Design, Synthesis, and Biological Evaluation of Novel Hydroxamic Acid-Based Organoselenium Hybrids
Abstract
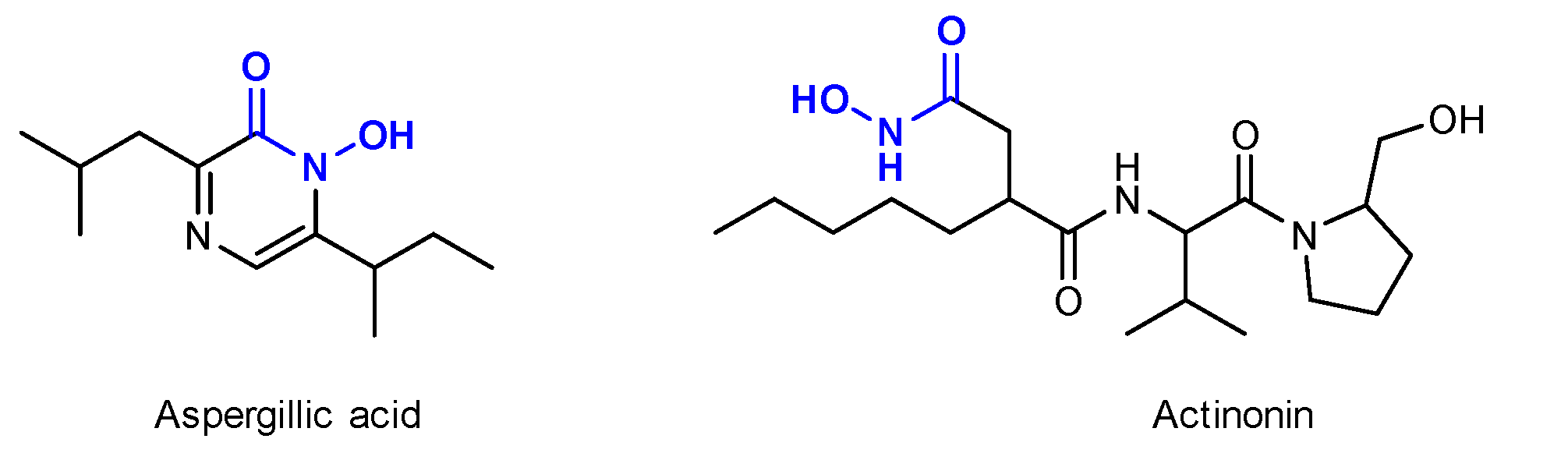
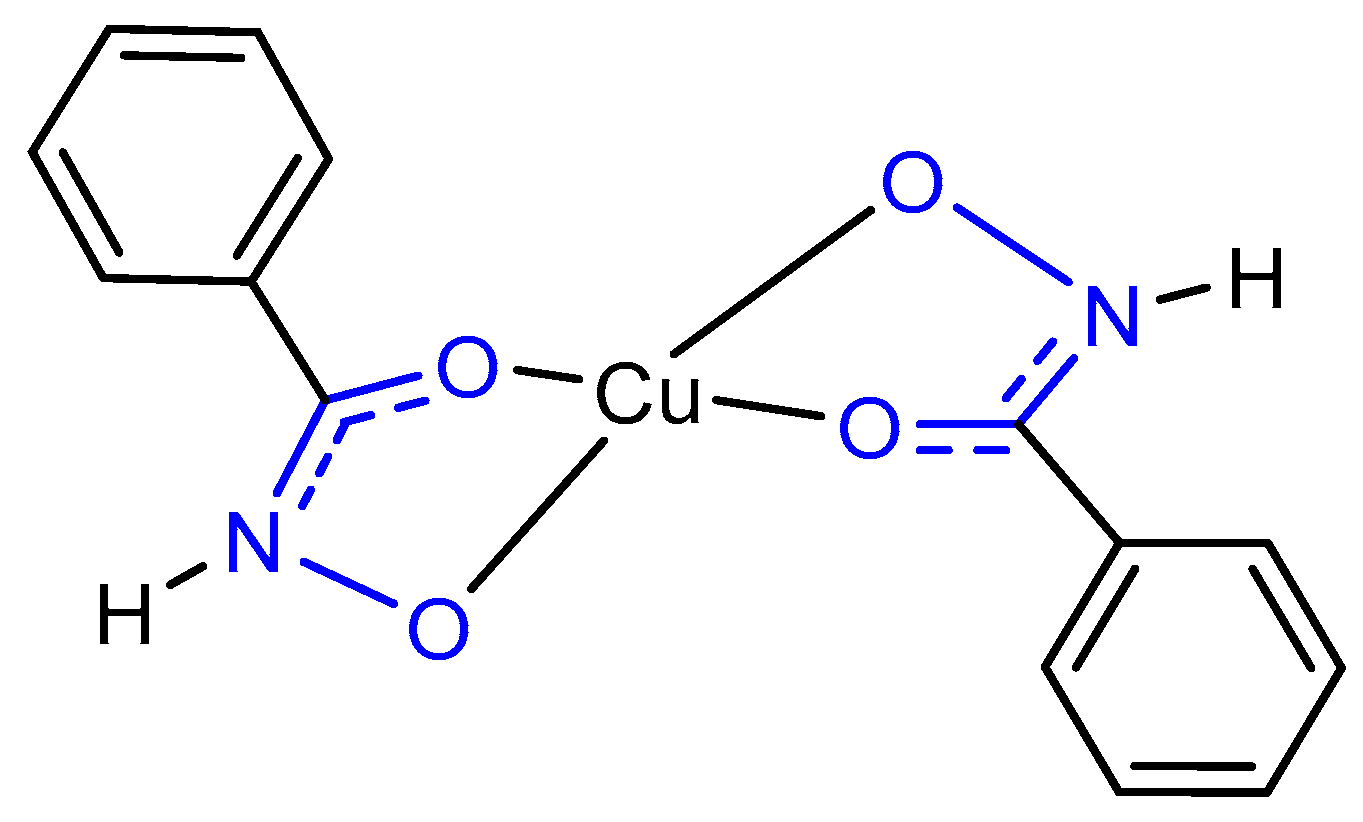
1. Introduction
2. Results and Discussions
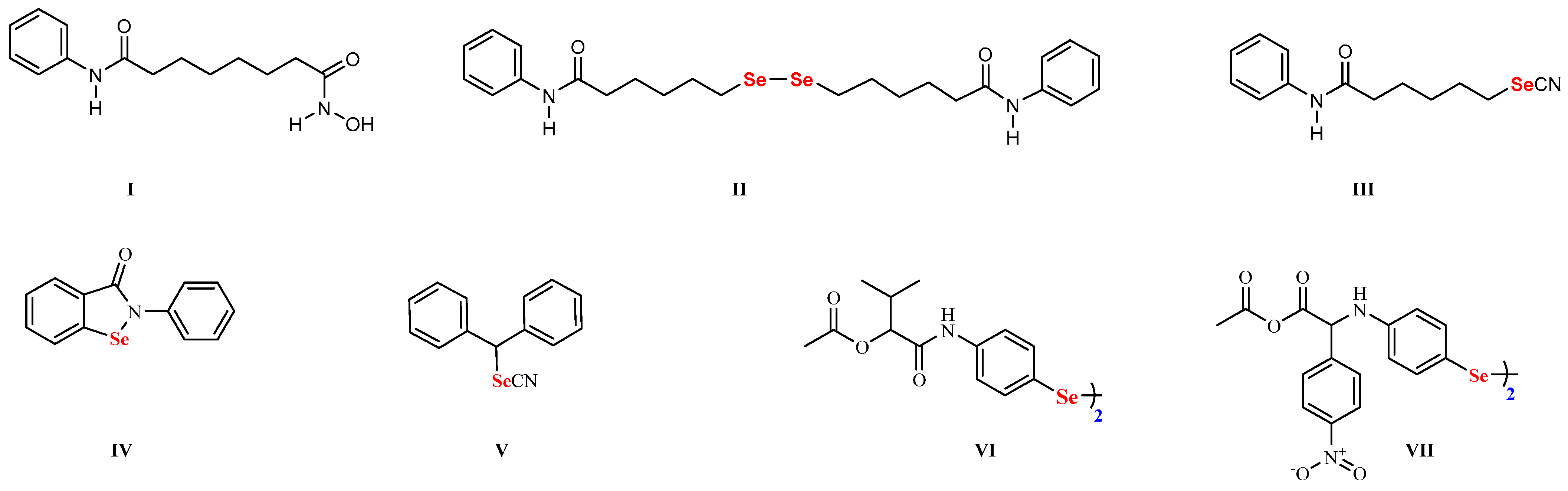
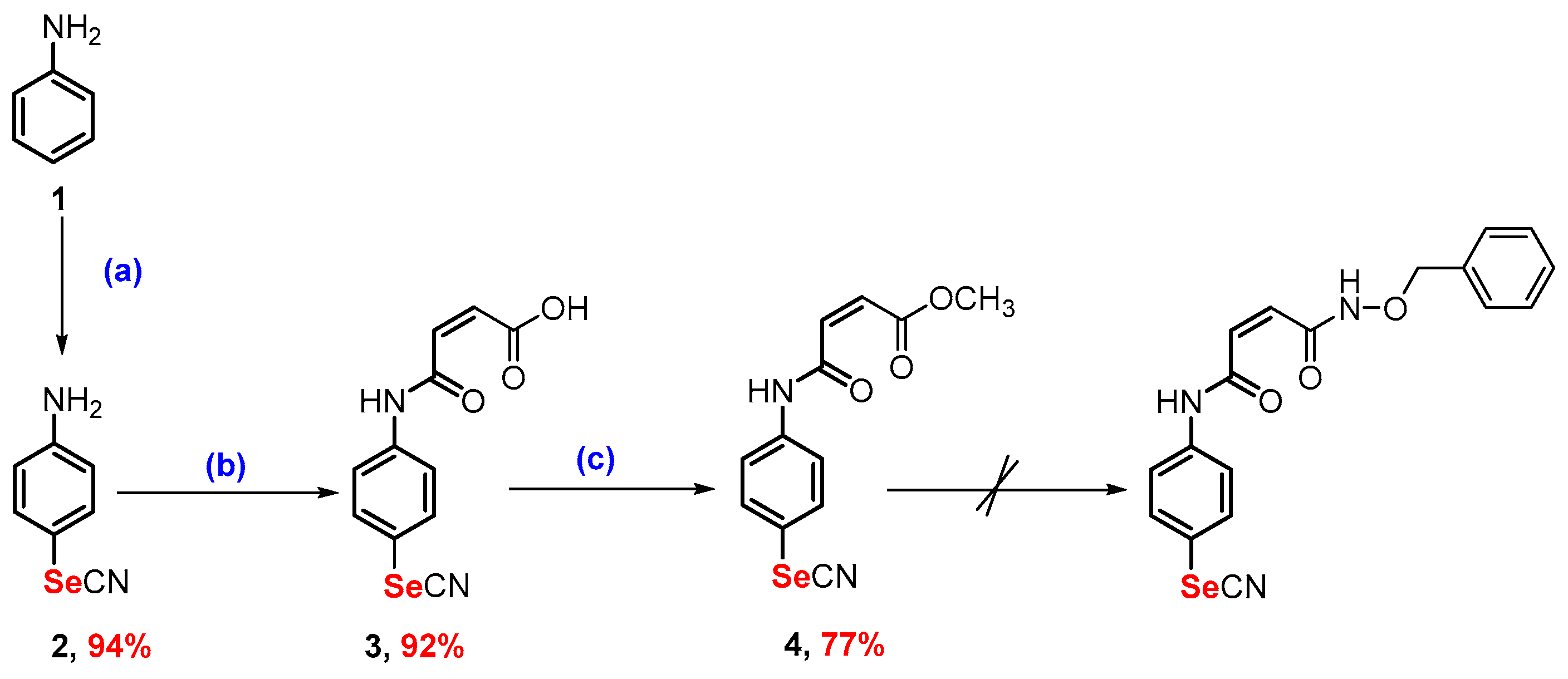
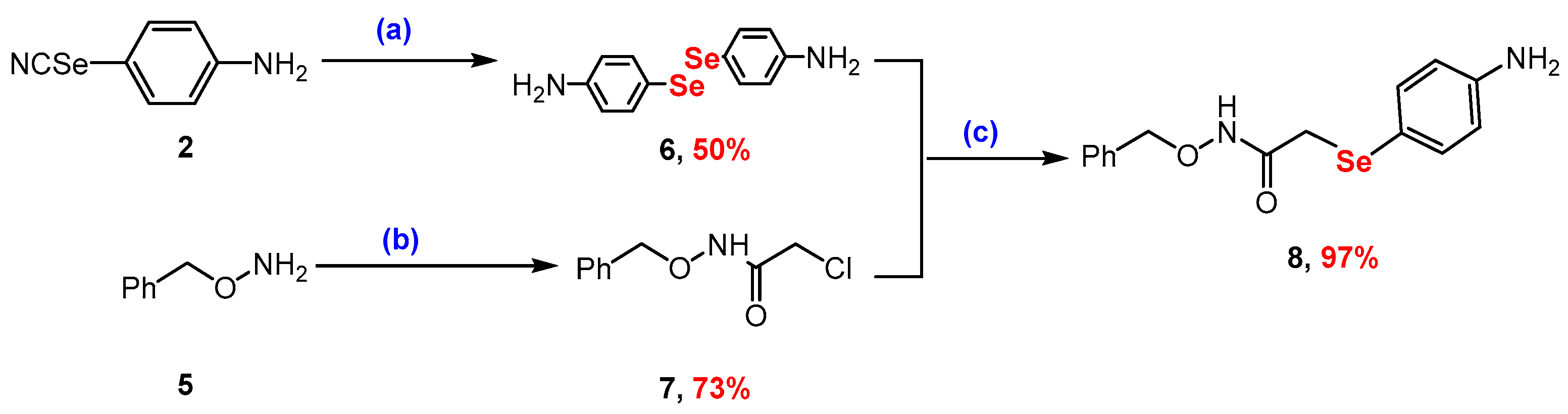
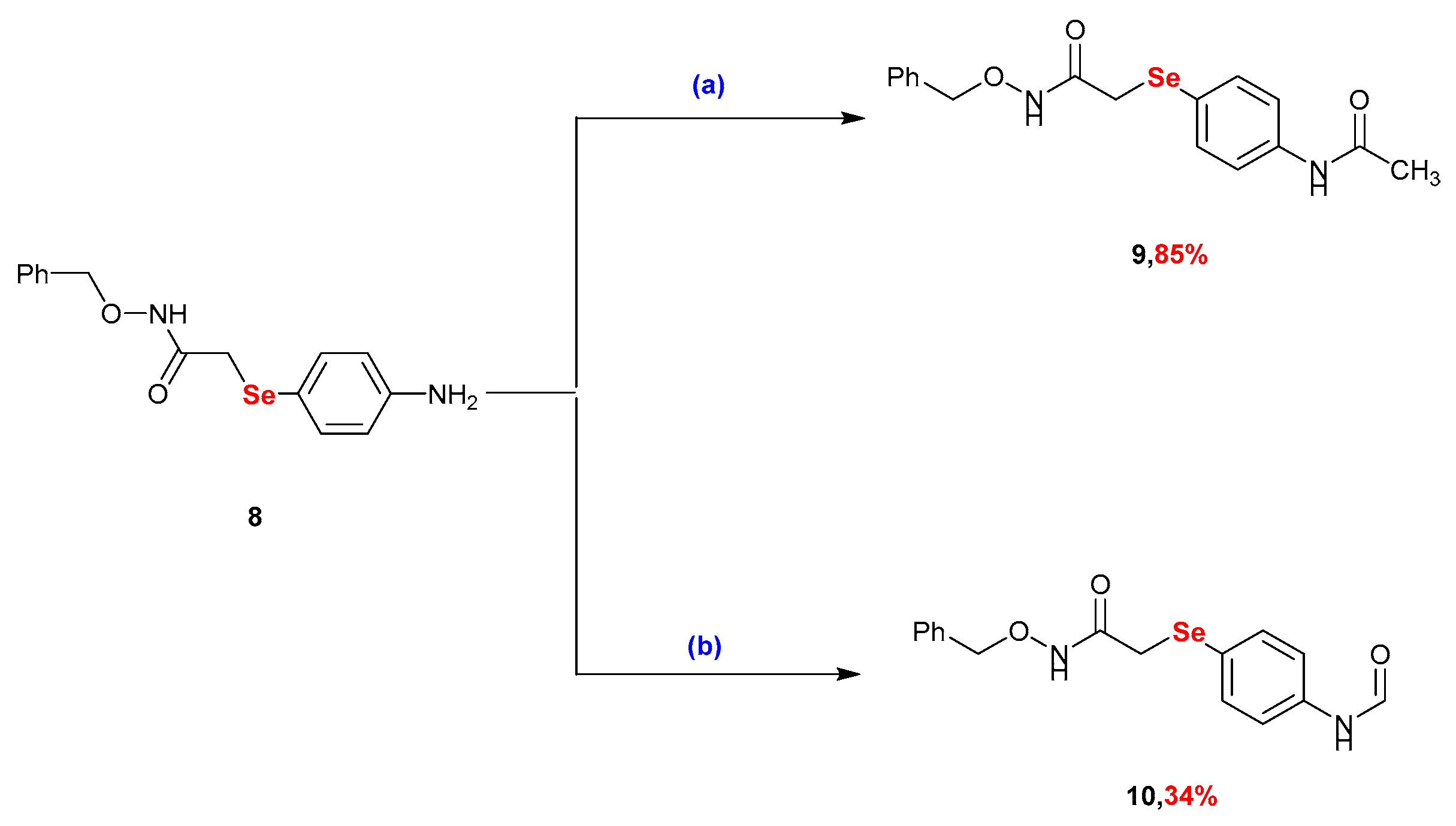
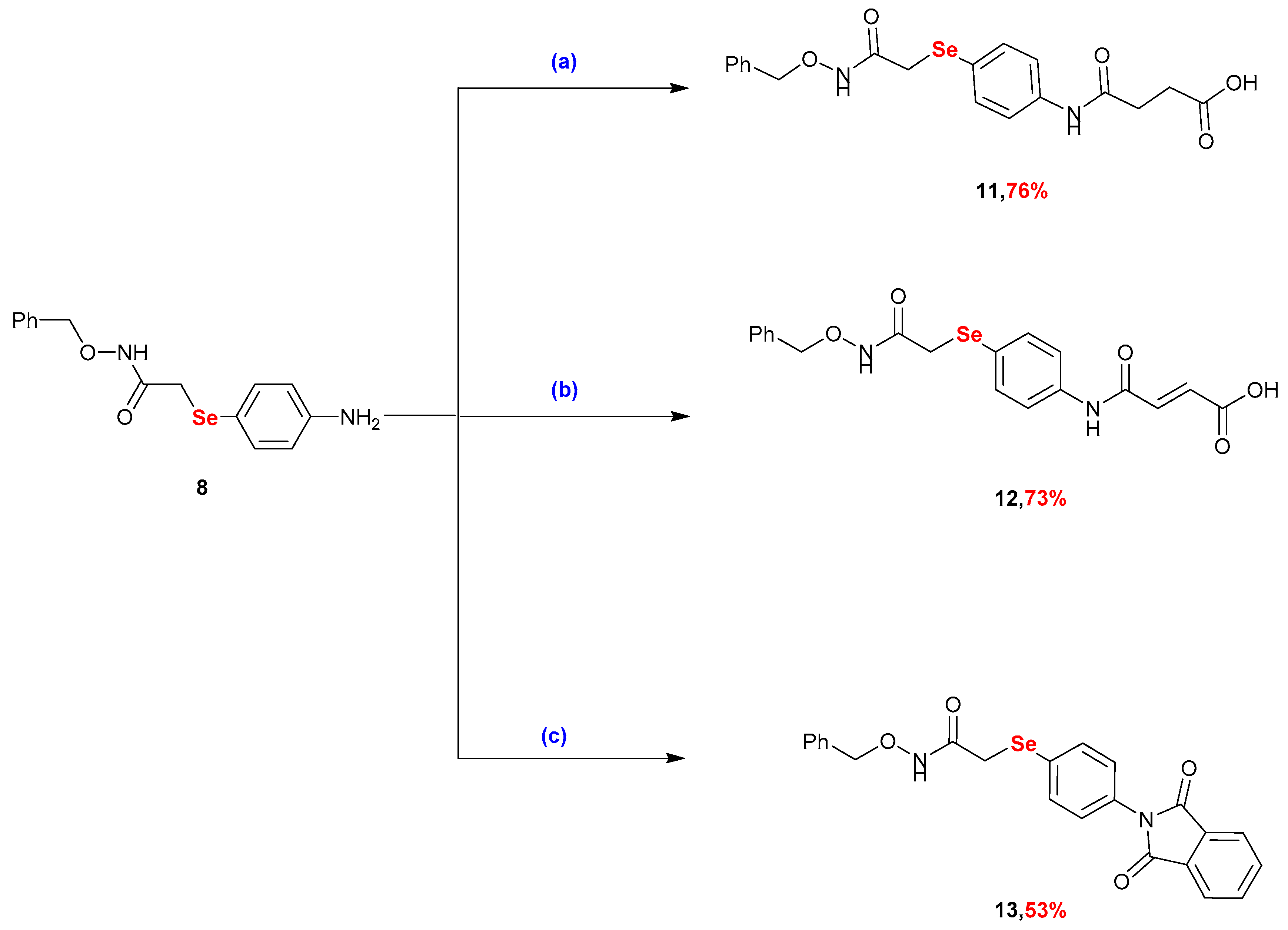
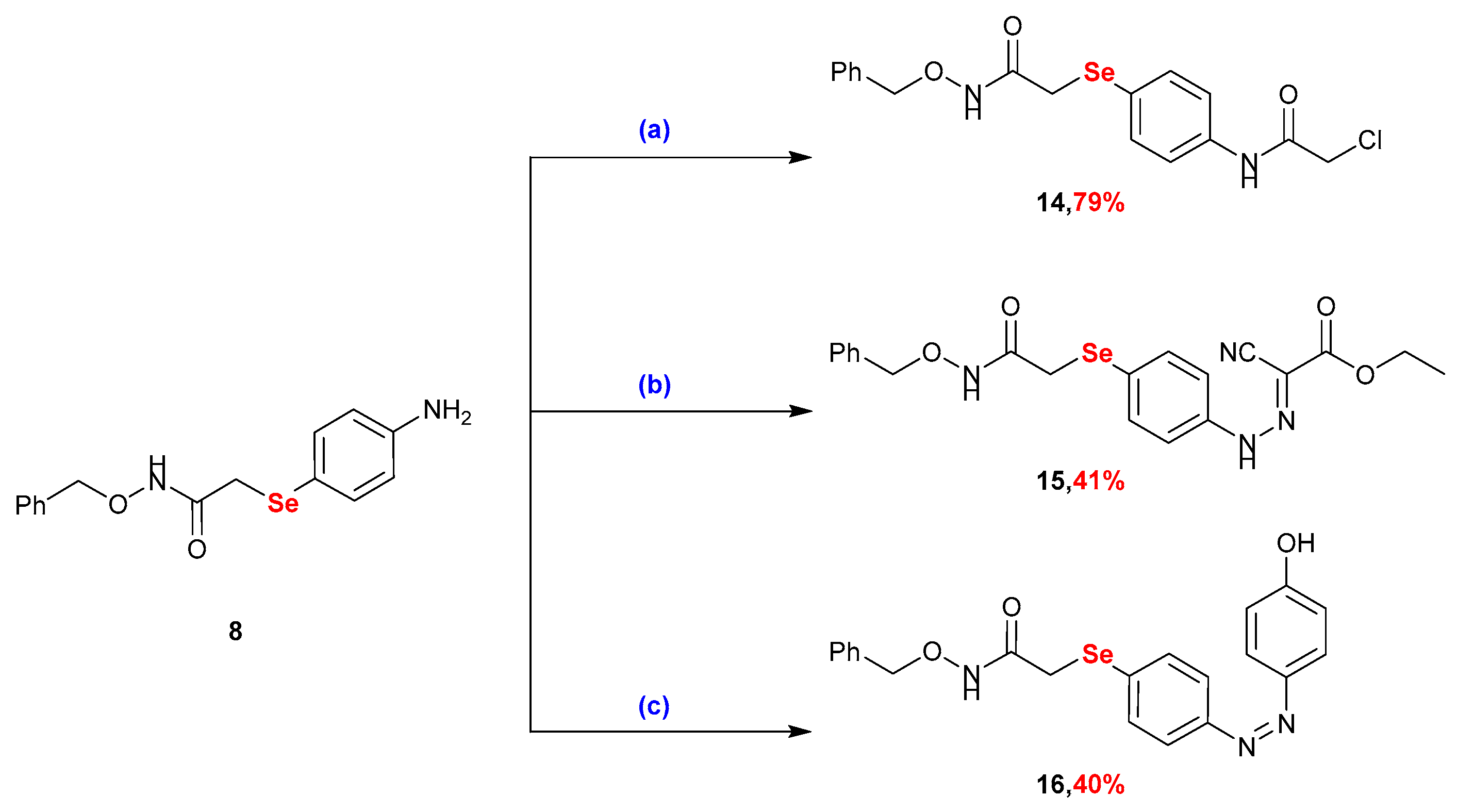
2.1. Synthesis
2.2. Biology
2.2.1. Evaluation of the Cytotoxicity of the OSe-Based HAs Compounds
2.2.2. Estimation of the Antimicrobial Properties of the OSe-Based HAs Compounds
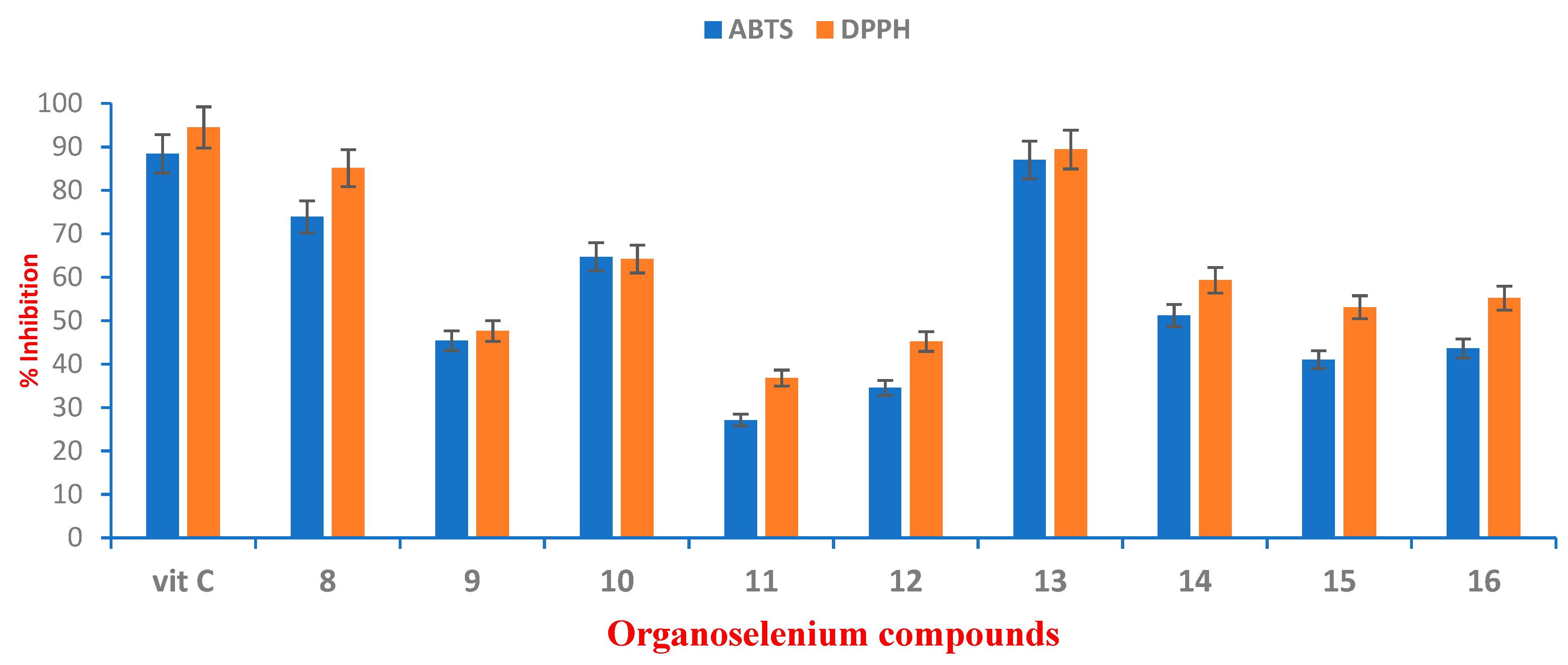
2.2.3. Evaluation of the Antioxidant Properties of the OSe-Based HAs Compounds
3. Materials and Methods
3.1. Experimental
3.2. Chemistry
3.2.1. Synthesis of Methyl (Z)-4-Oxo-4-((4-selenocyanatophenyl)amino)but-2-enoate (4)
3.2.2. Synthesis of N-(benzyloxy)-2-chloroacetamide (7)
3.2.3. Synthesis of 2-((4-Aminophenyl)selanyl)-N-(benzyloxy)acetamide (8)
3.2.4. Synthesis of 2-((4-Acetamidophenyl)selanyl)-N-(benzyloxy)acetamide (9)
3.2.5. Synthesis of N-(benzyloxy)-2-((4-formamidophenyl)selanyl)acetamide (10)
3.2.6. Synthesis of 4-((4-((2-((Benzyloxy)amino)-2-oxoethyl)selanyl)phenyl)amino)-4-oxobutanoic Acid (11)
3.2.7. Synthesis of 4-((4-((2-((Benzyloxy)amino)-2-oxoethyl)selanyl)phenyl)amino)-4-oxobut-2-enoic Acid (12)
3.2.8. Synthesis of N-(benzyloxy)-2-((4-(1,3-dioxoisoindolin-2-yl)phenyl)selanyl)acetamide (13)
3.2.9. Synthesis of N-(benzyloxy)-2-((4-(2-chloroacetamido)phenyl)selanyl)acetamide (14)
3.2.10. Synthesis of Ethyl 2-(2-(4-((2-((benzyloxy)amino)-2-oxoethyl)selanyl)phenyl)hydrazineylidene)-2-cyanoacetate (15)
3.2.11. Synthesis of N-(benzyloxy)-2-((4-((4-hydroxyphenyl)diazenyl)phenyl)selanyl)acetamide (16)
3.3. Biological Assays
3.3.1. Anticancer Activity
3.3.2. Antimicrobial Activity
3.3.3. Antioxidant Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gupta, S.P. Hydroxamic Acids: A Unique Family of Chemicals with Multiple Biological Activities; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar] [CrossRef]
- Taibi Houria, Z.S.; Said, Z.; Hafsa, B. Synthesis, characterisation and antimicrobial evaluation of hydroxamic acid. Eur. J. Biomed. Pharm. Sci. 2016, 3, 1–6. [Google Scholar] [CrossRef]
- Končić, M.Z.; Barbarić, M.; Perković, I.; Zorc, B. Antiradical, Chelating and Antioxidant Activities of Hydroxamic Acids and Hydroxyureas. Molecules 2011, 16, 6232–6242. [Google Scholar] [CrossRef]
- Tretyakov, B.; Gadomsky, S.; Terentiev, A. A Reaction of N-substituted Succinimides with Hydroxylamine as a Novel Approach to the Synthesis of Hydroxamic Acids. Beilstein Arch. 2023, 1, 6–19. [Google Scholar]
- Ali, D.B.; Abedullah, S.A. Preparation of Some Hydroxamic Acid Derivatives and Study of their Biological Activity as Anti-Cancer and Anti-Bacterial Agents. HIV Nurs. 2023, 23, 809–816. [Google Scholar]
- Al-Faiyz, Y.S.; Clark, A.J.; Filik, R.P.; Peacock, J.L.; Thomas, G.H. Rearrangements of activated O-acyl hydroxamic acid derivatives. Tetrahedron Lett. 1998, 39, 1269–1272. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Kumar, D.; Singh, S.K. Strategies for the Synthesis of Hydroxamic Acids. Curr. Org. Synth. 2018, 15, 154–165. [Google Scholar] [CrossRef]
- Adiguzel, E.; Yilmaz, F.; Emirik, M.; Ozil, M. Synthesis and characterisation of two new hydroxamic acids derivatives and their metal complexes. An investigation on the keto/enol, E/Z and hydroxamate/hydroximate forms Hydroxamic acid. J. Mol. Struct. 2017, 1127, 403–412. [Google Scholar] [CrossRef]
- Azeredo, N.F.B.; Borges, F.V.; Mathias, M.S. Effect of the hydroxamate group in the antitumoral activity and toxicity toward normal cells of new copper(II) complexes. BioMetals 2021, 34, 229–244. [Google Scholar] [CrossRef] [PubMed]
- Fazary, A.; Khalil, M.; Fahmy, A.; Tantawy, T. The role of hydroxamic acids in biochemical processes. Med. J. Islam. Acad. Sci. 2001, 14, 109–116. [Google Scholar]
- Saban, N.; Bujak, M. Hydroxyurea and hydroxamic acid derivatives as antitumor drugs. Cancer Chemother. Pharmacol. 2009, 64, 213–221. [Google Scholar] [CrossRef]
- Kalia, V.C. Green Synthesis of Hydroxamic Acid and Its Potential Industrial Applications. In Microbial Applications; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–336. [Google Scholar] [CrossRef]
- Bíró, L.; Buglyó, P.; Farkas, E. Diversity in the Interaction of Amino Acid- and Peptide-Based Hydroxamic Acids with Some Platinum Group Metals in Solution. Molecules 2022, 27, 669. [Google Scholar] [CrossRef]
- Al-Faiyz, Y.S.; Gouda, M. Multi-Walled Carbon Nanotubes Functionalized with Hydroxamic Acid Derivatives for the Removal of Lead from Wastewater: Kinetics, Isotherm, and Thermodynamic Studies. Polymers 2022, 14, 3870. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Bhardwaj, R.; Gautam, V.; Kohli, S.K.; Kaur, P.; Bali, R.S.; Saini, P.; Thukral, A.K.; Arora, S.; Vig, A.P. Microbial Siderophores in Metal Detoxification and Therapeutics: Recent Prospective and Applications. In Plant Microbiome: Stress Response; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar] [CrossRef]
- Gauglitz, J.M.; Butler, A. Amino acid variability in the peptide composition of a suite of amphiphilic peptide siderophores from an open ocean Vibrio species. J. Biol. Inorg. Chem. 2013, 18, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.B. Amino Acids, Peptides and Proteins in Organic Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Citarella, A.; Moi, D.; Pinzi, L.; Bonanni, D.; Rastelli, G. Hydroxamic Acid Derivatives: From Synthetic Strategies to Medicinal Chemistry Applications. ACS Omega 2021, 6, 21843–21849. [Google Scholar] [CrossRef] [PubMed]
- Al Shaer, D.; Al Musaimi, O.; de la Torre, B.G.; Albericio, F. Hydroxamate siderophores: Natural occurrence, chemical synthesis, iron-binding affinity and use as Trojan horses against pathogens. Eur. J. Med. Chem. 2020, 208, 112791. [Google Scholar] [CrossRef]
- Al-Faiyz, Y.S.S. Thoination of N-alkyl-O-acyl hydroxamic acid derivatives via Lawesson’s reagent. J. Mol. Struct. 2022, 1249, 131497. [Google Scholar] [CrossRef]
- Hwu, J.R.; Tsay, S.C. Counterattack reagents: Thiosilanes in the conversion of nitro compounds to thiohydroxamic acids and thiohydroximates. Tetrahedron 1990, 46, 7413–7428. [Google Scholar] [CrossRef]
- Bell, S.J.; Friedman, S.A.; Leong, J. Antibiotic action of N-methylthioformohydroxamate metal complexes. Antimicrob. Agents Chemother. 1979, 15, 384–391. [Google Scholar] [CrossRef]
- Ma, L.S.; Jiang, C.Y.; Cui, M.; Lu, R.; Liu, S.S.; Zheng, B.B.; Li, L.; Li, X. Fluopsin C induces oncosis of human breast adenocarcinoma cells. Acta Pharmacol. Sin. 2013, 34, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Phanstiel, O., IV. Synthesis of N-(Hydroxy)amide- and N-(Hydroxy) thioamide-containing peptides. J. Org. Chem. 2000, 65, 1442–1447. [Google Scholar] [CrossRef]
- Lemercier, B.C.; Pierce, J.G. Synthesis of thiohydroxamic acids and thiohydroximic acid derivatives. J. Org. Chem. 2014, 79, 2321–2330. [Google Scholar] [CrossRef]
- Cheshmedzhieva, D.; Toshev, N.; Gerova, M.; Petrov, O.; Dudev, T. Sulfur and selenium derivatives of suberoylanilide hydroxamic acid (SAHA) as a plausible HDAC inhibitor: A DFT study of their tautomerism and metal affinity/selectivity. Bulg. Chem. Commun. 2018, 50, 228–236. [Google Scholar]
- Tang, C.; Du, Y.; Liang, Q.; Cheng, Z.; Tian, J. A selenium-containing selective histone deacetylase inhibitor for targeted: In vivo breast tumor imaging and therapy. J. Mater. Chem. B 2019, 7, 3528–3536. [Google Scholar] [CrossRef]
- Wirth, T. Organoselenium chemistry in stereoselective reactions. Angew. Chem. Int. Ed. 2000, 39, 3740–3749. [Google Scholar] [CrossRef]
- Santoro, S.; Azeredo, J.B.; Nascimento, V.; Sancineto, L.; Braga, A.L.; Santi, C. The green side of the moon: Ecofriendly aspects of organoselenium chemistry. Rsc Adv. 2014, 4, 31521–31535. [Google Scholar] [CrossRef]
- Santi, C.; Santoro, S.; Battistelli, B. Organoselenium compounds as catalysts in nature and laboratory. Curr. Org. Chem. 2010, 14, 2442. [Google Scholar] [CrossRef]
- Bevinakoppamath, S.; Saleh Ahmed, A.M.; Ramachandra, S.C.; Vishwanath, P.; Prashant, A. Chemopreventive and Anticancer Property of Selenoproteins in Obese Breast Cancer. Front. Pharmacol. 2021, 12, 618172. [Google Scholar] [CrossRef]
- Naithani, R. Organoselenium compounds in cancer chemoprevention. Mini. Rev. Med. Chem. 2008, 8, 657–668. [Google Scholar] [CrossRef]
- Mugesh, G.; du Mont, W.W.; Sies, H. Chemistry of biologically important synthetic organoselenium compounds. Chem. Rev. 2001, 101, 2125–2179. [Google Scholar] [CrossRef]
- Shaaban, S.; Negm, A.; Ibrahim, E.E.; Elrazak, A.A. Chemotherapeutic agents for the treatment of hepatocellular carcinoma: Efficacy and mode of action. Oncol. Rev. 2014, 8, 246. [Google Scholar] [CrossRef]
- Refaay, D.A.; Ahmed, D.M.; Mowafy, A.M.; Shaaban, S. Evaluation of novel multifunctional organoselenium compounds as potential cholinesterase inhibitors against Alzheimer’s disease. Med. Chem. Res. 2022, 31, 894–904. [Google Scholar] [CrossRef]
- Makhal, P.N.; Nandi, A.; Kaki, V.R. Insights into the recent synthetic advances of organoselenium compounds. ChemistrySelect 2021, 6, 663–679. [Google Scholar] [CrossRef]
- Narajji, C.; Karvekar, M.D.; Das, A.K. Biological importance of organoselenium compounds. Indian J. Pharm. Sci. 2007, 69, 344–351. [Google Scholar]
- Soriano-Garcia, M. Organoselenium compounds as potential therapeutic and chemopreventive agents: A review. Curr. Med. Chem. 2004, 11, 1657–1669. [Google Scholar] [CrossRef]
- Chen, Z.; Lai, H.; Hou, L.; Chen, T. Rational design and action mechanisms of chemically innovative organoselenium in cancer therapy. Chem. Comm. 2020, 56, 179–196. [Google Scholar] [CrossRef]
- Liao, L.; Zhao, X. Modern organoselenium catalysis: Opportunities and challenges. Synlett 2021, 32, 1262–1268. [Google Scholar]
- Tian, X.; Schaich, K.M. Effects of molecular structure on kinetics and dynamics of the trolox equivalent antioxidant capacity assay with ABTS(+*). J. Agric. Food Chem. 2013, 61, 5511–5519. [Google Scholar] [CrossRef]
- Shaaban, S.; Zarrouk, A.; Vervandier-Fasseur, D.; S.Al-Faiyz, Y.; El-Sawy, H.; Althagafi, I.; Andreoletti, P.; Cherkaoui-Malki, M. Cytoprotective organoselenium compounds for oligodendrocytes. Arab. J. Chem. 2021, 14, 103051. [Google Scholar] [CrossRef]
- Sak, M.; Al-Faiyz, Y.S.; Elsawy, H.; Shaaban, S. Novel organoselenium redox modulators with potential anticancer, antimicrobial, and antioxidant activities. Antioxidants 2022, 11, 1231. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, S.; Sasse, F.; Burkholz, T.; Jacob, C. Sulfur, selenium and tellurium pseudo peptides: Synthesis and biological evaluation. Bioorg. Med. Chem. 2014, 22, 3610–3619. [Google Scholar] [CrossRef]
- Abdel-Motaal, M.; Almohawes, K.; Tantawy, M.A. Antimicrobial evaluation and docking study of some new substituted benzimidazole-2yl derivatives. Bioorg. Chem. 2020, 101, 103972. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Motaal, M.; Nabil, A. Biological activity of some newly synthesised hydrazone derivatives derived from (dicyclopropylmethylene) hydrazone. Eur. Chem. Bull. 2018, 7, 280–287. [Google Scholar] [CrossRef]
- El-Senduny, F.F.; Shabana, S.M.; Rösel, D.; Brabek, J.; Althagafi, I.; Angeloni, G.; Manolikakes, G.; Shaaban, S. Urea-functionalized organoselenium compounds as promising anti-HepG2 and apoptosis-inducing agents. Future Med. Chem. 2021, 13, 1655–1677. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, S.; Shabana, S.M.; Al-Faiyz, Y.S.; Manolikakes, G.; El-Senduny, F.F. Enhancing the chemosensitivity of HepG2 cells towards cisplatin by organoselenium pseudopeptides. Bioorg. Chem. 2021, 109, 104713. [Google Scholar] [CrossRef]
- Shaaban, S.; Negm, A.; Sobh, M.A.; Wessjohann, L.A. Expeditious Entry to Functionalized Pseudo-peptidic Organoselenide Redox Modulators via Sequential Ugi/SN Methodology. Anti-Cancer Agents Med. Chem. 2016, 16, 621–632. [Google Scholar] [CrossRef]
- Shaaban, S.; Negm, A.; Ashmawy, A.M.; Ahmed, D.M.; Wessjohann, L.A. Combinatorial synthesis, in silico, molecular and biochemical studies of tetrazole-derived organic selenides with increased selectivity against hepatocellular carcinoma. Eur. J. Med. Chem. 2016, 122, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, S.; Ashmawy, A.M.; Negm, A.; Wessjohann, L.A. Synthesis and biochemical studies of novel organic selenides with increased selectivity for hepatocellular carcinoma and breast adenocarcinoma. Eur. J. Med. Chem. 2019, 179, 515–526. [Google Scholar] [CrossRef] [PubMed]
| Compounds | MCF7 a | HepG2 a | WI38 a | ||
|---|---|---|---|---|---|
| IC50 (µM) a | TI c | IC50 (µM) a | TI c | IC50 (µM) a | |
| Adriamycin | 4.17 ± 0.2 | 1.6 | 4.50 ± 0.2 | 1.5 | 6.72 ± 0.5 |
| 8 | 9.86 ± 0.7 | 2.8 | 7.57 ± 0.5 | 3.6 | 27.42 ± 2.1 |
| 9 | 45.58 ± 2.9 | 1.9 | 53.04 ± 3.2 | 1.6 | 84.75 ± 4.8 |
| 10 | 49.29 ± 3.1 | 0.8 | 83.36 ± 4.2 | 0.5 | 37.39 ± 2.6 |
| 11 | 35.02 ± 2.5 | 1.5 | 48.78 ± 2.8 | 1.1 | 52.49 ± 3.2 |
| 12 | 63.02 ± 3.8 | 0.6 | 72.85 ± 3.7 | 0.5 | 36.53 ± 2.4 |
| 13 | 91.56 ± 4.6 | 0.8 | >100 b | 0.6 | 61.57 ± 3.8 |
| 14 | 87.41 ± 4.1 | 1.1 | >100 b | 1 | >100 b |
| 15 | 21.58 ± 1.6 | 3.8 | 15.83 ± 1.3 | 5.2 | 82.03 ± 4.6 |
| 16 | 33.73 ± 2.4 | 1.9 | 26.48 ± 2.0 | 2.4 | 64.16 ± 3.9 |
| Compound | E. coli | S. aureus | C. albicans | |||
|---|---|---|---|---|---|---|
| ZID (mm) a | IA% b | ZID (mm) a | IA% b | ZID (mm) a | IA% b | |
| 8 | 16 | 69.6 | 19 | 90.5 | 22 | 91.7 |
| 9 | 6 | 26.1 | 7 | 33.3 | 9 | 37.5 |
| 10 | NA | - | 6 | 28.6 | 7 | 29.2 |
| 11 | 7 | 30.4 | 10 | 47.6 | 13 | 54.2 |
| 12 | 4 | 17.4 | 5 | 23.8 | 10 | 41.7 |
| 13 | N.A. | - | NA | - | 2 | 8.3 |
| 14 | N.A. | - | NA | - | 4 | 16.7 |
| 15 | 12 | 52.2 | 15 | 71.4 | 20 | 83.3 |
| 16 | 9 | 39.1 | 11 | 52.4 | 16 | 66.7 |
| Ampicillin | 23 | 100 | 21 | 100 | - | - |
| Clotrimazole | - | - | - | - | 24 | 100 |
| Compounds | MIC (μM) | ||
|---|---|---|---|
| E. coli | S. aureus | C. albicans | |
| 8 | 8 | 4 | 4 |
| Ampicillin | 0.5 | 1 | - |
| Clotrimazole | - | - | 2 |
| Compounds | IC50 (µM) | |
|---|---|---|
| DPPH | ABTS | |
| Vitamin C | 19.18 ± 0.13 | 28.16 ± 0.19 |
| 8 | 23.67 ± 0.21 | 27.24 ± 0.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alotaibi, J.S.; Al-Faiyz, Y.S.; Shaaban, S. Design, Synthesis, and Biological Evaluation of Novel Hydroxamic Acid-Based Organoselenium Hybrids. Pharmaceuticals 2023, 16, 367. https://doi.org/10.3390/ph16030367
Alotaibi JS, Al-Faiyz YS, Shaaban S. Design, Synthesis, and Biological Evaluation of Novel Hydroxamic Acid-Based Organoselenium Hybrids. Pharmaceuticals. 2023; 16(3):367. https://doi.org/10.3390/ph16030367
Chicago/Turabian StyleAlotaibi, Jameelah S., Yasair S. Al-Faiyz, and Saad Shaaban. 2023. "Design, Synthesis, and Biological Evaluation of Novel Hydroxamic Acid-Based Organoselenium Hybrids" Pharmaceuticals 16, no. 3: 367. https://doi.org/10.3390/ph16030367
APA StyleAlotaibi, J. S., Al-Faiyz, Y. S., & Shaaban, S. (2023). Design, Synthesis, and Biological Evaluation of Novel Hydroxamic Acid-Based Organoselenium Hybrids. Pharmaceuticals, 16(3), 367. https://doi.org/10.3390/ph16030367







