Natural Ingredients to Improve Immunity
Abstract
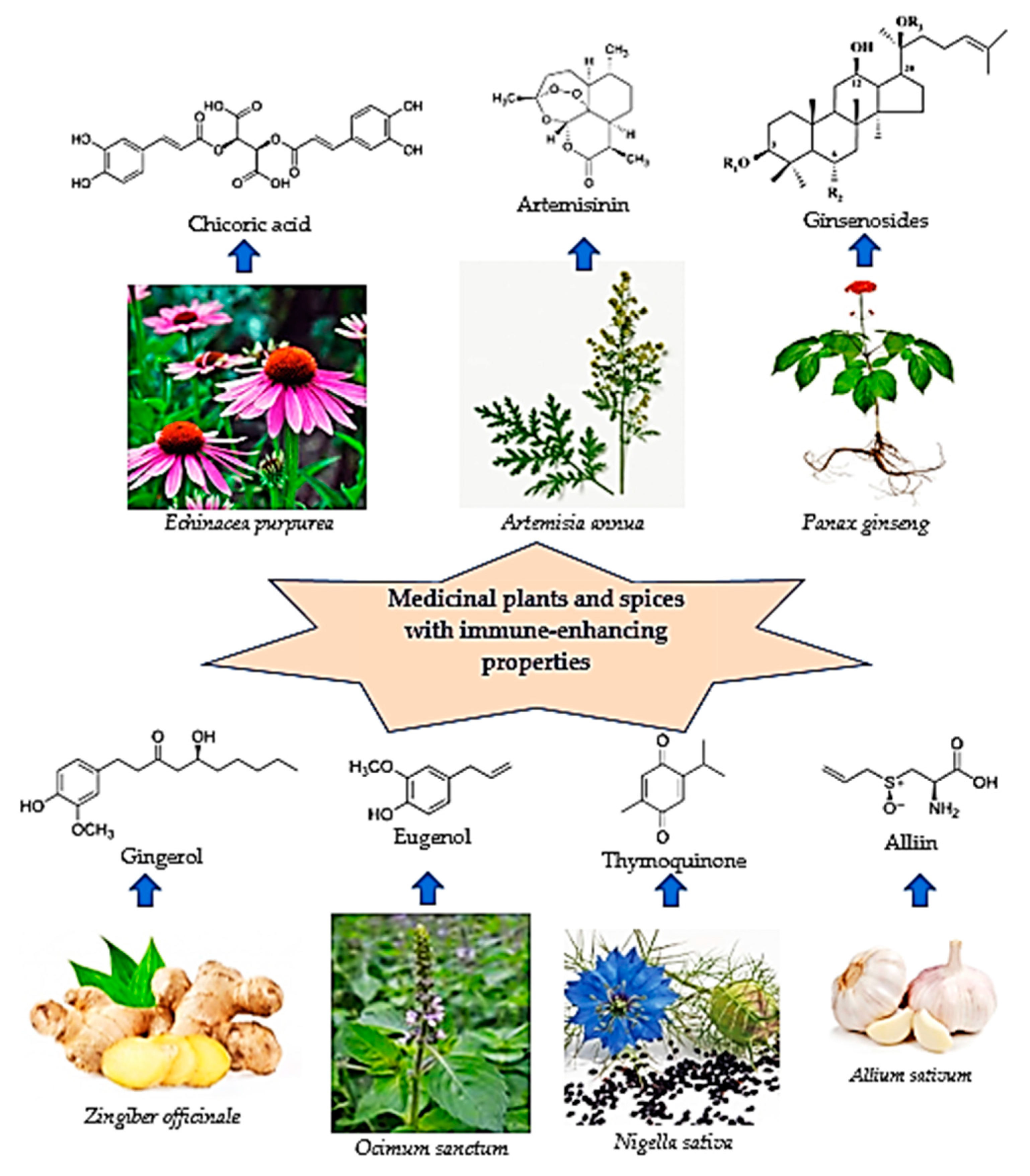
:1. Introduction
2. Medicinal Plants as Immunomodulators
2.1. Coneflower (Echinacea ssp.)
2.2. Artemisia afra/annua
2.3. Ginseng (Panax ginseng)
2.4. Spices
3. Selected Natural Compounds with Immunomodulating Properties
3.1. Quercetin
3.2. Propolis
3.3. Glucans
3.4. Melatonin
4. Micronutrients: Vitamins and Minerals
4.1. Vitamin C
4.2. Vitamin D
4.3. Folic Acid
4.4. Magnesium
4.5. Zinc
4.6. Selenium
5. Probiotics, Prebiotics, Synbiotics, and Immunity
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Catanzaro, M.; Corsini, E.; Rosini, M.; Racchi, M.; Lanni, C. Immunomodulators Inspired by Nature: A Review on Curcumin and Echinacea. Molecules 2018, 23, 2778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parkin, J.; Cohen, B. An overview of the immune system. Lancet 2001, 357, 1777–1789. [Google Scholar] [CrossRef] [PubMed]
- Raducanu, A.E.; Tihauan, B.M.; Marinas, I.C.; Ciuperca, O.T.; Tebrencu, C.E.; Ionescu, E.; Onisei, T. The Biological Effects of Novel Nutraceuticals with Curcuminoids and Other Plant-Derived Immunomodulators and Pre-Probiotics. Pharmaceutics 2021, 13, 666. [Google Scholar] [CrossRef]
- Tieu, S.; Charchoglyan, A.; Wagter-Lesperance, L.; Karimi, K.; Bridle, B.; Karrow, N.; Mallard, B. Immunoceuticals: Harnessing Their Immunomodulatory Potential to Promote Health and Wellness. Nutrients 2022, 14, 4075. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.S.; Warrington, R.; Watson, W.; Kim, H.L. An introduction to immunology and immunopathology. Allergy Asthma Clin. Immunol. 2018, 14 (Suppl. S2), 49. [Google Scholar] [CrossRef] [Green Version]
- Sultan, M.; Buttxs, M.; Qayyum, M.; Suleria, H. Immunity: Plants as Effective Mediators. Crit. Rev. Food Sci. Nutr. 2014, 54, 1298–1308. [Google Scholar] [CrossRef]
- Vishwakarma, S.; Panigrahi, C.; Barua, S.; Sahoo, M.; Mandliya, S. Food nutrients as inherent sources of immunomodulation during COVID-19 pandemic. Lebensm.-Wiss. Und-Technol. 2022, 158, 113154. [Google Scholar] [CrossRef]
- Wieczorek, P.P.; Hudz, N.; Yezerska, O.; Horcinova-Sedlackova, V.; Shanaida, M.; Korytniuk, O.; Jasicka-Misiak, I. Chemical Variability and Pharmacological Potential of Propolis as a Source for the Development of New Pharmaceutical Products. Molecules 2022, 27, 1600. [Google Scholar] [CrossRef]
- Ilina, T.; Kashpur, N.; Granica, S.; Bazylko, A.; Shinkovenko, I.; Kovalyova, A.; Goryacha, O.; Koshovyi, O. Phytochemical Profiles and In Vitro Immunomodulatory Activity of Ethanolic Extracts from Galium aparine L. Plants 2019, 8, 541. [Google Scholar] [CrossRef] [Green Version]
- Mahima; Rahal, A.; Deb, R.; Latheef, S.K.; Abdul Samad, H.; Tiwari, R.; Verma, A.K.; Kumar, A.; Dhama, K. Immunomodulatory and therapeutic potentials of herbal, traditional/indigenous and ethnoveterinary medicines. Pak. J. Biol. Sci. 2012, 15, 754–774. [Google Scholar]
- Hassoun, A.; Harastani, R.; Jagtap, S.; Trollman, H.; Garcia-Garcia, G.; Awad, N.; Zannou, O.; Galanakis, C.; Gökşen, G.; Nayik, G.; et al. Truths and myths about superfoods in the era of the COVID-19 pandemic. Crit. Rev. Food Sci. Nutr. 2022, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Khanna, K.; Kohli, S.K.; Kaur, R.; Bhardwaj, A.; Bhardwaj, V.; Ohri, P.; Sharma, A.; Ahmad, A.; Bhardwaj, R.; Ahmad, P. Herbal immune-boosters: Substantial warriors of pandemic COVID-19 battle. Phytomedicine 2021, 85, 153361. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, S.K.; Malaiya, A.; Mishra, G.; Jain, D.; Kesharwani, P.; Mody, N.; Ahmadi, A.; Paliwal, R.; Jain, A. An exhaustive comprehension of the role of herbal medicines in Pre- and Post-COVID manifestations. J. Ethnopharmacol. 2022, 296, 115420. [Google Scholar] [CrossRef]
- Rondanelli, M.; Miccono, A.; Lamburghini, S.; Avanzato, I.; Riva, A.; Allegrini, P.; Faliva, M.; Peroni, G.; Nichetti, M.; Perna, S. Self-Care for Common Colds: The Pivotal Role of Vitamin D, Vitamin C, Zinc, and Echinacea in Three Main Immune Interactive Clusters (Physical Barriers, Innate and Adaptive Immunity) Involved during an Episode of Common Colds—Practical Advice on Dosages and on the Time to Take These Nutrients/Botanicals in order to Prevent or Treat Common Colds. Evid.-Based Complement. Altern. Med. 2018, 2018, 5813095. [Google Scholar]
- Nantz, M.; Rowe, C.; Muller, C.; Creasy, R.; Stanilka, J.; Percival, S. Supplementation with aged garlic extract improves both NK and γδ-T cell function and reduces the severity of cold and flu symptoms: A randomized, double-blind, placebo-controlled nutrition intervention. Clin. Nutr. 2012, 31, 337–344. [Google Scholar] [CrossRef]
- de Oliveira, C.A.F.; Vetvicka, V.; Zanuzzo, F.S. Beta-Glucan successfully stimulated the immune system in different jawed vertebrate species. Comp. Immunol. Microbiol. Infect. Dis. 2019, 62, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Arshad, M.S.; Khan, U.; Sadiq, A.; Khalid, W.; Hussain, M.; Yasmeen, A.; Asghar, Z.; Rehana, H. Coronavirus disease (COVID-19) and immunity booster green foods: A mini review. Food Sci. Nutr. 2020, 8, 3971–3976. [Google Scholar] [CrossRef] [PubMed]
- Burkard, M.; Leischner, C.; Lauer, U.M.; Busch, C.; Venturelli, S.; Frank, J. Dietary flavonoids and modulation of natural killer cells: Implications in malignant and viral diseases. J. Nutr. Biochem. 2017, 46, 1–12. [Google Scholar] [CrossRef]
- Gasmi, A.; Mujawdiya, P.K.; Noor, S.; Lysiuk, R.; Darmohray, R.; Piscopo, S.; Lenchyk, L.; Antonyak, H.; Dehtiarova, K.; Shanaida, M.; et al. Polyphenols in Metabolic Diseases. Molecules 2022, 27, 6280. [Google Scholar] [CrossRef]
- Huang, R.Y.; Yu, Y.L.; Cheng, W.C.; OuYang, C.N.; Fu, E.; Chu, C.L. Immunosuppressive effect of quercetin on dendritic cell activation and function. J. Immunol. 2010, 184, 6815–6821. [Google Scholar] [CrossRef] [Green Version]
- Chaudhary, P.; Sharma, R.; Rawat, S.; Janmeda, P. Antipyretic Medicinal Plants, Phytocompounds, and Green Nanoparticles: An Updated Review. Curr. Pharm. Biotechnol. 2023, 24, 23–49. [Google Scholar] [PubMed]
- Quispe, C.; Cruz-Martins, N.; Manca, M.L.; Manconi, M.; Sytar, O.; Hudz, N.; Shanaida, M.; Kumar, M.; Taheri, Y.; Martorell, M.; et al. Nano-Derived Therapeutic Formulations with Curcumin in Inflammation-Related Diseases. Oxid Med. Cell Longev. 2021, 2021, 3149223. [Google Scholar] [CrossRef] [PubMed]
- Yücel, Ç.; Şeker Karatoprak, G.; Bahadir, O.; Akkol, E.; Barak, T.H.; Sobarzo-SÁNchez, E.; Aschner, M.; Shirooie, S. Immunomodulatory and anti-inflammatory therapeutic potential of gingerols and their nanoformulations. Front. Pharmacol. 2022, 13. [Google Scholar] [CrossRef]
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ota, K.; Dambaeva, S.; Kim, M.W.; Han, A.R.; Fukui, A.; Gilman-Sachs, A.; Beaman, K.; Kwak-Kim, J. 1,25-Dihydroxy-vitamin D3 regulates NK-cell cytotoxicity, cytokine secretion, and degranulation in women with recurrent pregnancy losses. Eur. J. Immunol. 2015, 45, 3188–3199. [Google Scholar] [CrossRef]
- Mitra, S.; Paul, S.; Roy, S.; Sutradhar, H.; Emran, T.; Nainu, F.; Khandaker, M.; Almalki, M.; Wilairatana, P.; Mubarak, M. Exploring the Immune-Boosting Functions of Vitamins and Minerals as Nutritional Food Bioactive Compounds: A Comprehensive Review. Molecules 2022, 27, 555. [Google Scholar] [CrossRef]
- Wagh, V.D. Propolis: A wonder bees product and its pharmacological potentials. Adv. Pharmacol. Sci. 2013, 2013, 308249. [Google Scholar] [CrossRef] [Green Version]
- Oleshchuk, O.; Ivankiv, Y.; Falfushynska, H.; Mudra, A.; Lisnychuk, N. Hepatoprotective Effect of Melatonin in Toxic Liver Injury in Rats. Medicina 2019, 55, 304. [Google Scholar] [CrossRef] [Green Version]
- Ivankiv, Y.; Oleshchuk, O. Immunomodulatory effect of melatonin supplementation in experimental diabetes. Pharmacia 2020, 67, 223–228. [Google Scholar] [CrossRef]
- Yates, C.R.; Bruno, E.J.; Yates, M.E.D. Tinospora Cordifolia: A review of its immunomodulatory properties. J. Diet. Suppl. 2022, 19, 271–285. [Google Scholar] [CrossRef]
- Ahmed, H.; Babakir-Mina, M. Investigation of rosemary herbal extracts (Rosmarinus officinalis) and their potential effects on immunity. Phytother. Res. 2020, 34, 1829–1837. [Google Scholar] [CrossRef] [PubMed]
- Miles, E.; Calder, P. Effects of Citrus Fruit Juices and Their Bioactive Components on Inflammation and Immunity: A Narrative Review. Front. Immunol. 2021, 12, 712608. [Google Scholar] [CrossRef]
- Shanaida, M.; Pryshlyak, A.; Golembiovska, O. Determination of triterpenoids in some Lamiaceae species. Res. J. Pharm. Technol. 2018, 7, 3113–3118. [Google Scholar] [CrossRef]
- Al-Ataby, I.A.; Talib, W.H. Daily Consumption of Lemon and Ginger Herbal Infusion Caused Tumor Regression and Activation of the Immune System in a Mouse Model of Breast Cancer. Front. Nutr. 2022, 9, 829101. [Google Scholar] [CrossRef]
- Aryaeian, N.; Shahram, F.; Mahmoudi, M.; Tavakoli, H.; Yousefi, B.; Arablou, T. The effect of ginger supplementation on some immunity and inflammation intermediate genes expression in patients with active Rheumatoid Arthritis. Gene 2019, 698, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Bałan, B.; Sokolnicka, I.; Skopińska-Różewska, E.; Skopiński, P. The modulatory influence of some Echinacea-based remedies on antibody production and cellular immunity in mice. Cent.-Eur. J. Immunol. 2016, 41, 12–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ilina, T.; Skowronska, W.; Kashpur, N.; Granica, S.; Bazylko, A.; Kovalyova, A.; Goryacha, O.; Koshovyi, O. Immunomodulatory Activity and Phytochemical Profile of Infusions from Cleavers Herb. Molecules 2020, 25, 3721. [Google Scholar] [CrossRef] [PubMed]
- Mykhailenko, O.; Lesyk, R.; Finiuk, N.; Rostyslav, S.; Tetyana, Y.; Anna, O.; Valentina, V.; Volodymyr, M.; Georgiyants, V. In vitro anticancer activity screening of Iridaceae plant extracts. J. Appl. Pharm. Sci. 2020, 10, 59–63. [Google Scholar]
- Williamson, E.M. Synergy and other interactions in phytomedicines. Phytomedicine 2001, 8, 401–409. [Google Scholar] [CrossRef]
- Thomford, N.E.; Senthebane, D.A.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural Products for Drug Discovery in the 21st Century: Innovations for Novel Drug Discovery. Int. J. Mol. Sci. 2018, 19, 1578. [Google Scholar] [CrossRef] [Green Version]
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug. Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boozari, M.; Hosseinzadeh, H. Natural products for COVID-19 prevention and treatment regarding to previous coronavirus infections and novel studies. Phytother. Res. 2020, 35, 864–876. [Google Scholar] [CrossRef] [PubMed]
- Shree, P.; Mishra, P.; Selvaraj, C.; Singh, S.K.; Chaube, R.; Garg, N.; Tripathi, Y.B. Targeting COVID-19 (SARS-CoV-2) main protease through active phytochemicals of ayurvedic medicinal plants—Withania somnifera (Ashwagandha), Tinospora cordifolia (Giloy) and Ocimum sanctum (Tulsi)—A molecular docking study. J. Biomol. Struct. Dyn. 2022, 40, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Francis, T.; Sooriyaarachchi, P.; Jayawardena, R. Usage of nutritional supplements to improve immunity during the COVID-19 pandemic: An online survey. Clin. Nutr. Open Sci. 2022, 43, 6–19. [Google Scholar] [CrossRef]
- Al Kury, L.T.; Taha, Z.; Mahmod, A.I.; Talib, W.H. Xanthium spinosum L. Extracts Inhibit Breast Cancer in Mice by Apoptosis Induction and Immune System Modulation. Pharmaceuticals 2022, 15, 1504. [Google Scholar] [CrossRef]
- Drenkhan, R.; Kaldmäe, H.; Silm, M.; Adamson, K.; Bleive, U.; Aluvee, A.; Erik, M.; Raal, A. Comparative Analyses of Bioactive Compounds in Inonotus obliquus Conks Growing on Alnus and Betula. Biomolecules 2022, 12, 1178. [Google Scholar] [CrossRef]
- Rios, J.L. Effects of triterpenes on the immune system. J. Ethnopharmacol. 2010, 128, 1–14. [Google Scholar] [CrossRef]
- Venter, C.; Eyerich, S.; Sarin, T.; Klatt, K.C. Nutrition and the Immune System: A Complicated Tango. Nutrients 2020, 12, 818. [Google Scholar] [CrossRef] [Green Version]
- Mangla, B.; Javed, S.; Sultan, M.H.; Kumar, P.; Kohli, K.; Najmi, A.; Alhazmi, H.A.; Al Bratty, M.; Ahsan, W. Sulforaphane: A review of its therapeutic potentials, advances in its nanodelivery, recent patents, and clinical trials. Phytother. Res. 2021, 35, 5440–5458. [Google Scholar] [CrossRef]
- Barrett, B. Medicinal properties of Echinacea: A critical review. Phytomedicine 2003, 10, 66–86. [Google Scholar] [CrossRef]
- Chicca, A.; Raduner, S.; Pellati, F.; Strompen, T.; Altmann, K.H.; Schoop, R.; Gertsch, J. Synergistic immunomopharmacological effects of N-alkylamides in Echinacea purpurea herbal extracts. Int. Immunopharmacol. 2009, 9, 850–858. [Google Scholar] [CrossRef]
- Luettig, B.; Steinmuller, C.; Gifford, G.E.; Wagner, H.; Lohmann-Matthes, M.L. Macrophage activation by the polysaccharide arabinogalactan isolated from plant cell cultures of Echinacea purpurea. J. Natl. Cancer Inst. 1989, 81, 669–675. [Google Scholar] [CrossRef]
- Fonseca, F.; Papanicolaou, G.; Lin, H.; Lau, C.; Kennelly, E.; Cassileth, B.; Cunningham-Rundles, S. Echinacea purpurea (L.) Moench modulates human T-cell cytokine response. Int. Immunopharmacol. 2014, 19, 94–102. [Google Scholar] [CrossRef] [Green Version]
- Signer, J.; Jonsdottir, H.R.; Albrich, W.C.; Strasser, M.; Zust, R.; Ryter, S.; Ackermann-Gaumann, R.; Lenz, N.; Siegrist, D.; Suter, A.; et al. Author Correction: In vitro virucidal activity of Echinaforce®, an Echinacea purpurea preparation, against coronaviruses, including common cold coronavirus 229E and SARS-CoV-2. Virol. J. 2020, 17, 172. [Google Scholar] [CrossRef]
- Vimalanathan, S.; Shehata, M.; Sadasivam, K.; Delbue, S.; Dolci, M.; Pariani, E.; D’Alessandro, S.; Pleschka, S. Broad Antiviral Effects of Echinacea purpurea against SARS-CoV-2 Variants of Concern and Potential Mechanism of Action. Microorganisms 2022, 10, 2145. [Google Scholar] [CrossRef] [PubMed]
- Kolev, E.; Mircheva, L.; Edwards, M.R.; Johnston, S.L.; Kalinov, K.; Stange, R.; Gancitano, G.; Berghe, W.V.; Kreft, S. Echinacea Purpurea For the Long-Term Prevention of Viral Respiratory Tract Infections During COVID-19 Pandemic: A Randomized, Open, Controlled, Exploratory Clinical Study. Front. Pharmacol. 2022, 13, 856410. [Google Scholar] [CrossRef]
- Fuzimoto, A.D. An overview of the anti-SARS-CoV-2 properties of Artemisia annua, its antiviral action, protein-associated mechanisms, and repurposing for COVID-19 treatment. J. Integr. Med. 2021, 19, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.Q.; van der Kooy, F.; Verpoorte, R. Artemisia afra: A potential flagship for African medicinal plants? S. Afr. J. Bot. 2009, 75, 185–195. [Google Scholar] [CrossRef] [Green Version]
- Ncube, T.; Muyima, N. Comparative evaluation of the antimicrobial activities of essential oils of Artemisia afra, Pteronia incana and Rosmarinus officinalis on selected Bacteria and yeast strains. Lett. Appl. Microbiol. 1999, 28, 291–296. [Google Scholar]
- More, G.; Lall, N.; Hussein, A.; Tshikalange, T.E. Antimicrobial Constituents of Artemisia afra Jacq. ex Willd. against Periodontal Pathogens. Evid.-Based Complement. Altern. Med. 2012, 2012, 252758. [Google Scholar] [CrossRef] [Green Version]
- Appalasamy, S.; Lo, K.Y.; Ch’ng, S.J.; Nornadia, K.; Othman, A.S.; Chan, L.K. Antimicrobial activity of artemisinin and precursor derived from in vitro plantlets of Artemisia annua L. Biomed. Res. Int. 2014, 2014, 215872. [Google Scholar] [CrossRef] [Green Version]
- Hunt, S.; Yoshida, M.; Davis, C.E.; Greenhill, N.S.; Davis, P.F. An extract of the medicinal plant Artemisia annua modulates production of inflammatory markers in activated neutrophils. J. Inflamm. Res. 2015, 8, 9–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruperti Repilado, F.J.; Haefliger, S.; Rehm, S.; Zweier, M.; Rentsch, K.; Blum, J.; Jetter, A.; Heim, M.; Leuppi-Taegtmeyer, A.; Terracciano, L.; et al. Danger of Herbal Tea: A Case of Acute Cholestatic Hepatitis Due to Artemisia annua Tea. Front. Med. 2019, 6, 221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- du Toit, A.; van der Kooy, F. Artemisia afra, a controversial herbal remedy or a treasure trove of new drugs? J. Ethnopharmacol. 2019, 244, 112127. [Google Scholar] [CrossRef] [PubMed]
- Ratan, Z.A.; Youn, S.H.; Kwak, Y.S.; Han, C.K.; Haidere, M.F.; Kim, J.K.; Min, H.; Jung, Y.J.; Hosseinzadeh, H.; Hyun, S.H.; et al. Adaptogenic effects of Panax ginseng on modulation of immune functions. J. Ginseng. Res. 2021, 45, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.G.; Shin, K.S.; Yoon, T.J.; Yu, K.W.; Ra, K.S.; Kim, J.M.; Kim, S.Y.; Suh, H.J. Fermentation of Korean red ginseng by Lactobacillus plantarum M-2 and its immunological activities. Appl. Biochem. Biotechnol. 2011, 165, 1107–1119. [Google Scholar] [CrossRef]
- Ma, J.; Liu, H.; Wang, X. Effect of ginseng polysaccharides and dendritic cells on the balance of Th1/Th2 T helper cells in patients with non-small cell lung cancer. J. Tradit. Chin. Med. 2014, 34, 641–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, Y.J.; Son, H.J.; Kim, K.S. A 14-week randomized, placebo-controlled, double-blind clinical trial to evaluate the efficacy and safety of ginseng polysaccharide (Y-75). J. Transl. Med. 2014, 12, 283. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Guo, M.; Feng, Y.; Zheng, H.; Lei, P.; Ma, X.; Han, X.; Guan, H.; Hou, D. Effect of ginseng polysaccharides on NK cell cytotoxicity in immunosuppressed mice. Exp. Ther. Med. 2016, 12, 3773–3777. [Google Scholar] [CrossRef] [Green Version]
- Karimi, N.; Dabidi Roshan, V.; Bayatiyani, Z. Individually and Combined Water-Based Exercise with Ginger Supplement, on Systemic Inflammation and Metabolic Syndrome Indices, Among the Obese Women with Breast Neoplasms. Iran. J. Cancer Prev. 2015, 8, e3856. [Google Scholar] [CrossRef] [Green Version]
- Khatoon, S.; Kalam, N.; Balasubramaniam, V.R.; Shaikh, M.F.; Ansari, M.T. Chemotherapeutic Role of Polyphenols Present in Ocimum sanctum. Anticancer Agents Med. Chem. 2022, 22, 3325–3342. [Google Scholar] [PubMed]
- Arreola, R.; Quintero-Fabian, S.; Lopez-Roa, R.I.; Flores-Gutierrez, E.O.; Reyes-Grajeda, J.P.; Carrera-Quintanar, L.; Ortuno-Sahagun, D. Immunomodulation and anti-inflammatory effects of garlic compounds. J. Immunol. Res 2015, 2015, 401630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, Y.; Zhu, X.; Wang, Q.; Jiang, Y.; Shang, H.; Cui, L.; Cao, Y. Allicin enhances host pro-inflammatory immune responses and protects against acute murine malaria infection. Malar. J. 2012, 11, 268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poles, J.; Karhu, E.; McGill, M.; McDaniel, H.R.; Lewis, J.E. The effects of twenty-four nutrients and phytonutrients on immune system function and inflammation: A narrative review. J. Clin. Transl. Res. 2021, 7, 333–376. [Google Scholar] [PubMed]
- Gasmi, A.; Mujawdiya, P.K.; Lysiuk, R.; Shanaida, M.; Peana, M.; Gasmi Benahmed, A.; Beley, N.; Kovalska, N.; Bjorklund, G. Quercetin in the Prevention and Treatment of Coronavirus Infections: A Focus on SARS-CoV-2. Pharmaceuticals 2022, 15, 1049. [Google Scholar] [CrossRef]
- Shakoor, H.; Feehan, J.; Apostolopoulos, V.; Platat, C.; Al Dhaheri, A.S.; Ali, H.I.; Ismail, L.C.; Bosevski, M.; Stojanovska, L. Immunomodulatory Effects of Dietary Polyphenols. Nutrients 2021, 13, 728. [Google Scholar] [CrossRef]
- Mollazadeh, H.; Cicero, A.F.G.; Blesso, C.N.; Pirro, M.; Majeed, M.; Sahebkar, A. Immune modulation by curcumin: The role of interleukin-10. Crit. Rev. Food Sci. Nutr. 2019, 59, 89–101. [Google Scholar] [CrossRef]
- Gasmi Benahmed, A.; Gasmi, A.; Arshad, M.; Shanaida, M.; Lysiuk, R.; Peana, M.; Pshyk-Titko, I.; Adamiv, S.; Shanaida, Y.; Bjorklund, G. Health benefits of xylitol. Appl. Microbiol. Biotechnol. 2020, 104, 7225–7237. [Google Scholar] [CrossRef]
- Salli, K.; Lehtinen, M.J.; Tiihonen, K.; Ouwehand, A.C. Xylitol’s Health Benefits beyond Dental Health: A Comprehensive Review. Nutrients 2019, 11, 1813. [Google Scholar] [CrossRef] [Green Version]
- Anand David, A.V.; Arulmoli, R.; Parasuraman, S. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar] [PubMed] [Green Version]
- Javadi, F.; Ahmadzadeh, A.; Eghtesadi, S.; Aryaeian, N.; Zabihiyeganeh, M.; Rahimi Foroushani, A.; Jazayeri, S. The Effect of Quercetin on Inflammatory Factors and Clinical Symptoms in Women with Rheumatoid Arthritis: A Double-Blind, Randomized Controlled Trial. J. Am. Coll. Nutr. 2017, 36, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, R.; Tamtaji, O.; Mirhosseini, N.; Lankarani, K.; Akbari, M.; Heydari, S.; Dadgostar, E.; Asemi, Z. The effects of quercetin supplementation on lipid profiles and inflammatory markers among patients with metabolic syndrome and related disorders: A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2019, 60, 1855–1868. [Google Scholar] [CrossRef] [PubMed]
- Konrad, M.; Nieman, D.C.; Henson, D.A.; Kennerly, K.M.; Jin, F.; Wallner-Liebmann, S.J. The acute effect of ingesting a quercetin-based supplement on exercise-induced inflammation and immune changes in runners. Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Heinz, S.; Henson, D.; Nieman, D.; Austin, M.; Jin, F. A 12-week supplementation with quercetin does not affect natural killer cell activity, granulocyte oxidative burst activity or granulocyte phagocytosis in female human subjects. Br. J. Nutr. 2010, 104, 849–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jalali, M.; Ranjbar, T.; Mosallanezhad, Z.; Mahmoodi, M.; Moosavian, S.P.; Ferns, G.A.; Jalali, R.; Sohrabi, Z. Effect of Propolis Intake on Serum C-Reactive Protein (CRP) and Tumor Necrosis Factor-alpha (TNF-alpha) Levels in Adults: A Systematic Review and Meta-Analysis of Clinical Trials. Complement. Ther. Med. 2020, 50, 102380. [Google Scholar] [CrossRef]
- Zakerkish, M.; Jenabi, M.; Zaeemzadeh, N.; Hemmati, A.A.; Neisi, N. The Effect of Iranian Propolis on Glucose Metabolism, Lipid Profile, Insulin Resistance, Renal Function and Inflammatory Biomarkers in Patients with Type 2 Diabetes Mellitus: A Randomized Double-Blind Clinical Trial. Sci. Rep. 2019, 9, 7289. [Google Scholar] [CrossRef] [Green Version]
- Zhu, A.; Wu, Z.; Zhong, X.; Ni, J.; Li, Y.; Meng, J.; Du, C.; Zhao, X.; Nakanishi, H.; Wu, S. Brazilian Green Propolis Prevents Cognitive Decline into Mild Cognitive Impairment in Elderly People Living at High Altitude. J. Alzheimer’s Dis. 2018, 63, 551–560. [Google Scholar] [CrossRef]
- Zhao, L.; Pu, L.; Wei, J.; Li, J.; Wu, J.; Zhonghao, X.; Gao, W.; Guo, C. Brazilian Green Propolis Improves Antioxidant Function in Patients with Type 2 Diabetes Mellitus. Int. J. Environ. Res. Public Health 2016, 13, 498. [Google Scholar] [CrossRef] [Green Version]
- Mujica, V.; Orrego, R.; Fuentealba, R.; Leiva, E.; Zuniga, J. Propolis as an Adjuvant in the Healing of Human Diabetic Foot Wounds Receiving Care in the Diagnostic and Treatment Centre from the Regional Hospital of Talca. J. Diabetes Res. 2019, 2019, 2507578. [Google Scholar] [CrossRef] [Green Version]
- Lardo, S.; Bagus, S.; Yongkie, I.; Djoko, W. The effect of a unique propolis compound (PropoelixTM) on clinical outcomes in patients with dengue hemorrhagic fever. Infect. Drug Resist. 2014, 7, 323–329. [Google Scholar] [CrossRef] [Green Version]
- Maheshwari, G.; Sowrirajan, S.; Joseph, B. Extraction and Isolation of beta-Glucan from Grain Sources-A Review. J. Food Sci. 2017, 82, 1535–1545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akramiene, D.; Kondrotas, A.; Didziapetriene, J.; Kevelaitis, E. Effects of beta-glucans on the immune system. Medicina 2007, 43, 597–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.-S.; Ho, S.-Y.; Nan, F.-H.; Chen, S.-N. Ganoderma lucidum beta 1,3/1,6 glucan as an immunomodulator in inflammation induced by a high-cholesterol diet. BMC Complement. Altern. Med. 2016, 16, 500. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Yuan, Y.; Yue, T. Immunostimulatory activities of beta-d-glucan from Ganoderma Lucidum. Carbohydr. Polym. 2014, 102, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Morales, D.; Rutckeviski, R.; Villalva, M.; Abreu, H.; Soler-Rivas, C.; Santoyo, S.; Iacomini, M.; Smiderle, F.R. Isolation and comparison of alpha- and beta-D-glucans from shiitake mushrooms (Lentinula edodes) with different biological activities. Carbohydr. Polym. 2020, 229, 115521. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.J.; Masterson, C.; Rezoagli, E.; O’Toole, D.; Major, I.; Stack, G.D.; Lynch, M.; Laffey, J.G.; Rowan, N.J. beta-Glucan extracts from the same edible shiitake mushroom Lentinus edodes produce differential in-vitro immunomodulatory and pulmonary cytoprotective effects—Implications for coronavirus disease (COVID-19) immunotherapies. Sci. Total Environ. 2020, 732, 139330. [Google Scholar] [CrossRef]
- Gaullier, J.M.; Sleboda, J.; Ofjord, E.S.; Ulvestad, E.; Nurminiemi, M.; Moe, C.; Tor, A.; Gudmundsen, O. Supplementation with a soluble beta-glucan exported from Shiitake medicinal mushroom, Lentinus edodes (Berk.) singer mycelium: A crossover, placebo-controlled study in healthy elderly. Int. J. Med. Mushrooms 2011, 13, 319–326. [Google Scholar] [CrossRef] [Green Version]
- Gordon, M.; Guralnik, M.; Kaneko, Y.; Mimura, T.; Goodgame, J.; DeMarzo, C.; Pierce, D.; Baker, M.; Lang, W. A phase II controlled study of a combination of the immune modulator, lentinan, with didanosine (ddI) in HIV patients with CD4 cells of 200–500/mm3. J. Med. 1995, 26, 193–207. [Google Scholar]
- Jung, S.J.; Jung, E.S.; Choi, E.K.; Sin, H.S.; Ha, K.C.; Chae, S.W. Immunomodulatory effects of a mycelium extract of Cordyceps (Paecilomyces hepiali; CBG-CS-2): A randomized and double-blind clinical trial. BMC Complement. Altern. Med. 2019, 19, 77. [Google Scholar] [CrossRef]
- Smiderle, F.; Baggio, C.; Borato, D.; Santana-Filho, A.; Sassaki, G.; Iacomini, M.; Van Griensven, L. Anti-Inflammatory Properties of the Medicinal Mushroom Cordyceps militaris Might Be Related to Its Linear (1→3)-β-D-Glucan. PLoS ONE 2014, 9, e110266. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.J.; Baik, H.W.; Kim, S.J.; Lee, S.G.; Ahn, H.Y.; Park, J.S.; Park, S.J.; Jang, E.J.; Park, S.W.; Choi, J.Y.; et al. Cordyceps militaris Enhances Cell-Mediated Immunity in Healthy Korean Men. J. Med. Food. 2015, 18, 1164–1172. [Google Scholar] [CrossRef] [PubMed]
- Lehne, G.; Haneberg, B.; Gaustad, P.; Johansen, P.; Preus, H.; Abrahamsen, T. Oral administration of a new soluble branched β-1,3-D-glucan is well tolerated and can lead to increased salivary concentrations of immunoglobulin A in healthy volunteers. Clin. Exp. Immunol. 2006, 143, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Nishimura, M.; Sato, Y.; Sato, H.; Nishihira, J. Enhancement of the Th1-phenotype immune system by the intake of Oyster mushroom (Tamogitake) extract in a double-blind, placebo-controlled study. J. Tradit. Complement. Med. 2016, 6, 424–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farnoosh, G.; Akbariqomi, M.; Badri, T.; Bagheri, M.; Izadi, M.; Saeedi-Boroujeni, A.; Rezaei, E.; Ghaleh, H.; Aghamollaei, H.; Fasihi-Ramandi, M.; et al. Efficacy of a Low Dose of Melatonin as an Adjunctive Therapy in Hospitalized Patients with COVID-19: A Randomized, Double-blind Clinical Trial. Arch. Med. Res. 2020, 53, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Bocheva, G.; Slominski, R.M.; Janjetovic, Z.; Kim, T.K.; Bohm, M.; Steinbrink, K.; Reiter, R.J.; Kleszczynski, K.; Slominski, A.T. Protective Role of Melatonin and Its Metabolites in Skin Aging. Int. J. Mol. Sci. 2022, 23, 1238. [Google Scholar] [CrossRef]
- Talib, W.H.; Alsayed, A.R.; Abuawad, A.; Daoud, S.; Mahmod, A.I. Melatonin in Cancer Treatment: Current Knowledge and Future Opportunities. Molecules 2021, 26, 2506. [Google Scholar] [CrossRef]
- Faridzadeh, A.; Tabashiri, A.; Heidarian Miri, H.; Mahmoudi, M. The role of melatonin as an adjuvant in the treatment of COVID-19: A systematic review. Heliyon 2022, 8, e10906. [Google Scholar] [CrossRef]
- Giovane, R.A.; Di Giovanni-Kinsley, S.; Keeton, E. Micronutrients for potential therapeutic use against COVID-19; a review. Clin. Nutr. ESPEN 2021, 46, 9–13. [Google Scholar] [CrossRef]
- Borges, L.; Gennari-Felipe, M.; Dias, B.B.; Hatanaka, E. Melatonin, Zinc, and Vitamin C: Potential Adjuvant Treatment for COVID-19 Patients. Front. Nutr. 2021, 8, 821824. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, X.; Ni, L.; Di, X.; Ma, B.; Niu, S.; Liu, C.; Reiter, R. COVID-19: Melatonin as a potential adjuvant treatment. Life Sci. 2020, 250, 117583. [Google Scholar] [CrossRef]
- Bjorklund, G.; Shanaida, M.; Lysiuk, R.; Antonyak, H.; Klishch, I.; Shanaida, V.; Peana, M. Selenium: An Antioxidant with a Critical Role in Anti-Aging. Molecules 2022, 27, 6613. [Google Scholar] [CrossRef]
- Castiglione, D.; Platania, A.; Conti, A.; Falla, M.; D’Urso, M.; Marranzano, M. Dietary Micronutrient and Mineral Intake in the Mediterranean Healthy Eating, Ageing, and Lifestyle (MEAL) Study. Antioxidants 2018, 7, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gombart, A.F.; Pierre, A.; Maggini, S. A Review of Micronutrients and the Immune System-Working in Harmony to Reduce the Risk of Infection. Nutrients 2020, 12, 236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rawat, D.; Roy, A.; Maitra, S.; Gulati, A.; Khanna, P.; Baidya, D.K. Vitamin C and COVID-19 treatment: A systematic review and meta-analysis of randomized controlled trials. Diabetes Metab. Syndr. 2021, 15, 102324. [Google Scholar] [CrossRef] [PubMed]
- Schencking, M.; Vollbracht, C.; Weiss, G.; Lebert, J.; Biller, A.; Goyvaerts, B.; Kraft, K. Intravenous vitamin C in the treatment of shingles: Results of a multicenter prospective cohort study. Med. Sci. Monit. 2012, 18, CR215–CR224. [Google Scholar] [CrossRef] [Green Version]
- Sezikli, M.; Cetinkaya, Z.A.; Guzelbulut, F.; Yesil, A.; Cosgun, S.; Kurdas, O.O. Supplementing vitamins C and E to standard triple therapy for the eradication of Helicobacter pylori. J. Clin. Pharm. Ther. 2012, 37, 282–285. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Salanti, G.; Belletti, A.; Bellomo, R.; Carr, A.; Furukawa, T.A.; Luethi, N.; Luo, Y.; Putzu, A.; Sartini, C.; et al. Effect of adjunctive vitamin C, glucocorticoids, and vitamin B1 on longer-term mortality in adults with sepsis or septic shock: A systematic review and a component network meta-analysis. Intensive Care Med. 2022, 48, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.W.; Lee, H.C. Vitamin D and health—The missing vitamin in humans. Pediatr. Neonatol. 2019, 60, 237–244. [Google Scholar] [CrossRef] [Green Version]
- Ao, T.; Kikuta, J.; Ishii, M. The Effects of Vitamin D on Immune System and Inflammatory Diseases. Biomolecules 2021, 11, 1624. [Google Scholar] [CrossRef]
- Griffin, T.P.; Wall, D.; Blake, L.; Griffin, D.G.; Robinson, S.M.; Bell, M.; Mulkerrin, E.C.; O’Shea, P.M. Vitamin D Status of Adults in the Community, in Outpatient Clinics, in Hospital, and in Nursing Homes in the West of Ireland. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2020, 75, 2418–2425. [Google Scholar] [CrossRef]
- Bae, M.; Kim, H. Mini-Review on the Roles of Vitamin C, Vitamin D, and Selenium in the Immune System against COVID-19. Molecules 2020, 25, 5346. [Google Scholar] [CrossRef] [PubMed]
- Soltani-Zangbar, M.S.; Mahmoodpoor, A.; Dolati, S.; Shamekh, A.; Valizadeh, S.; Yousefi, M.; Sanaie, S. Serum levels of vitamin D and immune system function in patients with COVID-19 admitted to intensive care unit. Gene Rep. 2022, 26, 101509. [Google Scholar] [CrossRef]
- Mailhot, G.; White, J.H. Vitamin D and Immunity in Infants and Children. Nutrients 2020, 12, 1233. [Google Scholar] [CrossRef] [PubMed]
- Eckard, A.R.; O’Riordan, M.A.; Rosebush, J.C.; Lee, S.T.; Habib, J.G.; Ruff, J.H.; Labbato, D.; Daniels, J.E.; Uribe-Leitz, M.; Tangpricha, V.; et al. Vitamin D supplementation decreases immune activation and exhaustion in HIV-1-infected youth. Antivir. Ther. 2018, 23, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Liberman, K.; Njemini, R.; Luiking, Y.; Forti, L.N.; Verlaan, S.; Bauer, J.M.; Memelink, R.; Brandt, K.; Donini, L.M.; Maggio, M.; et al. Thirteen weeks of supplementation of vitamin D and leucine-enriched whey protein nutritional supplement attenuates chronic low-grade inflammation in sarcopenic older adults: The PROVIDE study. Aging Clin. Exp. Res. 2019, 31, 845–854. [Google Scholar] [CrossRef] [Green Version]
- Folate, Folate—Fact Sheet for Health Professionals. U.S. Department of Health & Human Services: US National Institutes of Health. 2022. Available online: https://ods.od.nih.gov/factsheets/folate-HealthProfessional/ (accessed on 30 November 2022).
- Prietl, B.; Treiber, G.; Mader, J.; Hoeller, E.; Wolf, M.; Pilz, S.; Graninger, W.; Obermayer-Pietsch, B.; Pieber, T. High-dose cholecalciferol supplementation significantly increases peripheral CD4+ Tregs in healthy adults without negatively affecting the frequency of other immune cells. Eur. J. Nutr. 2013, 53, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Uribe-Diaz, S.; Nazeer, N.; Jaime, J.; Vargas-Bermudez, D.S.; Yitbarek, A.; Ahmed, M.; Rodriguez-Lecompte, J.C. Folic acid enhances proinflammatory and antiviral molecular pathways in chicken B-lymphocytes infected with a mild infectious bursal disease virus. Br. Poult. Sci. 2022, 63, 1–13. [Google Scholar] [CrossRef]
- Chen, Y.M.; Wei, J.L.; Qin, R.S.; Hou, J.P.; Zang, G.C.; Zhang, G.Y.; Chen, T.T. Folic acid: A potential inhibitor against SARS-CoV-2 nucleocapsid protein. Pharm. Biol. 2022, 60, 862–878. [Google Scholar] [CrossRef]
- Voelkle, M.; Gregoriano, C.; Neyer, P.; Koch, D.; Kutz, A.; Bernasconi, L.; Conen, A.; Mueller, B.; Schuetz, P. Prevalence of Micronutrient Deficiencies in Patients Hospitalized with COVID-19: An Observational Cohort Study. Nutrients 2022, 14, 1862. [Google Scholar] [CrossRef]
- Berger, M.M.; Shenkin, A.; Schweinlin, A.; Amrein, K.; Augsburger, M.; Biesalski, H.K.; Bischoff, S.C.; Casaer, M.P.; Gundogan, K.; Lepp, H.L.; et al. ESPEN micronutrient guideline. Clin. Nutr. 2022, 41, 1357–1424. [Google Scholar] [CrossRef]
- Razzaque, M. Magnesium: Are We Consuming Enough? Nutrients 2018, 10, 1863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathew, A.; Panonnummal, R. ‘Magnesium’-the master cation-as a drug—Possibilities and evidences. BioMetals 2021, 34, 955–986. [Google Scholar] [CrossRef] [PubMed]
- Eskander, M.; Razzaque, M. Can Maintaining Optimal Magnesium Balance Reduce the Disease Severity of COVID-19 Patients? Front. Endocrinol. 2022, 13, 843152. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.C. Combating COVID-19 and Building Immune Resilience: A Potential Role for Magnesium Nutrition? J. Am. Coll. Nutr. 2020, 39, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Maier, J.A.; Castiglioni, S.; Locatelli, L.; Zocchi, M.; Mazur, A. Magnesium and inflammation: Advances and perspectives. Semin. Cell Dev. Biol. 2021, 115, 37–44. [Google Scholar] [CrossRef]
- Trapani, V.; Rosanoff, A.; Baniasadi, S.; Barbagallo, M.; Castiglioni, S.; Guerrero-Romero, F.; Iotti, S.; Mazur, A.; Micke, O.; Pourdowlat, G.; et al. The relevance of magnesium homeostasis in COVID-19. Eur. J. Nutr. 2022, 61, 625–636. [Google Scholar] [CrossRef]
- Fiorentini, D.; Cappadone, C.; Farruggia, G.; Prata, C. Magnesium: Biochemistry, Nutrition, Detection, and Social Impact of Diseases Linked to Its Deficiency. Nutrients 2021, 13, 1136. [Google Scholar] [CrossRef]
- Weyh, C.; Kruger, K.; Peeling, P.; Castell, L. The Role of Minerals in the Optimal Functioning of the Immune System. Nutrients 2022, 14, 644. [Google Scholar] [CrossRef]
- Rangan, A.M.; Samman, S. Zinc intake and its dietary sources: Results of the 2007 Australian National Children’s Nutrition and Physical Activity Survey. Nutrients 2012, 4, 611–624. [Google Scholar] [CrossRef] [Green Version]
- Jafari, A.; Noormohammadi, Z.; Askari, M.; Daneshzad, E. Zinc supplementation and immune factors in adults: A systematic review and meta-analysis of randomized clinical trials. Crit. Rev. Food Sci. Nutr. 2022, 62, 3023–3041. [Google Scholar] [CrossRef]
- Kumar, A.; Kubota, Y.; Chernov, M.; Kasuya, H. Potential role of zinc supplementation in prophylaxis and treatment of COVID-19. Med. Hypotheses 2020, 144, 109848. [Google Scholar] [CrossRef]
- Li, Y.; Luo, W.; Liang, B. Circulating trace elements status in COVID-19 disease: A meta-analysis. Front. Nutr. 2022, 9, 982032. [Google Scholar] [CrossRef] [PubMed]
- Howie, S.; Bottomley, C.; Chimah, O.; Ideh, R.C.; Ebruke, B.; Okomo, U.; Onyeama, C.; Donkor, S.; Rodrigues, O.; Tapgun, M.; et al. Zinc as an adjunct therapy in the management of severe pneumonia among Gambian children: Randomized controlled trial. J. Glob. Health 2018, 8, 010418. [Google Scholar] [CrossRef] [PubMed]
- Kewcharoenwong, C.; Schuster, G.U.; Wessells, K.R.; Hinnouho, G.M.; Barffour, M.A.; Kounnavong, S.; Brown, K.H.; Hess, S.Y.; Samer, W.; Tussakhon, I.; et al. Daily Preventive Zinc Supplementation Decreases Lymphocyte and Eosinophil Concentrations in Rural Laotian Children from Communities with a High Prevalence of Zinc Deficiency: Results of a Randomized Controlled Trial. J. Nutr. 2020, 150, 2204–2213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saper, R.B.; Rash, R. Zinc: An essential micronutrient. Am. Fam. Physician 2009, 79, 768–772. [Google Scholar]
- Wessels, I.; Fischer, H.J.; Rink, L. Dietary and Physiological Effects of Zinc on the Immune System. Annu. Rev. Nutr. 2021, 41, 133–175. [Google Scholar] [CrossRef]
- Razaghi, A.; Poorebrahim, M.; Sarhan, D.; Bjornstedt, M. Selenium stimulates the antitumour immunity: Insights to future research. Eur. J. Cancer 2021, 155, 256–267. [Google Scholar] [CrossRef]
- Kieliszek, M.; Lipinski, B. Selenium supplementation in the prevention of coronavirus infections (COVID-19). Med. Hypotheses 2020, 143, 109878. [Google Scholar] [CrossRef]
- Khatiwada, S.; Subedi, A. A Mechanistic Link Between Selenium and Coronavirus Disease 2019 (COVID-19). Curr. Nutr. Rep. 2021, 10, 125–136. [Google Scholar] [CrossRef]
- Li, S.; Zhao, Q.; Zhang, K.; Sun, W.; Jia, X.; Yang, Y.; Yin, J.; Tang, C.; Zhang, J. Se deficiency induces renal pathological changes by regulating selenoprotein expression, disrupting redox balance, and activating inflammation. Metallomics 2020, 12, 1576–1584. [Google Scholar] [CrossRef]
- Fath, M.K.; Naderi, M.; Hamzavi, H.; Ganji, M.; Shabani, S.; Ghahroodi, F.N.; Khalesi, B.; Pourzardosht, N.; Hashemi, Z.S.; Khalili, S. Molecular mechanisms and therapeutic effects of different vitamins and minerals in COVID-19 patients. J. Trace. Elem. Med. Biol. 2022, 73, 127044. [Google Scholar] [CrossRef] [PubMed]
- Vahidi, H.; Barabadi, H.; Muthupandian, S. Emerging Selenium Nanoparticles to Combat Cancer: A Systematic Review. J. Clust. Sci. 2020, 31, 301–309. [Google Scholar] [CrossRef]
- Guarner, F.; Schaafsma, G.J. Probiotics. Int. J. Food Microbiol. 1998, 39, 237–238. [Google Scholar] [CrossRef] [PubMed]
- Schrezenmeir, J.; de Vrese, M. Probiotics, prebiotics, and synbiotics--approaching a definition. Am. J. Clin. Nutr. 2001, 73 (Suppl. S2), 361S–364S. [Google Scholar] [CrossRef] [Green Version]
- Batista, V.; Da Silva, T.; de Jesus, L.; Coelho Rocha, N.; Lima, F.; Tavares, L.; Azevedo, V.; Mancha-Agresti, P.; Drumond, M. Probiotics, Prebiotics, Synbiotics, and Paraprobiotics as a Therapeutic Alternative for Intestinal Mucositis. Front. Microbiol. 2020, 11, 544490. [Google Scholar] [CrossRef]
- Lubiech, K.; Twaruzek, M. Lactobacillus Bacteria in Breast Milk. Nutrients 2020, 12, 3783. [Google Scholar] [CrossRef]
- Kazemifard, N.; Dehkohneh, A.; Baradaran Ghavami, S. Probiotics and probiotic-based vaccines: A novel approach for improving vaccine efficacy. Front. Med. 2022, 9, 940454. [Google Scholar] [CrossRef]
- Kuchmak, O.B.; Klymnyuk, S.I.; Romanyuk, L.B.; Pokryshko, O.V. The Use of Probiotics in Patients with Rheumatoid Arthritis. Lik. Sprava 2014, 12, 63–65. [Google Scholar]
- Yeşilyurt, N.; Yılmaz, B.; Ağagündüz, D.; Capasso, R. Involvement of Probiotics and Postbiotics in the Immune System Modulation. Biologics 2021, 1, 89–110. [Google Scholar] [CrossRef]
- Lakshmi, B.; Viswanath, B.; Sai Gopal, D.V.R. Probiotics as Antiviral Agents in Shrimp Aquaculture. J. Pathog. 2013, 2013, 424123. [Google Scholar] [CrossRef] [Green Version]
- Naghibzadeh, N.; Salmani, F.; Nomiri, S.; Tavakoli, T. Investigating the effect of quadruple therapy with Saccharomyces boulardii or Lactobacillus reuteri strain (DSMZ 17648) supplements on eradication of Helicobacter pylori and treatments adverse effects: A double-blind placebo-controlled randomized clinical trial. BMC Gastroenterol. 2022, 22, 107. [Google Scholar]
- Kanmani, P.; Satish Kumar, R.; Yuvaraj, N.; Paari, K.A.; Pattukumar, V.; Arul, V. Probiotics and its functionally valuable products-a review. Crit. Rev. Food Sci. Nutr. 2013, 53, 641–658. [Google Scholar] [CrossRef] [PubMed]
- Schlee, M.; Wehkamp, J.; Altenhoefer, A.; Oelschlaeger, T.; Stange, E.; Fellermann, K. Induction of Human -Defensin 2 by the Probiotic Escherichia coli Nissle 1917 Is Mediated through Flagellin. Infect. Immun. 2007, 75, 2399–2407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hare, P.; Englander, H.; Mok, W. Probiotic Escherichia coli Nissle 1917 Inhibits Bacterial Persisters that Survive Fluoroquinolone Treatment. J. Appl. Microbiol. 2022, 132, 4020–4032. [Google Scholar] [CrossRef] [PubMed]
- Schlee, M.; Harder, J.; Koten, B.; Stange, E.F.; Wehkamp, J.; Fellermann, K. Probiotic lactobacilli and VSL#3 induce enterocyte beta-defensin 2. Clin. Exp. Immunol. 2008, 151, 528–535. [Google Scholar]
- Gupta, T.; Kaur, H.; Kapila, S.; Kapila, R. Lactobacillus fermentum (MTCC-5898) alleviates Escherichia coli-induced inflammatory responses in intestinal epithelial cells by modulating immune genes and NF-kappaB signalling. J. Appl. Microbiol. 2021, 131, 3008–3017. [Google Scholar] [CrossRef]
- Yousefi, B.; Eslami, M.; Ghasemian, A.; Kokhaei, P.; Salek Farrokhi, A.; Darabi, N. Probiotics importance and their immunomodulatory properties. J. Cell. Physiol. 2018, 234, 8008–8018. [Google Scholar] [CrossRef]
- Kim, J.M.; Kim, J.S.; Kim, Y.J.; Oh, Y.K.; Kim, I.Y.; Chee, Y.J.; Han, J.S.; Jung, H.C. Conjugated linoleic acids produced by Lactobacillus dissociates IKK-gamma and Hsp90 complex in Helicobacter pylori-infected gastric epithelial cells. Lab. Investig. 2008, 88, 541–552. [Google Scholar] [CrossRef] [Green Version]
- Lv, Z.; Wang, B.; Zhou, X.; Wang, F.; Xie, Y.; Zheng, H.; Lv, N. Efficacy and safety of probiotics as adjuvant agents for Helicobacter pylori infection: A meta-analysis. Exp. Ther. Med. 2015, 9, 707–716. [Google Scholar] [CrossRef] [Green Version]
- Kandasamy, S.; Chattha, K.S.; Vlasova, A.N.; Rajashekara, G.; Saif, L.J. Lactobacilli and Bifidobacteria enhance mucosal B cell responses and differentially modulate systemic antibody responses to an oral human rotavirus vaccine in a neonatal gnotobiotic pig disease model. Gut Microbes 2014, 5, 639–651. [Google Scholar] [CrossRef]
- Kawashima, T.; Ikari, N.; Kouchi, T.; Kowatari, Y.; Kubota, Y.; Shimojo, N.; Tsuji, N.M. The molecular mechanism for activating IgA production by Pediococcus acidilactici K15 and the clinical impact in a randomized trial. Sci. Rep. 2018, 8, 5065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chapman, C.; Gibson, G.R.; Rowland, I. In vitro evaluation of single- and multi-strain probiotics: Inter-species inhibition between probiotic strains, and inhibition of pathogens. Anaerobe 2012, 18, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Ropero, M.P.; Martin, R.; Sierra, S.; Lara-Villoslada, F.; Rodriguez, J.M.; Xaus, J.; Olivares, M. Two Lactobacillus strains, isolated from breast milk, differently modulate the immune response. J. Appl. Microbiol. 2007, 102, 337–343. [Google Scholar] [CrossRef]
- You, S.; Ma, Y.; Yan, B.; Pei, W.; Wu, Q.; Ding, C.; Huang, C. The promotion mechanism of prebiotics for probiotics: A review. Front. Nutr. 2022, 9, 1000517. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Xu, S.; Zhang, W.; Wu, D.; Yang, G. Probiotic Escherichia coli NISSLE 1917 for inflammatory bowel disease applications. Food Funct. 2022, 13, 5914–5924. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, D.; Xie, Y.; Yao, X.; Li, Y. Bifidobacterium infantis Induces Protective Colonic PD-L1 and Foxp3 Regulatory T Cells in an Acute Murine Experimental Model of Inflammatory Bowel Disease. Gut Liver 2019, 13, 430–439. [Google Scholar] [CrossRef] [Green Version]
- Xiao Joe, J.T.; Shi Sung, H.T.; Wu, J.L.; Lai, Y.S.; Lu, M.W. Dietary Administration of Novel Multistrain Probiotics from Healthy Grouper Intestines Promotes the Intestinal Immune Response against NNV Infection. Life 2021, 11, 1053. [Google Scholar] [CrossRef]
- Olaya, G.N.; Rubiano, J.; Velez, F.; Duarte, K.; Salas Cárdenas, S.; Fernandez, M. In vitro antiviral activity of Lactobacillus casei and Bifidobacterium adolescentis against rotavirus infection monitored by NSP4 protein production. J. Appl. Microbiol. 2016, 120, 1041–1051. [Google Scholar] [CrossRef] [Green Version]
- Ang, L.Y.; Too, H.K.; Tan, E.L.; Chow, T.V.; Shek, L.P.; Tham, E.H.; Alonso, S. Erratum to: Antiviral activity of Lactobacillus reuteri Protectis against Coxsackievirus A and Enterovirus 71 infection in human skeletal muscle and colon cell lines. Virol. J. 2016, 13, 186. [Google Scholar] [CrossRef] [Green Version]
- Loniewski, I.; Skonieczna-Zydecka, K.; Solek-Pastuszka, J.; Marlicz, W. Probiotics in the Management of Mental and Gastrointestinal Post-COVID Symptomes. J. Clin. Med. 2022, 11, 5155. [Google Scholar] [CrossRef]
- Xavier-Santos, D.; Padilha, M.; Fabiano, G.; Vinderola, G.; Cruz, A.; Sivieri, K.; Antunes, A. Evidences and perspectives of the use of probiotics, prebiotics, synbiotics, and postbiotics as adjuvants for prevention and treatment of COVID-19: A bibliometric analysis and systematic review. Trends Food Sci. Technol. 2022, 120, 174–192. [Google Scholar] [CrossRef] [PubMed]
- Watzl, B.; Girrbach, S.; Roller, M. Inulin, oligofructose and immunomodulation. Br. J. Nutr. 2005, 93 (Suppl. S1), S49–S55. [Google Scholar] [CrossRef]
- Pujari, R.; Banerjee, G. Impact of prebiotics on immune response: From the bench to the clinic. Immunol. Cell Biol. 2021, 99, 255–273. [Google Scholar] [CrossRef] [PubMed]
- Seifert, S.; Watzl, B. Inulin and oligofructose: Review of experimental data on immune modulation. J. Nutr. 2007, 137 (Suppl. S11), 2563S–2567S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poggi, A.; Benelli, R.; Vene, R.; Costa, D.; Ferrari, N.; Tosetti, F.; Zocchi, M.R. Human Gut-Associated Natural Killer Cells in Health and Disease. Front. Immunol. 2019, 10, 961. [Google Scholar] [CrossRef] [Green Version]
- Fuhrmann, L.; Vahjen, W.; Zentek, J.; Gunther, R.; Saliu, E.M. The Impact of Pre- and Probiotic Product Combinations on Ex vivo Growth of Avian Pathogenic Escherichia coli and Salmonella Enteritidis. Microorganisms 2022, 10, 121. [Google Scholar] [CrossRef]
- Zuntar, I.; Petric, Z.; Bursac Kovacevic, D.; Putnik, P. Safety of Probiotics: Functional Fruit Beverages and Nutraceuticals. Foods 2020, 9, 947. [Google Scholar] [CrossRef]
- Dronkers, T.M.G.; Ouwehand, A.C.; Rijkers, G.T. Data on global analysis of clinical trials with probiotics. Data Brief 2020, 32, 106269. [Google Scholar] [CrossRef]
- Dudek-Wicher, R.; Junka, A.; Paleczny, J.; Bartoszewicz, M. Clinical Trials of Probiotic Strains in Selected Disease Entities. Int. J. Microbiol. 2020, 2020, 8854119. [Google Scholar] [CrossRef]
- Mirashrafi, S.; Hejazi Taghanaki, S.Z.; Sarlak, F.; Moravejolahkami, A.R.; Hojjati Kermani, M.A.; Haratian, M. Effect of Probiotics Supplementation on Disease Progression, Depression, General Health and Anthropometric Measurements in Relapsing-Remitting Multiple Sclerosis Patients: A Systematic Review and Meta-analysis of Clinical Trials. Int. J. Clin. Pract. 2021, 75, e14724. [Google Scholar] [CrossRef]
- Boggio Marzet, C.; Burgos, F.; Del Compare, M.; Gerold, I.; Tabacco, O.; Vinderola, G. Approach to probiotics in pediatrics: The role of Lactobacillus rhamnosus GG. Arch. Argent. Pediatr. 2022, 120, e1–e7. [Google Scholar]
- Ceccherini, C.; Daniotti, S.; Bearzi, C.; Re, I. Evaluating the Efficacy of Probiotics in IBS Treatment Using a Systematic Review of Clinical Trials and Multi-Criteria Decision Analysis. Nutrients 2022, 14, 2689. [Google Scholar] [CrossRef]
- Zmora, N.; Zilberman-Schapira, G.; Suez, J.; Mor, U.; Dori-Bachash, M.; Bashiardes, S.; Kotler, E.; Zur, M.; Regev-Lehavi, D.; Ben Zeev, R.; et al. Personalized Gut Mucosal Colonization Resistance to Empiric Probiotics Is Associated with Unique Host and Microbiome Features. Cell 2018, 174, 1388–1405.e21. [Google Scholar] [CrossRef] [Green Version]
- Mikawlrawng, K.; Kumar, S.; Bhatnagar, K. Drug Interactions, Safety and Efficacy of Probiotics. Asian J. Med. Health 2016, 1, 1–8. [Google Scholar] [CrossRef]
- Shah, N.P. Probiotic bacteria: Selective enumeration and survival in dairy foods. J. Dairy Sci. 2000, 83, 894–907. [Google Scholar] [CrossRef]
- Bansal, S.; Mangal, M.; Sharma, S.K.; Gupta, R.K. Non-dairy Based Probiotics: A Healthy Treat for Intestine. Crit. Rev. Food Sci. Nutr. 2016, 56, 1856–1867. [Google Scholar] [CrossRef]
- Swanson, K.; Gibson, G.; Hutkins, R.; Reimer, R.; Reid, G.; Verbeke, K.; Scott, K.; Holscher, H.; Azad, M.; Delzenne, N.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef]
- FAO; WHO. Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food: London, Ontario, Canada, April 30–May 1, 2002; World Health Organization: Roma, Italy, 2006; pp. 34–50. Available online: http://www.who.int/foodsafety/publications/fs_management/probiotics2/en/ (accessed on 28 March 2023).
- Markowiak, P.; Slizewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef]
- van den Nieuwboer, M.; Claassen, E. Dealing with the remaining controversies of probiotic safety. Benef. Microbes 2019, 10, 605–616. [Google Scholar] [CrossRef]
- Yoha, K.S.; Nida, S.; Dutta, S.; Moses, J.A.; Anandharamakrishnan, C. Targeted Delivery of Probiotics: Perspectives on Research and Commercialization. Probiotics Antimicrob Proteins 2022, 14, 15–48. [Google Scholar] [CrossRef]
- Merenstein, D.; Pot, B.; Leyer, G.; Ouwehand, A.C.; Preidis, G.A.; Elkins, C.A.; Hill, C.; Lewis, Z.T.; Shane, A.L.; Zmora, N.; et al. Emerging issues in probiotic safety: 2023 perspectives. Gut Microbes 2023, 15, 2185034. [Google Scholar] [CrossRef]
- Suez, J.; Zmora, N.; Elinav, E. Probiotics in the next-generation sequencing era. Gut Microbes 2019, 11, 77–93. [Google Scholar] [CrossRef]
- Kothari, D.; Patel, S.; Kim, S.-K. Probiotic supplements might not be universally-effective and safe: A review. Biomed. Pharmacother. 2019, 111, 537–547. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gasmi, A.; Shanaida, M.; Oleshchuk, O.; Semenova, Y.; Mujawdiya, P.K.; Ivankiv, Y.; Pokryshko, O.; Noor, S.; Piscopo, S.; Adamiv, S.; et al. Natural Ingredients to Improve Immunity. Pharmaceuticals 2023, 16, 528. https://doi.org/10.3390/ph16040528
Gasmi A, Shanaida M, Oleshchuk O, Semenova Y, Mujawdiya PK, Ivankiv Y, Pokryshko O, Noor S, Piscopo S, Adamiv S, et al. Natural Ingredients to Improve Immunity. Pharmaceuticals. 2023; 16(4):528. https://doi.org/10.3390/ph16040528
Chicago/Turabian StyleGasmi, Amin, Mariia Shanaida, Oleksandra Oleshchuk, Yuliya Semenova, Pavan Kumar Mujawdiya, Yana Ivankiv, Olena Pokryshko, Sadaf Noor, Salva Piscopo, Stepan Adamiv, and et al. 2023. "Natural Ingredients to Improve Immunity" Pharmaceuticals 16, no. 4: 528. https://doi.org/10.3390/ph16040528
APA StyleGasmi, A., Shanaida, M., Oleshchuk, O., Semenova, Y., Mujawdiya, P. K., Ivankiv, Y., Pokryshko, O., Noor, S., Piscopo, S., Adamiv, S., & Bjørklund, G. (2023). Natural Ingredients to Improve Immunity. Pharmaceuticals, 16(4), 528. https://doi.org/10.3390/ph16040528







