In Silico Identification of Peptides with PPARγ Antagonism in Protein Hydrolysate from Rice (Oryza sativa)
Abstract
:1. Introduction
2. Results
2.1. Proximate Composition and In Vitro Digestibility
2.2. Electrophoresis (SDS-PAGE)
2.3. In Silico Digestion
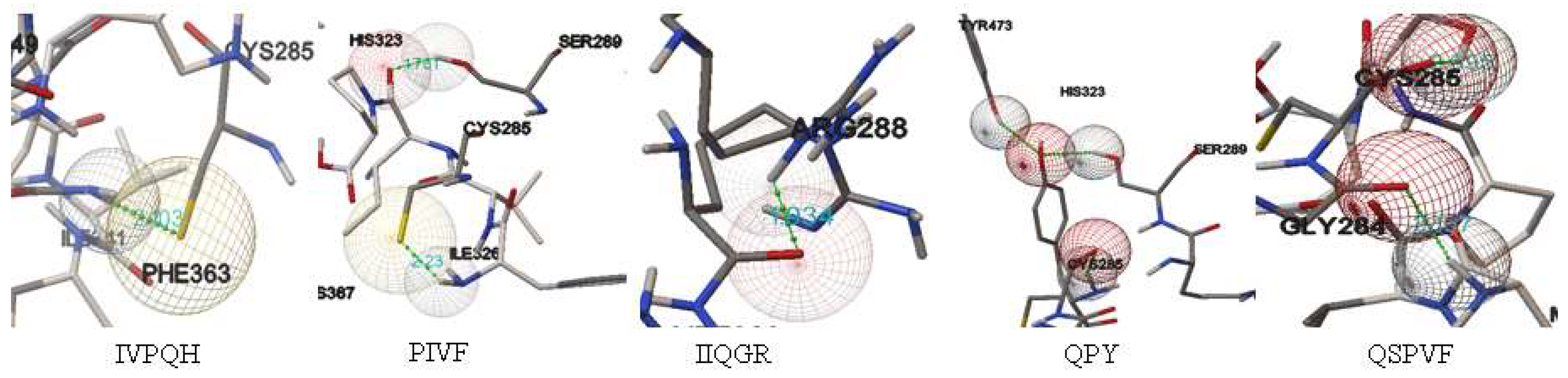
2.4. In Silico Screening for Bioactive Peptides against PPAR-γ
2.5. Pharmacokinetics and Drug-Likeness Prediction
3. Discussion
4. Materials and Methods
4.1. Novel Protein Concentrate (NPC) Obtention
4.2. Amino Acid Score
4.3. Electrophoresis (SDS-PAGE)
4.4. In Vitro Digestibility and Bioaccesibility
4.5. In Silico Digestive Process
4.6. Ligand Preparation
4.7. Receptor Preparation
4.8. ADME Prediction
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Obesity. Available online: https://www.who.int/health-topics/obesity (accessed on 27 December 2022).
- Singh, B.P.; Aluko, R.E.; Hati, S.; Solanki, D. Bioactive Peptides in the Management of Lifestyle-Related Diseases: Current Trends and Future Perspectives. Crit. Rev. Food Sci. Nutr. 2021, 62, 4593–4606. [Google Scholar] [CrossRef] [PubMed]
- Hruby, A.; Manson, J.E.; Qi, L.; Malik, V.S.; Rimm, E.B.; Sun, Q.; Willett, W.C.; Hu, F.B. Determinants and Consequences of Obesity. Am. J. Public Health 2016, 106, 1656–1662. [Google Scholar] [CrossRef] [PubMed]
- de Sá, P.M.; Richard, A.J.; Hang, H.; Stephens, J.M. Transcriptional Regulation of Adipogenesis. In Comprehensive Physiology; Terjung, R., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 635–674. ISBN 978-0-470-65071-4. [Google Scholar]
- Xue, P.; Hou, Y.; Zuo, Z.; Wang, Z.; Ren, S.; Dong, J.; Fu, J.; Wang, H.; Andersen, M.E.; Zhang, Q.; et al. Long Isoforms of NRF1 Negatively Regulate Adipogenesis via Suppression of PPARγ Expression. Redox Biol. 2020, 30, 101414. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Cao, Y.; Xiao, J.; Song, M.; Ho, C.-T. Molecular Mechanisms of the Anti-Obesity Effect of Bioactive Ingredients in Common Spices: A Review. Food Funct. 2018, 9, 4569–4581. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.R.; Barrick, C.; Kim, K.-A.; Lindner, J.; Blondeau, B.; Fujimoto, Y.; Shiota, M.; Kesterson, R.A.; Kahn, B.B.; Magnuson, M.A. Deletion of PPAR in Adipose Tissues of Mice Protects against High Fat Diet-Induced Obesity and Insulin Resistance. Proc. Natl. Acad. Sci. USA 2005, 102, 6207–6212. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.-S.; Park, J.; Choi, J.H. Revisiting PPARγ as a Target for the Treatment of Metabolic Disorders. BMB Rep. 2014, 47, 599–608. [Google Scholar] [CrossRef] [Green Version]
- Lebovitz, H.E. Thiazolidinediones: The Forgotten Diabetes Medications. Curr. Diabetes Rep. 2019, 19, 151. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.S. Peptides and Peptidomimetics as Potential Antiobesity Agents: Overview of Current Status. Front. Nutr. 2019, 6, 11. [Google Scholar] [CrossRef]
- Petsalaki, E.; Russell, R.B. Peptide-Mediated Interactions in Biological Systems: New Discoveries and Applications. Curr. Opin. Biotechnol. 2008, 19, 344–350. [Google Scholar] [CrossRef]
- Mazorra-Manzano, M.A.; Ramírez-Suarez, J.C.; Yada, R.Y. Plant Proteases for Bioactive Peptides Release: A Review. Crit. Rev. Food Sci. Nutr. 2018, 58, 2147–2163. [Google Scholar] [CrossRef]
- Minkiewicz, P.; Mattison, C.P.; Darewicz, M. Quantitative In Silico Evaluation of Allergenic Proteins from Anacardium occidentale, Carya illinoinensis, Juglans regia and Pistacia vera and Their Epitopes as Precursors of Bioactive Peptides. Curr. Issues Mol. Biol. 2022, 44, 3100–3117. [Google Scholar] [CrossRef]
- Oseguera Toledo, M.E.; Gonzalez de Mejia, E.; Sivaguru, M.; Amaya-Llano, S.L. Common Bean (Phaseolus vulgaris L.) Protein-Derived Peptides Increased Insulin Secretion, Inhibited Lipid Accumulation, Increased Glucose Uptake and Reduced the Phosphatase and Tensin Homologue Activation In Vitro. J. Funct. Foods 2016, 27, 160–177. [Google Scholar] [CrossRef]
- Tsou, M.-J.; Kao, F.-J.; Lu, H.-C.; Kao, H.-C.; Chiang, W.-D. Purification and Identification of Lipolysis-Stimulating Peptides Derived from Enzymatic Hydrolysis of Soy Protein. Food Chem. 2013, 138, 1454–1460. [Google Scholar] [CrossRef]
- Li-Chan, E.C. Bioactive Peptides and Protein Hydrolysates: Research Trends and Challenges for Application as Nutraceuticals and Functional Food Ingredients. Curr. Opin. Food Sci. 2015, 1, 28–37. [Google Scholar] [CrossRef] [Green Version]
- López-Ibarra, C.; Ruiz-López, F. de J.; Bautista-Villarreal, M.; Báez-González, J.G.; Rodríguez Romero, B.A.; González-Martínez, B.E.; López-Cabanillas Lomelí, M.; Vázquez-Rodríguez, J.A. Protein Concentrates on Tepary Bean (Phaseolus acutifolius Gray) as a Functional Ingredient: In Silico Docking of Tepary Bean Lectin to Peroxisome Proliferator-Activated Receptor Gamma. Front. Nutr. 2021, 8, 661463. [Google Scholar] [CrossRef]
- Fan, X.; Cui, Y.; Zhang, R.; Zhang, X. Purification and Identification of Anti-Obesity Peptides Derived from Spirulina platensis. J. Funct. Foods 2018, 47, 350–360. [Google Scholar] [CrossRef]
- Rani, S.; Pooja, K.; Kumar Pal, G. Exploration of Rice Protein Hydrolysates and Peptides with Special Reference to Antioxidant Potential: Computational Derived Approaches for Bio-Activity Determination. Trends Food Sci. Technol. 2018, 80, 61–70. [Google Scholar] [CrossRef]
- Pantoa, T.; Kubota, M.; Suwannaporn, P.; Kadowaki, M. Characterization and Bioactivities of Young Rice Protein Hydrolysates. J. Cereal Sci. 2020, 95, 103049. [Google Scholar] [CrossRef]
- Taniguchi, M.; Kameda, M.; Namae, T.; Ochiai, A.; Saitoh, E.; Tanaka, T. Identification and Characterization of Multifunctional Cationic Peptides Derived from Peptic Hydrolysates of Rice Bran Protein. J. Funct. Foods 2017, 34, 287–296. [Google Scholar] [CrossRef]
- Cavasotto, C.N. In Silico Drug Discovery and Design: Theory, Methods, Challenges, and Applications; CRC Press: Boca Raton, FL, USA, 2016; p. 539. [Google Scholar]
- Kalmykova, S.D.; Arapidi, G.P.; Urban, A.S.; Osetrova, M.S.; Gordeeva, V.D.; Ivanov, V.T.; Govorun, V.M. In Silico Analysis of Peptide Potential Biological Functions. Russ. J. Bioorganic Chem. 2018, 44, 367–385. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (Ed.) Dietary Protein Quality Evaluation in Human Nutrition: Report of an FAO Expert Consultation, 31 March–2 April, 2011, Auckland, New Zealand; FAO Food and Nutrition Paper; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013; ISBN 978-92-5-107417-6. [Google Scholar]
- Aito-Inoue, M.; Lackeyram, D.; Fan, M.Z.; Sato, K.; Mine, Y. Transport of a Tripeptide, Gly-Pro-Hyp, across the Porcine Intestinal Brush-Border Membrane. J. Pept. Sci. Off. Publ. Eur. Pept. Soc. 2007, 13, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Miner-Williams, W.M.; Stevens, B.R.; Moughan, P.J. Are Intact Peptides Absorbed from the Healthy Gut in the Adult Human? Nutr. Res. Rev. 2014, 27, 308–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, Y.; Zhu, Y.; Gao, Y.; Shi, Z.; Hu, Y.; Ren, G. Suppressive Effects of Saponin-Enriched Extracts from Quinoa on 3T3-L1 Adipocyte Differentiation. Food Funct. 2015, 6, 3282–3290. [Google Scholar] [CrossRef] [PubMed]
- Aranaz, P.; Navarro-Herrera, D.; Zabala, M.; Miguéliz, I.; Romo-Hualde, A.; López-Yoldi, M.; Martínez, J.A.; Vizmanos, J.L.; Milagro, F.I.; González-Navarro, C.J. Phenolic Compounds Inhibit 3T3-L1 Adipogenesis Depending on the Stage of Differentiation and Their Binding Affinity to PPARγ. Molecules 2019, 24, 1045. [Google Scholar] [CrossRef] [Green Version]
- Selamassakul, O.; Laohakunjit, N.; Kerdchoechuen, O.; Ratanakhanokchai, K. A Novel Multi-Biofunctional Protein from Brown Rice Hydrolysed by Endo/Endo-Exoproteases. Food Funct. 2016, 7, 2635–2644. [Google Scholar] [CrossRef]
- Amagliani, L. The Composition, Extraction, Functionality and Applications of Rice Proteins: A Review. Trends Food Sci. Technol. 2017, 64, 1–12. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, M.; Ouwerkerk, P.B.F. Molecular and Environmental Factors Determining Grain Quality in Rice. Food Energy Secur. 2012, 1, 111–132. [Google Scholar] [CrossRef]
- Mohan, V.R.; Tresina, P.S.; Daffodil, E.D. Antinutritional Factors in Legume Seeds: Characteristics and Determination. In Encyclopedia of Food and Health; Elsevier: Amsterdam, The Netherlands, 2016; pp. 211–220. ISBN 978-0-12-384953-3. [Google Scholar]
- Ganapathy, V.; Ganapathy, M.E.; Leibach, F.H. Protein Digestion and Assimilation. In Textbook of Gastroenterology; Yamada, T., Ed.; Blackwell Publishing Ltd.: Oxford, UK, 2008; pp. 464–477. ISBN 978-1-4443-0325-4. [Google Scholar]
- Zhou, P.; Jin, B.; Li, H.; Huang, S.-Y. HPEPDOCK: A Web Server for Blind Peptide–Protein Docking Based on a Hierarchical Algorithm. Nucleic Acids Res. 2018, 46, W443–W450. [Google Scholar] [CrossRef]
- Kandemir-Cavas, C.; Pérez-Sanchez, H.; Mert-Ozupek, N.; Cavas, L. In Silico Analysis of Bioactive Peptides in Invasive Sea Grass Halophila Stipulacea. Cells 2019, 8, 557. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Ye, L.; Wang, X.; Shi, W.; Liu, H.; Qian, X.; Zhu, Y.; Yu, H. In Silico Investigations of Anti-Androgen Activity of Polychlorinated Biphenyls. Chemosphere 2013, 92, 795–802. [Google Scholar] [CrossRef]
- Ye, F.; Zhang, Z.-S.; Luo, H.-B.; Shen, J.-H.; Chen, K.-X.; Shen, X.; Jiang, H.-L. The Dipeptide H-Trp-Glu-OH Shows Highly Antagonistic Activity against PPARγ: Bioassay with Molecular Modeling Simulation. ChemBioChem 2006, 7, 74–82. [Google Scholar] [CrossRef]
- Ohtera, A.; Miyamae, Y.; Yoshida, K.; Maejima, K.; Akita, T.; Kakizuka, A.; Irie, K.; Masuda, S.; Kambe, T.; Nagao, M. Identification of a New Type of Covalent PPARγ Agonist Using a Ligand-Linking Strategy. ACS Chem. Biol. 2015, 10, 2794–2804. [Google Scholar] [CrossRef]
- Seargent, J.M.; Yates, E.A.; Gill, J.H. GW9662, a Potent Antagonist of PPAR γ, Inhibits Growth of Breast Tumour Cells and Promotes the Anticancer Effects of the PPAR γ Agonist Rosiglitazone, Independently of PPAR γ Activation: Special Report. Br. J. Pharmacol. 2004, 143, 933–937. [Google Scholar] [CrossRef] [Green Version]
- Choe, J.; Seol, K.-H.; Son, D.-I.; Lee, H.J.; Lee, M.; Jo, C. Identification of Angiotensin I-Converting Enzyme Inhibitory Peptides from Enzymatic Hydrolysates of Pork Loin. Int. J. Food Prop. 2019, 22, 1112–1121. [Google Scholar] [CrossRef] [Green Version]
- Nongonierma, A.B.; FitzGerald, R.J. Features of Dipeptidyl Peptidase IV (DPP-IV) Inhibitory Peptides from Dietary Proteins. J. Food Biochem. 2019, 43, e12451. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zhou, M.; Wu, T.; Fang, L.; Liu, C.; Min, W. Novel Anti-Obesity Peptide (RLLPH) Derived from Hazelnut (Corylus heterophylla Fisch) Protein Hydrolysates Inhibits Adipogenesis in 3T3-L1 Adipocytes by Regulating Adipogenic Transcription Factors and Adenosine Monophosphate-Activated Protein Kinase (AMPK) Activation. J. Biosci. Bioeng. 2020, 129, 259–268. [Google Scholar] [CrossRef]
- Shi, Z.; Hao, Y.; Teng, C.; Yao, Y.; Ren, G. Functional Properties and Adipogenesis Inhibitory Activity of Protein Hydrolysates from Quinoa (Chenopodium Quinoa Willd.). Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/fsn3.1052 (accessed on 27 November 2019).
- Vij, R.; Reddi, S.; Kapila, S.; Kapila, R. Transepithelial Transport of Milk Derived Bioactive Peptide VLPVPQK. Food Chem. 2016, 190, 681–688. [Google Scholar] [CrossRef]
- Chothe, P.; Singh, N.; Ganapathy, V. Evidence for Two Different Broad-Specificity Oligopeptide Transporters in Intestinal Cell Line Caco-2 and Colonic Cell Line CCD841. Am. J. Physiol. Cell Physiol. 2011, 300, C1260–C1269. [Google Scholar] [CrossRef]
- Xia, N.; Wang, J.-M.; Gong, Q.; Yang, X.-Q.; Yin, S.-W.; Qi, J.-R. Characterization and In Vitro Digestibility of Rice Protein Prepared by Enzyme-Assisted Microfluidization: Comparison to Alkaline Extraction. J. Cereal Sci. 2012, 56, 482–489. [Google Scholar] [CrossRef]
- Vázquez-Ortiz, F.A.; Caire, G.; Higuera-Ciapara, I.; Hernández, G. High Performance Liquid Chromatographic Determination of Free Amino Acids in Shrimp. J. Liq. Chromatogr. 1995, 18, 2059–2068. [Google Scholar] [CrossRef]
- Betancur-Ancona, D.; Gallegos-Tintoré, S.; Chel-Guerrero, L. Wet-Fractionation of Phaseolus Lunatus Seeds: Partial Characterization of Starch and Protein. J. Sci. Food Agric. 2004, 84, 1193–1201. [Google Scholar] [CrossRef]
- Camacho Espinoza, M.K.; Peinado Guevara, L.I.; López Valenzuela, J.Á.; Valdez Ortiz, Á.; Salinas Pérez, R.A.; Moreno Herrera, C.G.; Medina Godoy, S. Caracterización proteómica de granos de frijol azufrado (Phamseolus vulgaris) cultivados en el estado de Sinaloa. Ra Ximhai 2010, 6, 23–36. [Google Scholar] [CrossRef]
- INFOGEST. Static in Vitro Simulation of Gastrointestinal Food Digestion|Nature Protocols. Available online: https://www.nature.com/articles/s41596-018-0119-1 (accessed on 3 August 2021).
- AOAC. Official Methods of Analysis, 16th ed.; Harla: St. Paul, MN, USA, 1999. [Google Scholar]
- Managa, M.G.; Akinola, S.A.; Remize, F.; Garcia, C.; Sivakumar, D. Physicochemical Parameters and Bioaccessibility of Lactic Acid Bacteria Fermented Chayote Leaf (Sechium edule) and Pineapple (Ananas comosus) Smoothies. Front. Nutr. 2021, 8, 649189. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Kariluoto, S.; Edelmann, M.; Piironen, V. Bioaccessibility of Folate in Faba Bean, Oat, Rye and Wheat Matrices. Food Chem. 2021, 350, 129259. [Google Scholar] [CrossRef] [PubMed]
- Minkiewicz, P.; Iwaniak, A.; Darewicz, M. Darewicz BIOPEP-UWM Database of Bioactive Peptides: Current Opportunities. Int. J. Mol. Sci. 2019, 20, 5978. [Google Scholar] [CrossRef] [Green Version]
- Dziuba, J.; Iwaniak, A.; Minkiewicz, P. Computer-Aided Characteristics of Proteins as Potential Precursors of Bioactive Peptides SO POLIMERY. Polimery 2003, 48, 50–53. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Thiessen, P.A.; Bolton, E.E.; Chen, J.; Fu, G.; Gindulyte, A.; Han, L.; He, J.; He, S.; Shoemaker, B.A.; et al. PubChem Substance and Compound Databases. Nucleic Acids Res. 2016, 44, D1202–D1213. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [Green Version]
- Isyaku, Y.; Uzairu, A.; Uba, S. Computational Studies of a Series of 2-Substituted Phenyl-2-Oxo-, 2-Hydroxyl- and 2-Acylloxyethylsulfonamides as Potent Anti-Fungal Agents. Heliyon 2020, 6, e03724. [Google Scholar] [CrossRef]


| Amino Acid (g/100 g Protein) | NPC 1 | FAO Ref + |
|---|---|---|
| ALA | 5.75 | -- |
| ARG | 8.36 | -- |
| ASP | 8.05 | -- |
| GLN | 18.39 | -- |
| GLY | 4.70 | -- |
| PRO | 4.19 | -- |
| SER | 3.66 | -- |
| HIS | 2.19 | 1.6 |
| ILE | 3.87 | 3.0 |
| LEU | 8.78 | 6.1 |
| LYS | 3.87 | 4.8 |
| CYS + MET | 8.46 | 2.3 |
| PHE + TYR | 5.22 | 4.1 |
| THR | 2.30 | 2.5 |
| TRP | 1.88 | 0.66 |
| TOTAL PROTEIN (%) | 83.40 | -- |
| IVPD (%) | 43.07 | -- |
| IVBA (%) | 35.92 | -- |
| Protein Fraction | Length of Peptides | Number of Peptides | % |
|---|---|---|---|
| Glutelin | 1 | 66 | 77.65 |
| 2 a 5 | 16 | 18.82 | |
| >5 | 3 | 3.53 | |
| Prolamin | 1 | 18 | 42.86 |
| 2 a 5 | 14 | 33.33 | |
| >5 | 10 | 23.81 |
| Fraction | Peptide | Length | Scoring Bonding HPEPDOCK | Bonding Energy (kcal/mol) | Intermolecular Bonds |
|---|---|---|---|---|---|
| Glutelin | IVPQH | 5 | −170.40 | −1.87 | CYS285 |
| PIVF | 4 | −188.22 | −2.18 | SER289 CYS285 | |
| IIQGR | 5 | −170.12 | −2.07 | ARG288 | |
| Prolamin | QPY | 3 | −168.94 | −5.61 | CYS285 SER289 TYR473 |
| QSPVF | 5 | −175.73 | −6.38 | CYS285 GLY284 | |
| Control | GW9662 | --- | --- | −7.98 | CYS285 |
| Fraction | Peptide | Molecular Weight (g/mol) | Ilogp | HBDs | HBAs |
|---|---|---|---|---|---|
| Glutelin | IVPQH | 592.69 | 2.25 | 7 | 9 |
| PIVF | 474.59 | 2.36 | 7 | 6 | |
| IIQGR | 585.70 | 1.28 | 10 | 9 | |
| Prolamin | QPY | 406.43 | 1.34 | 5 | 7 |
| QSPVF | 576.64 | 2.32 | 7 | 9 | |
| Control | GW9662 | 276.68 | 1.81 | 1 | 3 |
| Protein | Hydrolyzed Fractions |
|---|---|
| Glutelin | M-ASIN-R-PIVF-F-TVCL-F-L-L-CDGSL-AQQL-L-GQSTSQW-QSSR-R-GSPR-GCR-F-DR-L-QAF-EPIR-SVR-SQAGTTEF-F-DVSN-EL-F-QCTGVSVVR-R-VIEPR-GL-L-L-PH-Y-TN-GASL-VY-IIQGR-GITGPTF-PGCPETY-QQQF-QQSGQAQL-TESQSQSH-K-F-K-DEH-QK-IH-R-F-R-QGDVIAL-PAGVAH-W-CY-N-DGEVPVVAIY-VTDIN-N-GAN-QL-DPR-QR-DF-L-L-AGN-K-R-N-PQAY-R-R-EVEEW-SQN-IF-SGF-STEL-L-SEAF-GISN-QVAR-QL-QCQN-DQR-GEIVR-VER-GL-SL-L-QPY-ASL-QEQEQGQM-QSR-EH-Y-QEGGY-QQSQY-GSGCPN-GL-DETF-CTM-R-VR-QN-IDN-PN-R-ADTY-N-PR-AGR-VTN-L-N-SQN-F-PIL-N-L-VQM-SAVK-VN-L-Y-QN-AL-L-SPF-W-N-IN-AH-SIVY-ITQGR-AQVQVVN-N-N-GK-TVF-N-GEL-R-R-GQL-L-IVPQH-Y-VVVK-K-AQR-EGCAY-IAF-K-TN-PN-SM-VSH-IAGK-SSIF-R-AL-PTDVL-AN-AY-R-ISR-EEAQR-L-K-H-N-R-GDEF-GAF-TPL-QY-K-SY-QDVY-N-VAESS |
| Prolamin | M-K-IIF-F-F-AL-L-AEAACSASAQF-DAVTQVY-R-QY-QL-QQQM-L-SPCGEF-VR-QQCSTVATPF-F-QSPVF-QL-R-N-CQVM-QQQCCQQL-R-M-IAQQSH-CQAISSVQAIVQQL-QL-QQF-SGVY-F-DQAQAQAQAM-L-GL-N-L-PSICGIY-PSY-N-TVPEIPTVGGIW-Y |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-López, F.d.J.; Espinosa-Rodríguez, B.A.; Silva-Mares, D.A.; González-Martínez, B.E.; López-Cabanillas Lomelí, M.; Méndez-López, L.F.; Vázquez-Rodríguez, J.A. In Silico Identification of Peptides with PPARγ Antagonism in Protein Hydrolysate from Rice (Oryza sativa). Pharmaceuticals 2023, 16, 440. https://doi.org/10.3390/ph16030440
Ruiz-López FdJ, Espinosa-Rodríguez BA, Silva-Mares DA, González-Martínez BE, López-Cabanillas Lomelí M, Méndez-López LF, Vázquez-Rodríguez JA. In Silico Identification of Peptides with PPARγ Antagonism in Protein Hydrolysate from Rice (Oryza sativa). Pharmaceuticals. 2023; 16(3):440. https://doi.org/10.3390/ph16030440
Chicago/Turabian StyleRuiz-López, Felipe de Jesús, Bryan Alejandro Espinosa-Rodríguez, David Arturo Silva-Mares, Blanca Edelia González-Martínez, Manuel López-Cabanillas Lomelí, Luis Fernando Méndez-López, and Jesús Alberto Vázquez-Rodríguez. 2023. "In Silico Identification of Peptides with PPARγ Antagonism in Protein Hydrolysate from Rice (Oryza sativa)" Pharmaceuticals 16, no. 3: 440. https://doi.org/10.3390/ph16030440
APA StyleRuiz-López, F. d. J., Espinosa-Rodríguez, B. A., Silva-Mares, D. A., González-Martínez, B. E., López-Cabanillas Lomelí, M., Méndez-López, L. F., & Vázquez-Rodríguez, J. A. (2023). In Silico Identification of Peptides with PPARγ Antagonism in Protein Hydrolysate from Rice (Oryza sativa). Pharmaceuticals, 16(3), 440. https://doi.org/10.3390/ph16030440










