Synthesis, Anticancer, Antimicrobial and Antioxidant Potential of Novel 4-(Substituted phenyl-1,3,4-oxadiazol/thiadiazol-2-yl)-4-(4-substituted phenyl) Azetidin-2-One Derivatives
Abstract
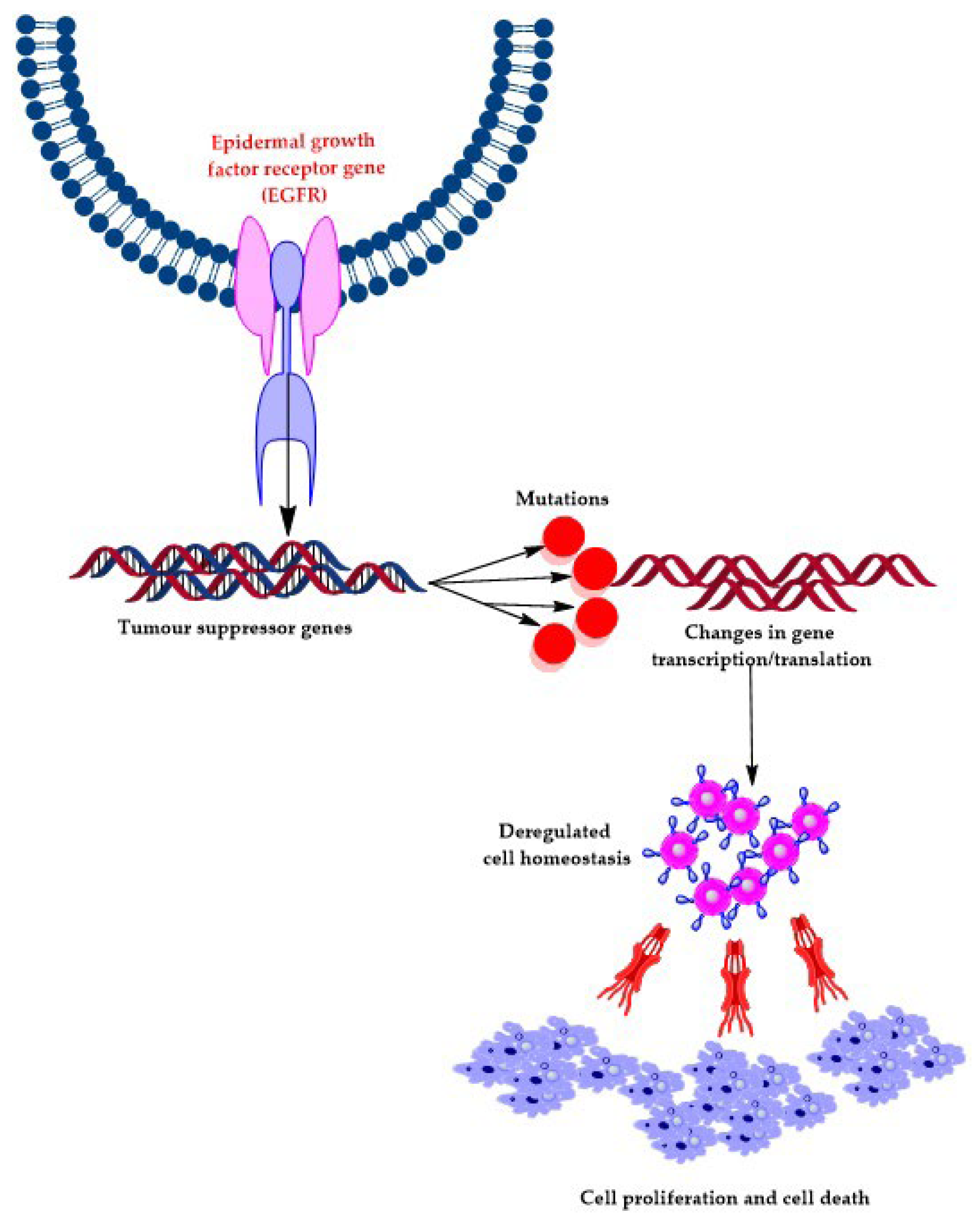
:1. Introduction
2. Results
2.1. Chemistry
2.2. Antimicrobial Screening
2.3. In-Vitro Cytotoxic Assay (MTT Assay)
2.4. In-Vitro Antioxidant Evaluation
2.5. Structure-Activity Relationship
- The different substitution and presence of pharmacophore ring in the final derivatives of 4-substitutedphenyl-1,3,4-oxadiazol/thiadiazol-2-yl)-4-(4-substituted phenyl) azetidin-2-one derivatives played an extremely crucial role in improving the overall biological potentials.
- The presence of EWGs (Cl/NO2/dichloro) at the para position in the synthesized compound AZ-5, AZ-9, AZ-10, and AZ-19 increases anticancer and antimicrobial potential.
- The presence of EWGs (Cl/dichloro) at the para position in the synthesized compounds showed significant antibacterial potential against gram (-ve) bacterial strains (AZ-5 and AZ-10).
- Similarly, the presence of the NH2 group at the para position in the synthesized compounds (AZ-15) showed remarkable antioxidant action.
- EWGs-Cl and Br at the para position in the synthesized compound AZ-19 and AZ-20 increased the antifungal potential against Aspergillus niger and T. harzianum.
- The presence of OCH3 in the synthesized AZ-8 and AZ-9 resulted in antibacterial activity against K. pneumoniae.
- The overall presence of a Cl and NO2 combination at the para position of synthesized conjugates showed remarkable anticancer activity against the MCF-7 cancer cell line and potent antimicrobial action (AZ-19).

3. Material and Methods
3.1. Biological Procedure (s): MTT Assay
- MTT Assay Protocol (Steps involved in cell culture) Cell lines were grown for different types such as MCF-7 in RPMI-1640 medium.
- 2.
- Media were supplemented with 10% FBS.
- 3.
- MCF-7 cell lines were cultured and maintained in DMEM medium supplemented with 10% FBS.
- 4.
- The culture was kept in a 5% CO2 atmosphere with controlled humidity at 37 °C.
- 5.
- A stock solution of test conjugates was made in DMSO and added to the cell culture at desired concentrations. The final culture was not diluted more than 1:1000 with DMSO.
3.2. In Vitro Antimicrobial Assay
Minimum Bactericidal Concentration (MBC)
3.3. In Vitro Antioxidant Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feng, Y.; Spezia, M.; Huang, S.; Yuan, C.; Zeng, Z.; Zhang, L.; Ji, X.; Liu, W.; Huang, B.; Luo, W.; et al. Breast cancer development and progression: Risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes Dis. 2018, 5, 77–106. [Google Scholar] [CrossRef] [PubMed]
- Breast Cancer Facts & Statistics. 2022. Available online: https://www.breastcancer.org/facts-statistics (accessed on 17 January 2023).
- Kumar, D.; Kumar, H.; Kumar, V.; Deep, A.; Sharma, A.; Marwaha, M.G.; Marwaha, R.K. Mechanism-based approaches of 1,3,4 thiadiazole scaffolds as potent enzyme inhibitors for cytotoxicity and antiviral activity. Med. Drug Discov. 2023, 17, 100150. [Google Scholar] [CrossRef]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer. Nat. Rev. Dis. Primers 2019, 5, 66. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global Cancer Incidence and Mortality Rates and Trends—An Update. Cancer Epidemiol. Biomark. Prev. 2016, 25, 16–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mariotto, A.B.; Etzioni, R.; Hurlbert, M.; Penberthy, L.; Mayer, M. Estimation of the Number of Women Living with Metastatic Breast Cancer in the United States. Cancer Epidemiol. Biomark. Prev. 2017, 26, 809–815. [Google Scholar] [CrossRef] [Green Version]
- Winters, S.; Martin, C.; Murphy, D.; Shokar, N.K. Breast Cancer Epidemiology, Prevention, and Screening. Prog. Mol. Biol. Transl. Sci. 2017, 151, 1–32. [Google Scholar] [CrossRef]
- Garcia-Recio, S.; Hinoue, T.; Wheeler, G.L.; Kelly, B.J.; Garrido-Castro, A.C.; Pascual, T.; De Cubas, A.A.; Xia, Y.; Felsheim, B.M.; McClure, M.B.; et al. Multiomics in primary and metastatic breast tumors from the AURORA US network finds microenvironment and epigenetic drivers of metastasis. Nat. Cancer 2022, 4, 128–147. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [Green Version]
- Menzel, A.; Samouda, H.; Dohet, F.; Loap, S.; Ellulu, M.S.; Bohn, T. Common and Novel Markers for Measuring Inflammation and Oxidative Stress Ex Vivo in Research and Clinical Practice—Which to Use Regarding Disease Outcomes? Antioxidants 2021, 10, 414. [Google Scholar] [CrossRef]
- Saeidnia, S.; Abdollahi, M. Antioxidants: Friends or foe in prevention or treatment of cancer: The debate of the century. Toxicol. Appl. Pharmacol. 2013, 271, 49–63. [Google Scholar] [CrossRef]
- Wahabi, K.; Perwez, A.; Rizvi, M.A. Antioxidant in Cancer. In Handbook of Oxidative Stress in Cancer: Therapeutic Aspects; Springer: Singapore, 2022; pp. 1–16. [Google Scholar] [CrossRef]
- Kumar, D.; Kumar, V.; Marwaha, R.; Singh, G. Oxadiazole-An Important Bioactive Scaffold for Drug Discovery and Development Process Against HIV and Cancer—A Review. Curr. Bioact. Compd. 2017, 15, 271–279. [Google Scholar] [CrossRef]
- Kumar, D.; Narang, R.; Judge, V.; Kumar, D.; Narasimhan, B. Antimicrobial evaluation of 4-methylsulfanyl benzylidene/3-hydroxy benzylidene hydrazides and QSAR studies. Med. Chem. Res. 2012, 21, 382–394. [Google Scholar] [CrossRef]
- Kumar, D.; Kumar, V.; Kumar, H.; Deep, A.; Kumar, R. 1,3,4-Oxadiazole as an emerging telomerase inhibitor—A promising anticancer motif. Cancer Adv. 2022, 5, e22018. [Google Scholar] [CrossRef]
- Kumar, D.; Kumar, H.; Deep, A.; Kumar, S.; Marwaha, R.K. Synthesis, Antimicrobial and Antioxidant Potential of 2-(Substitutedphenyl)-3-(5-(Furan-2-Yl)-1,3,4-Oxadiazol-2-Yl) Thiazolidin-4-One Conjugates. J. Pharm. Negat. Results 2022, 13, 3409–3425. [Google Scholar]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Liu, Z.; Ren, Z.; Zhang, J.; Chuang, C.-C.; Kandaswamy, E.; Zhou, T.; Zuo, L. Role of ROS and Nutritional Antioxidants in Human Diseases. Front. Physiol. 2018, 9, 477. [Google Scholar] [CrossRef] [Green Version]
- Stecoza, C.E.; Nitulescu, G.M.; Draghici, C.; Caproiu, M.T.; Olaru, O.T.; Bostan, M.; Mihaila, M. Synthesis and Anticancer Evaluation of New 1,3,4-Oxadiazole Derivatives. Pharmaceuticals 2021, 14, 438. [Google Scholar] [CrossRef]
- Mohan, C.D.; Anilkumar, N.C.; Rangappa, S.; Shanmugam, M.K.; Mishra, S.; Chinnathambi, A.; Alharbi, S.A.; Bhattacharjee, A.; Sethi, G.; Kumar, A.P.; et al. Novel 1,3,4-Oxadiazole Induces Anticancer Activity by Targeting NF-ΚB in Hepatocellular Carcinoma Cells. Front. Oncol. 2018, 8, 42. [Google Scholar] [CrossRef] [Green Version]
- Beniwal, M.; Jain, N.; Jain, S.; Aggarwal, N. Design, synthesis, anticancer evaluation and docking studies of novel 2-(1-isonicotinoyl-3-phenyl-1H-pyrazol-4-yl)-3-phenylthiazolidin-4-one derivatives as Aurora-A kinase inhibitors. BMC Chem. 2022, 16, 61. [Google Scholar] [CrossRef]
- Aggarwal, N.; Jain, S.; Chopra, N. Hybrids of Thiazolidin-4-Ones and 1,3,4-Thiadiazole: Synthesis and Biological Screening of A Potential New Class of Acetylcholinesterae Inhibitors. Biointerface Res. Appl. Chem. 2022, 12, 2800–2812. [Google Scholar] [CrossRef]
- Anthwal, T.; Paliwal, S.; Nain, S. Diverse Biological Activities of 1,3,4-Thiadiazole Scaffold. Chemistry 2022, 4, 1654–1671. [Google Scholar] [CrossRef]
- Kumar, H.; Aggarwal, N.; Marwaha, M.G.; Deep, A.; Chopra, H.; Matin, M.M.; Roy, A.; Bin Emran, T.; Mohanta, Y.K.; Ahmed, R.; et al. Thiazolidin-2,4-Dione Scaffold: An Insight into Recent Advances as Antimicrobial, Antioxidant, and Hypoglycemic Agents. Molecules 2022, 27, 6763. [Google Scholar] [CrossRef] [PubMed]
- Ranganatha, V.L.; Khanum, S.A. Synthesis and evaluation of in vitro antioxidant properties of novel 2,5-disubstituted 1,3,4-oxadiazoles. Russ. J. Bioorg. Chem. 2014, 40, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.T.H.; Choudhary, M.I.; Khan, K.M.; Rani, M.; Rahman, A.U. Structure–activity relationships of tyrosinase inhibitory combinatorial library of 2,5-disubstituted-1,3,4-oxadiazole analogues. Bioorg. Med. Chem. 2005, 13, 3385–3395. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.T.; Hirschbein, B.L.; Cheung, H.; McCarter, J.; Janc, J.W.; Yu, Z.W.; Wesolowski, G. Keto-1,3,4-oxadiazoles as cathepsin K inhibitors. Bioorg. Med. Chem. Lett. 2006, 16, 2909–2914. [Google Scholar] [CrossRef] [PubMed]
- Ningegowda, R.; Chandrashekharappa, S.; Singh, V.; Mohanlall, V.; Venugopala, K.N. Design, synthesis and characterization of novel 2-(2, 3-dichlorophenyl)-5-aryl-1,3,4-oxadiazole derivatives for their anti-tubercular activity against Mycobacterium tuberculosis. Chem. Data Collect. 2020, 28, 100431. [Google Scholar] [CrossRef]
- Gholap, S.; Tambe, M.; Nawale, L.; Sarkar, D.; Sangshetti, J.; Damale, M. Design, synthesis, and pharmacological evaluation of fluorinated azoles as anti-tubercular agents. Arch. Pharm. 2018, 351, 1700294. [Google Scholar] [CrossRef]
- Hamdani, S.S.; Khan, B.A.; Ahmed, M.N.; Hameed, S.; Akhter, K.; Ayub, K.; Mahmood, T. Synthesis, crystal structures, computational studies and α-amylase inhibition of three novel 1,3,4-oxadiazole derivatives. J. Mol. Struct. 2020, 1200, 127085. [Google Scholar] [CrossRef]
- Amir, M.; Javed, S.A.; Kumar, H. Synthesis of Some 1,3,4-Oxadiazole Derivatives as Potential Anti-Inflammatory Agents; CSIR: New Delhi, India, 2007; pp. 1014–1019. [Google Scholar] [CrossRef]
- Abd-Ellah, H.S.; Abdel-Aziz, M.; Shoman, M.E.; Beshr, E.A.; Kaoud, T.S.; Ahmed, A.-S.F. Novel 1,3,4-oxadiazole/oxime hybrids: Synthesis, docking studies and investigation of anti-inflammatory, ulcerogenic liability and analgesic activities. Bioorg. Chem. 2016, 69, 48–63. [Google Scholar] [CrossRef]
- Rathore, A.; Sudhakar, R.; Ahsan, M.J.; Ali, A.; Subbarao, N.; Jadav, S.S.; Umar, S.; Yar, M.S. In vivo anti-inflammatory activity and docking study of newly synthesized benzimidazole derivatives bearing oxadiazole and morpholine rings. Bioorg. Chem. 2017, 70, 107–117. [Google Scholar] [CrossRef]
- Abbas Tabatabai, S.; Lashkari, S.B.; Zarrindast, M.R.; Gholibeikian, M.; Shafiee, A. Design, Synthesis and Anticonvulsant Activity of 2-(2-Phenoxy) Phenyl-1,3,4-Oxadiazole Derivatives. Iran. J. Pharm. Res. 2013, 12, 105–111. [Google Scholar]
- Harish, K.; Mohana, K.; Mallesha, L.; Veeresh, B.; Reddy, B.; Kumar, N. Synthesis and Evaluation of In Vivo Anticonvulsant Activity of 2,5- Disubstituted-1,3,4-Oxadiazole Derivatives. Lett. Drug Des. Discov. 2013, 10, 783–791. [Google Scholar] [CrossRef]
- Bankar, G.R.; Nampurath, G.K.; Nayak, P.G.; Bhattacharya, S. A possible correlation between the correction of endothelial dysfunction and normalization of high blood pressure levels by 1,3,4-oxadiazole derivative, an L-type Ca2+ channel blocker in deoxycorticosterone acetate and NG-nitro-l-arginine hypertensive rats. Chem.-Biol. Interact. 2010, 183, 327–331. [Google Scholar] [CrossRef]
- Niu, P.; Kang, J.; Tian, X.; Song, L.; Liu, H.; Wu, J.; Yu, W.; Chang, J. Synthesis of 2-Amino-1,3,4-Oxadiazoles and 2-Amino-1,3,4-Thiadiazoles via Sequential Condensation and I2-Mediated Oxidative C-O/C-S Bond Formation. J. Org. Chem. 2015, 80, 1018–1024. [Google Scholar] [CrossRef]
- Van Merloo, J.; Kaspers, G.J.; Cloos, J. Cell sensitivity assays: The MTT assay. Methods Mol. Biol. 2011, 731, 237–245.5. [Google Scholar]
- Microbiology: A Laboratory Manual|WorldCat.Org. Available online: https://www.worldcat.org/title/microbiology-a-laboratory-manual/oclc/876232639 (accessed on 19 November 2022).
- Indian Pharmacopoeia Commission. Indian Pharmacopoeia 2007; The Indian Pharmacopoeia Commission Ghaziabad: Ghaziabad, India, 2007; Volume 1, pp. 36–41. [Google Scholar]
- Kumar, D.; Judge, V.; Narang, R.; Sangwan, S.; De Clercq, E.; Balzarini, J.; Narasimhan, B. Benzylidene/2-chlorobenzylidene hydrazides: Synthesis, antimicrobial activity, QSAR studies and antiviral evaluation. Eur. J. Med. Chem. 2010, 45, 2806–2816. [Google Scholar] [CrossRef]
- Sugiharto, S.; Yudiarti, T.; Isroli, I. Assay of Antioxidant Potential of Two Filamentous Fungi Isolated from the Indonesian Fermented Dried Cassava. Antioxidants 2016, 5, 6. [Google Scholar] [CrossRef] [Green Version]





| COMPOUNDs | R1 | R2 |
|---|---|---|
| AZ-1 | Cl | NO2 |
| AZ-2 | Cl | OCH3 |
| AZ-3 | Cl | Br |
| AZ-4 | NO2 | NO2 |
| AZ-5 | NO2 | 3,5 (Cl)2 |
| AZ-6 | OCH3 | 3,5 (Cl)2 |
| AZ-7 | OCH3 | Br |
| AZ-8 | OCH3 | NO2 |
| AZ-9 | OCH3 | Cl |
| AZ-10 | NO2 | Cl |
| AZ-11 | OCH3 | OH |
| AZ-12 | OCH3 | OCH3 |
| AZ-13 | NO2 | OCH3 |
| AZ-14 | Br | OH |
| AZ-15 | Br | NH2 |
| AZ-16 | OCH3 | 3,5 (Cl)2 |
| AZ-17 | OCH3 | NO2 |
| AZ-18 | OCH3 | Br |
| AZ-19 | Cl | NO2 |
| AZ-20 | Br | Cl |
| Antimicrobial Screening | |||||||
|---|---|---|---|---|---|---|---|
| Comp. | Gram-Positive Bacteria(s) | Gram Negative Bacteria(s) | Fungal Strains | ||||
| SA | EF | PA | EC | KP | TH | AN | |
| AZ-1 | 15.43 | 15.43 | 15.43 | 30.86 | 15.43 | 15.43 | 15.43 |
| AZ-2 | 32.05 | 32.05 | 32.05 | 64.10 | 32.05 | 32.05 | 16.03 |
| AZ-3 | 14.24 | 28.47 | 28.47 | 56.95 | 28.47 | 28.47 | 14.24 |
| AZ-4 | 7.51 | 15.02 | 15.02 | 7.51 | 30.05 | 7.51 | 15.02 |
| AZ-5 | 3.55 | 7.10 | 7.10 | 3.55 | 3.55 | 7.10 | 7.10 |
| AZ-6 | 14.71 | 14.71 | 14.71 | 14.71 | 14.71 | 7.35 | 29.41 |
| AZ-7 | 14.37 | 14.37 | 14.37 | 7.18 | 7.18 | 14.37 | 14.37 |
| AZ-8 | 15.59 | 15.59 | 15.59 | 7.79 | 7.79 | 7.79 | 15.59 |
| AZ-9 | 4.00 | 8.01 | 8.01 | 16.03 | 16.03 | 16.03 | 8.01 |
| AZ-10 | 3.85 | 7.72 | 7.72 | 7.72 | 7.72 | 30.86 | 7.72 |
| AZ-11 | 8.40 | 33.60 | 33.60 | 8.40 | 4.19 | 8.40 | 4.19 |
| AZ-12 | 32.38 | 32.38 | 32.38 | 32.38 | 32.38 | 32.38 | 32.38 |
| AZ-13 | 15.59 | 31.17 | 31.17 | 31.17 | 31.17 | 31.17 | 31.17 |
| AZ-14 | 7.15 | 28.60 | 14.30 | 14.30 | 14.30 | 7.15 | 28.60 |
| AZ-15 | 7.17 | 28.67 | 7.17 | 28.67 | 14.33 | 28.67 | 28.67 |
| AZ-16 | 7.09 | 14.17 | 14.17 | 28.34 | 28.34 | 14.17 | 28.34 |
| AZ-17 | 14.99 | 14.99 | 29.98 | 29.98 | 14.99 | 14.99 | 14.99 |
| AZ-18 | 13.86 | 13.86 | 13.86 | 13.86 | 13.86 | 6.93 | 13.86 |
| AZ-19 | 3.71 | 14.85 | 29.69 | 3.71 | 3.71 | 3.71 | 29.69 |
| AZ-20 | 6.87 | 3.43 | 3.43 | 6.87 | 6.87 | 6.87 | 3.43 |
| Std Drug | 4.29 * | 4.29 * | 4.29 * | 4.29 * | 4.29 * | 5.10 ** | 5.10 ** |
| Minimum Bactericidal Concentration (µM) Evaluation | |||||
|---|---|---|---|---|---|
| Comp. | Gram Positive Bacteria (s) | Gram Negative Bacteria (s) | |||
| SA | EF | PA | EC | KP | |
| AZ-1 | 30.86 | 30.86 | 30.86 | 30.86 | 30.86 |
| AZ-2 | 64.10 | 64.10 | 64.10 | 64.10 | 64.10 |
| AZ-3 | 28.47 | 56.95 | 56.95 | 56.95 | 56.95 |
| AZ-4 | 15.02 | 30.05 | 30.05 | 15.02 | 30.05 |
| AZ-5 | 7.10 | 7.10 | 7.10 | 14.40 | 14.20 |
| AZ-6 | 29.42 | 14.71 | 29.42 | 29.42 | 29.42 |
| AZ-7 | 14.37 | 14.37 | 14.37 | 14.37 | 14.37 |
| AZ-8 | 15.59 | 15.59 | 15.59 | 31.18 | 31.18 |
| AZ-9 | 8.01 | 16.03 | 16.03 | 16.03 | 16.03 |
| AZ-10 | 7.72 | 14.30 | 14.30 | 28.67 | 28.67 |
| AZ-11 | 8.40 | 33.60 | 33.60 | 16.80 | 8.40 |
| AZ-12 | 32.38 | 32.38 | 32.38 | 32.38 | 32.38 |
| AZ-13 | 15.59 | 31.17 | 62.34 | 62.34 | 31.17 |
| AZ-14 | 14.30 | 28.60 | 14.30 | 14.30 | 14.30 |
| AZ-15 | 14.33 | 28.67 | 14.33 | 43.10 | 14.33 |
| AZ-16 | 14.17 | 28.34 | 28.34 | 28.34 | 28.34 |
| AZ-17 | 14.99 | 14.99 | 29.98 | 29.98 | 14.99 |
| AZ-18 | 13.86 | 13.86 | 13.86 | 13.86 | 13.86 |
| AZ-19 | 14.85 | 14.85 | 29.69 | 14.85 | 14.85 |
| AZ-20 | 6.87 | 6.87 | 6.87 | 13.74 | 13.74 |
| Amox. | 4.27 | 4.27 | 4.27 | 4.27 | 4.27 |
| Comp. | Conc. | Absorbance | % of Inhibition | IC50 | Comp. | Conc. | Absorbance | % of Inhibition | IC50 |
|---|---|---|---|---|---|---|---|---|---|
| AZ-1 | 0 | 1.74 ± 0.02 | 4.89 | AZ-11 | 0 | 2.44 ± 0.02 | 2.25 | ||
| 0.1 | 1.41 ± 0.03 | 18.77 | 0.1 | 1.76 ± 0.02 | 27.84 | ||||
| 0.5 | 1.31 ± 0.04 | 24.57 | 0.5 | 1.14 ± 0.04 | 53.32 | ||||
| 1 | 1.21 ± 0.05 | 30.40 | 1 | 0.92 ± 0.03 | 62.09 | ||||
| 2 | 0.95 ± 0.03 | 45.08 | 2 | 0.72 ± 0.07 | 70.36 | ||||
| AZ-2 | 0 | 1.67 ± 0.02 | 2.16 | AZ-12 | 0 | 1.91 ± 0.02 | 2.55 | ||
| 0.1 | 1.26 ± 0.04 | 24.65 | 0.1 | 1.44 ± 0.04 | 24.52 | ||||
| 0.5 | 0.94 ± 0.03 | 43.78 | 0.5 | 1.22 ± 0.01 | 35.98 | ||||
| 1 | 0.41 ± 0.03 | 75.43 | 1 | 0.82 ± 0.02 | 57.06 | ||||
| 2 | 0.26 ± 0.02 | 84.55 | 2 | 0.42 ± 0.05 | 78.29 | ||||
| AZ-3 | 0 | 1.66 ± 0.02 | 3.72 | AZ-13 | 0 | 2.65 ± 0.02 | 1.76 | ||
| 0.1 | 1.55 ± 0.04 | 6.69 | 0.1 | 1.87 ± 0.03 | 29.29 | ||||
| 0.5 | 1.11 ± 0.02 | 33.20 | 0.5 | 1.01 ± 0.02 | 61.79 | ||||
| 1 | 0.88 ± 0.02 | 46.84 | 1 | 0.59 ± 0.06 | 77.83 | ||||
| 2 | 0.88 ± 0.01 | 47.23 | 2 | 0.40 ± 0.05 | 84.87 | ||||
| AZ-4 | 0 | 1.73 ± 0.02 | 4.49 | AZ-14 | 0 | 3.40 ± 0.03 | 1.96 | ||
| 0.1 | 1.40 ± 0.02 | 19.31 | 0.1 | 2.27 ± 0.01 | 33.27 | ||||
| 0.5 | 1.22 ± 0.01 | 29.64 | 0.5 | 1.74 ± 0.01 | 48.68 | ||||
| 1 | 1.09 ± 0.02 | 37.07 | 1 | 1.05 ± 0.01 | 69.06 | ||||
| 2 | 0.95 ± 0.05 | 45.43 | 2 | 0.35 ± 0.01 | 89.85 | ||||
| AZ-5 | 0 | 1.73 ± 0.05 | 2.01 | AZ-15 | 0 | 2.83 ± 0.02 | 1.93 | ||
| 0.1 | 1.49 ± 0.03 | 14.18 | 0.1 | 1.87 ± 0.02 | 34.13 | ||||
| 0.5 | 0.66 ± 0.04 | 61.66 | 0.5 | 1.22 ± 0.01 | 57.00 | ||||
| 1 | 0.31 ± 0.04 | 82.32 | 1 | 1.05 ± 0.02 | 63.02 | ||||
| 2 | 0.12 ± 0.07 | 93.29 | 2 | 0.70 ± 0.02 | 75.20 | ||||
| AZ-6 | 0 | 2.30 ± 0.04 | 2.04 | AZ-16 | 0 | 2.07 ± 0.02 | 2.22 | ||
| 0.1 | 1.56 ± 0.04 | 32.29 | 0.1 | 1.63 ± 0.01 | 21.50 | ||||
| 0.5 | 1.09 ± 0.03 | 52.59 | 0.5 | 1.05 ± 0.01 | 49.29 | ||||
| 1 | 0.90 ± 0.02 | 60.80 | 1 | 0.68 ± 0.02 | 67.35 | ||||
| 2 | 0.37 ± 0.03 | 84.12 | 2 | 0.32 ± 0.02 | 84.55 | ||||
| AZ-7 | 0 | 1.89 ± 0.04 | 2.70 | AZ-17 | 0 | 1.72 ± 0.02 | 2.91 | ||
| 0.1 | 1.27 ± 0.04 | 33.05 | 0.1 | 1.41 ± 0.01 | 18.45 | ||||
| 0.5 | 1.08 ± 0.03 | 42.97 | 0.5 | 1.10 ± 0.01 | 36.49 | ||||
| 1 | 0.89 ± 0.03 | 53.04 | 1 | 0.78 ± 0.01 | 54.50 | ||||
| 2 | 0.70 ± 0.06 | 62.85 | 2 | 0.61 ± 0.02 | 64.67 | ||||
| AZ-8 | 0 | 2.65 ± 0.02 | 1.76 | AZ-18 | 0 | 1.78 ± 0.02 | 2.89 | ||
| 0.1 | 1.87 ± 0.03 | 29.29 | 0.1 | 1.49 ± 0.02 | 16.13 | ||||
| 0.5 | 1.01 ± 0.02 | 61.79 | 0.5 | 1.09 ± 0.01 | 38.57 | ||||
| 1 | 0.59 ± 0.07 | 77.83 | 1 | 0.91 ± 0.02 | 48.53 | ||||
| 2 | 0.40 ± 0.04 | 84.87 | 2 | 0.54 ± 0.02 | 69.63 | ||||
| AZ-9 | 0 | 2.84 ± 0.03 | 1.62 | AZ-19 | 0 | 2.29 ± 0.02 | 2.70 | ||
| 0.1 | 1.80 ± 0.03 | 36.80 | 0.1 | 1.60 ± 0.02 | 30.18 | ||||
| 0.5 | 1.09 ± 0.03 | 61.50 | 0.5 | 1.17 ± 0.02 | 48.84 | ||||
| 1 | 0.82 ± 0.05 | 70.99 | 1 | 0.96 ± 0.03 | 58.12 | ||||
| 2 | 0.27 ± 0.05 | 90.56 | 2 | 0.12 ± 0.04 | 94.76 | ||||
| AZ-10 | 0 | 2.89 ± 0.04 | 1.03 | AZ-20 | 0 | 2.28 ± 0.02 | 2.71 | ||
| 0.1 | 1.40 ± 0.02 | 51.46 | 0.1 | 1.81 ± 0.01 | 20.44 | ||||
| 0.5 | 1.07 ± 0.02 | 62.87 | 0.5 | 1.64 ± 0.03 | 28.05 | ||||
| 1 | 0.88 ± 0.03 | 69.64 | 1 | 1.02 ± 0.02 | 55.27 | ||||
| 2 | 0.20 ± 0.08 | 93.15 | 2 | 0.48 ± 0.03 | 79.11 | ||||
| Dox. (Ref. Drug) | 0 | 3.28 ± 0.02 | 0.05 | ||||||
| 0.1 | 1.73 ± 0.01 | 48.88 | |||||||
| 0.5 | 1.03 ± 0.02 | 65.58 | |||||||
| 1 | 0.76 ± 0.02 | 83.12 | |||||||
| 2 | 0.09 ± 0.03 | 98.76 | |||||||
| 3-chloro-1-(5-(4-chlorophenyl)-1,3,4-oxadiazol-2-yl)-4-(4-methoxyphenyl)azetidin-2-one. Mol. Formula C18H13Cl2N3O3, Mol.Wt: 390, Color Dark Yellowish, % Yield 59%, M. Pt 243–245 °C, Rf Value 0.7 *. IR (KBr pellets) cm−1: (C-H stretching, aromatic), 3077.30, (C=O stretching, azetidine ring), 1730.62 (C=N stretching, imine group), 1644.93, (C=C stretching, aromatic ring), 1526.07, (C-O-C stretching, Oxadiazole ring), 1022.24, (C=C stretching, methylene group), 1596.85, (N=O stretching, Nitro Group), 1461.89, (C-Cl stretching, azetidine ring). 710.86, (C-Cl stretching, benzene ring). 811.7. 1H NMR (DMSO-d6, 400 MHz, δ ppm) δ 7.50–7.46 (m, 2H), 7.44–7.40 (m, 2H), 7.28–7.24 (m, 2H), 6.93–6.89 (m, 2H), 5.97 (s, 1H), 5.44 (s, 1H), 3.83–3.79 (m, 3H). CHN (Elemental analysis) calculated: C, 55.40; H, 3.36; N, 10.77. Found: C, 56.01; H, 3.06; N, 09.17. |
| 4-(4-bromophenyl)-3-chloro-1-(5-(4-chlorophenyl)-1,3,4-oxadiazol-2-yl)azetidin-2-one. Mol. Formula C17H10BrCl2N3O2, Mol.Wt: 439, Color Reddish, % Yield 47%, M. Pt 213–215 °C, Rf Value 0.5 *. IR (KBr pellets) cm−1: (C-H stretching, aromatic), 3104.92, (C=O stretching, azetidine ring), 1719.27 (C=N stretching, imine group), 1640.54, (C=C stretching, aromatic ring), 1566.02, (C-O-C stretching, Oxadiazole ring), 1077.07, (C=C stretching, methylene group), 1623.75, (N=O stretching, Nitro Group), 1482.73, (C-N stretching, Oxadiazole ring), 1623.75, (C-Cl stretching, azetidine ring). 759.76, (C-Cl stretching, benzene ring). 591.54. 1H NMR (DMSO-d6, 400 MHz, δ ppm) δ 7.50–7.46 (m, 4H), 7.44–7.40 (m, 2H), 7.20–7.16 (m, 2H), 6.03 (s, 1H), 5.44 (s, 1H). CHN (Elemental analysis) calculated: C, 46.50; H, 2.30; N, 9.57. Found: C, 45.78; H, 2.10; N, 9.37. |
| 3-chloro-4-(4-nitrophenyl)-1-(5-(4-nitrophenyl)-1,3,4-oxadiazol-2-yl)azetidin-2-one, Mol. Formula C17H10ClN5O6, Mol.Wt: 416, Color Light green, % Yield 61%, M. Pt 260–262 °C, Rf Value 0.7 * (IR (KBr pellets) cm−1: (C-H stretching, aromatic), 3094.57, (C=O stretching, azetidine ring), 1719.01 (C=N stretching, imine group), 1624.49, (C=C stretching, aromatic ring), 1513.82, (C-O-C stretching, Oxadiazole ring), 994.19, (C=C stretching, methylene group), 1513.82, (N=O stretching, Nitro Group), 1478.35, (C-Cl stretching, azetidine ring). 817.24 cm−1. 1H NMR (DMSO-d6, 400 MHz, δ ppm) δ 8.34–8.30 (m, 2H), 8.22–8.18 (m, 2H), 7.88–7.84 (m, 2H), 7.59–7.55 (m, 2H), 5.99 (s, 1H), 5.44 (s, 1H).). CHN (Elemental analysis) calculated: C, 49.11; H, 2.42; N, 16.85. Found: C, 48.91; H, 2.12; N, 16.05. |
| 3-chloro-4-(3,5-dichlorophenyl)-1-(5-(4-nitrophenyl)-1,3,4-oxadiazol-2-yl)azetidin-2-one. Mol. Formula C17H9Cl3N4O4, Mol.Wt: 440, Color Yellowish, % Yield 78%, M. Pt 216–218 °C, Rf Value 0.8 *. (IR (KBr pellets) cm−1: (C-H stretching, aromatic), 3077.02, (C=O stretching, azetidine ring), 1717.83 (C=N stretching, imine group), 1638.32, (C=C stretching, aromatic ring), 1541.00, (C-O-C stretching, Oxadiazole ring), 994.83, (C=C stretching, methylene group), 1514.92, (N=O stretching, Nitro Group), 1478.35, (C-Cl stretching, azetidine ring). 711.63, (C-Cl stretching, Benzene ring). 581.02. 1H NMR (DMSO-d6, 400 MHz, δ ppm) δ 8.34–8.30 (m, 2H), 7.88–7.84 (m, 2H), 7.33–7.26 (m, 3H), 5.92 (s, 1H), 5.44 (s, 1H).). CHN (Elemental analysis) calculated: C, 46.44; H, 2.06; N, 12.74. Found: C, 46.04; H, 2.26; N, 12.34. |
| 3-chloro-4-(3,5-dichlorophenyl)-1-(5-(4-methoxyphenyl)-1,3,4-oxadiazol-2-yl)azetidin-2-one. Mol. Formula C18H12Cl3N3O3, Mol.Wt: 425, Color White, % Yield 56%, M. Pt 273–275 °C Rf Value 0.7 *. IR (KBr pellets) cm−1: (C-H stretching, aromatic), 3080.90, (C=O stretching, azetidine ring), 1696.15 (C=N stretching, imine group), 1642.73, (C=C stretching, aromatic ring), 1604.64, (C-O-C stretching, Oxadiazole ring), 991.01, (C-N stretching, imine group), 1241.96, (N=O stretching, Nitro Group), 1511.02, (C-Cl stretching, azetidine ring). 609.30, (C-Cl stretching, benzene ring). 824.03. 1H NMR (DMSO-d6, 400 MHz, δ ppm) δ 7.60–7.56 (m, 2H), 7.33–7.26 (m, 3H), 7.06–7.02 (m, 2H), 5.93 (s, 1H), 5.44 (s, 1H), 3.83–3.79 (m, 3H). CHN (Elemental analysis) calculated: C, 50.91; H, 2.85; N, 9.90. Found: C,49.89; H, 2.45; N, 9.67. |
| 4-(4-bromophenyl)-3-chloro-1-(5-(4-methoxyphenyl)-1,3,4-oxadiazol-2-yl)azetidin-2-one, Mol. Formula C18H13BrClN3O3, Mol.Wt: 435, Color Reddish, % Yield 70%, M. Pt 256–258 °C, Rf Value 0.8 **. IR (KBr pellets) cm−1: (C-H stretching, aromatic), 3090.44, (C=O stretching, azetidine ring), 1728.20 (C=N stretching, imine group), 1628.34, (C=C stretching, aromatic ring), 1600.08, (C-O-C stretching, Oxadiazole ring), 993.30, (C=C stretching, methylene group), 1646.09, (C-N stretching, imine group), 1230.74, (N=O stretching, Nitro Group), 1525.68 (C-N stretching, Oxadiazole ring), 1160.29, (C-Cl stretching, azetidine ring). 833.21, (C-Br stretching, benzene ring). 535.73. 1H NMR (DMSO-d6, 400 MHz, δ ppm) δ 7.60–7.56 (m, 2H), 7.49–7.45 (m, 2H), 7.22–7.18 (m, 2H), 7.06–7.02 (m, 2H), 5.89 (s, 1H), 5.44 (s, 1H), 3.83–3.79 (m, 3H).). CHN (Elemental analysis) calculated: C, 49.74; H, 3.01; N, 9.67. Found: C, 49.14; H, 2.71; N, 9.27. |
| 3-chloro-1-(5-(4-methoxyphenyl)-1,3,4-oxadiazol-2-yl)-4-(4-nitrophenyl)azetidin-2-one, Mol. Formula C18H13ClN4O5, Mol.Wt: 401, Color Cream, % Yield 49%, M. Pt 210–203 °C, Rf Value 0.7 *. IR (KBr pellets) cm−1: (C-H stretching, aromatic), 3094.12, (C=O stretching, azetidine ring), 1724.33, C=N stretching, imine group), 1627.23, (C=C stretching, aromatic ring), 1519.86, (C-O-C stretching, Oxadiazole ring), 1466.14, (C=C stretching, methylene group), 1590.50, (C-N stretching, imine group), 1083.45, (N=O stretching, Nitro Group), 1519.86, (C-Cl stretching, azetidine ring). 831.78, (C-Cl stretching, benzene ring). 715 1H NMR (DMSO-d6, 400 MHz, δ ppm) δ 8.22–8.18 (m, 2H), 7.60–7.55 (m, 4H), 7.06–7.02 (m, 2H), 6.00 (s, 1H), 5.44 (s, 1H), 3.83–3.79 (m, 3H). CHN (Elemental analysis) calculated: C, 53.94; H, 3.27; N, 13.98. Found: C, 53.14; H, 3.07; N, 13.38. |
| 3-chloro-4-(4-chlorophenyl)-1-(5-(4-methoxyphenyl)-1,3,4-oxadiazol-2-yl)azetidin-2-one. Mol. Formula C18H13Cl2N3O3, Mol.Wt: 390, Color Brownish, % Yield 54%, M. Pt 226–228 °C, Rf Value 0.5 **. IR (KBr pellets) cm−1: (C-H stretching, aromatic), 3080.56, (C=O stretching, azetidine ring), 1696.14 (C=N stretching, imine group), 1651.18, (C=C stretching, aromatic ring), 1603.86, (C-O-C stretching, Oxadiazole ring), 1019.52, (C-N stretching, imine group), 1163.73, (N=O stretching, Nitro Group), 1511.19, (C-N stretching, Oxadiazole ring), 1242.50, (C-Cl stretching, azetidine ring). 824.64, (C-Cl stretching, benzene ring). 610.60. 1H NMR (DMSO-d6, 400 MHz, δ ppm) δ 7.60–7.56 (m, 2H), 7.33–7.29 (m, 2H), 7.27–7.23 (m, 2H), 7.06–7.02 (m, 2H), 5.89 (s, 1H), 5.44 (s, 1H), 3.83–3.79 (m, 3H). CHN (Elemental analysis) calculated: C, 55.40; H, 3.36; N, 10.77. Found: C, 54.99; H, 3.06; N, 10.17. |
| 3-chloro-4-(4-chlorophenyl)-1-(5-(4-nitrophenyl)-1,3,4-oxadiazol-2-yl)azetidin-2-one,. Mol. Formula C17H10Cl2N4O4, Mol.Wt: 405, Color Cream, % Yield 78%, M. Pt 267–269 °C, Rf Value 0.6 *. IR (KBr pellets) cm−1: (C-H stretching, aromatic), 3088.79, (C=O stretching, azetidine ring), 1727.68 (C=N stretching, imine group), 1646.65, (C=C stretching, aromatic ring), 1628.03, (C-O-C stretching, Oxadiazole ring), 1011.50, (C=C stretching, methylene group), 1598.06, (C-N stretching, imine group), 1230.57, (N=O stretching, Nitro Group), 1525.01, (C-Cl stretching, azetidine ring). 832.40. 1H NMR (DMSO-d6, 400 MHz, δ ppm) δ 8.34–8.30 (m, 2H), 7.88–7.84 (m, 2H), 7.33–7.29 (m, 2H), 7.27–7.23 (m, 2H), 5.88 (s, 1H), 5.44 (s, 1H).). CHN (Elemental analysis) calculated: C, 50.39; H, 2.49; N, 13.83. Found: C, 50.11; H, 2.07; N, 13.22. |
| 3-chloro-4-(4-hydroxyphenyl)-1-(5-(4-methoxyphenyl)-1,3,4-oxadiazol-2-yl)azetidin-2-one. Mol. Formula C18H14ClN3O4, Mol.Wt: 372. Color Cream, % Yield 67%, M. Pt 213–215 °C, Rf Value 0.6 **. IR (KBr pellets) cm−1: (C-H stretching, aromatic), 3047.54, (C-OH stretching, benzene ring). 3369.92 (C=O stretching, azetidine ring), 1708.16, (C=N stretching, imine group), 1672.71, (C=C stretching, aromatic ring), 1543.59, (C-O-C stretching, Oxadiazole ring), 1078.82, (C-N stretching, imine group), 1153.83, (C-N stretching, Oxadiazole ring), 1272.32, (C-Cl stretching, azetidine ring). 616.31. 1H NMR (DMSO-d6, 400 MHz, δ ppm) δ 7.60–7.56 (m, 2H), 7.16–7.12 (m, 2H), 7.06–7.02 (m, 2H), 6.80–6.76 (m, 2H), 5.82 (s, 1H), 5.44 (s, 1H), 3.83–3.79 (m, 3H), 3.68 (s, 1H). CHN (Elemental analysis) calculated: C, 58.15; H, 3.80; N, 11.30. Found: C, 57.73; H, 3.43; N, 11.01. |
| 3-chloro-4-(4-methoxyphenyl)-1-(5-(4-methoxyphenyl)-1,3,4-oxadiazol-2-yl)azetidin-2-one, Mol. Formula C19H16ClN3O4 Mol.Wt: 386. Color White, % Yield 61%, M. Pt 243–245 °C, Rf Value0.6 *. IR (KBr pellets) cm−1: (C-H stretching, aromatic), 3120.65, (C=O stretching, azetidine ring), 1727.66 (C=N stretching, imine group), 1644.26, (C=C stretching, aromatic ring), 1610.28, (C-O-C stretching, Oxadiazole ring), 1020.66, (C-N stretching, imine group), 1120.32, (N=O stretching, Nitro Group), 1542.66, (C-Cl stretching, azetidine ring). 767.53. 1H NMR (DMSO-d6, 400 MHz, δ ppm) δ 7.60–7.56 (m, 2H), 7.30–7.26 (m, 2H), 7.06–7.02 (m, 2H), 6.92–6.88 (m, 2H), 5.82 (s, 1H), 5.44 (s, 1H), 3.83–3.79 (m, 6H). CHN (Elemental analysis) calculated: C, 59.15; H, 4.18; N, 10.89. Found: C, 58.92; H, 4.02; N, 10.11. |
| 3-chloro-4-(4-methoxyphenyl)-1-(5-(4-nitrophenyl)-1,3,4-oxadiazol-2-yl)azetidin-2-one. Mol. Formula C18H13ClN4O5, Mol.Wt: 401, Color Brown, % Yield 59%, M. Pt 219–221 °C, Rf Value 0.7 *. IR (KBr pellets) cm−1: (C-H stretching, aromatic), 3056, (C=O stretching, azetidine ring), 1717.65 (C=N stretching, imine group), 1638.15, (C=C stretching, aromatic ring), 1598.12, (C-O-C stretching, Oxadiazole ring), 1092.55, (C-N stretching, imine group), 1152.79, (N=O stretching, Nitro Group), 1500.83, (C-N stretching, Oxadiazole ring), 1179.03, (C-Cl stretching, azetidine ring). 753.62. 1H NMR (DMSO-d6, 400 MHz, δ ppm) δ 8.34–8.30 (m, 2H), 7.88–7.84 (m, 2H), 7.30–7.26 (m, 2H), 6.92–6.88 (m, 2H), 5.81 (s, 1H), 5.44 (s, 1H), 3.83–3.79 (m, 3H). CHN (Elemental analysis) calculated: C, 53.94; H, 3.27; N, 13.98. Found: C, 53.12; H, 3.32; N, 13.16. |
| 1-(5-(4-bromophenyl)-1,3,4-thiadiazol-2-yl)-3-chloro-4-(4-hydroxyphenyl)azetidin-2-one, Mol. Formula C17H11BrClN3O2S, Mol.Wt:: 437, Color Brown, % Yield 56%, M. Pt 278–280 °C, Rf Value 0.6 *. IR (KBr pellets) cm−1: (C-OH stretching, benzene ring). 3363.10 cm−1 (C=O stretching, azetidine ring), 1746.76 (C=N stretching, imine group), 1698.51, (C=C stretching, aromatic ring), 1675.56, (C-S-C stretching, Thiadiazole ring), 688.12, (C=C stretching, methylene group), 1617.24, (C-N stretching, imine group), 1205.08, (N=O stretching, Nitro Group), (C-N stretching, Oxadiazole ring), 1374.44 cm−1, (C-Cl stretching, azetidine ring). 682.89, (C-Cl stretching, benzene ring) 715. 1H NMR (DMSO-d6, 400 MHz, δ ppm) δ 7.61–7.57 (m, 2H), 7.48–7.44 (m, 2H), 7.13–7.09 (m, 2H), 6.80–6.76 (m, 2H), 5.86 (s, 1H), 5.44 (s, 1H), 3.66 (s, 1H).). CHN (Elemental analysis) calculated: C, 46.76; H, 2.54; N, 9.62. Found: C, 46.31; H, 2.29; N, 9.77. |
| 4-(4-aminophenyl)-1-(5-(4-bromophenyl)-1,3,4-thiadiazol-2-yl)-3-chloroazetidin-2-one, Mol. Formula C17H12BrClN4OS, Mol.Wt:: 436, Color White, % Yield 71%, M. Pt 212–214 °C, Rf Value 0.7 ** IR (KBr pellets) cm−1: (C-H stretching, aromatic), 3099.76, (C=O stretching, azetidine ring), 1717.65 (C=N stretching, imine group), 1638.31, (C=C stretching, aromatic ring), 1595.68, (C-S-C stretching, Thiadiazole ring), 689.88, (C=C stretching, methylene group), 1660, (C-N stretching, imine group), 1228.95, (N=O stretching, Nitro Group), 1514.26, (C-N stretching, Oxadiazole ring), 1170.60, (C-Cl stretching, azetidine ring). 711.65, (C-Br stretching, benzene ring) 581.74. 1H NMR (DMSO-d6, 400 MHz, δ ppm) δ 7.61–7.57 (m, 2H), 7.48–7.44 (m, 2H), 7.09–7.05 (m, 2H), 6.62–6.58 (m, 2H), 5.81 (s, 1H), 5.44 (s, 1H), 3.68–3.65 (m, 2H).). CHN (Elemental analysis) calculated: C, 46.86; H, 2.78; N, 12.86. Found: C, 46.23; H, 2.11; N, 12.34. |
| 3-chloro-4-(3,5-dichlorophenyl)-1-(5-(4-methoxyphenyl)-1,3,4-thiadiazol-2-yl)azetidin-2-one, Mol. Formula C18H12Cl3N3O2S, Mol.Wt:: 441, Color Orange, % Yield 61%, M. Pt 229–231 °C. Rf Value 0.7 *. IR (KBr pellets) cm−1: (C-H stretching, aromatic), 3026.81, (C=O stretching, azetidine ring), 1770.90 (C=N stretching, imine group), 1717.54, (C=C stretching, aromatic ring), 1583.98, (C-S-C stretching, Thiadiazole ring), 687.78, (C-N stretching, imine group), 1147.52, (N=O stretching, Nitro Group), 1505.88, (C-N stretching, Oxadiazole ring), 1252.85, (C-Cl stretching, azetidine ring). 614.10, (C-Cl stretching, benzene ring). 748.51. 1H NMR (DMSO-d6, 400 MHz, δ ppm) δ 7.59–7.55 (m, 2H), 7.33–7.26 (m, 3H), 7.06–7.02 (m, 2H), 5.94 (s, 1H), 5.44 (s, 1H), 3.82–3.78 (m, 3H).). CHN (Elemental analysis) calculated: C, 49.06; H, 2.74; N, 9.53. Found: C, 48.73; H, 2.14; N, 9.13. |
| 3-chloro-1-(5-(4-methoxyphenyl)-1,3,4-thiadiazol-2-yl)-4-(4-nitrophenyl)azetidin-2-one, Mol. Formula C18H13ClN4O4S, Mol.Wt: 417, Color White, % Yield 42%, M. Pt 256–258 °C, Rf Value 0.6 * IR (KBr pellets) cm−1: (C-H stretching, aromatic), 3081.85, (C-H stretching, aliphatic), 2927.47, (C=O stretching, azetidine ring), 1724.03, (C=N stretching, imine group), 1642.73, (C=C stretching, aromatic ring), 1625.24, (C-S-C stretching, Thiadiazole ring), 686.12, (C-N stretching, imine group), 1202.49, (N=O stretching, Nitro Group), 1514.13, (C-N stretching, Oxadiazole ring), 1232.21, (C-Cl stretching, azetidine ring). 569.75. 1H NMR (DMSO-d6, 400 MHz, δ ppm) δ 8.22–8.18 (m, 2H), 7.59–7.55 (m, 4H), 7.06–7.02 (m, 2H), 6.00 (s, 1H), 5.44 (s, 1H), 3.82–3.78 (m, 3H).). CHN (Elemental analysis) calculated: C, 51.87; H, 3.14; N, 13.44. Found: C, 51.24; H, 3.02; N, 13.09 |
| 4-(4-bromophenyl)-3-chloro-1-(5-(4-methoxyphenyl)-1,3,4-thiadiazol-2-yl)azetidin-2-one. Mol. Formula C18H13BrClN3O2S, Mol.Wt: 451, Color Brown, % Yield 59%, M. Pt 232–234 °C. Rf Value 0.6 *. IR (KBr pellets) cm−1: (C-H stretching, aromatic), 3251.73, (C=O stretching, azetidine ring), 1691.23, (C=N stretching, imine group), 1652.07, (C=C stretching, aromatic ring), 1593.04, (C-S-C stretching, Thiadiazole ring), 690.03, (C-N stretching, imine group), 1159.74, (N=O stretching, Nitro Group), 1444.77, (C-N stretching, Oxadiazole ring), 1333.87, (C-Cl stretching, azetidine ring). 682.38, (C-Br stretching, benzene ring). 539.20. 1H NMR (DMSO-d6, 400 MHz, δ ppm) δ 7.59–7.55 (m, 2H), 7.49–7.45 (m, 2H), 7.22–7.18 (m, 2H), 7.06–7.02 (m, 2H), 5.89 (s, 1H), 5.44 (s, 1H), 3.82–3.78 (m, 3H). CHN (Elemental analysis) calculated: C, 47.97; H, 2.91; N, 9.32. Found: C, 47.23; H, 2.11; N, 9.05. |
| 3-chloro-1-(5-(4-chlorophenyl)-1,3,4-thiadiazol-2-yl)-4-(4-nitrophenyl)azetidin-2-one.Mol. Formula C17H10Cl2N4O3S, Mol. Wt: 421, Color Light red, % Yield 43%, M. Pt 199–201 °C, Rf Value 0.7 *. IR (KBr pellets) cm−1: (C-H stretching, aromatic), 3056.78, (C=O stretching, azetidine ring), 1684.47 (C=N stretching, imine group), 1579.22, (C=C stretching, aromatic ring), 1517, (C-S-C stretching, Thiadiazole ring), 633.15, (C=C stretching, methylene group), 1660, (C-N stretching, imine group), (N=O stretching, Nitro Group), 1419.43, (C-N stretching, Oxadiazole ring), 1296.91 (C-Cl stretching, azetidine ring). 766.98, (C-Cl stretching, benzene ring). 823.45. 1H NMR (DMSO-d6, 400 MHz, δ ppm) δ 8.22–8.18 (m, 2H), 7.59–7.51 (m, 4H), 7.46–7.42 (m, 2H), 5.99 (s, 1H), 5.44 (s, 1H)). CHN (Elemental analysis) calculated: C, 48.47; H, 2.39; N, 13.30. Found: C, 48.22; H, 2.01; N, 13.11. |
| 1-(5-(4-bromophenyl)-1,3,4-thiadiazol-2-yl)-3-chloro-4-(4-chlorophenyl)azetidin-2-one, Mol. Formula C17H10BrCl2N3OS, Mol. Wt: 455, Color Brownish, % Yield 43%, M. Pt 240–242 °C, Rf Value 0.5 *. IR (KBr pellets) cm−1: (C-H str, aromatic), 3088.54 (C=O stretching, azetidine ring), 1710.74 (C=N stretching, imine group), 1673.39, (C=C stretching, aromatic ring), (C-S-C stretching, Thiadiazole ring), 689.16, (C-N stretching, imine group), 1278.44, (N=O stretching, Nitro Group), 1543.61, (C-N stretching, Oxadiazole ring), 1175.76, (C-Cl stretching, azetidine ring). 616.78, (C-Br stretching, benzene ring). 519.45, (C-Cl stretching, benzene ring). 759.66. 1H NMR (DMSO-d6, 400 MHz, δ ppm) δ 7.61–7.57 (m, 2H), 7.48–7.44 (m, 2H), 7.34–7.30 (m, 2H), 7.24–7.20 (m, 2H), 5.93 (s, 1H), 5.44 (s, 1H)). CHN (Elemental analysis) calculated: C, 44.86; H, 2.21; N, 9.23. Found: C, 44.02; H, 2.11; N, 9.01. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, D.; Aggarwal, N.; Kumar, V.; Kumar, H.; Deep, A.; Bibi, S.; Chopra, H.; Kumar Marwaha, R.; Alshammari, A.; Alharbi, M.; et al. Synthesis, Anticancer, Antimicrobial and Antioxidant Potential of Novel 4-(Substituted phenyl-1,3,4-oxadiazol/thiadiazol-2-yl)-4-(4-substituted phenyl) Azetidin-2-One Derivatives. Pharmaceuticals 2023, 16, 517. https://doi.org/10.3390/ph16040517
Kumar D, Aggarwal N, Kumar V, Kumar H, Deep A, Bibi S, Chopra H, Kumar Marwaha R, Alshammari A, Alharbi M, et al. Synthesis, Anticancer, Antimicrobial and Antioxidant Potential of Novel 4-(Substituted phenyl-1,3,4-oxadiazol/thiadiazol-2-yl)-4-(4-substituted phenyl) Azetidin-2-One Derivatives. Pharmaceuticals. 2023; 16(4):517. https://doi.org/10.3390/ph16040517
Chicago/Turabian StyleKumar, Davinder, Navidha Aggarwal, Virender Kumar, Harsh Kumar, Aakash Deep, Shabana Bibi, Hitesh Chopra, Rakesh Kumar Marwaha, Abdulrahman Alshammari, Metab Alharbi, and et al. 2023. "Synthesis, Anticancer, Antimicrobial and Antioxidant Potential of Novel 4-(Substituted phenyl-1,3,4-oxadiazol/thiadiazol-2-yl)-4-(4-substituted phenyl) Azetidin-2-One Derivatives" Pharmaceuticals 16, no. 4: 517. https://doi.org/10.3390/ph16040517
APA StyleKumar, D., Aggarwal, N., Kumar, V., Kumar, H., Deep, A., Bibi, S., Chopra, H., Kumar Marwaha, R., Alshammari, A., Alharbi, M., & Hayee, A. (2023). Synthesis, Anticancer, Antimicrobial and Antioxidant Potential of Novel 4-(Substituted phenyl-1,3,4-oxadiazol/thiadiazol-2-yl)-4-(4-substituted phenyl) Azetidin-2-One Derivatives. Pharmaceuticals, 16(4), 517. https://doi.org/10.3390/ph16040517







