de Mello, M.C.; de Mello, F.G. Dopaminergic signaling in the developing retina. Brain Res. Rev. 2007, 54, 181–188. [Google Scholar] [CrossRef]
- Witkovsky, P. Dopamine and retinal function. Doc. Ophthalmol. Adv. Ophthalmol. 2004, 108, 17–40. [Google Scholar] [CrossRef] [Green Version]
- Gastinger, M.J.; Tian, N.; Horvath, T.; Marshak, D.W. Retinopetal axons in mammals: Emphasis on histamine and serotonin. Curr. Eye Res. 2006, 31, 655–667. [Google Scholar] [CrossRef] [Green Version]
- de Souza, C.F.; Acosta, M.L.; Polkinghorne, P.J.; McGhee, C.N.; Kalloniatis, M. Amino acid immunoreactivity in normal human retina and after brachytherapy. Clin. Exp. Optom. 2013, 96, 504–507. [Google Scholar] [CrossRef]
- Peppe, A.; Stanzione, P.; Pierantozzi, M.; Semprini, R.; Bassi, A.; Santilli, A.M.; Formisano, R.; Piccolino, M.; Bernardi, G. Does pattern electroretinogram spatial tuning alteration in Parkinson’s disease depend on motor disturbances or retinal dopaminergic loss? Electroencephalogr. Clin. Neurophysiol. 1998, 106, 374–382. [Google Scholar] [CrossRef]
- Schwitzer, T.; Schwan, R.; Angioi-Duprez, K.; Ingster-Moati, I.; Lalanne, L.; Giersch, A.; Laprevote, V. The cannabinoid system and visual processing: A review on experimental findings and clinical presumptions. Eur. Neuropsychopharmacol. 2015, 25, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Schwitzer, T.; Lavoie, J.; Giersch, A.; Schwan, R.; Laprevote, V. The emerging field of retinal electrophysiological measurements in psychiatric research: A review of the findings and the perspectives in major depressive disorder. J. Psychiatr. Res. 2015, 70, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Chaves, G.P.; Nogueira, T.C.A.; Britto, L.R.G.; Bordin, S.; Torrão, A.S. Retinal removal up-regulates cannabinoid CB1 receptors in the chick optic tectum. J. Neurosci. Res. 2008, 86, 1626–1634. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.B.; Merzouki, A.; Mesa, J.M.; Frey, K.A.; Bach, P.J. Cannabis improves night vision: A case study of dark adaptometry and scotopic sensitivity in kif smokers of the Rif mountains of northern Morocco. J. Ethnopharmacol. 2004, 93, 99–104. [Google Scholar] [CrossRef]
- Cécyre, B.; Bachand, I.; Papineau, F.; Brochu, C.; Casanova, C.; Bouchard, J.-F. Cannabinoids affect the mouse visual acuity via the cannabinoid receptor type 2. Sci. Rep. 2020, 10, 15819. [Google Scholar] [CrossRef]
- Danser, A.H.; Derkx, F.H.; Admiraal, P.J.; Deinum, J.; de Jong, P.T.; Schalekamp, M.A. Angiotensin levels in the eye. Investig. Ophthalmol. Vis. Sci. 1994, 35, 1008–1018. [Google Scholar]
- Giese, M.J.; Speth, R.C. The ocular renin–angiotensin system: A therapeutic target for the treatment of ocular disease. Pharmacol. Ther. 2014, 142, 11–32. [Google Scholar] [CrossRef]
- Paul, M.; Poyan Mehr, A.; Kreutz, R. Physiology of local renin-angiotensin systems. Physiol. Rev. 2006, 86, 747–803. [Google Scholar] [CrossRef]
- Ribeiro-Oliveira, A.N., Jr.; Nogueira, A.I.; Pereira, R.M.; Boas, W.W.V.; dos Santos, R.A.S.; e Silva, A.C.S.E. The renin–angiotensin system and diabetes: An update. Vasc. Health Risk Manag. 2008, 4, 787. [Google Scholar]
- Guang, C.; Phillips, R.D.; Jiang, B.; Milani, F. Three key proteases–angiotensin-I-converting enzyme (ACE), ACE2 and renin–within and beyond the renin-angiotensin system. Arch. Cardiovasc. Dis. 2012, 105, 373–385. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, G.; Delarue, F.O.; Burcklé, C.; Bouzhir, L.; Giller, T.; Sraer, J.-D. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J. Clin. Investig. 2002, 109, 1417–1427. [Google Scholar] [CrossRef]
- Boucher, R.; Demassieux, S.; Garcia, R.; Genest, J. Tonin, angiotensin II system: A review. Circ. Res. 1977, 41, 26–29. [Google Scholar] [CrossRef]
- Arakawa, K. Serine protease angiotensin II systems. J. Hypertens. Suppl. Off. J. Int. Soc. Hypertens. 1996, 14, S3–S7. [Google Scholar]
- Maruta, H.; Arakawa, K. Confirmation of direct angiotensin formation by kallikrein. Biochem. J. 1983, 213, 193–200. [Google Scholar] [CrossRef] [Green Version]
- Tonnesen, M.G.; Klempner, M.S.; Austen, K.F.; Wintroub, B.U. Identification of a human neutrophil angiotension II-generating protease as cathepsin G. J. Clin. Investig. 1982, 69, 25–30. [Google Scholar] [CrossRef] [Green Version]
- Becari, C.; Oliveira, E.B.; Salgado, M.C.O. Alternative pathways for angiotensin II generation in the cardiovascular system. Braz. J. Med. Biol. Res. 2011, 44, 914–919. [Google Scholar] [CrossRef] [Green Version]
- Urata, H.; Healy, B.; Stewart, R.W.; Bumpus, F.M.; Husain, A. Angiotensin II-forming pathways in normal and failing human hearts. Circ. Res. 1990, 66, 883–890. [Google Scholar] [CrossRef] [Green Version]
- Izzo, J.L., Jr.; Weir, M.R. Angiotensin-converting enzyme inhibitors. J. Clin. Hypertens. 2011, 13, 667. [Google Scholar] [CrossRef]
- Wagenaar, L.J.; Voors, A.A.; Buikema, H.; van Gilst, W.H. Angiotensin Receptors in the Cardiovascular System; University Library Groningen: Groningen, The Netherlands, 2003. [Google Scholar]
- Cheng, Z.J.; Vapaatalo, H.; Mervaala, E. Angiotensin II and vascular inflammation. Med. Sci. Monit. 2005, 11, RA194–RA205. [Google Scholar]
- Culman, J.; Höhle, S.; Qadri, F.; Edling, O.; Blume, A.; Lebrun, C.; Unger, T. Angiotensin as neuromodulator/neurotransmitter in central control of body fluid and electrolyte homeostasis. Clin. Exp. Hypertens. 1995, 17, 281–293. [Google Scholar] [CrossRef]
- Silva, A.F.; Torres, M.D.T.; Silva, L.S.; Alves, F.L.; de Sá Pinheiro, A.A.; Miranda, A.; Capurro, M.L.; de la Fuente-Nunez, C.; Oliveira, V.X. Angiotensin II-derived constrained peptides with antiplasmodial activity and suppressed vasoconstriction. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waghe, P.; Sarath, T.S.; Gupta, P.; Kandasamy, K.; Choudhury, S.; Kutty, H.S.; Mishra, S.K.; Sarkar, S.N. Arsenic causes aortic dysfunction and systemic hypertension in rats: Augmentation of angiotensin II signaling. Chem.-Biol. Interact. 2015, 237, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Luque, M.; Martin, P.; Martell, N.; Fernandez, C.; Brosnihan, K.B.; Ferrario, C.M. Effects of captopril related to increased levels of prostacyclin and angiotensin-(1-7) in essential hypertension. J. Hypertens. 1996, 14, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Holappa, M.; Valjakka, J.; Vaajanen, A. Angiotensin (1-7) and ACE2, “the hot spots” of renin-angiotensin system, detected in the human aqueous humor. Open Ophthalmol. J. 2015, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Vaajanen, A.; Luhtala, S.; Oksala, O.; Vapaatalo, H. Does the renin-angiotensin system also regulate intra-ocular pressure? Ann. Med. 2008, 40, 418–427. [Google Scholar] [CrossRef]
- Danser, A.H.J.; Van Den Dorpel, M.A.; Deinum, J.; Derkx, F.H.M.; Franken, A.A.M.; Peperkamp, E.; De Jong, P.; Schalekamp, M. Renin, prorenin, and immunoreactive renin in vitreous fluid from eyes with and without diabetic retinopathy. J. Clin. Endocrinol. Metab. 1989, 68, 160–167. [Google Scholar] [CrossRef] [Green Version]
- Vaajanen, A.; Vapaatalo, H. Local ocular renin–angiotensin system–a target for glaucoma therapy? Basic Clin. Pharmacol. Toxicol. 2011, 109, 217–224. [Google Scholar] [CrossRef]
- Cullinane, A.B.; Leung, P.S.; Ortego, J.; Coca-Prados, M.; Harvey, B.J. Renin-angiotensin system expression and secretory function in cultured human ciliary body non-pigmented epithelium. Br. J. Ophthalmol. 2002, 86, 676–683. [Google Scholar] [CrossRef]
- Shah, G.B.; Sharma, S.; Mehta, A.A.; Goyal, R.K. Oculohypotensive effect of angiotensin-converting enzyme inhibitors in acute and chronic models of glaucoma. J. Cardiovasc. Pharmacol. 2000, 36, 169–175. [Google Scholar] [CrossRef]
- Costagliola, C.; Verolino, M.; De Rosa, M.L.; Iaccarino, G.; Ciancaglini, M.; Mastropasqua, L. Effect of oral losartan potassium administration on intraocular pressure in normotensive and glaucomatous human subjects. Exp. Eye Res. 2000, 71, 167–171. [Google Scholar] [CrossRef]
- Wang, R.-F.; Podos, S.M.; Mittag, T.W.; Yokoyoma, T. Effect of CS-088, an angiotensin AT1 receptor antagonist, on intraocular pressure in glaucomatous monkey eyes. Exp. Eye Res. 2005, 80, 629–632. [Google Scholar] [CrossRef]
- Giardina, W.J.; Kleinert, H.D.; Ebert, D.M.; Wismer, C.T.; Chekal, M.A.; Stein, H.H. Intraocular pressure lowering effects of the renin inhibitor ABBOTT-64662 diacetate in animals. J. Ocul. Pharmacol. Ther. 1990, 6, 75–83. [Google Scholar] [CrossRef]
- Choudhary, R.; Kapoor, M.S.; Singh, A.; Bodakhe, S.H. Therapeutic targets of renin-angiotensin system in ocular disorders. J. Curr. Ophthalmol. 2017, 29, 7–16. [Google Scholar] [CrossRef] [Green Version]
- Feibel, R.M. Sympathectomy for glaucoma: Its rise and fall (1898–1910). Surv. Ophthalmol. 2015, 60, 500–507. [Google Scholar] [CrossRef]
- Lanigan, L.P.; Clark, C.V.; Hill, D.W. Retinal circulation responses to systemic autonomic nerve stimulation. Eye 1988, 2, 412–417. [Google Scholar] [CrossRef]
- Boland, M.V.; Ervin, A.M.; Friedman, D.S.; Jampel, H.D.; Hawkins, B.S.; Vollenweider, D.; Chelladurai, Y.; Ward, D.; Suarez-Cuervo, C.; Robinson, K.A. Comparative effectiveness of treatments for open-angle glaucoma: A systematic review for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2013, 158, 271–279. [Google Scholar] [CrossRef]
- Chan, K.K.W.; Tang, F.; Tham, C.C.Y.; Young, A.L.; Cheung, C.Y. Retinal vasculature in glaucoma: A review. BMJ Open Ophthalmol. 2017, 1, e000032. [Google Scholar] [CrossRef]
- Yu, H.G.; Chung, H.; Yoon, T.G.; Yum, K.W.; Kim, H.J. Stellate ganglion block increases blood flow into the optic nerve head and the peripapillary retina in human. Auton. Neurosci. 2003, 109, 53–57. [Google Scholar] [CrossRef]
- Rübsam, A.; Parikh, S.; Fort, P.E. Role of Inflammation in Diabetic Retinopathy. Int. J. Mol. Sci. 2018, 19, 942. [Google Scholar] [CrossRef] [Green Version]
- McDougal, D.H.; Gamlin, P.D. Autonomic control of the eye. Compr. Physiol. 2015, 5, 439–473. [Google Scholar] [CrossRef] [Green Version]
- Alvarado, J.A.; Murphy, C.G.; Franse-Carman, L.; Chen, J.; Underwood, J.L. Effect of beta-adrenergic agonists on paracellular width and fluid flow across outflow pathway cells. Investig. Ophthalmol. Vis. Sci. 1998, 39, 1813–1822. [Google Scholar]
- Zhou, E.H.; Krishnan, R.; Stamer, W.D.; Perkumas, K.M.; Rajendran, K.; Nabhan, J.F.; Lu, Q.; Fredberg, J.J.; Johnson, M. Mechanical responsiveness of the endothelial cell of Schlemm’s canal: Scope, variability and its potential role in controlling aqueous humour outflow. J. R. Soc. Interface 2012, 9, 1144–1155. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Schieber, A.T.; Camras, L.J.; Harasymowycz, P.J.; Herndon, L.W.; Allingham, R.R. Mathematical Modeling of Outflow Facility Increase With Trabecular Meshwork Bypass and Schlemm Canal Dilation. J. Glaucoma 2016, 25, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Howlett, A.C.; Blume, L.C.; Dalton, G.D. CB(1) cannabinoid receptors and their associated proteins. Curr. Med. Chem. 2010, 17, 1382–1393. [Google Scholar] [CrossRef] [Green Version]
- Howlett, A.C.; Song, C.; Berglund, B.A.; Wilken, G.H.; Pigg, J.J. Characterization of CB1 cannabinoid receptors using receptor peptide fragments and site-directed antibodies. Mol. Pharmacol. 1998, 53, 504–510. [Google Scholar] [CrossRef] [Green Version]
- Mukhopadhyay, S.; Howlett, A.C. CB1 receptor-G protein association. Subtype selectivity is determined by distinct intracellular domains. Eur. J. Biochem. 2001, 268, 499–505. [Google Scholar] [CrossRef]
- Howlett, A.C. Cannabinoid receptor signaling. Handb. Exp. Pharmacol. 2005, 168, 53–79. [Google Scholar] [CrossRef]
- Mann, D.L.; Kent, R.L.; Parsons, B.; Cooper, G. Adrenergic effects on the biology of the adult mammalian cardiocyte. Circulation 1992, 85, 790–804. [Google Scholar] [CrossRef] [Green Version]
- Pacher, P.; Steffens, S.; Haskó, G.; Schindler, T.H.; Kunos, G. Cardiovascular effects of marijuana and synthetic cannabinoids: The good, the bad, and the ugly. Nat. Rev. Cardiol. 2018, 15, 151–166. [Google Scholar] [CrossRef]
- Stanley, C.; O’Sullivan, S.E. Vascular targets for cannabinoids: Animal and human studies. Br. J. Pharmacol. 2014, 171, 1361–1378. [Google Scholar] [CrossRef] [Green Version]
- Girgih, A.T.; Alashi, A.; He, R.; Malomo, S.; Aluko, R.E. Preventive and treatment effects of a hemp seed (Cannabis sativa L.) meal protein hydrolysate against high blood pressure in spontaneously hypertensive rats. Eur. J. Nutr. 2014, 53, 1237–1246. [Google Scholar] [CrossRef]
- Schaich, C.L.; Grabenauer, M.; Thomas, B.F.; Shaltout, H.A.; Gallagher, P.E.; Howlett, A.C.; Diz, D.I. Medullary Endocannabinoids Contribute to the Differential Resting Baroreflex Sensitivity in Rats with Altered Brain Renin-Angiotensin System Expression. Front. Physiol. 2016, 7, 207. [Google Scholar] [CrossRef] [Green Version]
- Haspula, D.; Clark, M.A. Heterologous regulation of the cannabinoid type 1 receptor by angiotensin II in astrocytes of spontaneously hypertensive rats. J. Neurochem. 2016, 139, 523–536. [Google Scholar] [CrossRef]
- Szekeres, M.; Nádasy, G.L.; Turu, G.; Soltész-Katona, E.; Tóth, Z.E.; Balla, A.; Catt, K.J.; Hunyady, L. Angiotensin II induces vascular endocannabinoid release, which attenuates its vasoconstrictor effect via CB1 cannabinoid receptors. J. Biol. Chem. 2012, 287, 31540–31550. [Google Scholar] [CrossRef] [Green Version]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alswailmi, F.K. A Cross Talk between the Endocannabinoid System and Different Systems Involved in the Pathogenesis of Hypertensive Retinopathy. Pharmaceuticals 2023, 16, 345. https://doi.org/10.3390/ph16030345
Alswailmi FK. A Cross Talk between the Endocannabinoid System and Different Systems Involved in the Pathogenesis of Hypertensive Retinopathy. Pharmaceuticals. 2023; 16(3):345. https://doi.org/10.3390/ph16030345
Chicago/Turabian StyleAlswailmi, Farhan Khashim. 2023. "A Cross Talk between the Endocannabinoid System and Different Systems Involved in the Pathogenesis of Hypertensive Retinopathy" Pharmaceuticals 16, no. 3: 345. https://doi.org/10.3390/ph16030345
APA StyleAlswailmi, F. K. (2023). A Cross Talk between the Endocannabinoid System and Different Systems Involved in the Pathogenesis of Hypertensive Retinopathy. Pharmaceuticals, 16(3), 345. https://doi.org/10.3390/ph16030345




