Drug Candidate BGP-15 Prevents Isoproterenol-Induced Arrhythmias and Alters Heart Rate Variability (HRV) in Telemetry-Implanted Rats
Abstract
:1. Introduction
2. Results
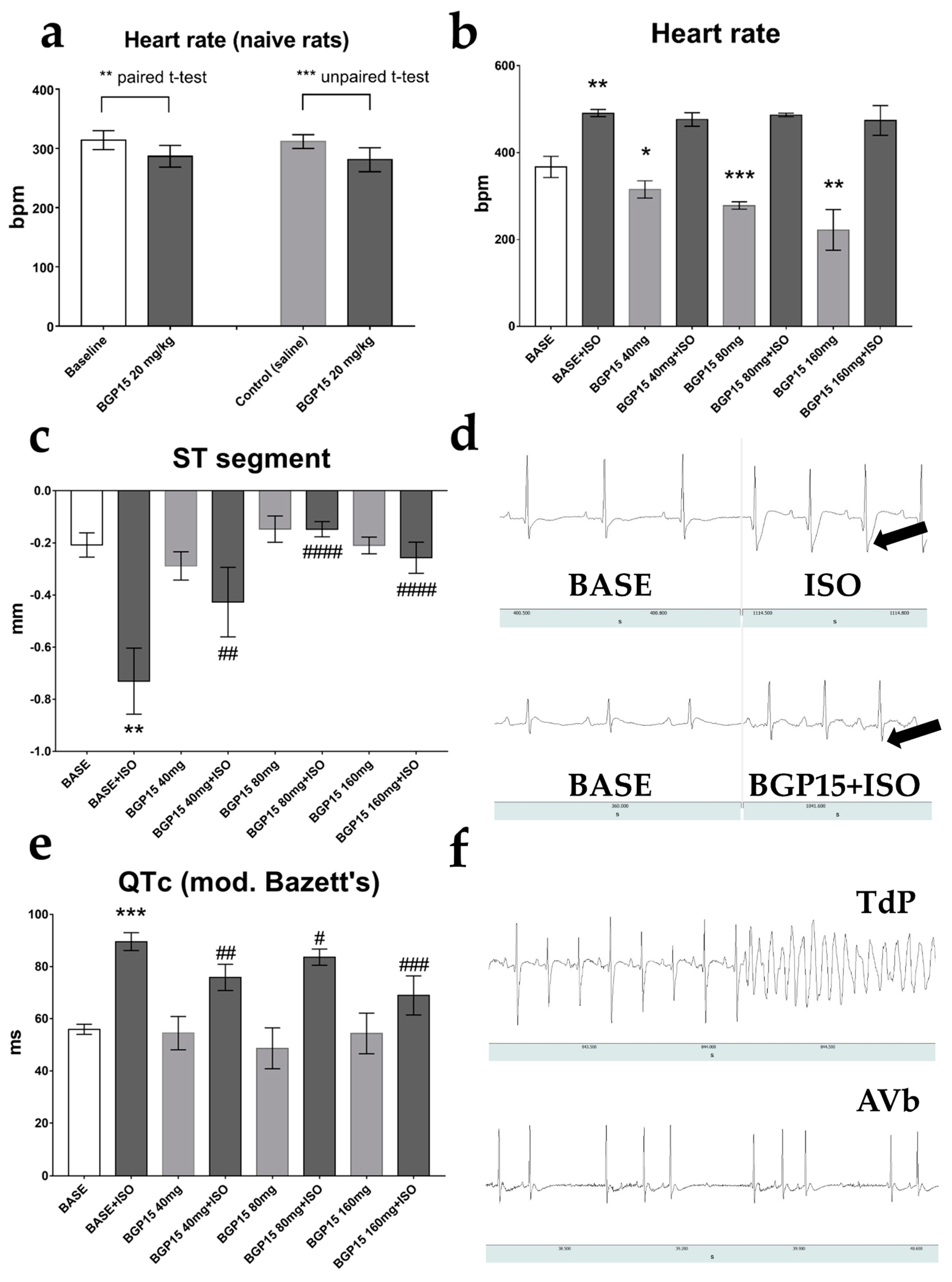
2.1. Effects of BGP-15 Dose-Escalation on Heart Rate and ECG Parameters of Naïve Rats
2.2. Effects of BGP-15 Dose-Escalation on ECG Parameters of ISO-Treated Rats
2.3. Effects of Single-Dose BGP-15 on 24-h HRV Parameters
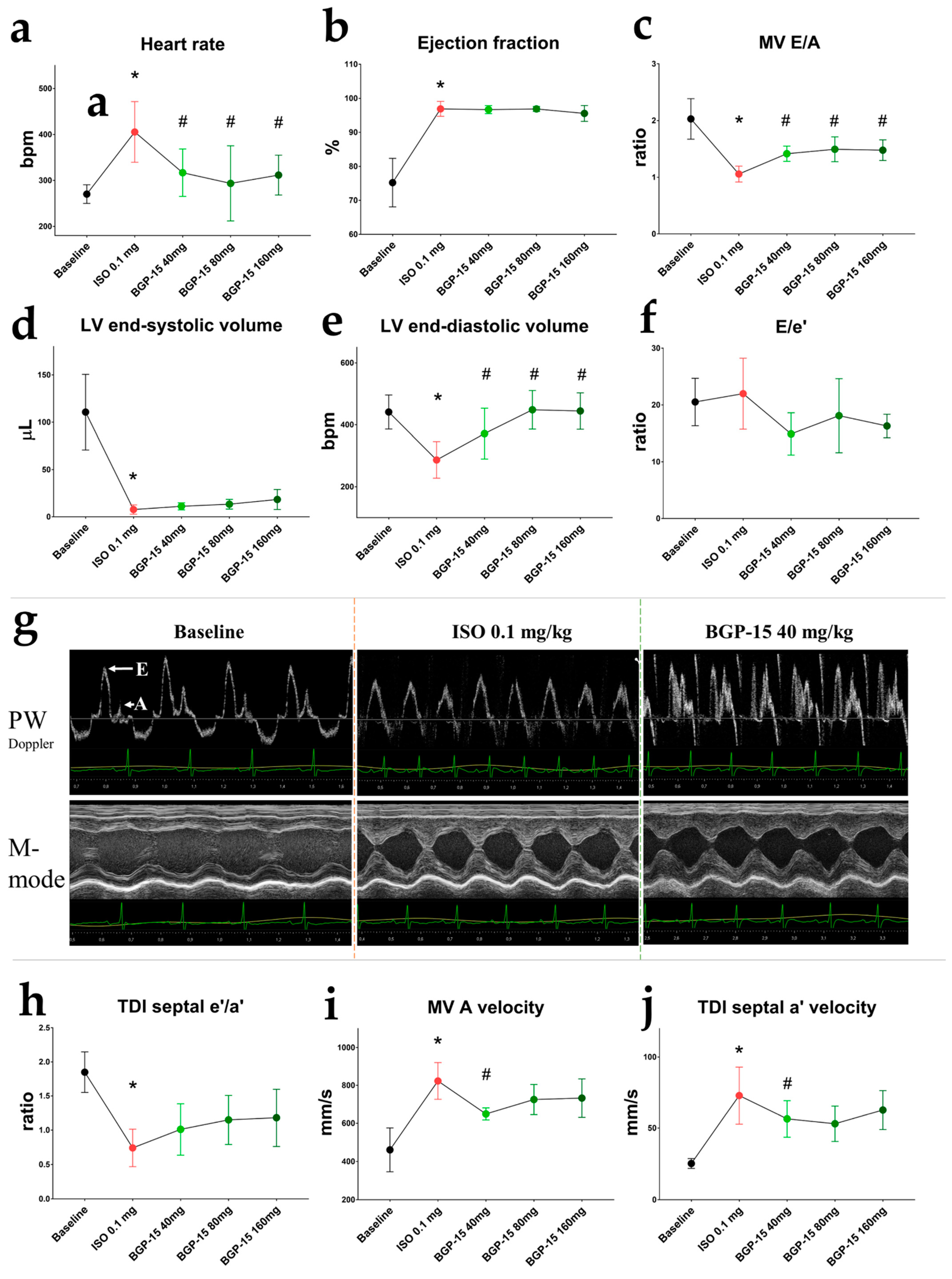
2.4. Ehocardiographic Results of the Acute Treatment Groups
2.5. Effects of 2-Week ISO and BGP-15-Treatments on 2-h HRV Parameters
2.6. Effects of the 2-Week-Long ISO and BGP-15 Treatments on Echocardiographic Parameters
2.7. Effects of BGP-15 on ISO-Induced Arrhythmogenesis
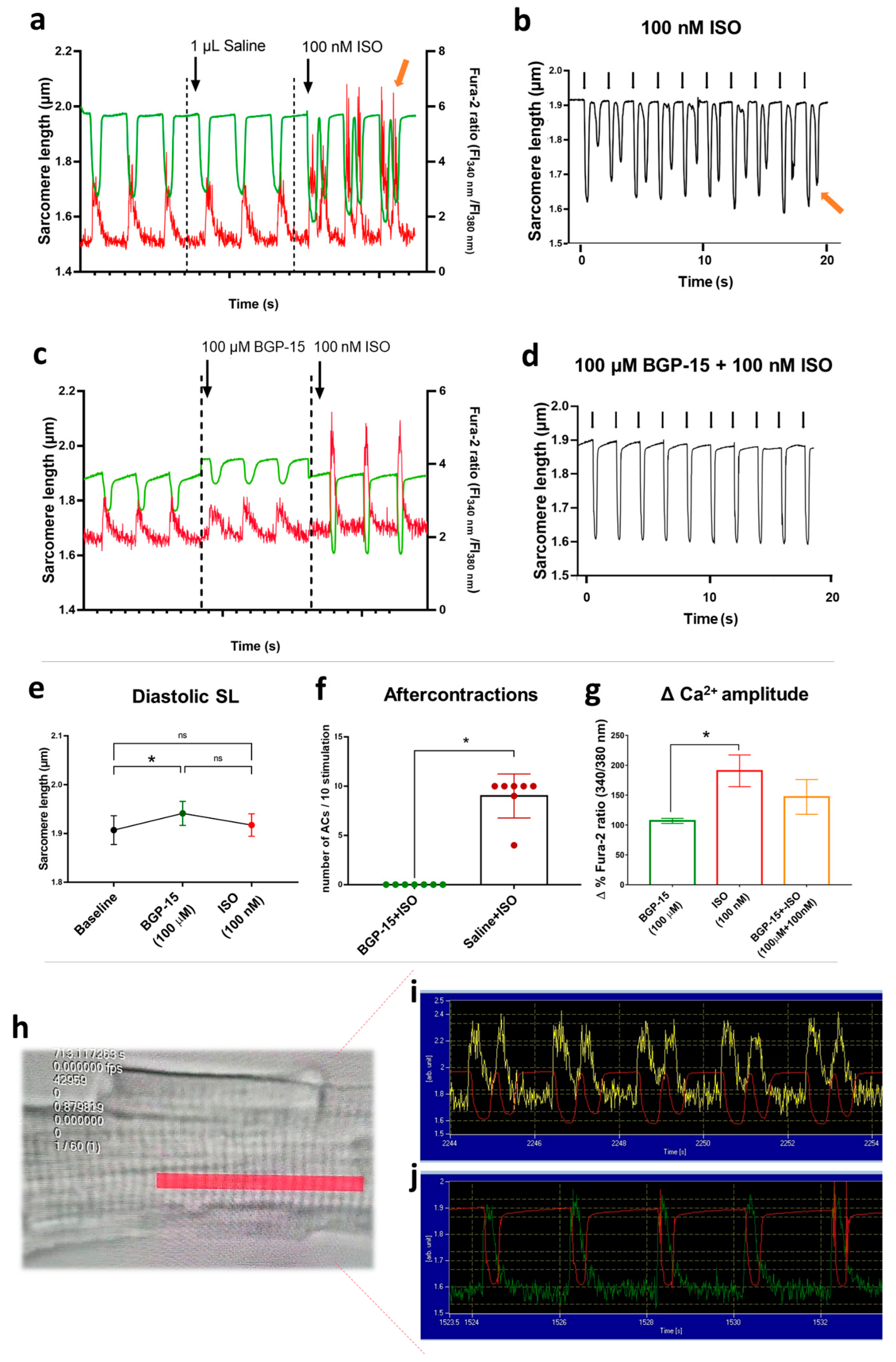
2.8. Results of Isolated Cardiomyocyte Experiments
3. Discussion
4. Materials and Methods
4.1. Animal Model and Chemicals
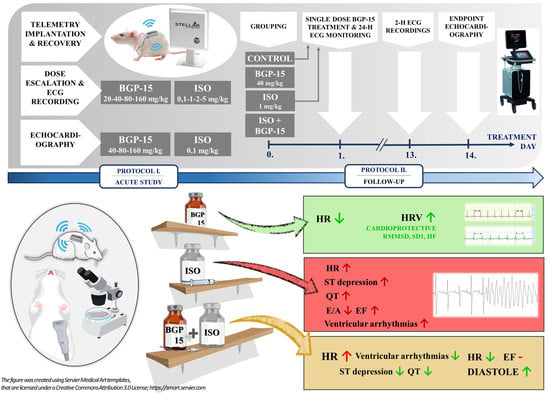
4.2. Study Design
4.3. Surgical Implantation of Radiotelemetry Transmitters
4.4. ECG Monitoring and Detection of Arrhythmias
4.5. Heart Rate Variability (HRV) Analyses
4.6. Echocardiography
4.7. Isolated Canine Cardiomyocyte Experiments
4.8. Statistics and Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Adabag, A.S.; Luepker, R.V.; Roger, V.L.; Gersh, B.J. Sudden cardiac death: Epidemiology and risk factors. Nat. Rev. Cardiol. 2010, 7, 216–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heijman, J.; Ghezelbash, S.; Dobrev, D. Investigational antiarrhythmic agents: Promising drugs in early clinical development. Expert Opin. Investig. Drugs 2017, 26, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Fordyce, C.B.; Roe, M.T.; Ahmad, T.; Libby, P.; Borer, J.S.; Hiatt, W.R.; Califf, R.M. Cardiovascular drug development: Is it dead or just hibernating? J. Am. Coll. Cardiol. 2015, 65, 1567–1582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowey, P.R.; Robinson, V.M. The Relentless Pursuit of New Drugs to Treat Cardiac Arrhythmias. Circulation 2020, 141, 1507–1509. [Google Scholar] [CrossRef]
- Novak, V.; Saul, J.P.; Eckberg, D.L. Task Force report on heart rate variability. Circulation 1997, 96, 1056–1057. [Google Scholar]
- Srinivasan, N.T.; Schilling, R.J.; Centre, S.B.H.B.H. Sudden Cardiac Death and Arrhythmias. Arrhythmia Electrophysiol. Rev. 2018, 7, 111–117. [Google Scholar] [CrossRef]
- Bilchick, K.; Fetics, B.; Djoukeng, R.; Fisher, S.G.; Fletcher, R.D.; Singh, S.N.; Nevo, E.; Berger, R.D. Prognostic value of heart rate variability in chronic congestive heart failure (Veterans Affairs’ Survival Trial of Antiarrhythmic Therapy in Congestive Heart Failure). Am. J. Cardiol. 2002, 90, 24–28. [Google Scholar] [CrossRef]
- La Rovere, M.T.; Pinna, G.D.; Maestri, R.; Mortara, A.; Capomolla, S.; Febo, O.; Ferrari, R.; Franchini, M.; Gnemmi, M.; Opasich, C.; et al. Short-Term Heart Rate Variability Strongly Predicts Sudden Cardiac Death in Chronic Heart Failure Patients. Circulation 2003, 107, 565–570. [Google Scholar] [CrossRef] [Green Version]
- Frenneaux, M.P. Autonomic changes in patients with heart failure and in post-myocardial infarction patients. Heart 2004, 90, 1248–1255. [Google Scholar] [CrossRef] [Green Version]
- Tsuji, H.; Venditti, F.J.; Manders, E.S.; Evans, J.C.; Larson, M.G.; Feldman, C.L.; Levy, D. Reduced heart rate variability and mortality risk in an elderly cohort. The Framingham Heart Study. Circulation 1994, 90, 878–883. [Google Scholar] [CrossRef] [Green Version]
- Chang, S.C.; Ren, S.; Rau, C.D.; Wang, J.J. Isoproterenol-Induced Heart Failure Mouse Model Using Osmotic Pump Implantation. Methods Mol. Biol. 2018, 1816, 207–220. [Google Scholar] [CrossRef]
- Patel, P.J.; Segar, R.; Patel, J.K.; Padanilam, B.J.; Prystowsky, E.N. Arrhythmia induction using isoproterenol or epinephrine during electrophysiology study for supraventricular tachycardia. J. Cardiovasc. Electrophysiol. 2018, 29, 1635–1640. [Google Scholar] [CrossRef]
- Thireau, J.; Zhang, B.L.; Poisson, D.; Babuty, D. Heart rate variability in mice: A theoretical and practical guide. Exp. Physiol. 2007, 93, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Gehrig, S.M.; Van Der Poel, C.; Sayer, T.A.; Schertzer, J.D.; Henstridge, D.C.; Church, J.E.; Lamon, S.; Russell, A.P.; Davies, K.E.; Febbraio, M.A.; et al. Hsp72 preserves muscle function and slows progression of severe muscular dystrophy. Nature 2012, 484, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Pető, Á.; Kósa, D.; Fehér, P.; Ujhelyi, Z.; Sinka, D.; Vecsernyés, M.; Szilvássy, Z.; Juhász, B.; Csanádi, Z.; Vígh, L.; et al. Pharmacological Overview of the BGP-15 Chemical Agent as a New Drug Candidate for the Treatment of Symptoms of Metabolic Syndrome. Molecules 2020, 25, 429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sapra, G.; Tham, Y.K.; Cemerlang, N.; Matsumoto, A.; Kiriazis, H.; Bernardo, B.C.; Henstridge, D.C.; Ooi, J.Y.Y.; Pretorius, L.; Boey, E.J.H.; et al. The small-molecule BGP-15 protects against heart failure and atrial fibrillation in mice. Nat. Commun. 2014, 5, 5705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szabados, E.; Literati-Nagy, P.; Farkas, B.; Sumegi, B. BGP-15, a nicotinic amidoxime derivate protecting heart from ischemia reperfusion injury through modulation of poly(ADP-ribose) polymerase. Biochem. Pharmacol. 2000, 59, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Hu, X.; Li, J.; Liu, J.; Bulte, L.B.-T.; Wiersma, M.; Malik, N.-U.; van Marion, D.M.S.; Tolouee, M.; Hoogstra-Berends, F.; et al. DNA damage-induced PARP1 activation confers cardiomyocyte dysfunction through NAD+ depletion in experimental atrial fibrillation. Nat. Commun. 2019, 10, 1307. [Google Scholar] [CrossRef] [Green Version]
- Halmosi, R.; Berente, Z.; Osz, E.; Toth, K.; Literati-Nagy, P.; Sumegi, B. Effect of Poly(ADP-Ribose) Polymerase Inhibitors on the Ischemia-Reperfusion-Induced Oxidative Cell Damage and Mitochondrial Metabolism in Langendorff Heart Perfusion System. Mol. Pharmacol. 2001, 59, 1497–1505. [Google Scholar] [CrossRef] [Green Version]
- Crul, T.; Toth, N.; Piotto, S.; Literati-Nagy, P.; Tory, K.; Haldimann, P.; LHooper, P. Hydroximic acid derivatives: Pleiotropic HSP co-inducers restoring homeostasis and robustness. Curr. Pharm. Des. 2013, 19, 309–346. [Google Scholar] [CrossRef]
- Horvath, O.; Ordog, K.; Bruszt, K.; Deres, L.; Gallyas, F.; Sumegi, B.; Toth, K.; Halmosi, R. BGP-15 Protects against Heart Failure by Enhanced Mitochondrial Biogenesis and Decreased Fibrotic Remodelling in Spontaneously Hypertensive Rats. Oxidative Med. Cell. Longev. 2021, 2021, 1250858. [Google Scholar] [CrossRef]
- Kozma, M.; Bombicz, M.; Varga, B.; Priksz, D.; Gesztelyi, R.; Tarjanyi, V.; Kiss, R.; Szekeres, R.; Takacs, B.; Menes, A.; et al. Cardioprotective Role of BGP-15 in Ageing Zucker Diabetic Fatty Rat (ZDF) Model: Extended Mitochondrial Longevity. Pharmaceutics 2022, 14, 226. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.A.; O’Rourke, B. Cardiac mitochondria and arrhythmias. Cardiovasc. Res. 2010, 88, 241–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Handklo-Jamal, R.; Meisel, E.; Yakubovich, D.; Vysochek, L.; Beinart, R.; Glikson, M.; McMullen, J.R.; Dascal, N.; Nof, E.; Oz, S. Andersen–Tawil Syndrome Is Associated with Impaired PIP2 Regulation of the Potassium Channel Kir2.1. Front. Pharmacol. 2020, 11, 672. [Google Scholar] [CrossRef] [PubMed]
- Bombicz, M.; Priksz, D.; Gesztelyi, R.; Kiss, R.; Hollos, N.; Varga, B.; Nemeth, J.; Toth, A.; Papp, Z.; Szilvassy, Z.; et al. The Drug Candidate BGP-15 Delays the Onset of Diastolic Dysfunction in the Goto-Kakizaki Rat Model of Diabetic Cardiomyopathy. Molecules 2019, 24, 586. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Scholz, P.M.; He, Y.; Tse, J.; Weiss, H.R. Cyclic GMP signaling and regulation of SERCA activity during cardiac myocyte contraction. Cell Calcium 2005, 37, 259–266. [Google Scholar] [CrossRef]
- Kennedy, T.L.; Swiderski, K.; Murphy, K.T.; Gehrig, S.M.; Curl, C.L.; Chandramouli, C.; Febbraio, M.A.; Delbridge, L.M.; Koopman, R.; Lynch, G.S. BGP-15 Improves Aspects of the Dystrophic Pathology in mdx and dko Mice with Differing Efficacies in Heart and Skeletal Muscle. Am. J. Pathol. 2016, 186, 3246–3260. [Google Scholar] [CrossRef] [Green Version]
- Lampé, N.; Priksz, D.; Erdei, T.; Bombicz, M.; Kiss, R.; Varga, B.; Zsuga, J.; Szerafin, T.; Csanádi, Z.; Balla, G.; et al. Negative Inotropic Effect of BGP-15 on the Human Right Atrial Myocardium. J. Clin. Med. 2020, 9, 1434. [Google Scholar] [CrossRef]
- Priksz, D.; Lampe, N.; Kovacs, A.; Herwig, M.; Bombicz, M.; Varga, B.; Wilisicz, T.; Szilvassy, J.; Posa, A.; Kiss, R.; et al. Nicotinic-acid derivative BGP-15 improves diastolic function in a rabbit model of atherosclerotic cardiomyopathy. Br. J. Pharmacol. 2022, 179, 2240–2258. [Google Scholar] [CrossRef]
- Literati-Nagy, B.; Kulcsar, E.; Literati-Nagy, Z.; Buday, B.; Peterfai, E.; Horvath, T.; Tory, K.; Kolonics, A.; Fleming, A.; Mandl, J.; et al. Improvement of Insulin Sensitivity by a Novel Drug, BGP-15, in Insulin-resistant Patients: A Proof of Concept Randomized Double-blind Clinical Trial. Horm. Metab. Res. 2009, 41, 374–380. [Google Scholar] [CrossRef]
- Chu, V.; Otero, J.M.; Lopez, O.; Morgan, J.P.; Amende, I.; Hampton, T.G. Method for non-invasively recording electrocardiograms in conscious mice. BMC Physiol. 2001, 1, 6. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Ito, T.; Yamada, S.; Kuniyoshi, N.; Shiomi, M. Electrocardiograms corresponding to the development of myocardial infarction in anesthetized WHHLMI rabbits (Oryctolagus cuniculus), an animal model for familial hypercholesterolemia. Comp. Med. 2012, 62, 409–418. [Google Scholar] [PubMed]
- Krenek, P.; Kmecova, J.; Kucerova, D.; Bajuszova, Z.; Musil, P.; Gazova, A.; Ochodnicky, P.; Klimas, J.; Kyselovic, J. Isoproterenol-induced heart failure in the rat is associated with nitric oxide-dependent functional alterations of cardiac function. Eur. J. Heart Fail. 2009, 11, 140–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konopelski, P.; Ufnal, M. Electrocardiography in Rats: A Comparison to Human. Physiol. Res. 2016, 65, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, C.K.; Dilaveris, P.; Manolakou, P.; Galanakos, S.; Magkas, N.; Gatzoulis, K.; Tousoulis, D. QT Prolongation and Malignant Arrhythmia: How Serious a Problem? Eur. Cardiol. 2017, 12, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mccraty, R.; Shaffer, F. Heart Rate Variability: New Perspectives on Physiological Mechanisms, Assessment of Self-regulatory Capacity, and Health Risk. Glob. Adv. Health Med. 2015, 4, 46–61. [Google Scholar] [CrossRef] [Green Version]
- Curtis, A.B.; Karki, R.; Hattoum, A.; Sharma, U.C. Arrhythmias in Patients≥ 80 Years of Age: Pathophysiology, Management, and Outcomes. J. Am. Coll. Cardiol. 2018, 71, 2041–2057. [Google Scholar] [CrossRef]
- Chow, G.V.; Marine, J.E.; Fleg, J.L. Epidemiology of Arrhythmias and Conduction Disorders in Older Adults. Clin. Geriatr. Med. 2012, 28, 539–553. [Google Scholar] [CrossRef] [Green Version]
- Buchheit, M.; Simon, C.; Piquard, F.; Ehrhart, J.; Brandenberger, G. Effects of increased training load on vagal-related indexes of heart rate variability: A novel sleep approach. Am. J. Physiol. Circ. Physiol. 2004, 287, H2813–H2818. [Google Scholar] [CrossRef] [Green Version]
- Samniang, B.; Shinlapawittayatorn, K.; Chunchai, T.; Pongkan, W.; Kumfu, S.; Chattipakorn, S.C.; KenKnight, B.H.; Chattipakorn, N. Vagus Nerve Stimulation Improves Cardiac Function by Preventing Mitochondrial Dysfunction in Obese-Insulin Resistant Rats. Sci. Rep. 2016, 6, 19749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carnevali, L.; Trombini, M.; Graiani, G.; Madeddu, D.; Quaini, F.; Landgraf, R.; Neumann, I.D.; Nalivaiko, E.; Sgoifo, A. Low vagally-mediated heart rate variability and increased susceptibility to ventricular arrhythmias in rats bred for high anxiety. Physiol. Behav. 2014, 128, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, M.; Yayou, K.-I.; Ishii, K.; Hashimoto, S.-I.; Tsubone, H.; Sugano, S. Power spectral analysis of heart rate variability as a new method for assessing autonomic activity in the rat. J. Electrocardiol. 1994, 27, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Lampert, R.; Ickovics, J.R.; Viscoli, C.J.; Horwitz, R.I.; Lee, A.F. Effects of propranolol on recovery of heart rate variability following acute myocardial infarction and relation to outcome in the Beta-Blocker Heart Attack Trial. Am. J. Cardiol. 2003, 91, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Rote, W.E., 2nd; Connor, J. Autonomic mechanisms in heart rate variability after isoproterenol-induced myocardial damage in rats. J. Auton. Nerv. Syst. 1992, 38, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.C.; Thomas, L.; Leung, D. Tissue Doppler echocardiography. Minerva Cardioangiol. 2010, 58, 357–378. [Google Scholar] [PubMed]
- George, J.C.; Liner, A.; Hoit, B.D. Isoproterenol-Induced Myocardial Injury: A Systematic Comparison of Subcutaneous versus Intraperitoneal Delivery in a Rat Model. Echocardiography 2010, 27, 716–721. [Google Scholar] [CrossRef]
- Zygmunt, A.C.; Goodrow, R.; Weigel, C. INaCa and ICl(Ca) contribute to isoproterenol-induced delayed after depolarizations in midmyocardial cells. Am. J. Physiol. 1998, 275, H1979–H1992. [Google Scholar]
- Freestone, N.S.; Ribaric, S.; Scheuermann, M.; Mauser, U.; Paul, M.; Vetter, R. Differential lusitropic responsiveness to beta-adrenergic stimulation in rat atrial and ventricular cardiac myocytes. Pflugers Arch. 2000, 441, 78–87. [Google Scholar] [CrossRef]
- Volders, P.G.; Kulcsár, A.; Vos, A.M.; Sipido, K.R.; Wellens, H.J.; Lazzara, R.; Szabo, B. Similarities between early and delayed afterdepolarizations induced by isoproterenol in canine ventricular myocytes. Cardiovasc. Res. 1997, 34, 348–359. [Google Scholar] [CrossRef] [Green Version]
- Priori, S.G.; Corr, P.B. Mechanisms underlying early and delayed afterdepolarizations induced by catecholamines. Am. J. Physiol. Circ. Physiol. 1990, 258, H1796–H1805. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Ko, C.Y.; Nivala, M.; Weiss, J.N.; Qu, Z. Calcium-Voltage Coupling in the Genesis of Early and Delayed Afterdepolarizations in Cardiac Myocytes. Biophys. J. 2015, 108, 1908–1921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ginsburg, K.S.; Bers, D.M. Modulation of excitation-contraction coupling by isoproterenol in cardiomyocytes with controlled SR Ca2+ load and Ca2+ current trigger. J. Physiol. 2004, 556, 463–480. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Shaw, S.A.; Naami, R.; Vuong, C.L.; Basheer, W.A.; Guo, X.; Hong, T. Isoproterenol Promotes Rapid Ryanodine Receptor Movement to Bridging Integrator 1 (BIN1)-Organized Dyads. Circulation 2016, 133, 388–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egdell, R.; MacLeod, K. Calcium Extrusion During Aftercontractions in Cardiac Myocytes: The Role of the Sodium-calcium Exchanger in the Generation of the Transient Inward Current. J. Mol. Cell. Cardiol. 2000, 32, 85–93. [Google Scholar] [CrossRef]
- O’Connell, T.D.; Rodrigo, M.C.; Simpson, P.C. Isolation and Culture of Adult Mouse Cardiac Myocytes. Methods Mol. Biol. 2007, 357, 271–296. [Google Scholar] [CrossRef] [PubMed]
- Van Heerebeek, L.; Hamdani, N.; Falcão-Pires, I.; Leite-Moreira, A.F.; Begieneman, M.P.; Bronzwaer, J.G.; Paulus, W.J. Low myocardial protein kinase G activity in heart failure with preserved ejection fraction. Circulation 2012, 126, 830–839. [Google Scholar] [CrossRef] [Green Version]
- Chai, Y.; Zhang, D.M.; Lin, Y.F. Activation of cGMP-dependent protein kinase stimulates cardiac ATP-sensitive potassium channels via a ROS/calmodulin/CaMKII signaling cascade. PLoS ONE 2011, 6, e18191. [Google Scholar] [CrossRef] [Green Version]
- Abdallah, Y.; Gkatzoflia, A.; Pieper, H.; Zoga, E.; Walther, S.; Kasseckert, S.; Schäfer, C. Mechanism of cGMP-mediated protection in a cellular model of myocardial reperfusion injury. Cardiovasc. Res. 2005, 66, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Costa, A.D.; Garlid, K.D.; West, I.C.; Lincoln, T.M.; Downey, J.M.; Cohen, M.V.; Critz, S.D. Protein Kinase G Transmits the Cardioprotective Signal from Cytosol to Mitochondria. Circ. Res. 2005, 97, 329–336. [Google Scholar] [CrossRef] [Green Version]
- Sumegi, K.; Fekete, K.; Antus, C.; Debreceni, B.; Hocsak, E.; Gallyas, F., Jr.; Sumegi, B.; Szabo, A. BGP-15 Protects against Oxidative Stress- or Lipopolysaccharide-Induced Mitochondrial Destabilization and Reduces Mitochondrial Production of Reactive Oxygen Species. PLoS ONE 2017, 12, e0169372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salazar-Ramírez, F.; Ramos-Mondragón, R.; García-Rivas, G. Mitochondrial and Sarcoplasmic Reticulum Interconnection in Cardiac Arrhythmia. Front. Cell Dev. Biol. 2021, 8, 623381. [Google Scholar] [CrossRef] [PubMed]
- Xie, A.; Song, Z.; Liu, H.; Zhou, A.; Shi, G.; Wang, Q.; Gu, L.; Liu, M.; Xie, L.; Qu, Z.; et al. Mitochondrial Ca2+ Influx Contributes to Arrhythmic Risk in Nonischemic Cardiomyopathy. J. Am. Heart Assoc. 2018, 7, e007805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, C.; Li, M.; Inagaki, M.; Kawada, T.; Sunagawa, K.; Sugimachi, M. Vagal stimulation markedly suppresses arrhythmias in conscious rats with chronic heart failure after myocardial infarction. In Proceedings of the 2005 IEEE Engineering in Medicine and Biology 27th Annual Conference, Shanghai, China, 17–18 January 2006; pp. 7072–7075. [Google Scholar] [CrossRef]
- Lilley, E.; Stanford, S.C.; Kendall, D.E.; Alexander, S.P.; Cirino, G.; Docherty, J.R.; George, C.H.; Insel, P.A.; Izzo, A.A.; Ji, Y.; et al. ARRIVE 2.0 and the British Journal of Pharmacology: Updated guidance for 2020. Br. J. Pharmacol. 2020, 177, 3611–3616. [Google Scholar] [CrossRef] [PubMed]
- Jelinek, M.; Wallach, C.; Ehmke, H.; Schwoerer, A.P. Genetic background dominates the susceptibility to ventricular arrhythmias in a murine model of beta-adrenergic stimulation. Sci. Rep. 2018, 8, 2312. [Google Scholar] [CrossRef] [Green Version]
- York, M.; Scudamore, C.; Brady, S.; Chen, C.; Wilson, S.; Curtis, M.; Evans, G.; Griffiths, W.; Whayman, M.; Williams, T.; et al. Characterization of Troponin Responses in Isoproterenol-Induced Cardiac Injury in the Hanover Wistar Rat. Toxicol. Pathol. 2007, 35, 606–617. [Google Scholar] [CrossRef]
- Curtis, M.J.; Hancox, J.C.; Farkas, A.; Wainwright, C.L.; Stables, C.L.; Saint, D.A.; Clements-Jewery, H.; Lambiase, P.D.; Billman, G.E.; Janse, M.J.; et al. The Lambeth Conventions (II): Guidelines for the study of animal and human ventricular and supraventricular arrhythmias. Pharmacol. Ther. 2013, 139, 213–248. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [Green Version]
- Horváth, B.; Szentandrássy, N.; Veress, R.; Almássy, J.; Magyar, J.; Bányász, T.; Tóth, A.; Papp, Z.; Nánási, P.P. Frequency-dependent effects of omecamtiv mecarbil on cell shortening of isolated canine ventricular cardiomyocytes. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2017, 390, 1239–1246. [Google Scholar] [CrossRef]


| Parameter | BASE 24 h | 40 mg/kg BGP-15 24 h | Significance |
|---|---|---|---|
| Heart rate (bpm) | 372 ± 32 | 322 ± 28 | * |
| SDNN (ms) | 2.185 ± 0.5572 | 2.627 ± 0.5348 | ns |
| RMSSD (ms) | 1.819 ± 0.7211 | 2.485 ± 0.6293 | * |
| pNN10 (ms) | 0.1055 ± 0.1698 | 0.5351 ± 0.4455 | ns |
| SD1 (ms) | 1.274 ± 0.5263 | 1.761 ± 0.4447 | * |
| SD2 (ms) | 2.785 ± 0.6812 | 3.205 ± 0.7143 | ns |
| LF % | 23.09 ± 24.48 | 12.62 ± 5.244 | ns |
| HF % | 27.95 ± 14.9 | 54.32 ± 13.41 | * |
| VLF % | 38.78 ± 11.9 | 39.54 ± 10.56 | ns |
| Parameter | Control | BGP-15 | ISO | ISO+BGP-15 |
|---|---|---|---|---|
| LA/Ao ratio | 1.164 ± 0.37 | 0.814 ± 0.244 | 0.645 ± 0.112 | 0.887 ± 0.315 |
| Heart Rate (bpm) | 258 ± 29 | 254 ± 17 | 250 ± 30 | 252 ± 40 |
| LV Vol d (µL) | 372.4 ± 79.28 | 352.5 ± 40.81 | 329.7 ± 100.2 | 362.2 ± 77.27 |
| LV Vol s (µL) | 98.54 ± 47.0 | 73.28 ± 24.13 | 77.15 ± 54.61 | 75.81 ± 29.86 |
| SV (µL) | 273.8 ± 45.07 | 279.2 ± 28.06 | 252.5 ± 52.36 | 286.3 ± 63.03 |
| EF (%) | 77.61 ± 7.51 | 79.47 ± 5.348 | 76.38 ± 11.02 | 79.32 ± 7.121 |
| CO (ml/min) | 70.69 ± 13.94 | 70.88 ± 9.278 | 63.49 ± 16.94 | 71.44 ± 16.07 |
| LV mass corr. (mg) | 1089 ± 189.5 | 1262 ± 193.9 | 1385 ± 266.5 * | 1335 ± 205.4 * |
| E/A ratio | 1.841 ± 0.273 | 2.046 ± 0.39 | 1.907 ± 0.377 | 1.907 ± 0.331 |
| DecT (ms) | 42.59 ± 12.18 | 44.91 ± 14.77 | 61.5 ± 18.71 * | 47.69 ± 12.59 |
| TDI s’ (mm/s) | 43.3 ± 13.17 | 65.68 ± 10.14 * | 52.39 ± 9.155 | 57.32 ± 6.569 * |
| TDI e′ (mm/s) | 36.53 ± 10.62 | 44.07 ± 7.595 | 33.4 ± 9.435 | 44.74 ± 9.092 |
| TDI a′ (mm/s) | 32.06 ± 10.49 | 43.51 ± 18.13 | 41.22 ± 11.49 | 34.95 ± 8.726 |
| e′/a′ ratio | 1.205 ± 0.305 | 1.116 ± 0.5303 | 0.7586 ± 0.357 | 1.338 ± 0.244 # |
| E/e′ ratio | 26.44 ± 6.565 | 18.55 ± 3.237 * | 25.66 ± 4.967 | 18.14 ± 4.88 *,# |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernat, B.; Erdelyi, R.; Fazekas, L.; Garami, G.; Szekeres, R.M.; Takacs, B.; Bombicz, M.; Varga, B.; Sarkany, F.; Raduly, A.P.; et al. Drug Candidate BGP-15 Prevents Isoproterenol-Induced Arrhythmias and Alters Heart Rate Variability (HRV) in Telemetry-Implanted Rats. Pharmaceuticals 2023, 16, 359. https://doi.org/10.3390/ph16030359
Bernat B, Erdelyi R, Fazekas L, Garami G, Szekeres RM, Takacs B, Bombicz M, Varga B, Sarkany F, Raduly AP, et al. Drug Candidate BGP-15 Prevents Isoproterenol-Induced Arrhythmias and Alters Heart Rate Variability (HRV) in Telemetry-Implanted Rats. Pharmaceuticals. 2023; 16(3):359. https://doi.org/10.3390/ph16030359
Chicago/Turabian StyleBernat, Brigitta, Rita Erdelyi, Laszlo Fazekas, Greta Garami, Reka Maria Szekeres, Barbara Takacs, Mariann Bombicz, Balazs Varga, Fruzsina Sarkany, Arnold Peter Raduly, and et al. 2023. "Drug Candidate BGP-15 Prevents Isoproterenol-Induced Arrhythmias and Alters Heart Rate Variability (HRV) in Telemetry-Implanted Rats" Pharmaceuticals 16, no. 3: 359. https://doi.org/10.3390/ph16030359
APA StyleBernat, B., Erdelyi, R., Fazekas, L., Garami, G., Szekeres, R. M., Takacs, B., Bombicz, M., Varga, B., Sarkany, F., Raduly, A. P., Romanescu, D. D., Papp, Z., Toth, A., Szilvassy, Z., Juhasz, B., & Priksz, D. (2023). Drug Candidate BGP-15 Prevents Isoproterenol-Induced Arrhythmias and Alters Heart Rate Variability (HRV) in Telemetry-Implanted Rats. Pharmaceuticals, 16(3), 359. https://doi.org/10.3390/ph16030359