High Baseline Neutrophil-to-Lymphocyte Ratio Could Serve as a Biomarker for Tumor Necrosis Factor-Alpha Blockers and Their Discontinuation in Patients with Ankylosing Spondylitis
Abstract
1. Introduction
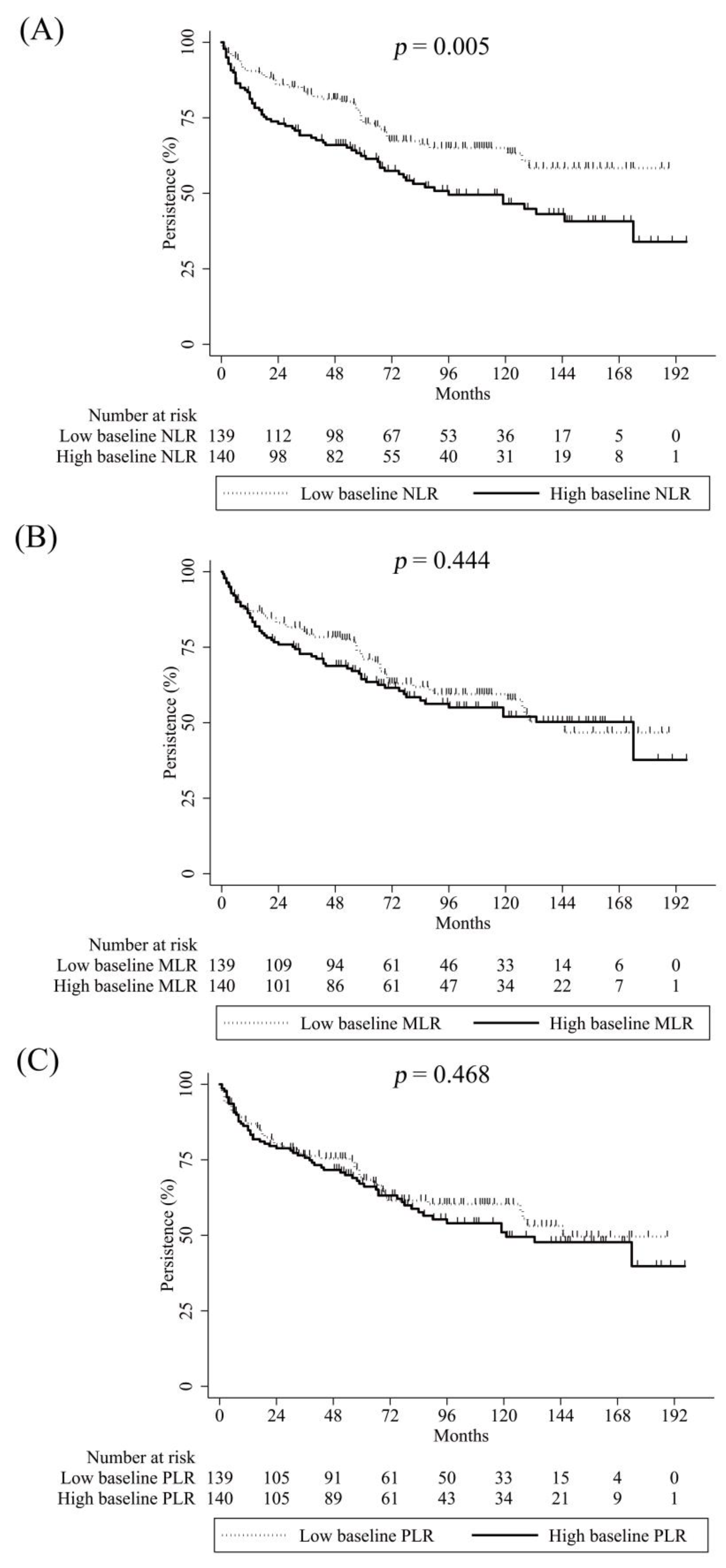
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Designs and Participants
4.2. Covariates
4.3. Study Outcomes
4.4. Statistical Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kwon, S.-R.; Kim, T.-H.; Kim, T.-J.; Park, W.; Shim, S.C. The Epidemiology and Treatment of Ankylosing Spondylitis in Korea. J. Rheum. Dis. 2022, 29, 193–199. [Google Scholar] [CrossRef]
- Ward, M.M.; Deodhar, A.; Gensler, L.S.; Dubreuil, M.; Yu, D.; Khan, M.A.; Haroon, N.; Borenstein, D.; Wang, R.; Biehl, A.; et al. 2019 Update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network Recommendations for the Treatment of Ankylosing Spondylitis and Nonradiographic Axial Spondyloarthritis. Arthritis Rheumatol. 2019, 71, 1599–1613. [Google Scholar] [CrossRef] [PubMed]
- Lata, M.; Hettinghouse, A.S.; Liu, C.J. Targeting tumor necrosis factor receptors in ankylosing spondylitis. Ann. N. Y. Acad. Sci. 2019, 1442, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.L.; Yang, C.H.; Chi, C.C. Drug Survival of Biologics in Treating Ankylosing Spondylitis: A Systematic Review and Meta-analysis of Real-World Evidence. BioDrugs 2020, 34, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Ma, Y.; Wu, M.; Zhang, X.; Yang, J.; Deng, J.; Guan, S.; Gao, X.; Xu, S.; Shuai, Z.; et al. Neutrophil lymphocyte ratio in patients with ankylosing spondylitis: A systematic review and meta-analysis. Mod. Rheumatol. 2020, 30, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Khorrampazhouh, N.; Omranzadeh, A.; Fazeli, B.; Zarifian, A.; Ghodsi, A.; Amirkhanlou, F.; Saberi, A.; Arekhi, S.; Tork, M.A.B.; Goudarzi, Z.; et al. A Systematic Review and Meta-analysis of Clinical Studies on Ankylosing Spondylitis and Neutrophil to Lymphocyte Ratio. Curr. Rheumatol. Rev. 2022, 18, 160–167. [Google Scholar] [CrossRef]
- Lee, H.N.; Kim, Y.K.; Kim, G.T.; Ahn, E.; So, M.W.; Sohn, D.H.; Lee, S.G. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratio as predictors of 12-week treatment response and drug persistence of anti-tumor necrosis factor-alpha agents in patients with rheumatoid arthritis: A retrospective chart review analysis. Rheumatol. Int. 2019, 39, 859–868. [Google Scholar] [CrossRef]
- Kim, A.; Kim, Y.; Kim, G.T.; Ahn, E.; So, M.W.; Sohn, D.H.; Lee, S.G. Platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio as potential makers for digital ulcers and interstitial lung disease in patients with systemic sclerosis: Cross-sectional analysis of data from a prospective cohort study. Rheumatol. Int. 2020, 40, 1071–1079. [Google Scholar] [CrossRef]
- Song, B.W.; Kim, A.R.; Moon, D.H.; Kim, Y.K.; Kim, G.T.; Ahn, E.Y.; So, M.W.; Lee, S.G. Associations of Neutrophil-to-Lymphocyte Ratio, Platelet-to-Lymphocyte Ratio and Monocyte-to-Lymphocyte Ratio with Osteoporosis and Incident Vertebral Fracture in Postmenopausal Women with Rheumatoid Arthritis: A Single-Center Retrospective Cohort Study. Medicina 2022, 58, 852. [Google Scholar] [CrossRef]
- Drugescu, A.; Roca, M.; Zota, I.M.; Costache, A.D.; Gavril, O.I.; Gavril, R.S.; Vasilcu, T.F.; Mitu, O.; Esanu, I.M.; Roca, I.C.; et al. Value of the Neutrophil to Lymphocyte Ratio and Platelet to Lymphocyte Ratio in Predicting CPET Performance in Patients with Stable CAD and Recent Elective PCI. Medicina 2022, 58, 814. [Google Scholar] [CrossRef]
- Hoppner, J.; Casteleyn, V.; Biesen, R.; Rose, T.; Windisch, W.; Burmester, G.R.; Siegert, E. SIGLEC-1 in Systemic Sclerosis: A Useful Biomarker for Differential Diagnosis. Pharmaceuticals 2022, 15, 1198. [Google Scholar] [CrossRef] [PubMed]
- Soldano, S.; Trombetta, A.C.; Contini, P.; Tomatis, V.; Ruaro, B.; Brizzolara, R.; Montagna, P.; Sulli, A.; Paolino, S.; Pizzorni, C.; et al. Increase in circulating cells coexpressing M1 and M2 macrophage surface markers in patients with systemic sclerosis. Ann. Rheum. Dis. 2018, 77, 1842–1845. [Google Scholar] [CrossRef] [PubMed]
- Eakin, A.J.; Ahmed, T.; McGeough, C.M.; Drain, S.; Alexander, H.D.; Wright, G.D.; Gardiner, P.V.; Small, D.; Bjourson, A.J.; Gibson, D.S. CD169+ Monocyte and Regulatory T Cell Subsets Are Associated with Disease Activity in Rheumatoid Arthritis. J. Pers. Med. 2022, 12, 1875. [Google Scholar] [CrossRef]
- Kucuk, A.; Uslu, A.U.; Ugan, Y.; Bagcaci, S.; Karahan, A.Y.; Akarmut, A.; Sahin, A.; Kucuksen, S. Neutrophil-to-lymphocyte ratio is involved in the severity of ankylosing spondylitis. Bratisl. Med. J.-Bratisl. Lek. Listy 2015, 116, 722–725. [Google Scholar] [CrossRef]
- Boyraz, I.; Koc, B.; Cogalgil, S. Assessment of Neutrophil-Lymphocyte and Platelet- Lymphocyte Ratios in Ankylosing Spondylitis. Arch. Rheumatol. 2016, 31, 199–200. [Google Scholar] [CrossRef] [PubMed]
- Al-Osami, M.H.; Awadh, N.I.; Khalid, K.B.; Awadh, A.I. Neutrophil/lymphocyte and platelet/lymphocyte ratios as potential markers of disease activity in patients with Ankylosing spondylitis: A case-control study. Adv. Rheumatol. 2020, 60, 13. [Google Scholar] [CrossRef]
- Coskun, B.N.; Oksuz, M.F.; Ermurat, S.; Tufan, A.N.; Orucoglu, N.; Dogan, A.; Dalkilic, E.; Pehlivan, Y. Neutrophil lymphocyte ratio can be a valuable marker in defining disease activity in patients who have started anti-tumor necrosis factor (TNF) drugs for ankylosing spondylitis. Eur. J. Rheumatol. 2014, 1, 101–105. [Google Scholar] [CrossRef]
- Liang, T.; Chen, J.; Xu, G.; Zhang, Z.; Xue, J.; Zeng, H.; Jiang, J.; Chen, T.; Qin, Z.; Li, H.; et al. Platelet-to-Lymphocyte Ratio as an Independent Factor Was Associated With the Severity of Ankylosing Spondylitis. Front. Immunol. 2021, 12, 760214. [Google Scholar] [CrossRef]
- Bozan, N.; Alpayci, M.; Aslan, M.; Cankaya, H.; Kiroglu, A.F.; Turan, M.; Ayral, A.; Senkoy, E.; Ilter, S. Mean platelet volume, red cell distribution width, platelet-to-lymphocyte and neutrophil-to-lymphocyte ratios in patients with ankylosing spondylitis and their relationships with high-frequency hearing thresholds. Eur. Arch. Otorhinolaryngol. 2016, 273, 3663–3672. [Google Scholar] [CrossRef]
- Gokmen, F.; Akbal, A.; Resorlu, H.; Gokmen, E.; Guven, M.; Aras, A.B.; Erbag, G.; Komurcu, E.; Akbal, E.; Cosar, M. Neutrophil-Lymphocyte Ratio Connected to Treatment Options and Inflammation Markers of Ankylosing Spondylitis. J. Clin. Lab. Anal. 2015, 29, 294–298. [Google Scholar] [CrossRef]
- Huang, Y.; Deng, W.; Zheng, S.; Feng, F.; Huang, Z.; Huang, Q.; Guo, X.; Huang, Z.; Huang, X.; Pan, X.; et al. Relationship between monocytes to lymphocytes ratio and axial spondyloarthritis. Int. Immunopharmacol. 2018, 57, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Ruof, J.; Stucki, G. Validity aspects of erythrocyte sedimentation rate and C-reactive protein in ankylosing spondylitis: A literature review. J. Rheumatol. 1999, 26, 966–970. [Google Scholar] [PubMed]
- Sheehan, N.J.; Slavin, B.M.; Donovan, M.P.; Mount, J.N.; Mathews, J.A. Lack of correlation between clinical disease activity and erythrocyte sedimentation rate, acute phase proteins or protease inhibitors in ankylosing spondylitis. Br. J. Rheumatol. 1986, 25, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Arends, S.; Brouwer, E.; van der Veer, E.; Groen, H.; Leijsma, M.K.; Houtman, P.M.; Th, A.J.T.L.; Kallenberg, C.G.; Spoorenberg, A. Baseline predictors of response and discontinuation of tumor necrosis factor-alpha blocking therapy in ankylosing spondylitis: A prospective longitudinal observational cohort study. Arthritis Res. Ther. 2011, 13, R94. [Google Scholar] [CrossRef]
- Jeong, H.; Eun, Y.H.; Kim, I.Y.; Kim, H.; Ahn, J.K.; Lee, J.; Koh, E.M.; Cha, H.S. Drug survival of tumor necrosis factor alpha inhibitors in patients with ankylosing spondylitis in Korea. Korean J. Intern. Med. 2018, 33, 407–416. [Google Scholar] [CrossRef]
- Rotar, Z.; Tomsic, M.; Praprotnik, S.; Slovenian, R. The persistence of golimumab compared to other tumour necrosis factor-alpha inhibitors in daily clinical practice for the treatment of rheumatoid arthritis, ankylosing spondylitis and psoriatic arthritis: Observations from the Slovenian nation-wide longitudinal registry of patients treated with biologic disease-modifying antirheumatic drugs-BioRx.si. Clin. Rheumatol. 2019, 38, 297–305. [Google Scholar] [CrossRef]
- Lee, J.W.; Kang, J.H.; Yim, Y.R.; Kim, J.E.; Wen, L.; Lee, K.E.; Park, D.J.; Kim, T.J.; Park, Y.W.; Lee, S.S. Predictors of Switching Anti-Tumor Necrosis Factor Therapy in Patients with Ankylosing Spondylitis. PLoS ONE 2015, 10, e0131864. [Google Scholar] [CrossRef]
- Kang, J.H.; Park, D.J.; Lee, J.W.; Lee, K.E.; Wen, L.; Kim, T.J.; Park, Y.W.; Lee, S.S. Drug survival rates of tumor necrosis factor inhibitors in patients with rheumatoid arthritis and ankylosing spondylitis. J. Korean Med. Sci. 2014, 29, 1205–1211. [Google Scholar] [CrossRef]
- Sieper, J.; Landewe, R.; Magrey, M.; Anderson, J.K.; Zhong, S.; Wang, X.; Lertratanakul, A. Predictors of remission in patients with non-radiographic axial spondyloarthritis receiving open-label adalimumab in the ABILITY-3 study. RMD Open 2019, 5, e000917. [Google Scholar] [CrossRef]
- Pina Vegas, L.; Sbidian, E.; Wendling, D.; Goupille, P.; Ferkal, S.; Le Corvoisier, P.; Ghaleh, B.; Luciani, A.; Claudepierre, P. Factors associated with remission at 5-year follow-up in recent-onset axial spondyloarthritis: Results from the DESIR cohort. Rheumatology 2022, 61, 1487–1495. [Google Scholar] [CrossRef]
- Shimabuco, A.Y.; Goncalves, C.R.; Moraes, J.C.B.; Waisberg, M.G.; Ribeiro, A.C.M.; Sampaio-Barros, P.D.; Goldenstein-Schainberg, C.; Bonfa, E.; Saad, C.G.S. Factors associated with ASDAS remission in a long-term study of ankylosing spondylitis patients under tumor necrosis factor inhibitors. Adv. Rheumatol. 2018, 58, 40. [Google Scholar] [CrossRef]
- Menegatti, S.; Bianchi, E.; Rogge, L. Anti-TNF Therapy in Spondyloarthritis and Related Diseases, Impact on the Immune System and Prediction of Treatment Responses. Front. Immunol. 2019, 10, 382. [Google Scholar] [CrossRef]
- Mills, K.H.G. IL-17 and IL-17-producing cells in protection versus pathology. Nat. Rev. Immunol. 2022, 23, 38–54. [Google Scholar] [CrossRef] [PubMed]
- Appel, H.; Maier, R.; Wu, P.; Scheer, R.; Hempfing, A.; Kayser, R.; Thiel, A.; Radbruch, A.; Loddenkemper, C.; Sieper, J. Analysis of IL-17(+) cells in facet joints of patients with spondyloarthritis suggests that the innate immune pathway might be of greater relevance than the Th17-mediated adaptive immune response. Arthritis Res. Ther. 2011, 13, R95. [Google Scholar] [CrossRef] [PubMed]
- Xueyi, L.; Lina, C.; Zhenbiao, W.; Qing, H.; Qiang, L.; Zhu, P. Levels of circulating Th17 cells and regulatory T cells in ankylosing spondylitis patients with an inadequate response to anti-TNF-alpha therapy. J. Clin. Immunol. 2013, 33, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Ramos, S.; Rafael-Vidal, C.; Pego-Reigosa, J.M.; Garcia, S. Monocytes and Macrophages in Spondyloarthritis: Functional Roles and Effects of Current Therapies. Cells 2022, 11, 515. [Google Scholar] [CrossRef]
- Van der Linden, S.; Valkenburg, H.A.; Cats, A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984, 27, 361–368. [Google Scholar] [CrossRef]
- Lee, S.H.; Park, W.; Won Lee, S.; Kim, H.A.; Choe, J.Y.; Lee, S.H.; Lee, S.S.; Park, S.H.; Park, M.C.; Sheen, D.H.; et al. Frequency of peripheral diseases in Korean patients with ankylosing spondylitis and the effectiveness of adalimumab. Int. J. Rheum. Dis. 2020, 23, 1175–1183. [Google Scholar] [CrossRef]
- Yang, M.; Xu, M.; Pan, X.; Hu, Z.; Li, Q.; Wei, Y.; Zhang, Y.; Rong, J.; Zhai, J.; He, P.; et al. Epidemiological comparison of clinical manifestations according to HLA-B*27 carrier status of Chinese ankylosing spondylitis patients. Tissue Antigens 2013, 82, 338–343. [Google Scholar] [CrossRef]
- Lennard-Jones, J.E. Classification of inflammatory bowel disease. Scand. J. Gastroenterol. Suppl. 1989, 170, 2–6; discussion 16–19. [Google Scholar] [CrossRef]
- Braun, J.; Davis, J.; Dougados, M.; Sieper, J.; van der Linden, S.; van der Heijde, D.; Group, A.W. First update of the international ASAS consensus statement for the use of anti-TNF agents in patients with ankylosing spondylitis. Ann. Rheum. Dis. 2006, 65, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Briars, L.; Lee, T.A.; Calip, G.S.; Suda, K.J.; Schumock, G.T. Use of Tumor Necrosis Factor-Alpha Inhibitors in Children and Young Adults With Juvenile Idiopathic Arthritis or Rheumatoid Arthritis. Pharmacotherapy 2016, 36, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
- Sauer, B.C.; Teng, C.C.; Tang, D.; Leng, J.; Curtis, J.R.; Mikuls, T.R.; Harrison, D.J.; Cannon, G.W. Persistence with Conventional Triple Therapy Versus a Tumor Necrosis Factor Inhibitor and Methotrexate in US Veterans with Rheumatoid Arthritis. Arthritis Care Res. 2017, 69, 313–322. [Google Scholar] [CrossRef]
- Wilke, T.; Mueller, S.; Lee, S.C.; Majer, I.; Heisen, M. Drug survival of second biological DMARD therapy in patients with rheumatoid arthritis: A retrospective non-interventional cohort analysis. BMC Musculoskelet. Disord. 2017, 18, 332. [Google Scholar] [CrossRef] [PubMed]
- Ornbjerg, L.M.; Brahe, C.H.; Askling, J.; Ciurea, A.; Mann, H.; Onen, F.; Kristianslund, E.K.; Nordstrom, D.; Santos, M.J.; Codreanu, C.; et al. Treatment response and drug retention rates in 24 195 biologic-naive patients with axial spondyloarthritis initiating TNFi treatment: Routine care data from 12 registries in the EuroSpA collaboration. Ann. Rheum. Dis. 2019, 78, 1536–1544. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Kim, Y.; Kim, G.T.; Ahn, E.; So, M.W.; Lee, S.G. Comparison of persistence rates between allopurinol and febuxostat as first-line urate-lowering therapy in patients with gout: An 8-year retrospective cohort study. Clin. Rheumatol. 2020, 39, 3769–3776. [Google Scholar] [CrossRef] [PubMed]
| Patients with AS (n = 279) | Healthy Controls (n = 171) | p-Value | |
|---|---|---|---|
| Age, years, mean ± SD | 34.5 ± 11.1 | 34.9 ± 6.4 | 0.655 |
| Female, n (%) | 48 (17.2) | 30 (17.5) | 0.926 |
| NLR, median (IQR) | 1.99 (1.36–2.72) | 1.45 (1.17–1.83) | <0.001 |
| MLR, median (IQR) | 0.24 (0.18–0.33) | 0.16 (0.13–0.2) | <0.001 |
| PLR, median (IQR) | 117.29 (94.91–158.91) | 115.11 (90.94–137.37) | 0.036 |
| CRP, mg/dL, median (IQR) | 0.93 (0.24–2.57) | 0.03 (0.02–0.05) | <0.001 |
| ESR, mm/h, median (IQR) | 25.5 (9.3–52.8) | ||
| Disease duration, months, median (IQR) | 8 (4–43) | ||
| BASDAI, mean ± SD | 6.8 ± 1.4 | ||
| Ant–TNF–α agents | |||
| Adalimumab, n (%) | 185 (66.3) | ||
| Etanercept, n (%) | 65 (23.3) | ||
| Infliximab, n (%) | 29 (10.4) | ||
| HLA–B27, n (%) | 221 (88) | ||
| Peripheral arthritis, n (%) | 125 (44.8) | ||
| Hip joint involvement, n (%) | 89 (31.9) | ||
| Uveitis, n (%) | 60 (21.5) | ||
| Psoriasis, n (%) | 11 (3.9) | ||
| IBD, n (%) | 7 (2.5) | ||
| Concomitant medications | |||
| NSAIDs, n (%) | 200 (71.7) | ||
| Methotrexate, n (%) | 60 (21.5) | ||
| Sulfasalazine, n (%) | 88 (31.5) | ||
| Glucocorticoids s, n (%) | 101 (36.2) |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|
| 1. Baseline NLR | - | - | - | - | - | - | - |
| 2. Baseline MLR | 0.712 ** | - | - | - | - | - | - |
| 3. Baseline PLR | 0.703 ** | 0.613 ** | - | - | - | - | - |
| 4. Baseline ESR | 0.33 ** | 0.288 ** | 0.364 ** | - | - | - | - |
| 5. Baseline CRP | 0.358 ** | 0.358 ** | 0.346 ** | 0.766 ** | - | - | - |
| 6. Baseline BASDAI | 0.132 * | 0.041 | 0.154 ** | 0.172 ** | 0.091 | - | - |
| 7. 3-month BASDAI | 0.137 * | 0.119 | 0.142 * | 0.098 | 0.033 | 0.439 ** | - |
| 8. 3-month changes in BASDAI | −0.004 | −0.069 | 0.027 | 0.09 | 0.035 | 0.601 ** | −0.364 ** |
| Crude OR (95% CI) | p-Value | Adjusted OR * (95% CI) | p-Value | Adjusted OR * (95% CI) | p-Value | Adjusted OR * (95% CI) | p-Value | |
|---|---|---|---|---|---|---|---|---|
| High baseline NLR | 9.65 (1.21–77.25) | 0.033 | 12.3 (1.37–110.49) | 0.025 | ||||
| High baseline MLR | 4.16 (0.87–19.96) | 0.075 | 4.8 (0.94–24.53) | 0.059 | ||||
| High baseline PLR | 4.09 (0.85–19.65) | 0.078 | 4.63 (0.89–24.18) | 0.069 | ||||
| Disease duration, month | 1.01 (0.99–1.02) | 0.064 | 1.01 (0.99–1.03) | 0.069 | – | – | 1.01 (0.99–1.02) | 0.088 |
| BASDAI | 0.59 (0.35–0.97) | 0.038 | 0.59 (0.34–1.02) | 0.059 | 0.57 (0.33–0.97) | 0.038 | 0.61 (0.36–1.02) | 0.059 |
| IBD | 12.7 (2.13–75.65) | 0.005 | 10.24 (1.45–72.37) | 0.02 | 9.22 (1.32–64.61) | 0.025 | 9.95 (1.45–68.51) | 0.02 |
| Age | 0.99 (0.94–1.05) | 0.856 | ||||||
| Female | 1.16 (0.24–5.63) | 0.856 | ||||||
| CRP, mg/dL | 0.96 (0.74–1.24) | 0.731 | ||||||
| Uveitis | 1.52 (0.38–6.06) | 0.554 | ||||||
| Peripheral arthritis | 3.06 (0.78–12.1) | 0.111 | ||||||
| Hip involvement | 1.44 (0.4–5.24) | 0.581 | ||||||
| NSAIDs | 0.6 (0.17–2.19) | 0.439 | ||||||
| Methotrexate | 0.93 (0.19–4.5) | 0.925 | ||||||
| Sulfasalazine | 2.28 (0.64–8.09) | 0.203 | ||||||
| Glucocorticoids | 0.43 (0.09–2.07) | 0.294 |
| Crude HR (95% CI) | p-Value | Adjusted HR * (95% CI) | p-Value | |
|---|---|---|---|---|
| High baseline NLR | 1.7 (1.17–2.48) | 0.006 | 1.66 (1.13–2.44) | 0.01 |
| High baseline MLR | 1.16 (0.8–1.67) | 0.446 | ||
| High baseline PLR | 1.14 (0.79–1.66) | 0.47 | ||
| Female | 1.46 (0.95–2.26) | 0.085 | 1.56 (1.01–2.41) | 0.048 |
| Uveitis | 0.66 (0.4–1.08) | 0.094 | 0.63 (0.39–1.03) | 0.067 |
| Psoriasis | 2.32 (1.07–4.99) | 0.032 | 2.11 (0.98–4.57) | 0.057 |
| Hip involvement | 1.6 (1.1–2.32) | 0.014 | 1.48 (1.01–2.16) | 0.042 |
| TNF-α inhibitors Adalimumab Etanercept Infliximab (ref.) | 0.89 (0.58–1.37) 0.8 (0.41–1.56) | 0.599 0.513 | ||
| Sulfasalazine | 1.47 (1.01–2.14) | 0.049 | - | - |
| Age | 0.99 (0.98–1.01) | 0.408 | ||
| Disease duration, month | 1 (0.99–1) | 0.371 | ||
| BASDAI | 0.91 (0.79–1.04) | 0.155 | ||
| CRP, mg/dL | 1.03 (0.98–1.08) | 0.289 | ||
| Peripheral arthritis | 0.97 (0.67–1.4) | 0.856 | ||
| IBD | 0.97 (0.31–3.05) | 0.956 | ||
| NSAIDs | 1.39 (0.91–2.12) | 0.124 | ||
| Methotrexate | 0.97 (0.62–1.51) | 0.882 | ||
| Glucocorticoids | 1.33 (0.91–1.93) | 0.136 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, D.-H.; Kim, A.; Song, B.-W.; Kim, Y.-K.; Kim, G.-T.; Ahn, E.-Y.; So, M.-W.; Lee, S.-G. High Baseline Neutrophil-to-Lymphocyte Ratio Could Serve as a Biomarker for Tumor Necrosis Factor-Alpha Blockers and Their Discontinuation in Patients with Ankylosing Spondylitis. Pharmaceuticals 2023, 16, 379. https://doi.org/10.3390/ph16030379
Moon D-H, Kim A, Song B-W, Kim Y-K, Kim G-T, Ahn E-Y, So M-W, Lee S-G. High Baseline Neutrophil-to-Lymphocyte Ratio Could Serve as a Biomarker for Tumor Necrosis Factor-Alpha Blockers and Their Discontinuation in Patients with Ankylosing Spondylitis. Pharmaceuticals. 2023; 16(3):379. https://doi.org/10.3390/ph16030379
Chicago/Turabian StyleMoon, Dong-Hyuk, Aran Kim, Byung-Wook Song, Yun-Kyung Kim, Geun-Tae Kim, Eun-Young Ahn, Min-Wook So, and Seung-Geun Lee. 2023. "High Baseline Neutrophil-to-Lymphocyte Ratio Could Serve as a Biomarker for Tumor Necrosis Factor-Alpha Blockers and Their Discontinuation in Patients with Ankylosing Spondylitis" Pharmaceuticals 16, no. 3: 379. https://doi.org/10.3390/ph16030379
APA StyleMoon, D.-H., Kim, A., Song, B.-W., Kim, Y.-K., Kim, G.-T., Ahn, E.-Y., So, M.-W., & Lee, S.-G. (2023). High Baseline Neutrophil-to-Lymphocyte Ratio Could Serve as a Biomarker for Tumor Necrosis Factor-Alpha Blockers and Their Discontinuation in Patients with Ankylosing Spondylitis. Pharmaceuticals, 16(3), 379. https://doi.org/10.3390/ph16030379






