First Evidence of Activity of Enfortumab Vedotin on Brain Metastases in Urothelial Cancer Patients
Abstract
1. Introduction
- (1)
- CNS metastases have been clinically stable for at least six weeks prior to screening.
- (2)
- If steroid treatment was required for CNS metastases, the subject should be on a stable dose (≤20 mg/day) of prednisone or equivalent for at least two weeks.
- (3)
- Baseline scans show no evidence of new or enlarged brain metastases.
- (4)
- The subject does not have leptomeningeal disease [10].
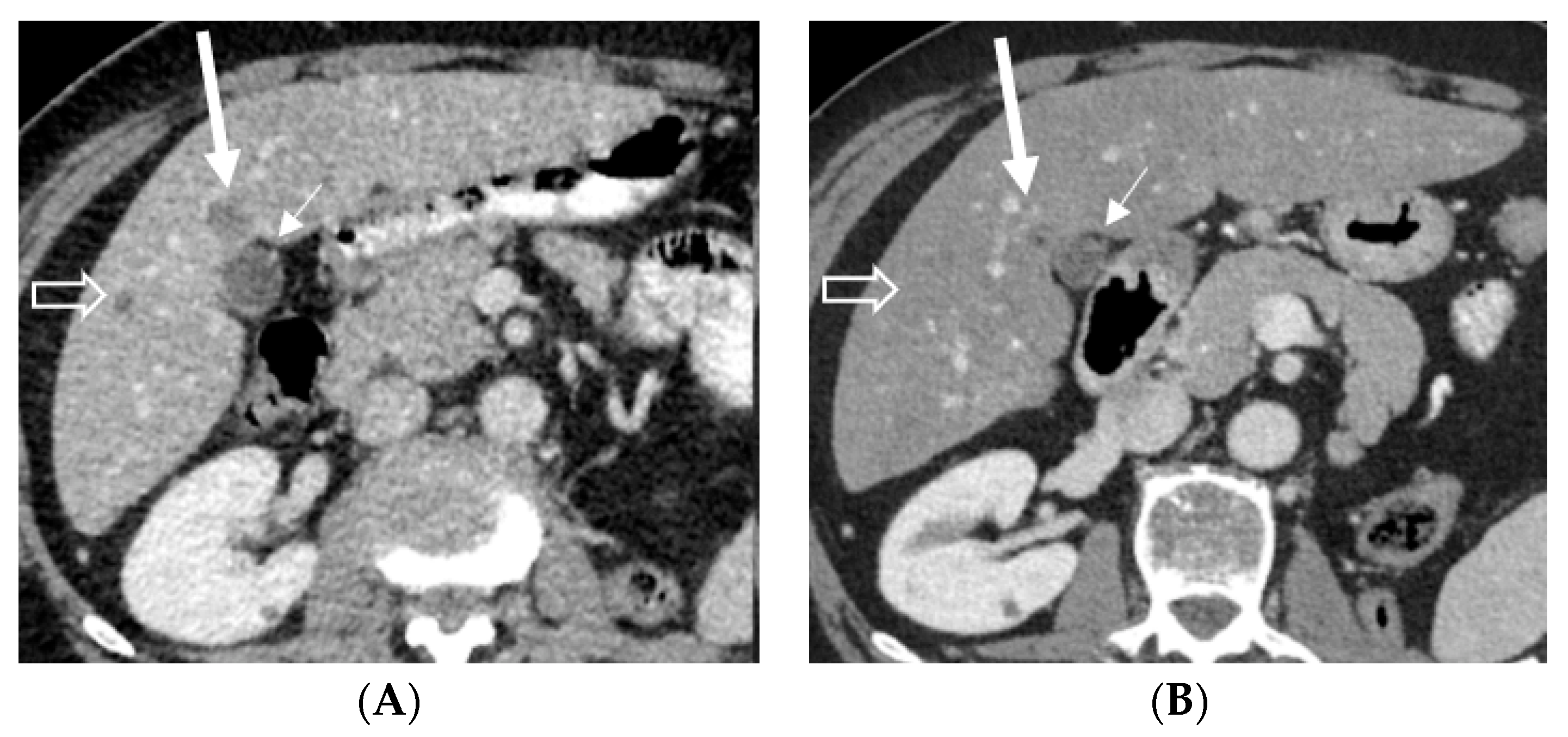
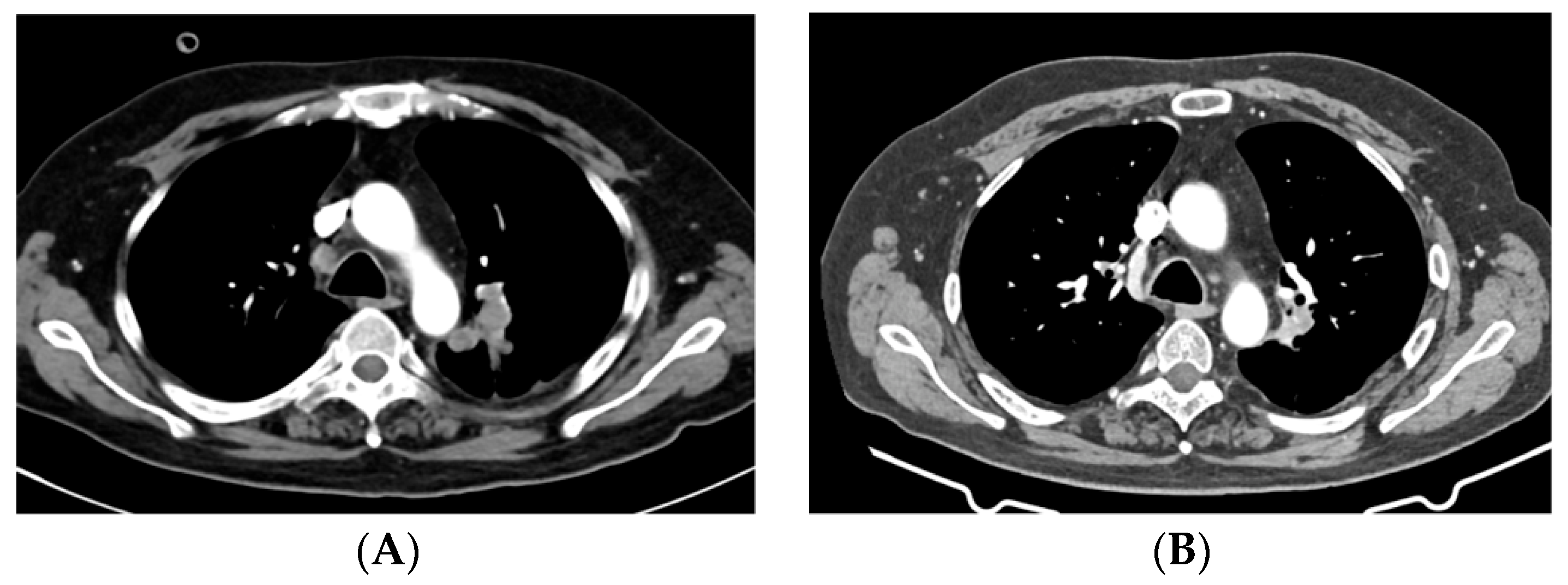
2. Case Presentations
2.1. Case 1
2.2. Case 2
2.3. Case 3
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bellmunt, J.; Orsola, A.; Leow, J.J.; Wiegel, T.; De Santis, M.; Horwich, A. Bladder cancer: ESMO practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2014, 25, iii40–iii48. [Google Scholar] [CrossRef]
- Warren, M.; Kolinsky, M.; Canil, C.M.; Czaykowski, P.; Sridhar, S.S.; Black, P.C.; Booth, C.M.; Kassouf, W.; Eapen, L.; Mukherjee, S.D.; et al. Canadian Urological Association/Genitourinary Medical Oncologists of Canada consensus statement: Management of unresectable locally advanced and metastatic urothelial carcinoma. Can. Urol. Assoc. J. 2019, 13, 318–327. [Google Scholar] [CrossRef]
- Kamat, A.M.; Bellmunt, J.; Galsky, M.D.; Konety, B.R.; Lamm, D.L.; Langham, D.; Lee, C.T.; Milowsky, M.I.; O’Donnell, M.A.; O’Donnell, P.H.; et al. Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of bladder carcinoma.n.d. J. Immunother. Cancer 2017, 5, 68. [Google Scholar] [CrossRef]
- Powles, T.; Rosenberg, J.E.; Sonpavde, G.P.; Loriot, Y.; Durán, I.; Lee, J.L.; Matsubara, N.; Vulsteke, C.; Castellano, D.; Wu, C.; et al. Enfortumab Vedotin in Previously Treated Advanced Urothelial Carcinoma. N. Engl. J. Med. 2021, 384, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- FDA Grants Regular Approval to Enfortumab Vedotin-Ejfv for Locally Advanced or Metastatic Urothelial Cancer|FDA n.d. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-regular-approval-enfortumab-vedotin-ejfv-locally-advanced-or-metastatic-urothelial-cancer (accessed on 30 January 2023).
- Padcev|European Medicines Agency n.d. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/padcev (accessed on 30 January 2023).
- Challita-Eid, P.M.; Satpayev, D.; Yang, P.; An, Z.; Morrison, K.; Shostak, Y.; Raitano, A.; Nadell, R.; Liu, W.; Lortie, D.R.; et al. Therapeutics, Targets, and Chemical Biology Enfortumab Vedotin Antibody-Drug Conjugate Targeting Nectin-4 Is a Highly Potent Therapeutic Agent in Multiple Preclinical Cancer Models n.d. Cancer Res. 2016, 76, 3003–3013. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, J.; Sridhar, S.S.; Zhang, J.; Smith, D.; Ruether, D.; Flaig, T.W.; Baranda, J.; Lang, J.; Plimack, E.R.; Sangha, R.; et al. EV-101: A phase I study of single-agent enfortumab vedotin in patients with nectin-4–positive solid tumors, including metastatic urothelial carcinoma. J. Clin. Oncol. 2020, 38, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Vulsteke, C.; Grivas, P.; Tagawa, S.T.; Bellmunt, J.; Santis, M.D.; Duran, I.; Goebell, P.J.; Necchi, A.; Sridhar, S.S.; Sternberg, C.N.; et al. TROPiCS-04: Study of sacituzumab govitecan (SG) in patients (pts) with locally advanced (LA) unresectable or metastatic urothelial cancer (mUC) that has progressed after prior platinum (PLT) and checkpoint inhibitor (CPI) therapy. J. Clin. Oncol. 2022, 40, TPS582. [Google Scholar] [CrossRef]
- A Study to Evaluate Enfortumab Vedotin Versus (vs) Chemotherapy in Subjects with Previously Treated Locally Advanced or Metastatic Urothelial Cancer (EV-301)-Full Text View-ClinicalTrials.gov n.d. Available online: https://clinicaltrials.gov/ct2/show/NCT03474107 (accessed on 30 January 2023).
- Boyle, H.J.; Lavergne, E.; Droz, J.P.; Bonnin, N.; Flechon, A. Brain metastases in patients with urothelial carcinoma. Tzu Chi Med. J. 2013, 31, 282. [Google Scholar] [CrossRef]
- Anami, Y.; Otani, Y.; Xiong, W.; Ha, S.Y.Y.; Yamaguchi, A.; Rivera-Caraballo, K.A.; Zhang, N.; An, Z.; Kaur, B.; Tsuchikama, K. Homogeneity of antibody-drug conjugates critically impacts the therapeutic efficacy in brain tumors. Cell Rep. 2022, 39, 110839. [Google Scholar] [CrossRef]
- Van Den Bent, M.; Eoli, M.; Sepulveda, J.M.; Smits, M.; Walenkamp, A.; Frenel, J.S.; Franceschi, E.; Clement, P.M.; Chinot, O.; De Vos, F.; et al. INTELLANCE 2/EORTC 1410 randomized phase II study of Depatux-M alone and with temozolomide vs temozolomide or lomustine in recurrent EGFR amplified glioblastoma. Neuro-Oncology 2020, 22, 684–693. [Google Scholar] [CrossRef]
- Rosenthal, M.; Curry, R.; Reardon, D.A.; Rasmussen, E.; Upreti, V.V.; Damore, M.A.; Henary, H.A.; Hill, J.S.; Cloughesy, T. Safety, tolerability, and pharmacokinetics of anti-EGFRvIII antibody–drug conjugate AMG 595 in patients with recurrent malignant glioma expressing EGFRvIII. Cancer Chemother. Pharm. 2019, 84, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J. The “Utility” of Highly Toxic Marine-Sourced Compounds. Mar. Drugs 2019, 17, 324. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.U. Reevaluating the role of antibody–drug conjugates in the treatment of patients with brain metastases. Ann. Oncol. 2020, 31, 1279–1281. [Google Scholar] [CrossRef] [PubMed]
- Bardia, A.; Hurvitz, S.A.; Tolaney, S.M.; Loirat, D.; Punie, K.; Oliveira, M.; Brufsky, A.; Sardesai, S.D.; Kalinsky, K.; Zelnak, A.B.; et al. Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer. N. Engl. J. Med. 2021, 384, 1529–1541. [Google Scholar] [CrossRef] [PubMed]
- Diéras, V.; Weaver, R.; Tolaney, S.M.; Bardia, A.; Punie, K.; Brufsky, A.; Rugo, H.S.; Kalinsky, K.; Traina, T.; Klein, L.; et al. Abstract PD13-07: Subgroup analysis of patients with brain metastases from the phase 3 ASCENT study of sacituzumab govitecan versus chemotherapy in metastatic triple-negative breast cancer. Cancer Res. 2021, 81, PD13-07. [Google Scholar] [CrossRef]
- Neuro/Sacituzumab Govitecan/Breast Brain Metastasis/Glioblastoma/Ph 0—Full Text View—ClinicalTrials.gov n.d. Available online: https://clinicaltrials.gov/ct2/show/NCT03995706 (accessed on 12 February 2023).
- Camidge, D.R.; Lee, E.Q.; Lin, N.U.; Margolin, K.; Ahluwalia, M.S.; Bendszus, M.; Chang, S.M.; Dancey, J.; de Vries, E.G.E.; Harris, G.J.; et al. Clinical trial design for systemic agents in patients with brain metastases from solid tumours: A guideline by the Response Assessment in Neuro-Oncology Brain Metastases working group. Lancet Oncol. 2018, 19, e20–e32. [Google Scholar] [CrossRef] [PubMed]
- Federal Register: Cancer Clinical Trial Eligibility Criteria: Brain Metastases; Draft Guidance for Industry; Availability n.d. Available online: https://www.federalregister.gov/documents/2019/03/13/2019-04584/cancer-clinical-trial-eligibility-criteria-brain-metastases-draft-guidance-for-industry-availability (accessed on 12 February 2023).





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vulsteke, C.; De Cocker, L.; Gómez de Liaño, A.; Montesdeoca, C.; De Meulenaere, A.; Croes, L.; Delombaerde, D.; Szabados, B.; Powles, T. First Evidence of Activity of Enfortumab Vedotin on Brain Metastases in Urothelial Cancer Patients. Pharmaceuticals 2023, 16, 375. https://doi.org/10.3390/ph16030375
Vulsteke C, De Cocker L, Gómez de Liaño A, Montesdeoca C, De Meulenaere A, Croes L, Delombaerde D, Szabados B, Powles T. First Evidence of Activity of Enfortumab Vedotin on Brain Metastases in Urothelial Cancer Patients. Pharmaceuticals. 2023; 16(3):375. https://doi.org/10.3390/ph16030375
Chicago/Turabian StyleVulsteke, Christof, Laurens De Cocker, Alfonso Gómez de Liaño, Cristina Montesdeoca, Astrid De Meulenaere, Lieselot Croes, Danielle Delombaerde, Bernadett Szabados, and Thomas Powles. 2023. "First Evidence of Activity of Enfortumab Vedotin on Brain Metastases in Urothelial Cancer Patients" Pharmaceuticals 16, no. 3: 375. https://doi.org/10.3390/ph16030375
APA StyleVulsteke, C., De Cocker, L., Gómez de Liaño, A., Montesdeoca, C., De Meulenaere, A., Croes, L., Delombaerde, D., Szabados, B., & Powles, T. (2023). First Evidence of Activity of Enfortumab Vedotin on Brain Metastases in Urothelial Cancer Patients. Pharmaceuticals, 16(3), 375. https://doi.org/10.3390/ph16030375






