Design, Synthesis, and Antiviral Activities of New Benzotriazole-Based Derivatives
Abstract
:1. Introduction
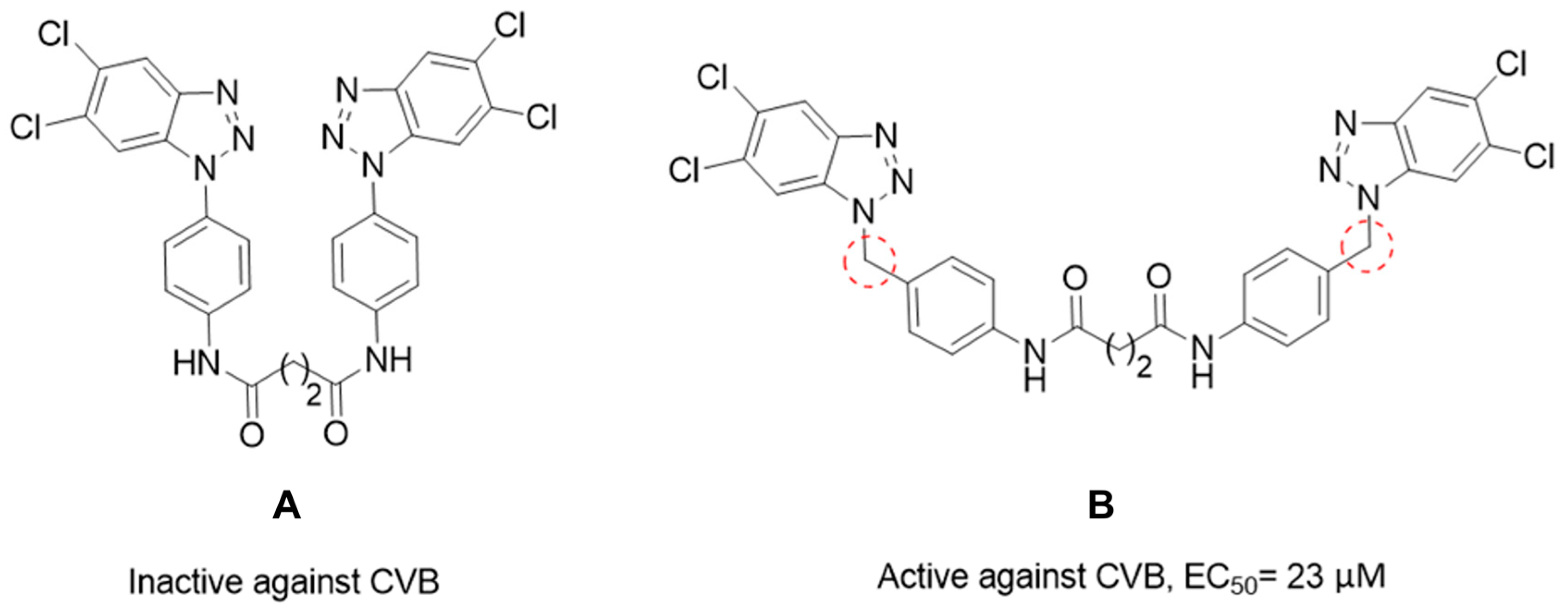
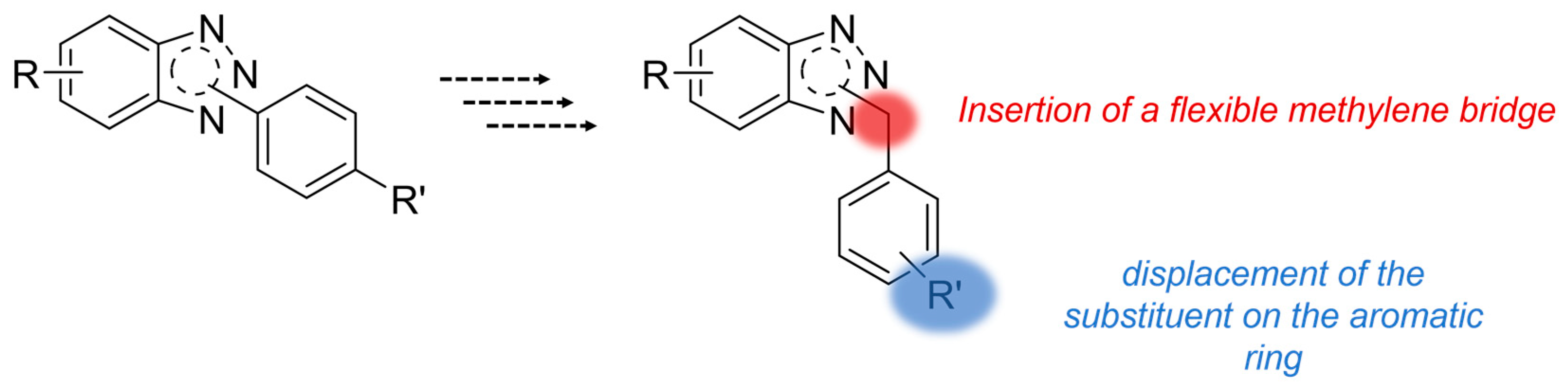
Rationale
2. Results
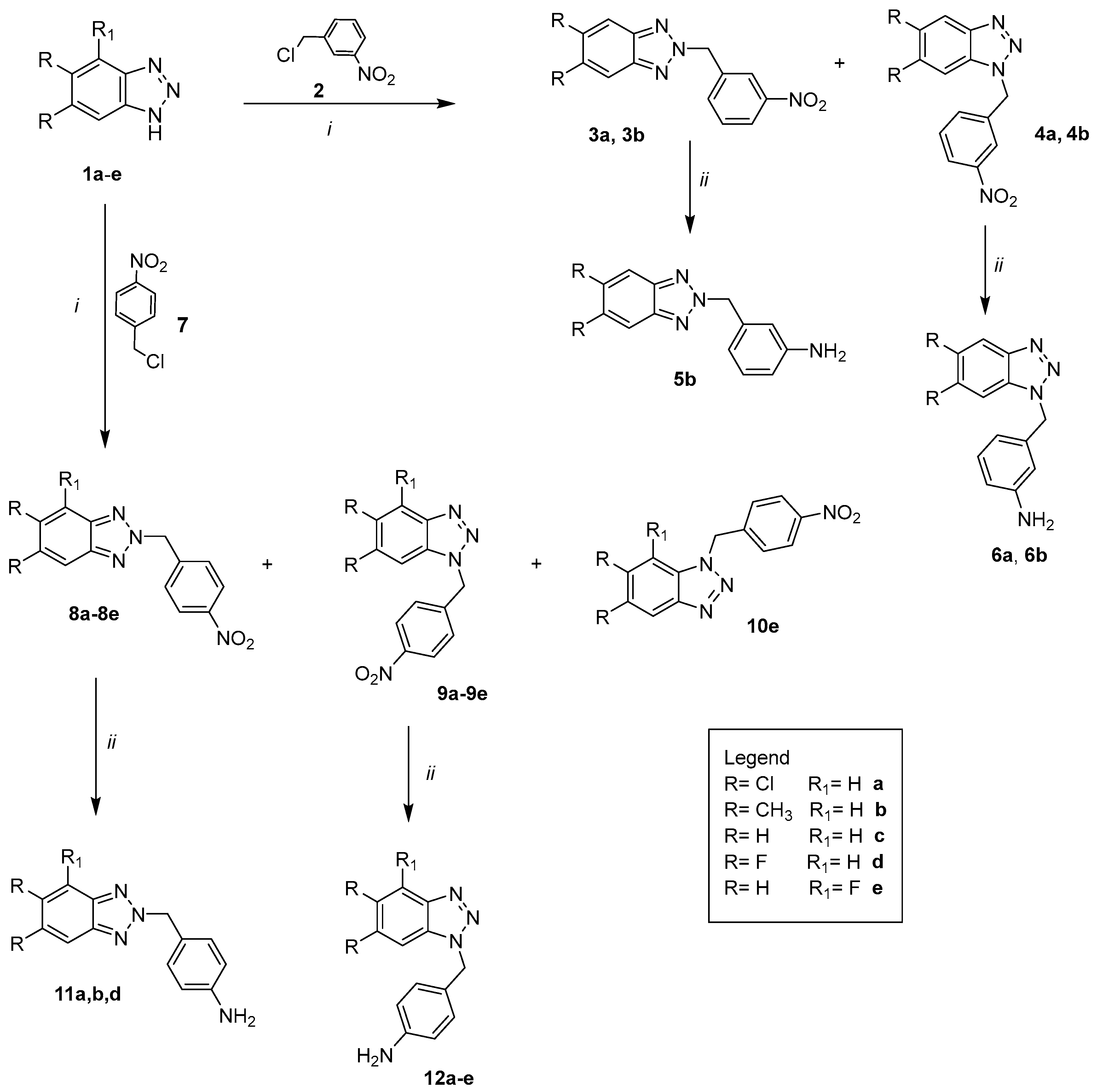
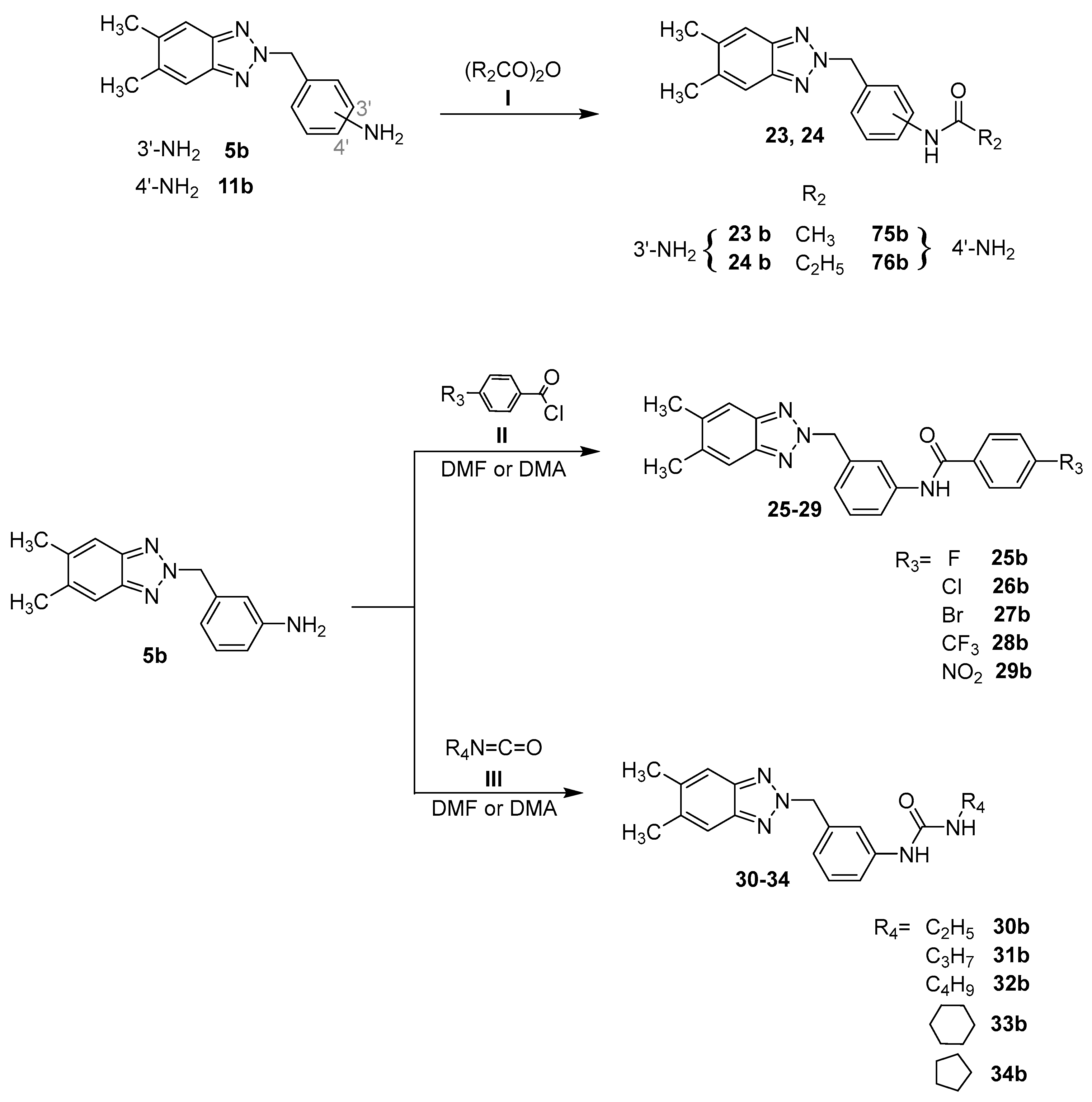
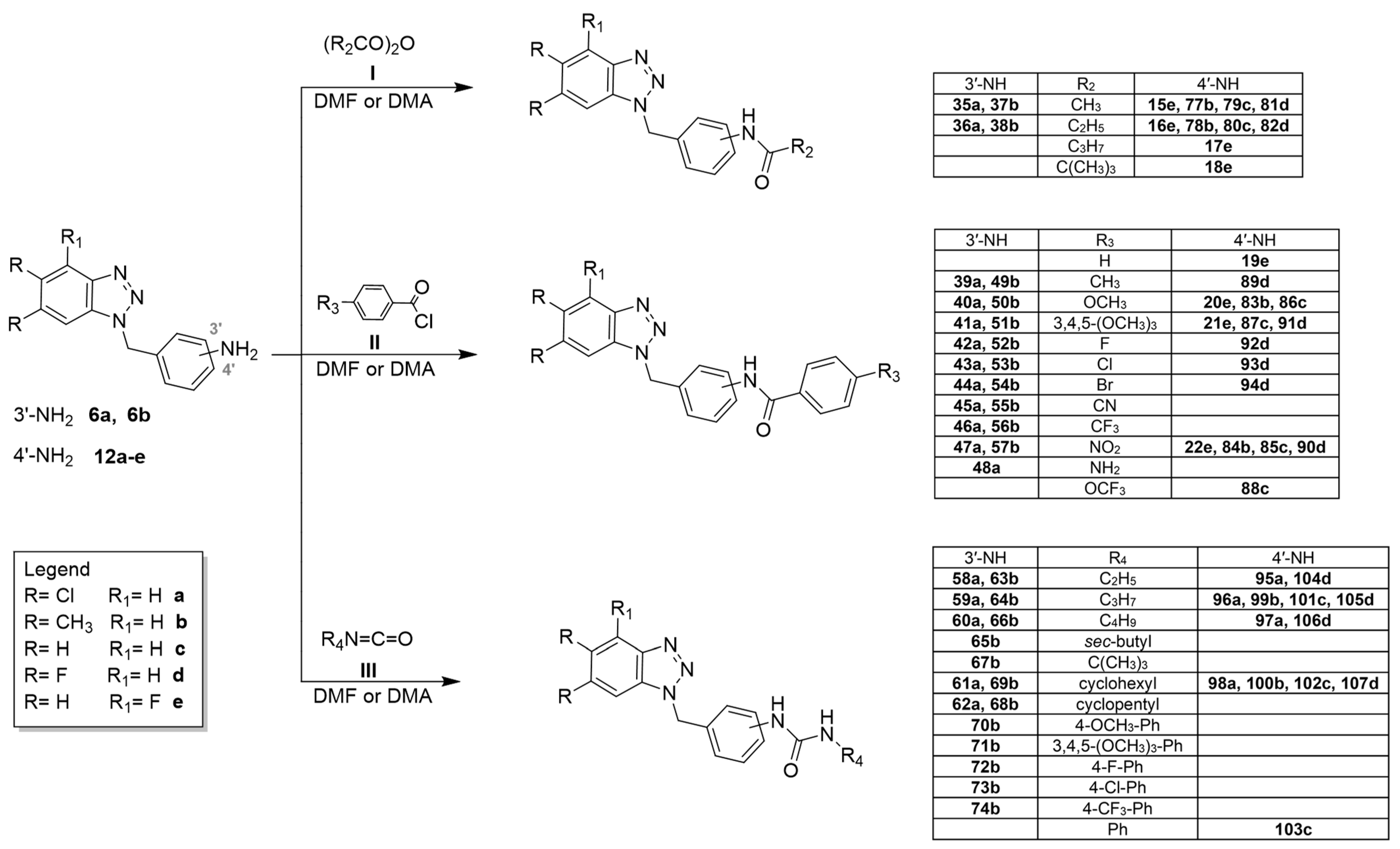
2.1. Chemistry
- i.
- The proper anhydride I (acetic anhydride, propionic anhydride, butyric anhydride and pivalic anhydride) at room temperature or for 1–72 h. The crude products were in turn obtained pure or required purification by flash chromatography;
- ii.
- The required benzoyl chloride derivatives II in N,N-dimethylacetamide (DMA) or N,N-dimethylformamide (DMF) at 80 °C from 3 h to 7 days. The purification of the compounds was carried out by recrystallization from ethanol or by flash chromatography;
- iii.
- The appropriate isocyanate R2N=C=O III in DMF, stirring the mixture at 100 °C from 2 to 9 days. The crude products were triturated with diethyl ether to obtain solids that were purified through recrystallization from ethanol or by flash chromatography.
2.2. Biology
2.2.1. Antiviral Assay
2.2.2. Transepithelial–Transendothelial Electrical Resistance (TEER) Test
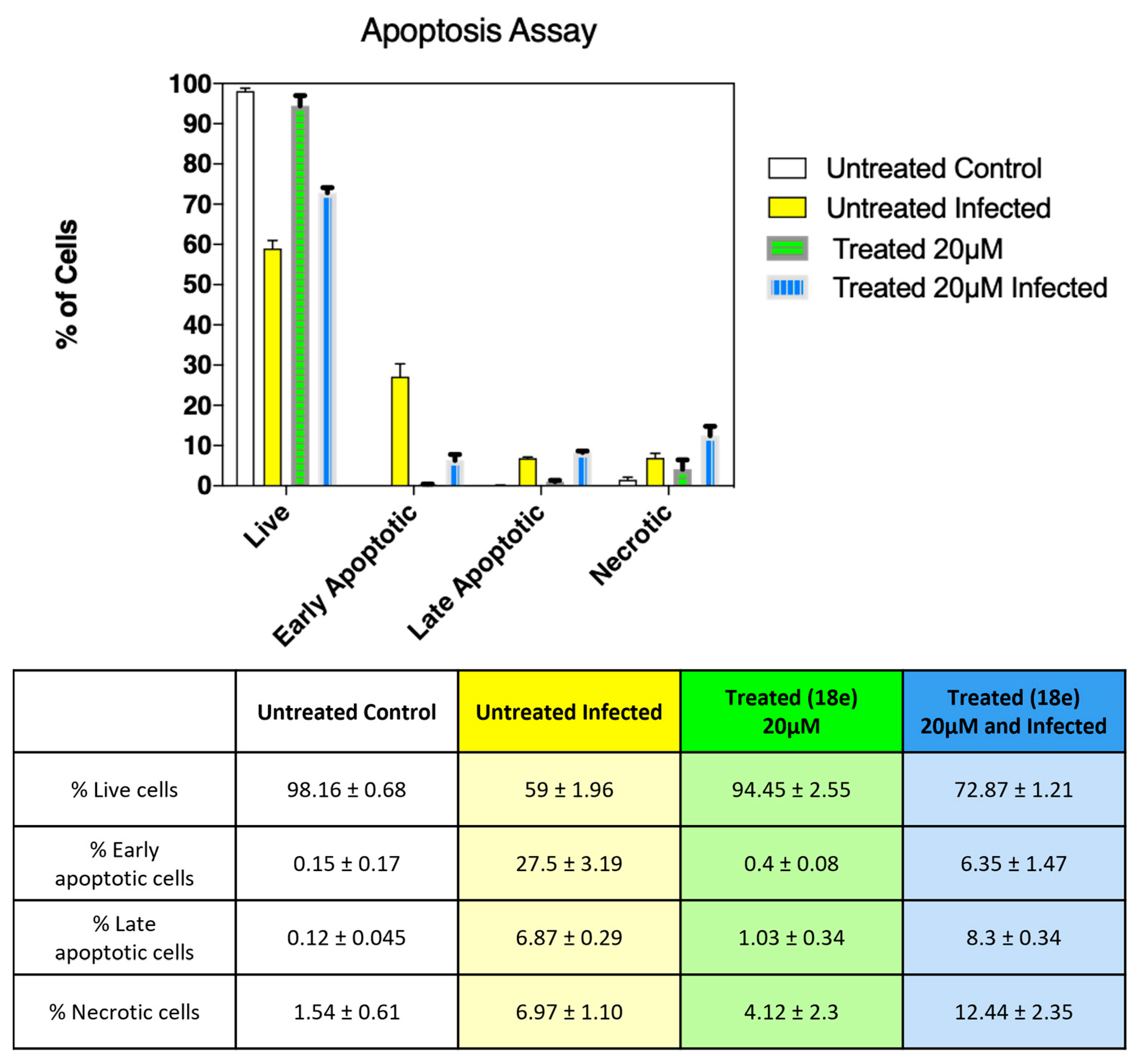
2.2.3. Protective Effect of 18e on Vero-76 Cell from CVB5 Infection
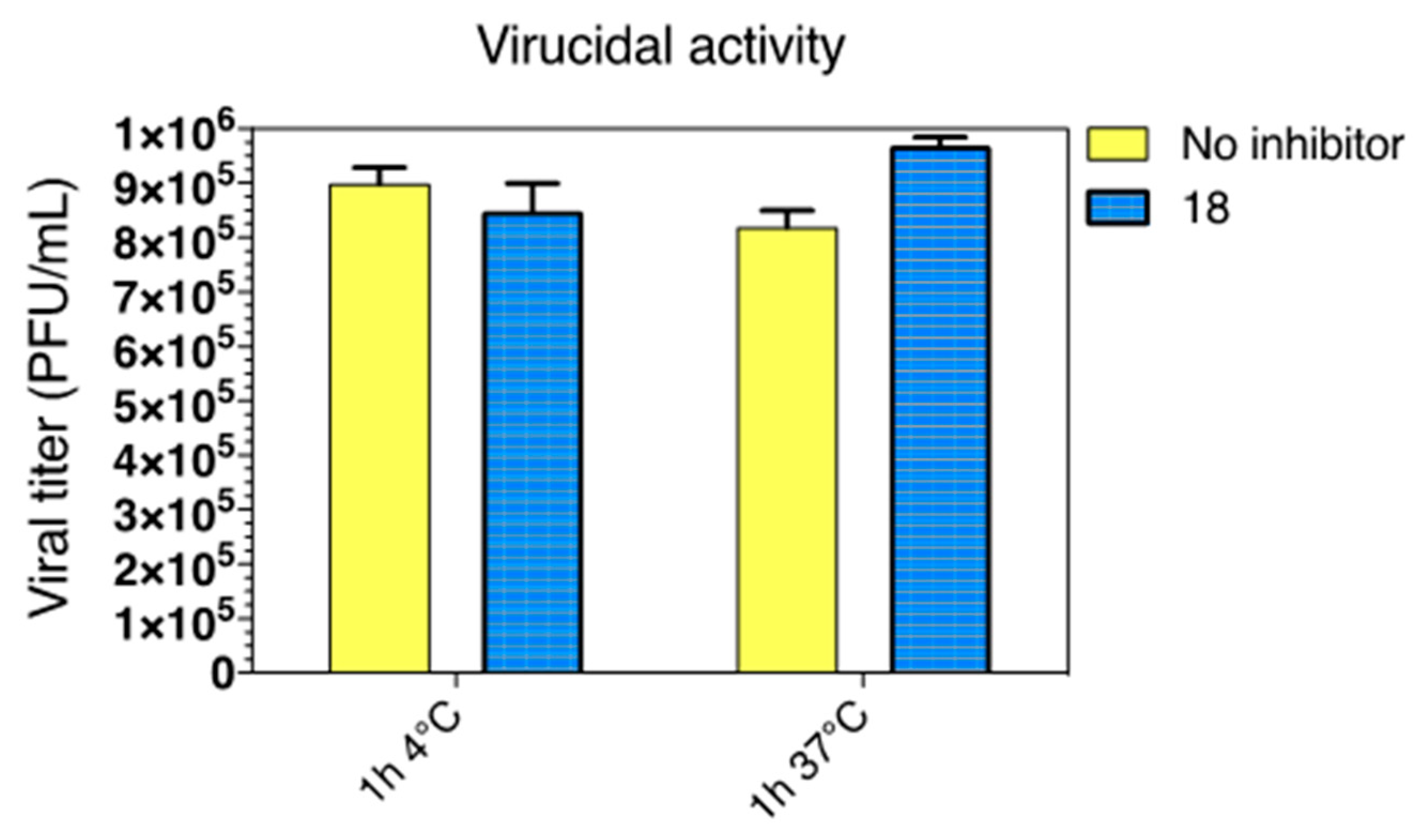
2.2.4. Virucidal Activity
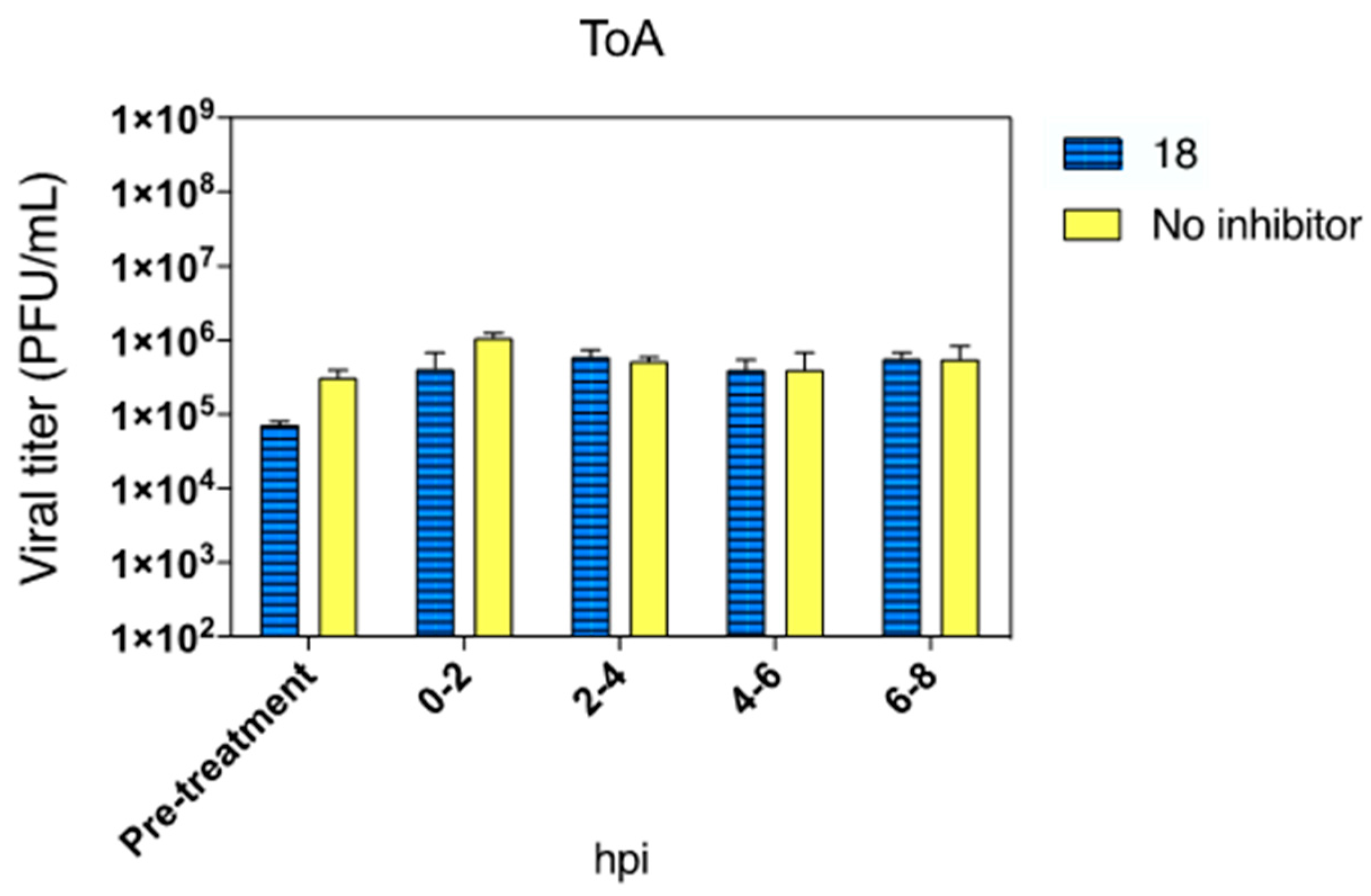
2.2.5. Time of Addition (ToA)
3. Materials and Methods
3.1. Chemistry
3.1.1. Starting Material and Known Intermediates
3.1.2. General Procedure to Obtain (5,6-R (4R))-1(2)-(3(4)-nitrobenzyl)-1(2)H-benzo[d][1,2,3]triazole (3a–b; 4a–b; 8a–e; 9a–e and 10e)
3.1.3. General Procedure to Obtain 4-((5,6-R-1H-benzo[d][1,2,3]triazol-1-yl)methyl)aniline 5b, 6b, 11b, 11d, 12b, 12c, 12e
3.1.4. General Procedure to Obtain 4(3)-((5,6-dichloro-1(2)H-Benzo[d][1,2,3]Triazol-1(2)-yl)methyl)aniline 6a, 11a, 12a, and 12d
3.1.5. General Procedure for the Preparation of Amides 15e–18e, 23b, 24b, 35a, 36a, 37b, 38b, 75b–78b, 79c, 80c, 81d, 82d
3.1.6. General Procedure for the Preparation of Amides 19e–22e, 25b–29b, 39a–48a, 49b–57b, 83b, 84b, 85c–88c, 89d–94d
3.1.7. General Procedure for the Preparation of Urea-Derivatives 30b–34b, 58a–62a, 63b–74b, 95a–98a, 99b–100b, 101c–103c, 104d–107d
3.2. Biology
3.2.1. Cells and Viruses
3.2.2. Cytotoxicity Assay
3.2.3. Transepithelial Electrical Resistance (TEER) Assay
3.2.4. Apoptosis Assay
3.2.5. Antiviral Assay
3.2.6. Virucidal Activity Assay
3.2.7. Cell Pre-Treatment Assay
3.2.8. Time of Addition Assay
3.2.9. Statistical Analysis
3.3. Experimental
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnson, N.P.A.S.; Mueller, J. Updating the Accounts: Global Mortality of the 1918-1920 “Spanish” Influenza Pandemic. Bull. Hist. Med. 2002, 76, 105–115. [Google Scholar] [CrossRef]
- Rajagopal, S.; Treanor, J. Pandemic (Avian) Influenza. Semin. Respir. Crit. Care Med. 2007, 28, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Viboud, C.; Simonsen, L.; Fuentes, R.; Flores, J.; Miller, M.A.; Chowell, G. Global Mortality Impact of the 1957–1959 Influenza Pandemic. J. Infect. Dis. 2016, 213, 738–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- 1968 Pandemic (H3N2 Virus)|Pandemic Influenza (Flu)|CDC. Available online: https://www.cdc.gov/flu/pandemic-resources/1968-pandemic.html (accessed on 13 January 2023).
- Global HIV Programme. Available online: https://www.who.int/teams/global-hiv-hepatitis-and-stis-programmes/hiv/strategic-information/hiv-data-and-statistics (accessed on 13 January 2023).
- Ruan, L.; Zeng, G. SARS Epidemic: SARS Outbreaks in Inner-Land of China. In Emerging Infections in Asia; Springer US: Boston, MA, USA, 2008; pp. 75–96. [Google Scholar]
- CDC Novel H1N1 Flu|The 2009 H1N1 Pandemic: Summary Highlights, April 2009–April 2010. Available online: https://www.cdc.gov/h1n1flu/cdcresponse.htm (accessed on 13 January 2023).
- de Wit, E.; van Doremalen, N.; Falzarano, D.; Munster, V.J. SARS and MERS: Recent Insights into Emerging Coronaviruses. Nat. Rev. Microbiol. 2016, 14, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Holmes, E.C.; Dudas, G.; Rambaut, A.; Andersen, K.G. The Evolution of Ebola Virus: Insights from the 2013–2016 Epidemic. Nature 2016, 538, 193–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adil, M.T.; Rahman, R.; Whitelaw, D.; Jain, V.; Al-Taan, O.; Rashid, F.; Munasinghe, A.; Jambulingam, P. SARS-CoV-2 and the Pandemic of COVID-19. Postgrad. Med. J. 2021, 97, 110–116. [Google Scholar] [CrossRef]
- Murray, C.J.L.; Ortblad, K.F.; Guinovart, C.; Lim, S.S.; Wolock, T.M.; Roberts, D.A.; Dansereau, E.A.; Graetz, N.; Barber, R.M.; Brown, J.C.; et al. Global, Regional, and National Incidence and Mortality for HIV, Tuberculosis, and Malaria during 1990–2013: A Systematic Analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 1005–1070. [Google Scholar] [CrossRef] [Green Version]
- Kujawski, S.A.; Midgley, C.M.; Rha, B.; Lively, J.Y.; Nix, W.A.; Curns, A.T.; Payne, D.C.; Englund, J.A.; Boom, J.A.; Williams, J.V.; et al. Enterovirus D68–Associated Acute Respiratory Illness—New Vaccine Surveillance Network, United States, July–October, 2017 and 2018. MMWR Morb. Mortal Wkly. Rep. 2019, 68, 277–280. [Google Scholar] [CrossRef] [Green Version]
- Messacar, K.; Asturias, E.J.; Hixon, A.M.; van Leer-Buter, C.; Niesters, H.G.M.; Tyler, K.L.; Abzug, M.J.; Dominguez, S.R. Enterovirus D68 and Acute Flaccid Myelitis—Evaluating the Evidence for Causality. Lancet Infect. Dis. 2018, 18, e239–e247. [Google Scholar] [CrossRef] [Green Version]
- Greninger, A.L.; Naccache, S.N.; Messacar, K.; Clayton, A.; Yu, G.; Somasekar, S.; Federman, S.; Stryke, D.; Anderson, C.; Yagi, S.; et al. A Novel Outbreak Enterovirus D68 Strain Associated with Acute Flaccid Myelitis Cases in the USA (2012–14): A Retrospective Cohort Study. Lancet Infect. Dis. 2015, 15, 671–682. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, H.; Tang, J.; He, Y.; Xiong, T.; Li, W.; Qu, Y.; Mu, D. Clinical Characteristics of Severe Neonatal Enterovirus Infection: A Systematic Review. BMC Pediatr. 2021, 21, 127. [Google Scholar] [CrossRef] [PubMed]
- Smuts, H.; Cronje, S.; Thomas, J.; Brink, D.; Korsman, S.; Hardie, D. Molecular Characterization of an Outbreak of Enterovirus-Associated Meningitis in Mossel Bay, South Africa, December 2015–January 2016. BMC. Infect. Dis. 2018, 18, 709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.H.P.; Chong, Y.M.; Tay, C.G.; Koh, M.T.; Chem, Y.K.; Noordin, N.; Jahis, R.; Sam, I.C.; Chan, Y.F. Detection of Enteroviruses during a 2018 Hand, Foot and Mouth Disease Outbreak in Malaysia. Trop. Biomed. 2021, 38, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Messacar, K.; Abzug, M.J.; Dominguez, S.R. 2014 Outbreak of Enterovirus D68 in North America. J. Med. Virol. 2016, 88, 739–745. [Google Scholar] [CrossRef]
- Kahrs, C.R.; Chuda, K.; Tapia, G.; Stene, L.C.; Mårild, K.; Rasmussen, T.; Rønningen, K.S.; Lundin, K.E.A.; Kramna, L.; Cinek, O.; et al. Enterovirus as Trigger of Coeliac Disease: Nested Case-Control Study within Prospective Birth Cohort. BMJ 2019, 364, l231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez Bergamin, C.; Atala Dib, S. Enterovirus and Type 1 Diabetes: What Is the Matter? World J. Diabetes 2015, 6, 828. [Google Scholar] [CrossRef]
- Mirand, A.; le Sage, F.V.; Pereira, B.; Cohen, R.; Levy, C.; Archimbaud, C.; Peigue-Lafeuille, H.; Bailly, J.-L.; Henquell, C. Ambulatory Pediatric Surveillance of Hand, Foot and Mouth Disease as Signal of an Outbreak of Coxsackievirus A6 Infections, France, 2014–2015. Emerg. Infect. Dis. 2016, 22, 1884–1893. [Google Scholar] [CrossRef] [PubMed]
- Keeren, K.; Böttcher, S.; Diedrich, S. Enterovirus Surveillance (EVSurv) in Germany. Microorganisms 2021, 9, 2005. [Google Scholar] [CrossRef]
- Kamau, E.; Nguyen, D.; Celma, C.; Blomqvist, S.; Horby, P.; Simmonds, P.; Harvala, H. Seroprevalence and Virologic Surveillance of Enterovirus 71 and Coxsackievirus A6, United Kingdom, 2006–2017. Emerg. Infect. Dis. 2021, 27, 2261–2268. [Google Scholar] [CrossRef]
- Yi, E.-J.; Shin, Y.-J.; Kim, J.-H.; Kim, T.-G.; Chang, S.-Y. Enterovirus 71 Infection and Vaccines. Clin. Exp. Vaccine. Res. 2017, 6, 4. [Google Scholar] [CrossRef]
- Nikonov, O.S.; Chernykh, E.S.; Garber, M.B.; Nikonova, E.Y. Enteroviruses: Classification, Diseases They Cause, and Approaches to Development of Antiviral Drugs. Biochemistry 2017, 82, 1615–1631. [Google Scholar] [CrossRef]
- Pevear, D.C.; Tull, T.M.; Seipel, M.E.; Groarke, J.M. Activity of Pleconaril against Enteroviruses. Antimicrob. Agents Chemother. 1999, 43, 2109–2115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egorova, A.; Ekins, S.; Schmidtke, M.; Makarov, V. Back to the Future: Advances in Development of Broad-Spectrum Capsid-Binding Inhibitors of Enteroviruses. Eur. J. Med. Chem. 2019, 178, 606–622. [Google Scholar] [CrossRef] [PubMed]
- Vitaku, E.; Smith, D.T.; Njardarson, J.T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar] [CrossRef] [PubMed]
- Labbozzetta, M.; Barreca, M.; Spanò, V.; Raimondi, M.V.; Poma, P.; Notarbartolo, M.; Barraja, P.; Montalbano, A. Novel Insights on [1,2]Oxazolo[5,4- e ]Isoindoles on Multidrug Resistant Acute Myeloid Leukemia Cell Line. Drug Dev. Res. 2022, 83, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Kerru, N.; Gummidi, L.; Maddila, S.; Gangu, K.K.; Jonnalagadda, S.B. A Review on Recent Advances in Nitrogen-Containing Molecules and Their Biological Applications. Molecules 2020, 25, 1909. [Google Scholar] [CrossRef] [Green Version]
- Cisar, J.S.; Pietsch, C.; DeRatt, L.G.; Jacoby, E.; Kazmi, F.; Keohane, C.; Legenski, K.; Matico, R.; Shaffer, P.; Simonnet, Y.; et al. N-Heterocyclic 3-Pyridyl Carboxamide Inhibitors of DHODH for the Treatment of Acute Myelogenous Leukemia. J. Med. Chem. 2022, 65, 11241–11256. [Google Scholar] [CrossRef] [PubMed]
- Cilibrasi, V.; Spanò, V.; Bortolozzi, R.; Barreca, M.; Raimondi, M.V.; Rocca, R.; Maruca, A.; Montalbano, A.; Alcaro, S.; Ronca, R.; et al. Synthesis of 2H-Imidazo[2′,1’:2,3] [1,3]Thiazolo[4,5-e]Isoindol-8-Yl-Phenylureas with Promising Therapeutic Features for the Treatment of Acute Myeloid Leukemia (AML) with FLT3/ITD Mutations. Eur. J. Med. Chem. 2022, 235, 114292. [Google Scholar] [CrossRef]
- Corona, P.; Ibba, R.; Piras, S.; Molicotti, P.; Bua, A.; Carta, A. Quinoxaline-based Efflux Pump Inhibitors Restore Drug Susceptibility in Drug-resistant Nontuberculous Mycobacteria. Arch. Pharm. 2022, 355, 2100492. [Google Scholar] [CrossRef]
- Madeddu, S.; Ibba, R.; Sanna, G.; Piras, S.; Riu, F.; Marongiu, A.; Ambrosino, A.; Caria, P.; Onnis, V.; Franci, G.; et al. Human Enterovirus B: Selective Inhibition by Quinoxaline Derivatives and Bioinformatic RNA-Motif Identification as New Targets. Pharmaceuticals 2022, 15, 181. [Google Scholar] [CrossRef]
- Perera, N.; Brun, J.; Alonzi, D.S.; Tyrrell, B.E.; Miller, J.L.; Zitzmann, N. Antiviral Effects of Deoxynojirimycin (DNJ)-Based Iminosugars in Dengue Virus-Infected Primary Dendritic Cells. Antiviral. Res. 2022, 199, 105269. [Google Scholar] [CrossRef] [PubMed]
- Barreca, M.; Ingarra, A.M.; Raimondi, M.V.; Spanò, V.; de Franco, M.; Menilli, L.; Gandin, V.; Miolo, G.; Barraja, P.; Montalbano, A. Insight on Pyrimido[5,4-g]Indolizine and Pyrimido[4,5-c]Pyrrolo[1,2-a]Azepine Systems as Promising Photosensitizers on Malignant Cells. Eur. J. Med. Chem 2022, 237, 114399. [Google Scholar] [CrossRef] [PubMed]
- Barreca, M.; Spanò, V.; Rocca, R.; Bivacqua, R.; Abel, A.-C.; Maruca, A.; Montalbano, A.; Raimondi, M.V.; Tarantelli, C.; Gaudio, E.; et al. Development of [1,2]Oxazoloisoindoles Tubulin Polymerization Inhibitors: Further Chemical Modifications and Potential Therapeutic Effects against Lymphomas. Eur. J. Med. Chem. 2022, 243, 114744. [Google Scholar] [CrossRef] [PubMed]
- Briguglio, I.; Piras, S.; Corona, P.; Gavini, E.; Nieddu, M.; Boatto, G.; Carta, A. Benzotriazole: An Overview on Its Versatile Biological Behavior. Eur. J. Med. Chem. 2015, 97, 612–648. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-Z.; Gan, L.-L.; Wang, H.; Zhou, C.-H. New Progress in Azole Compounds as Antimicrobial Agents. Mini-Rev. Med. Chem. 2016, 17, 122–166. [Google Scholar] [CrossRef] [PubMed]
- Kelemen, H.; Orgovan, G.; Szekely-Szentmiklosi, B. The Pharmaceutical Chemistry of Azole Antifungals. Acta Pharm. Hung. 2016, 86, 85–98. [Google Scholar]
- Carta, A.; Palomba, M.; Briguglio, I.; Corona, P.; Piras, S.; Jabes, D.; Guglierame, P.; Molicotti, P.; Zanetti, S. Synthesis and Anti-Mycobacterial Activities of Triazoloquinolones. Eur. J. Med. Chem. 2011, 46, 320–326. [Google Scholar] [CrossRef]
- Carta, A.; Bua, A.; Corona, P.; Piras, S.; Briguglio, I.; Molicotti, P.; Zanetti, S.; Laurini, E.; Aulic, S.; Fermeglia, M.; et al. Design, Synthesis and Antitubercular Activity of 4-Alkoxy-Triazoloquinolones Able to Inhibit the M. Tuberculosis DNA Gyrase. Eur. J. Med. Chem. 2019, 161, 399–415. [Google Scholar] [CrossRef]
- Ibba, R.; Piras, S.; Corona, P.; Riu, F.; Loddo, R.; Delogu, I.; Collu, G.; Sanna, G.; Caria, P.; Dettori, T.; et al. Synthesis, Antitumor and Antiviral In Vitro Activities of New Benzotriazole-Dicarboxamide Derivatives. Front. Chem. 2021, 9, 660424. [Google Scholar] [CrossRef]
- Riu, F.; Sanna, L.; Ibba, R.; Piras, S.; Bordoni, V.; Scorciapino, M.A.; Lai, M.; Sestito, S.; Bagella, L.; Carta, A. A Comprehensive Assessment of a New Series of 5′,6′-Difluorobenzotriazole-Acrylonitrile Derivatives as Microtubule Targeting Agents (MTAs). Eur. J. Med. Chem. 2021, 222, 113590. [Google Scholar] [CrossRef]
- Riu, F.; Ibba, R.; Zoroddu, S.; Sestito, S.; Lai, M.; Piras, S.; Sanna, L.; Bordoni, V.; Bagella, L.; Carta, A. Design, Synthesis, and Biological Screening of a Series of 4′-Fluoro-Benzotriazole-Acrylonitrile Derivatives as Microtubule-Destabilising Agents (MDAs). J. Enzyme. Inhib. Med. Chem. 2022, 37, 2223–2240. [Google Scholar] [CrossRef] [PubMed]
- Loddo, R.; Novelli, F.; Sparatore, A.; Tasso, B.; Tonelli, M.; Boido, V.; Sparatore, F.; Collu, G.; Delogu, I.; Giliberti, G.; et al. Antiviral Activity of Benzotriazole Derivatives. 5-[4-(Benzotriazol-2-Yl)Phenoxy]-2,2-Dimethylpentanoic Acids Potently and Selectively Inhibit Coxsackie Virus B5. Bioorg. Med. Chem. 2015, 23, 7024–7034. [Google Scholar] [CrossRef] [PubMed]
- Plewe, M.B.; Gantla, V.R.; Sokolova, N.v.; Shin, Y.-J.; Naik, S.; Brown, E.R.; Fetsko, A.; Zhang, L.; Kalveram, B.; Freiberg, A.N.; et al. Discovery of a Novel Highly Potent Broad-Spectrum Heterocyclic Chemical Series of Arenavirus Cell Entry Inhibitors. Bioorg. Med. Chem. Lett. 2021, 41, 127983. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.-L.; Zhang, Y.; Civiello, R.L.; Kadow, K.F.; Cianci, C.; Krystal, M.; Meanwell, N.A. Fundamental Structure–Activity Relationships Associated with a New Structural Class of Respiratory Syncytial Virus Inhibitor. Bioorg. Med. Chem. Lett. 2003, 13, 2141–2144. [Google Scholar] [CrossRef] [PubMed]
- Ibba, R.; Carta, A.; Madeddu, S.; Caria, P.; Serreli, G.; Piras, S.; Sestito, S.; Loddo, R.; Sanna, G. Inhibition of Enterovirus A71 by a Novel 2-Phenyl-Benzimidazole Derivative. Viruses 2021, 13, 58. [Google Scholar] [CrossRef] [PubMed]
- Bivacqua, R.; Barreca, M.; Spanò, V.; Raimondi, M.V.; Romeo, I.; Alcaro, S.; Andrei, G.; Barraja, P.; Montalbano, A. Insight into Non-Nucleoside Triazole-Based Systems as Viral Polymerases Inhibitors. Eur. J. Med. Chem. 2023, 249, 115136. [Google Scholar] [CrossRef]
- Corona, P.; Piras, S.; Ibba, R.; Riu, F.; Murineddu, G.; Sanna, G.; Madeddu, S.; Delogu, I.; Loddo, R.; Carta, A. Antiviral Activity of Benzotriazole Based Derivatives. Open Med. Chem. J. 2020, 14, 83–98. [Google Scholar] [CrossRef]
- Sanna, G.; Piras, S.; Madeddu, S.; Busonera, B.; Klempa, B.; Corona, P.; Ibba, R.; Murineddu, G.; Carta, A.; Loddo, R. 5,6-Dichloro-2-Phenyl-Benzotriazoles: New Potent Inhibitors of Orthohantavirus. Viruses 2020, 12, 122. [Google Scholar] [CrossRef] [Green Version]
- Piras, S.; Corona, P.; Ibba, R.; Riu, F.; Murineddu, G.; Sanna, G.; Madeddu, S.; Delogu, I.; Loddo, R.; Carta, A. Preliminary Anti-Coxsackie Activity of Novel 1-[4-(5,6-Dimethyl(H)-1H(2H)-Benzotriazol-1(2)-Yl)Phenyl]-3-Alkyl(Aryl)Ureas. Med. Chem. 2019, 16, 677–688. [Google Scholar] [CrossRef]
- Piras, S.; Sanna, G.; Carta, A.; Corona, P.; Ibba, R.; Loddo, R.; Madeddu, S.; Caria, P.; Aulic, S.; Laurini, E.; et al. Dichloro-Phenyl-Benzotriazoles: A New Selective Class of Human Respiratory Syncytial Virus Entry Inhibitors. Front. Chem. 2019, 7, 247. [Google Scholar] [CrossRef]
- Carta, A.; Loriga, M.; Piras, S.; Paglietti, G.; Ferrone, M.; Fermeglia, M.; Pricl, S.; Colla, P.; Collu, G.; Sanna, T.; et al. Synthesis and Anti-Picornaviridae In Vitro Activity of a New Class of Helicase Inhibitors the N,N-Bis[4-(1H(2H)-Benzotriazol-1(2)-Yl)Phenyl] Alkyldicarboxamides. Med. Chem. 2007, 3, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Kirk, K.L.; Cohen, L.A. Synthesis of Some Fluoronitrobenzimidazoles and Their Reactivities toward Peptide Nucleophiles. J. Org. Chem. 1969, 34, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Liedholm B Copper (I) Catalysed Replacement of Bromine by Chloride Ion in Halonitrobenzenes. Part II. Acta Chem. Scand. 1971, 25, 106–112.
- Sanna, G.; Madeddu, S.; Giliberti, G.; Piras, S.; Struga, M.; Wrzosek, M.; Kubiak-Tomaszewska, G.; Koziol, A.; Savchenko, O.; Lis, T.; et al. Synthesis and Biological Evaluation of Novel Indole-Derived Thioureas. Molecules 2018, 23, 2554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deiana, M.; Montoro, P.; Jerković, I.; Atzeri, A.; Marijanović, Z.; Serreli, G.; Piacente, S.; Tuberoso, C.I.G. First Characterization of Pompia Intrea Candied Fruit: The Headspace Chemical Profile, Polar Extract Composition and Its Biological Activities. Food Res. Int. 2019, 120, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Serreli, G.; Melis, M.P.; Zodio, S.; Naitza, M.R.; Casula, E.; Peñalver, P.; Lucas, R.; Loi, R.; Morales, J.C.; Deiana, M. Altered Paracellular Permeability in Intestinal Cell Monolayer Challenged with Lipopolysaccharide: Modulatory Effects of Pterostilbene Metabolites. Food Chem. Toxicol. 2020, 145, 111729. [Google Scholar] [CrossRef]
- Sanna, G.; Farci, P.; Busonera, B.; Murgia, G.; la Colla, P.; Giliberti, G. Antiviral Properties from Plants of the Mediterranean Flora. Nat. Prod. Res. 2015, 29, 2065–2070. [Google Scholar] [CrossRef]




| Compounds | MDBK | BVDV | BHK-21 | YFV | Reo-1 | Vero76 | CVB5 | Sb-1 | VV | HSV-1 | VSV | RSV | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a CC50 | b EC50 | c CC50 | d EC50 | e CC50 | f EC50 | ||||||||
| Amines | 6a | >100 | >100 | 57 | <57 | <57 | >100 | 52 ± 3.6 | >100 | 62.5 ± 16.2 | >100 | >100 | >100 |
| 11b | >100 | >100 | >100 | >100 | >100 | >100 | 6 | >100 | >100 | >100 | >100 | >100 | |
| 11d | >100 | >100 | >100 | >100 | >100 | >100 | 7.6 ± 1.9 | >100 | >100 | >100 | >100 | >100 | |
| Amides | 18e | >100 | >100 | >100 | >100 | >100 | >100/9 | 12.4 ± 0.6 | >100 | >100 | 52 ± 9.2 | >100 | >9 |
| 21e | 72 | <72 | 60 | <60 | <60 | >100 | >100 | >100 | >100 | >100 | >100 | 20 ± 2 | |
| 25b | >100 | >100 | >100 | >100 | >100 | >100 | 47 ± 2.2 | >100 | >100 | >100 | >100 | >100 | |
| 41a | >100 | >100 | >100/7 | >100 | >7 | >100/10.5 | 18.5 ± 2.1 | >100 | >100 | >100 | >100 | >10.5 | |
| 43a | >100 | >100 | >100 | >100 | >100 | >100/9.2 | 9 ± 1.6 | >100 | >100 | >100 | >100 | >9.2 | |
| 75b | >100 | >100 | >100 | >100 | >100 | >100 | 80 | >100 | >100 | >100 | >100 | >100 | |
| 77b | >100 | >100 | >100 | >100 | >100 | >100 | 85 | >100 | >100 | >100 | >100 | >100 | |
| 79c | 44 | >44 | 86 | >86 | >86 | 72/9 | >72 | >72 | >72 | >72 | >72 | >9 | |
| 86c | 87 | 3 | 95 | <95 | <95 | 85 | <85 | <85 | <85 | <85 | <85 | <85 | |
| Ureas | 32b | >100 | <100 | >100 | >100 | >100 | >100 | >100 | 85 | >100 | >100 | >100 | >100 |
| 99b | >100 | >100 | >100 | >100 | >100 | >100 | 16 | >100 | >100 | >100 | >100 | >100 | |
| 100b | >100 | >100 | >100 | >100 | >100 | >100 | 50 | >100 | <100 | >100 | >100 | >100 | |
| Ref cmps | NM107 | >100 | 6 ± 2.1 | ||||||||||
| NM108 | >100 | 1.5 | >100 | 1.0 | |||||||||
| Ribav | 35/52 | 12/18 | |||||||||||
| 6az-ur | >100 | 46 ± 2.5 | 9.3 ± 1.6 | 1.1 ± 0.4 | |||||||||
| ACG | s | >100 | 2.4 ± 0.6 | ||||||||||
| Plec | 77 ± 6.8 | 0.005 ± 0.002 | 2 ± 0.62 | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibba, R.; Corona, P.; Nonne, F.; Caria, P.; Serreli, G.; Palmas, V.; Riu, F.; Sestito, S.; Nieddu, M.; Loddo, R.; et al. Design, Synthesis, and Antiviral Activities of New Benzotriazole-Based Derivatives. Pharmaceuticals 2023, 16, 429. https://doi.org/10.3390/ph16030429
Ibba R, Corona P, Nonne F, Caria P, Serreli G, Palmas V, Riu F, Sestito S, Nieddu M, Loddo R, et al. Design, Synthesis, and Antiviral Activities of New Benzotriazole-Based Derivatives. Pharmaceuticals. 2023; 16(3):429. https://doi.org/10.3390/ph16030429
Chicago/Turabian StyleIbba, Roberta, Paola Corona, Francesca Nonne, Paola Caria, Gabriele Serreli, Vanessa Palmas, Federico Riu, Simona Sestito, Maria Nieddu, Roberta Loddo, and et al. 2023. "Design, Synthesis, and Antiviral Activities of New Benzotriazole-Based Derivatives" Pharmaceuticals 16, no. 3: 429. https://doi.org/10.3390/ph16030429
APA StyleIbba, R., Corona, P., Nonne, F., Caria, P., Serreli, G., Palmas, V., Riu, F., Sestito, S., Nieddu, M., Loddo, R., Sanna, G., Piras, S., & Carta, A. (2023). Design, Synthesis, and Antiviral Activities of New Benzotriazole-Based Derivatives. Pharmaceuticals, 16(3), 429. https://doi.org/10.3390/ph16030429









