Drug Repurposing of FDA Compounds against α-Glucosidase for the Treatment of Type 2 Diabetes: Insights from Molecular Docking and Molecular Dynamics Simulations
Abstract
1. Introduction
2. Results and Discussion
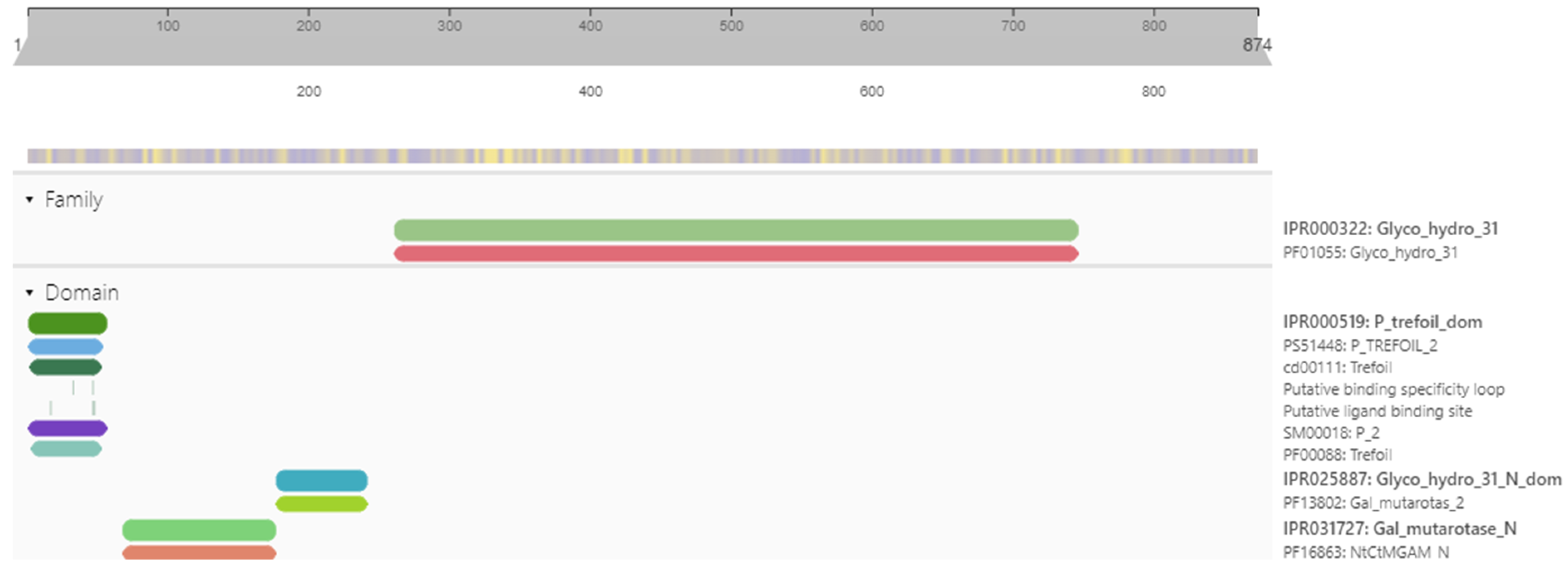
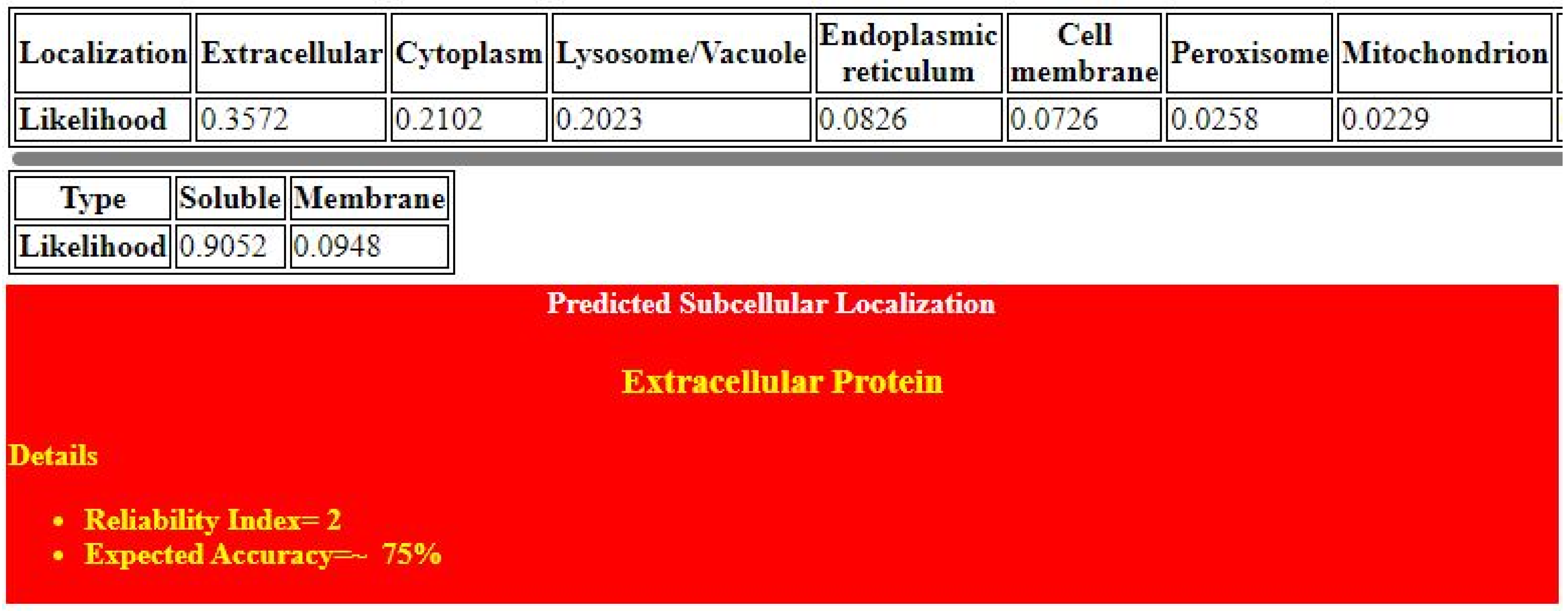
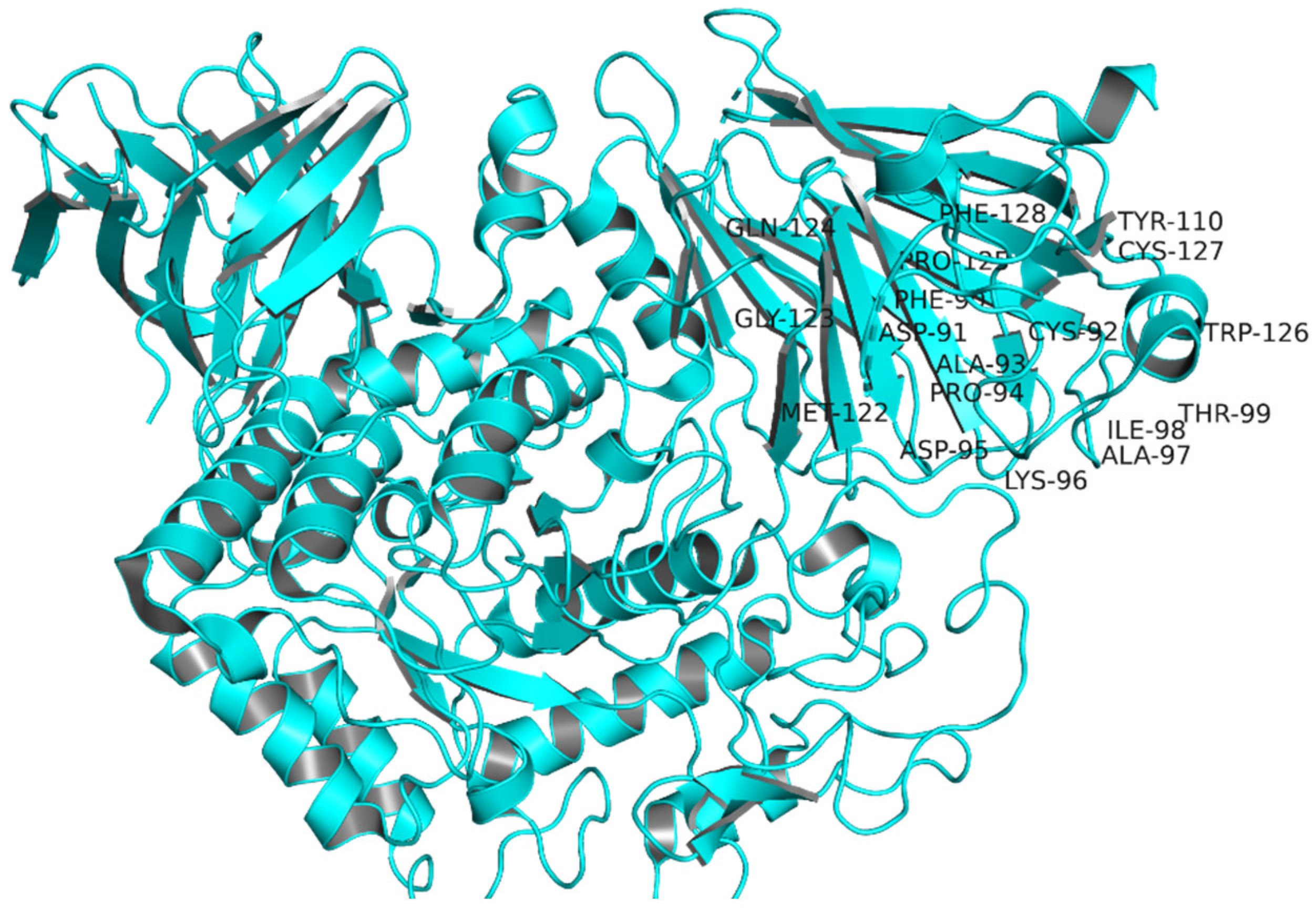
2.1. Target Protein Domains and Physicochemical Properties
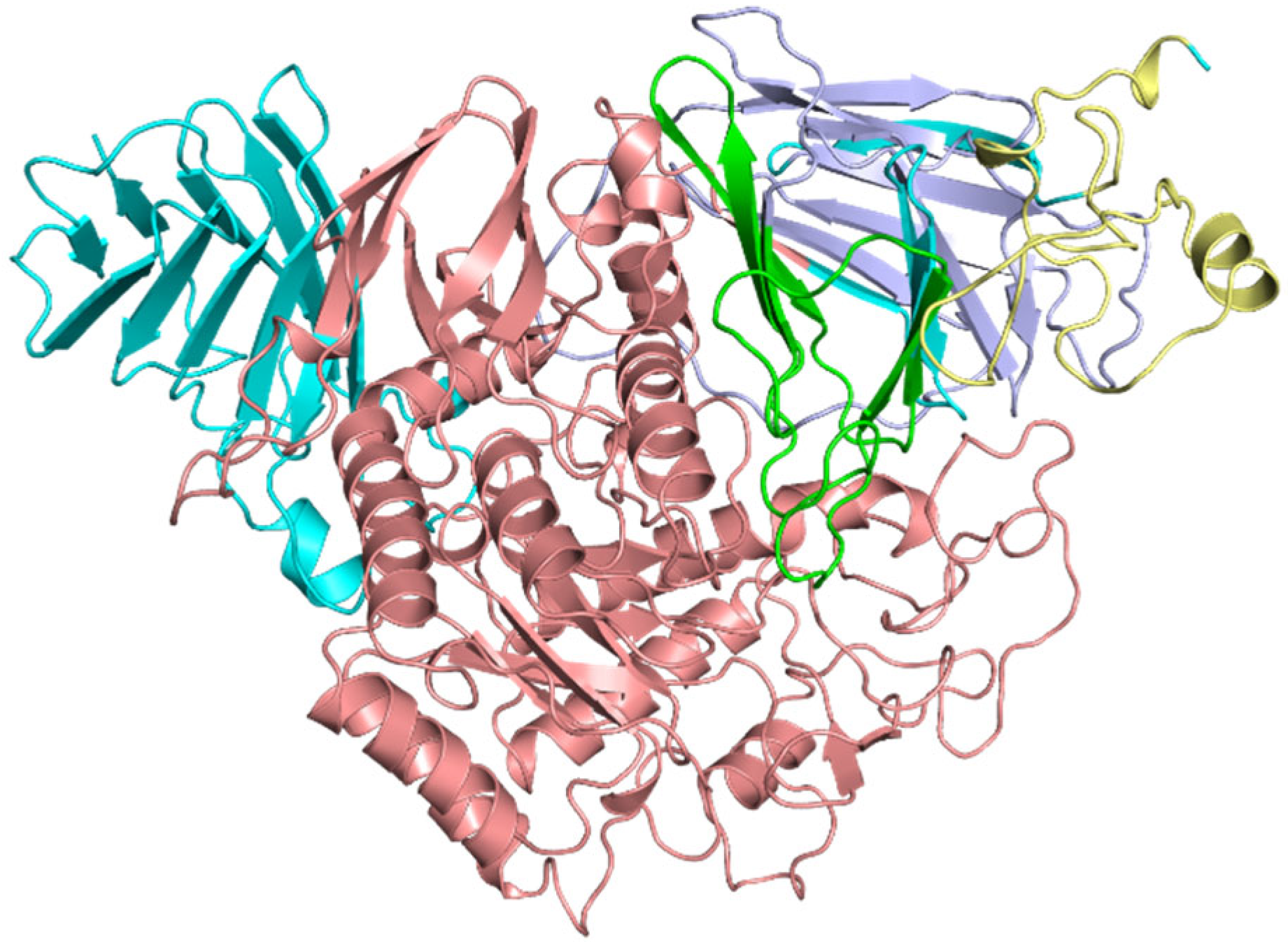
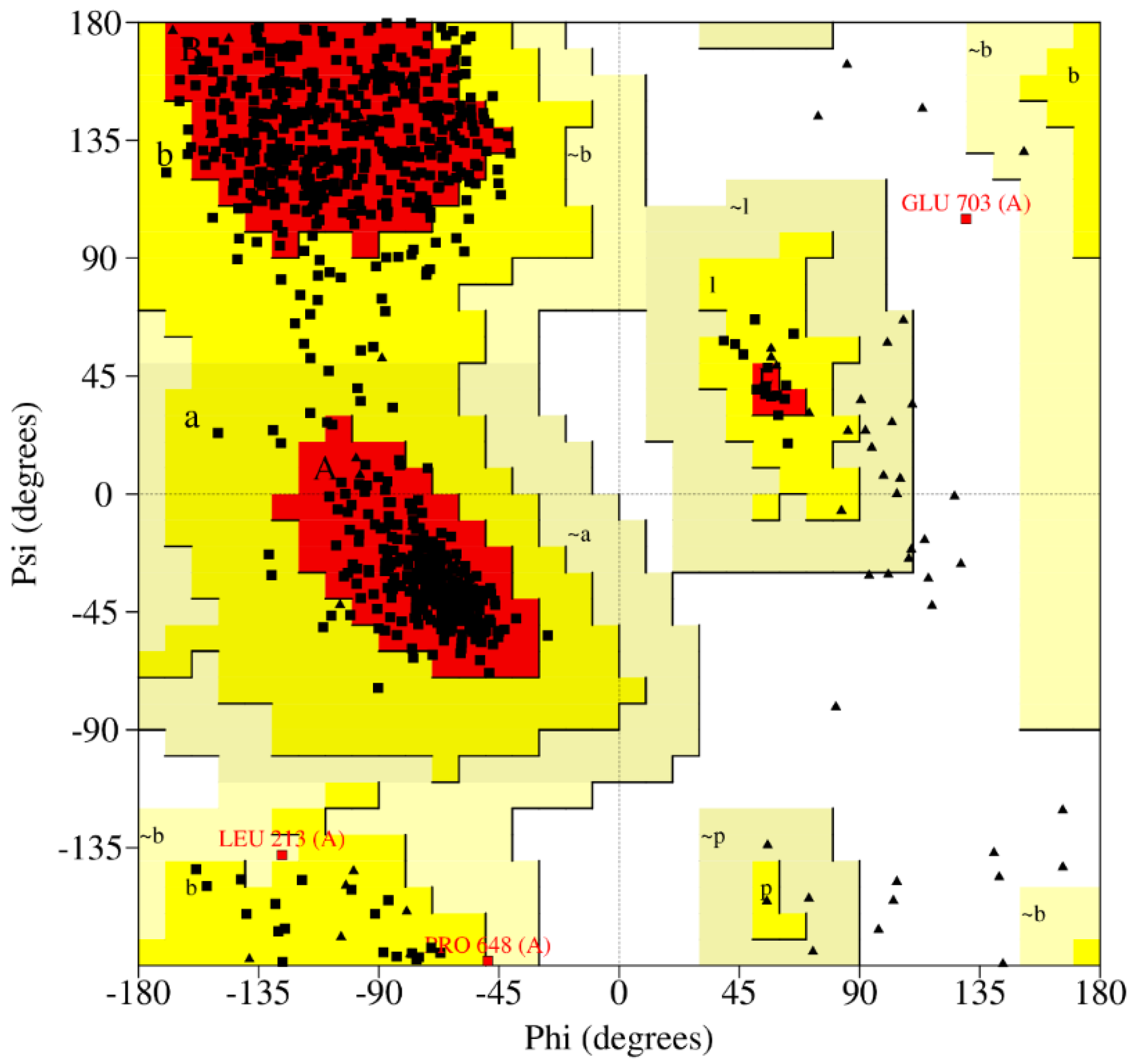
2.2. Protein Structure Refinement, Optimization, and Minimization
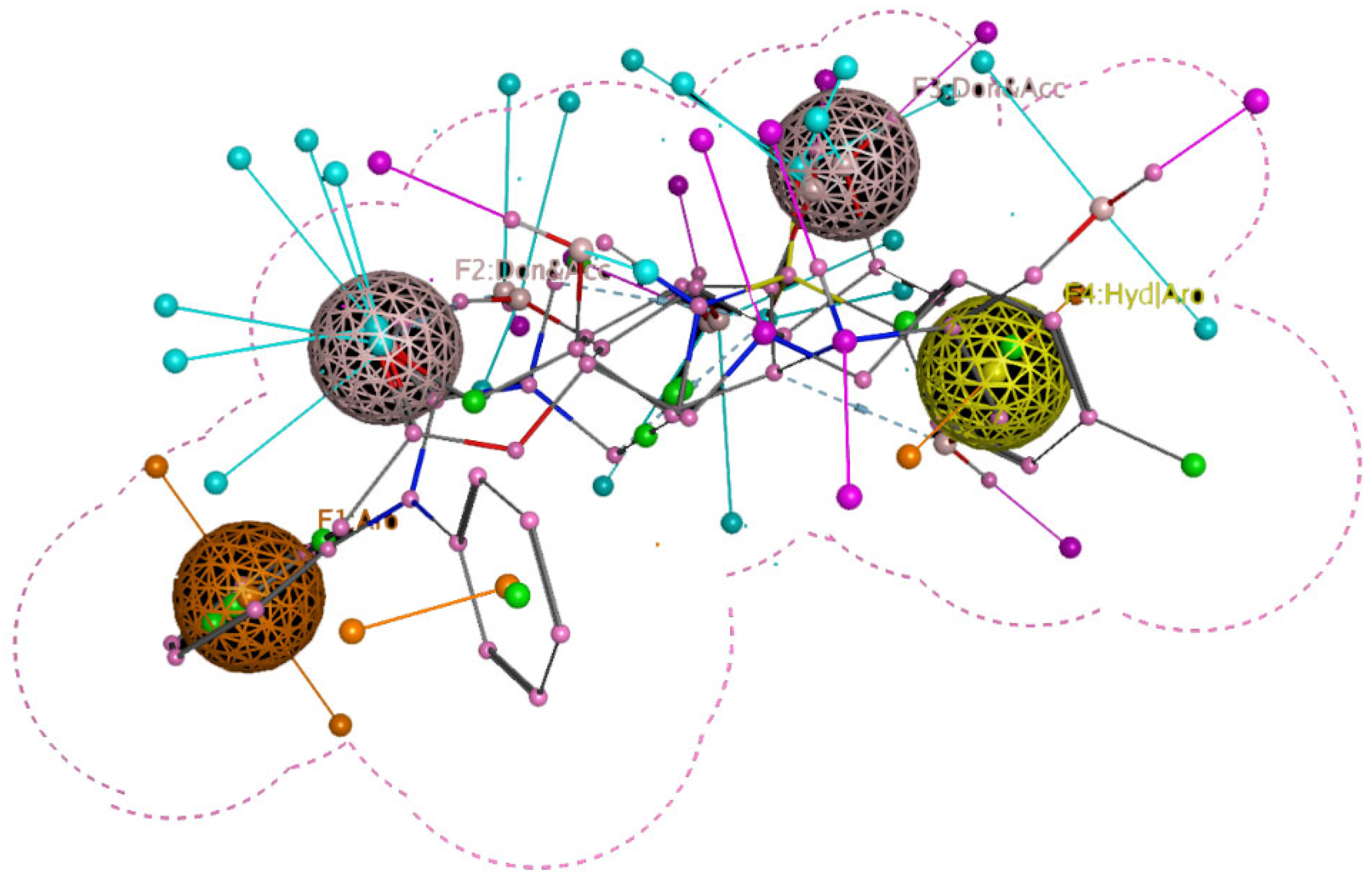
2.3. Reported Inhibitors Docking and Pharmacophore Query
2.4. Database Preparation and Virtual Screening
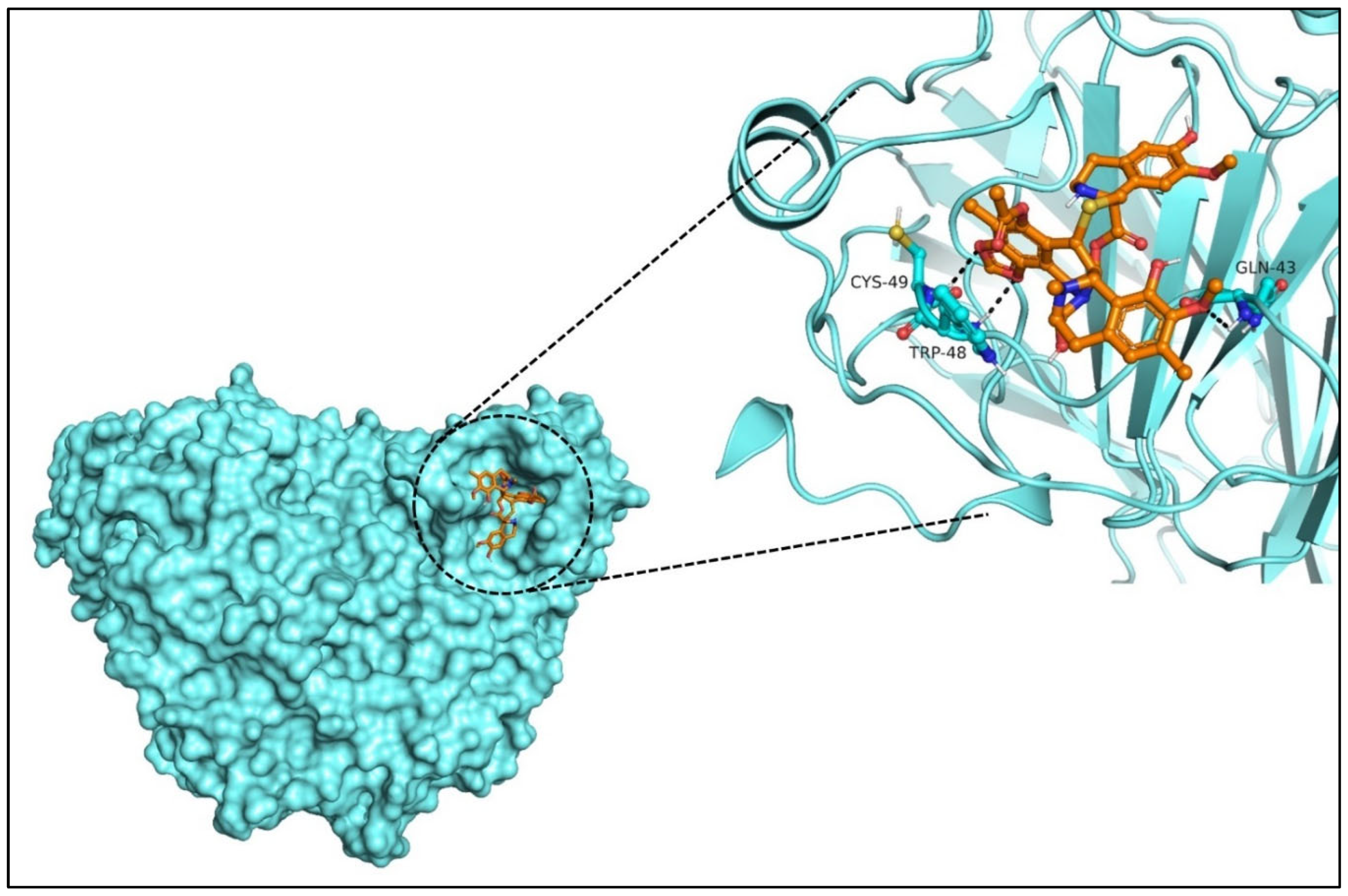
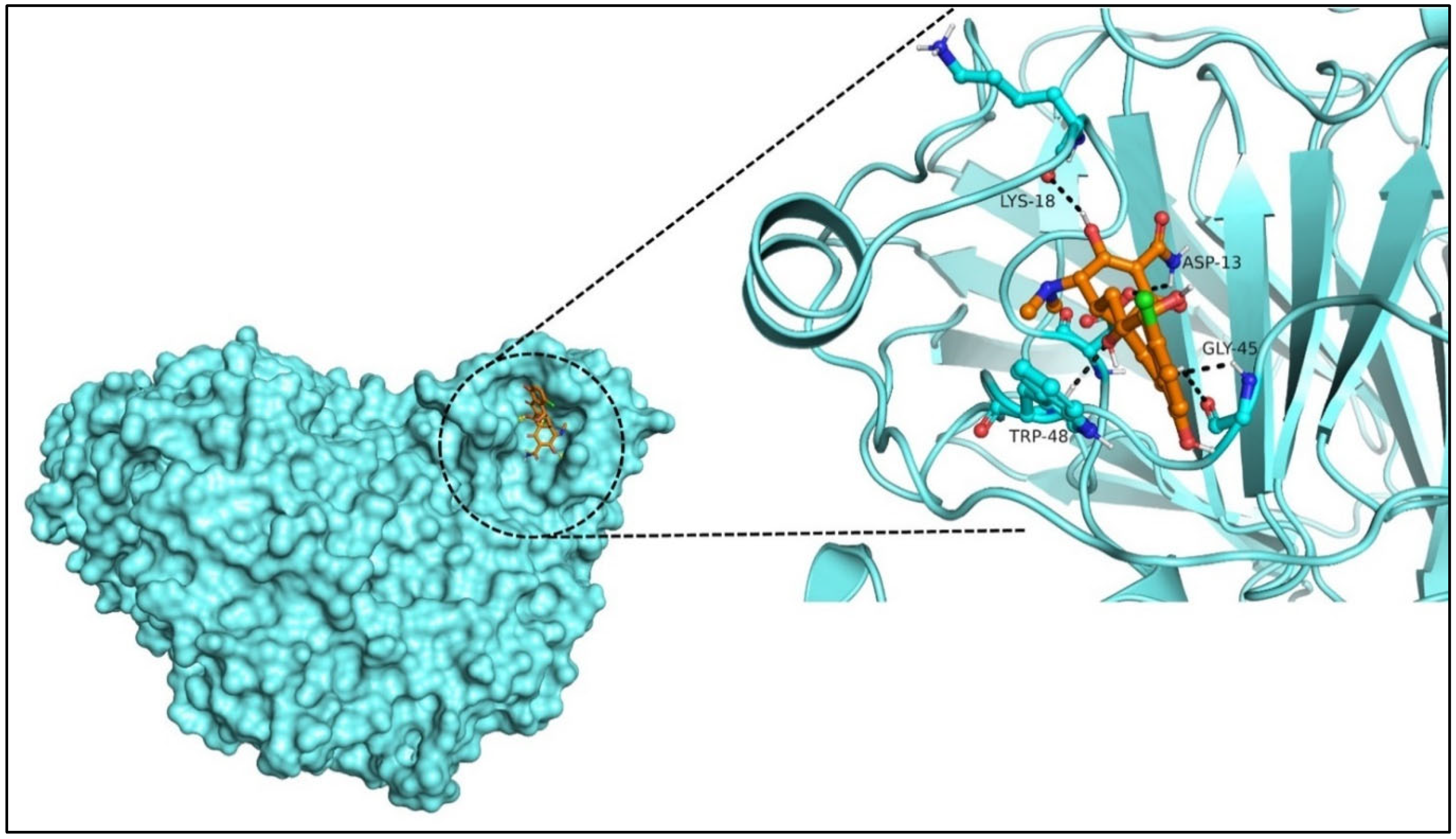
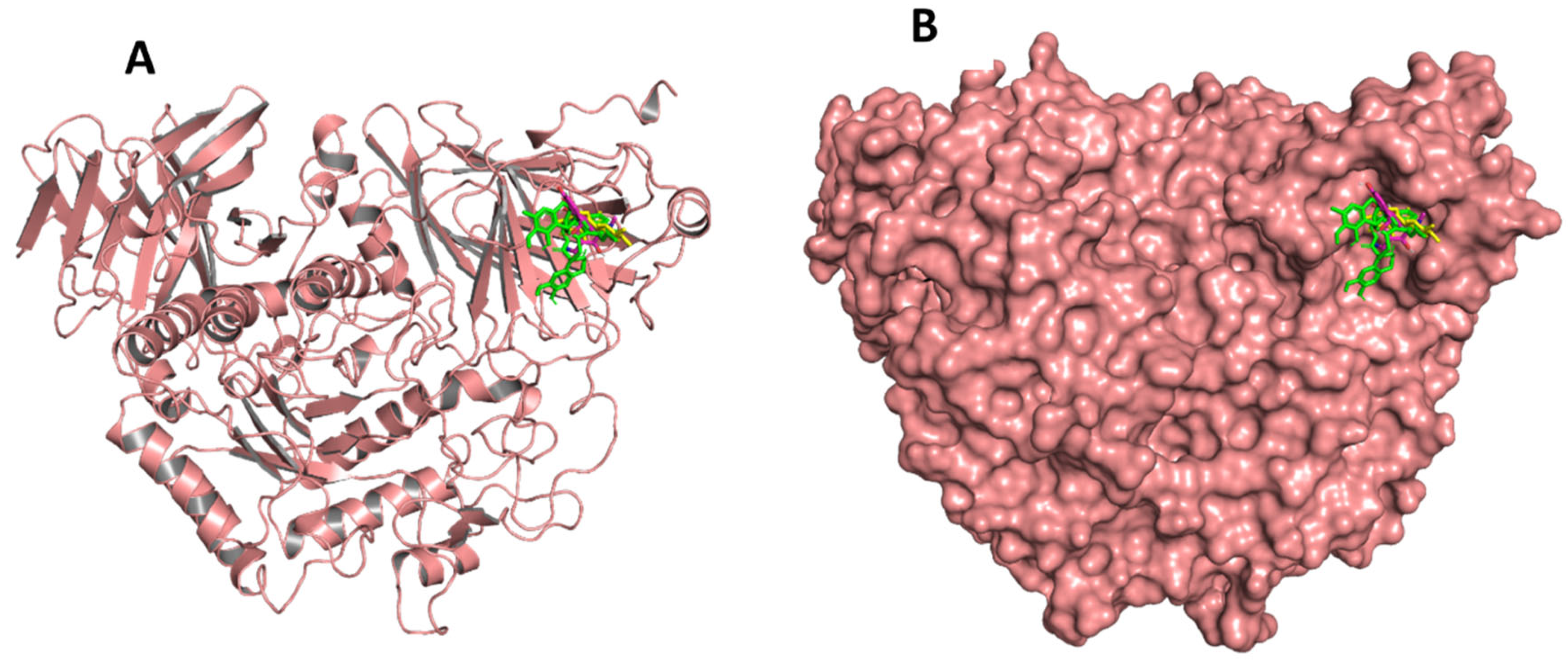
2.5. Molecular Docking and Lead Identification
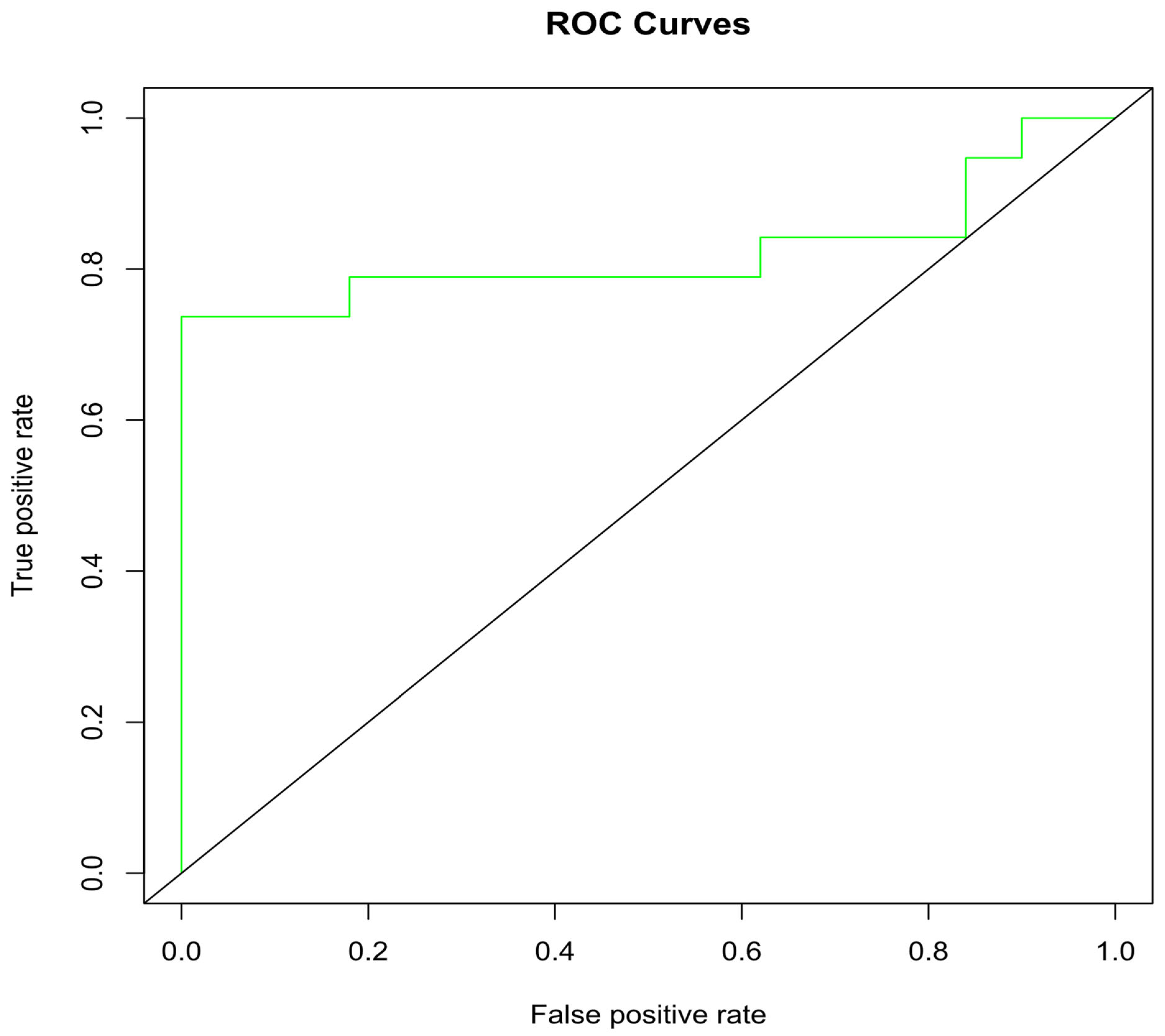
2.6. Docking Validation via ROC Curve
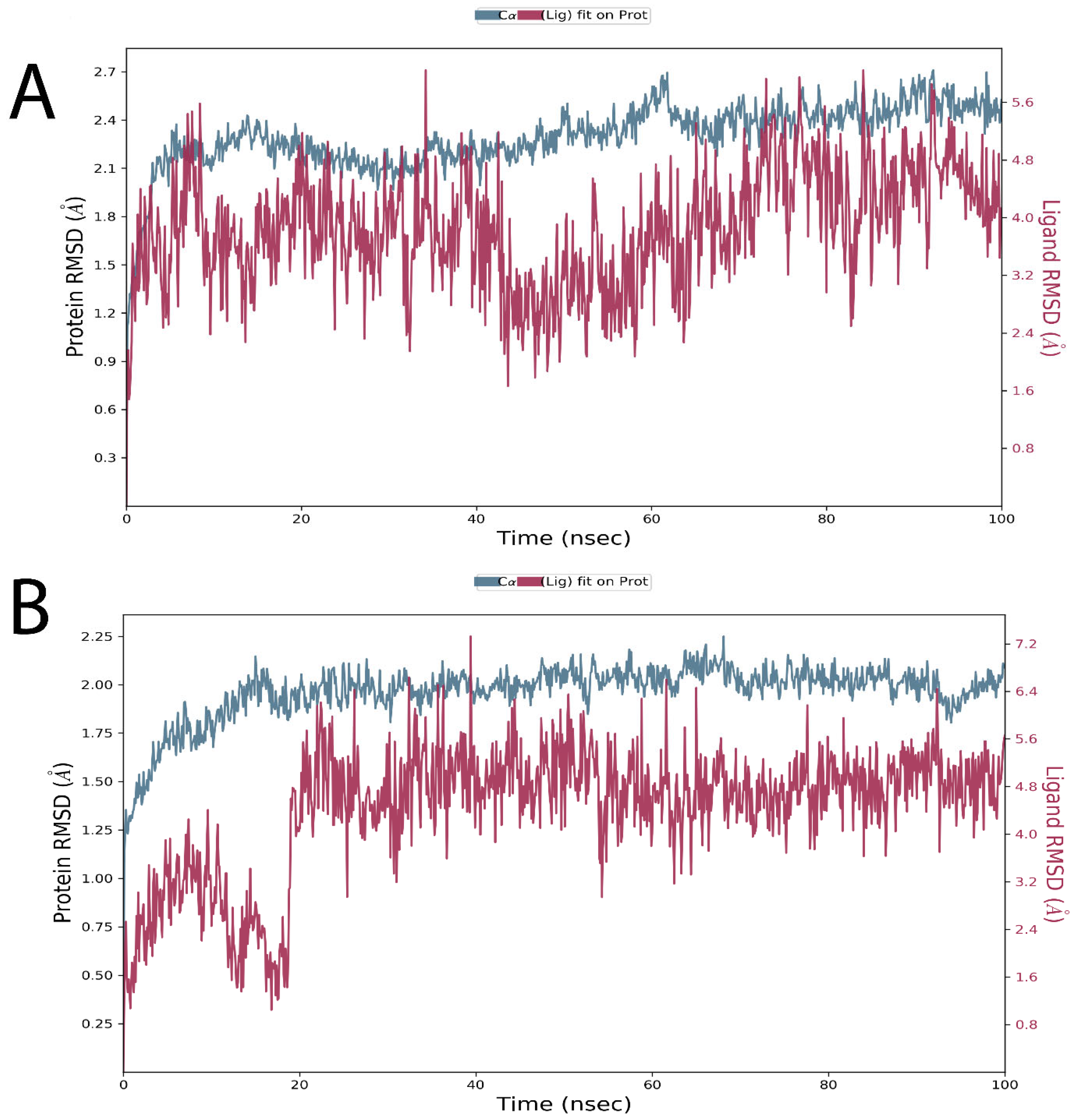
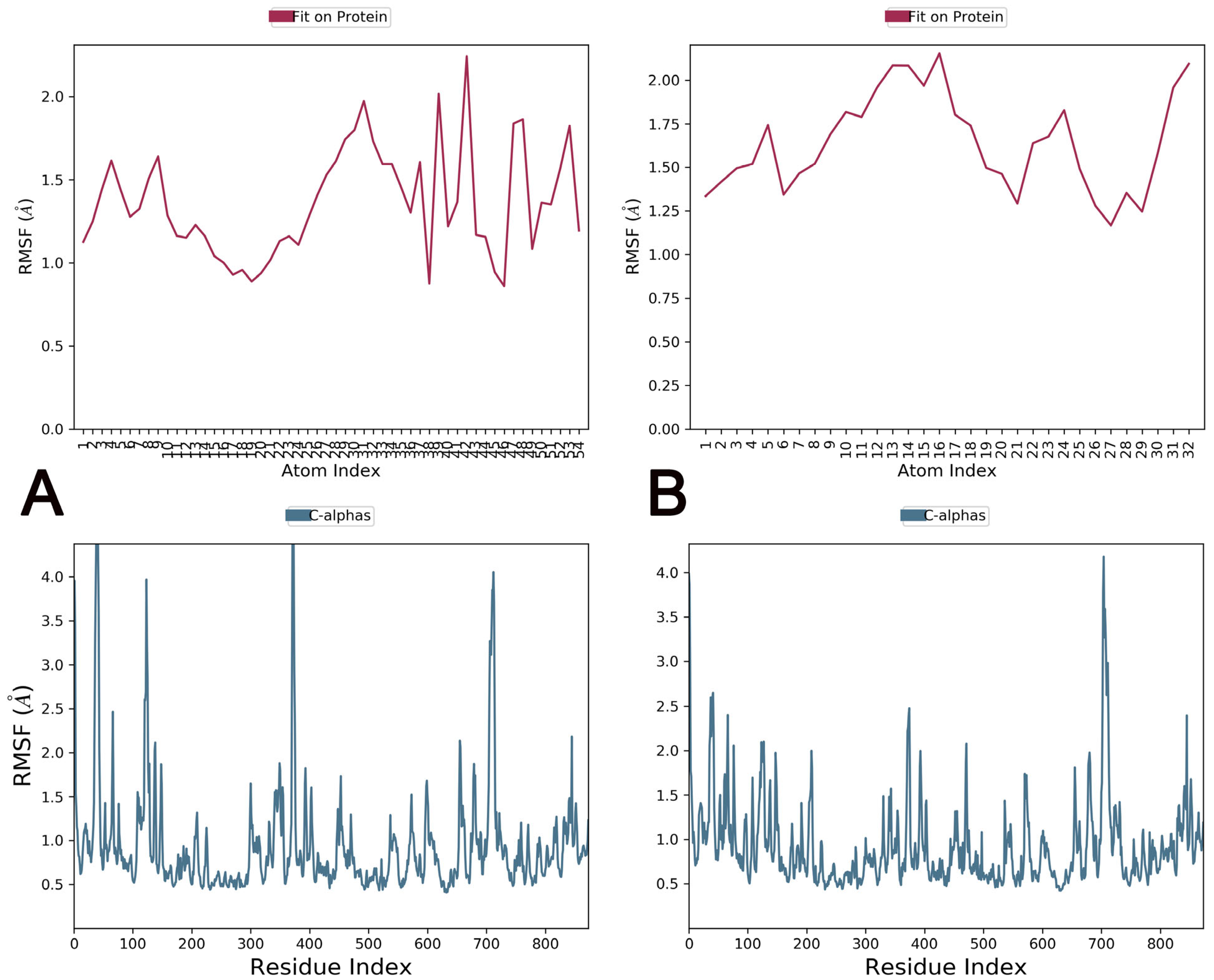
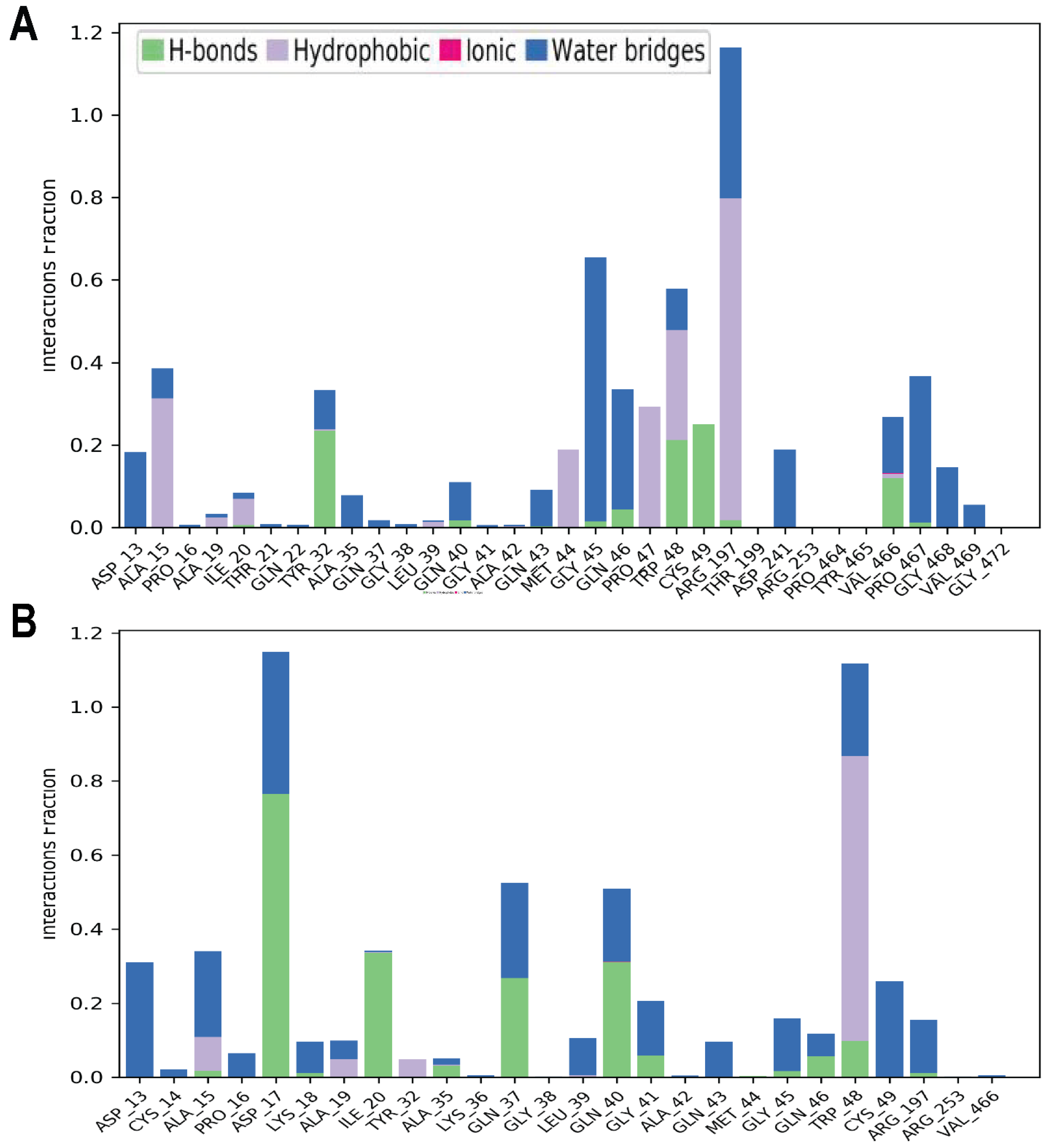
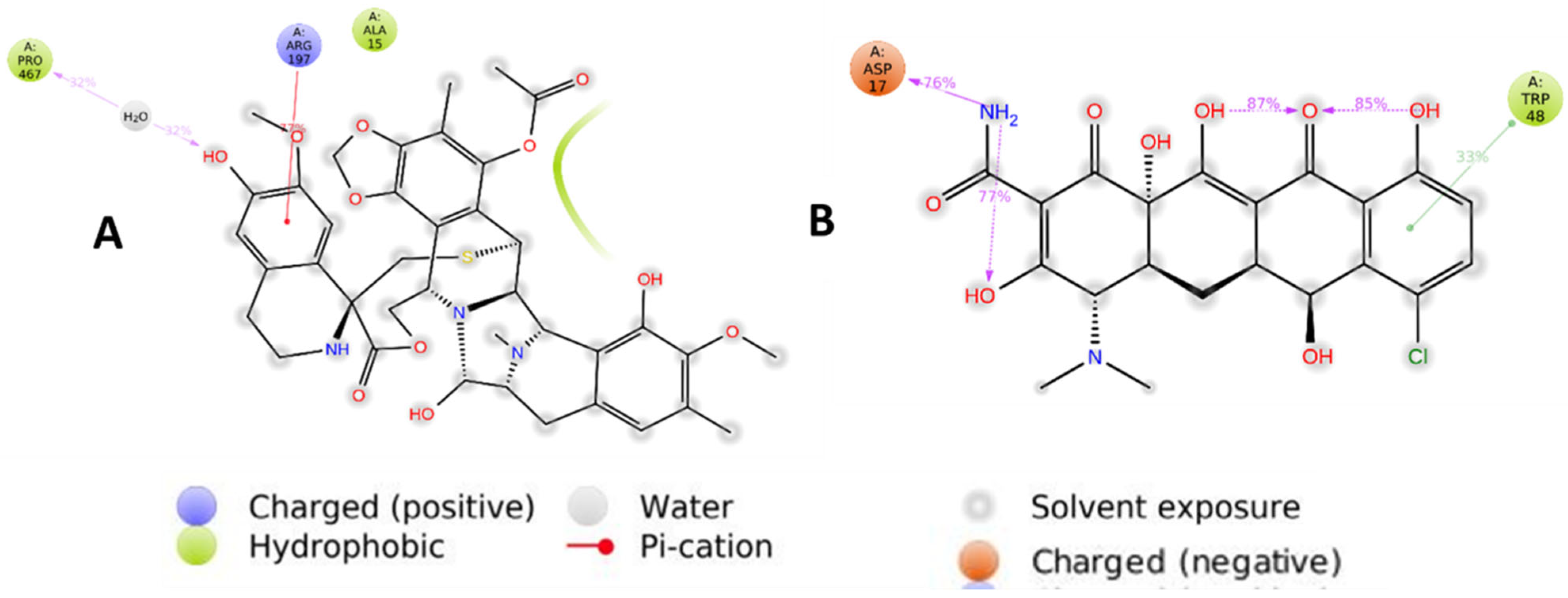
2.7. Molecular Dynamics Simulations
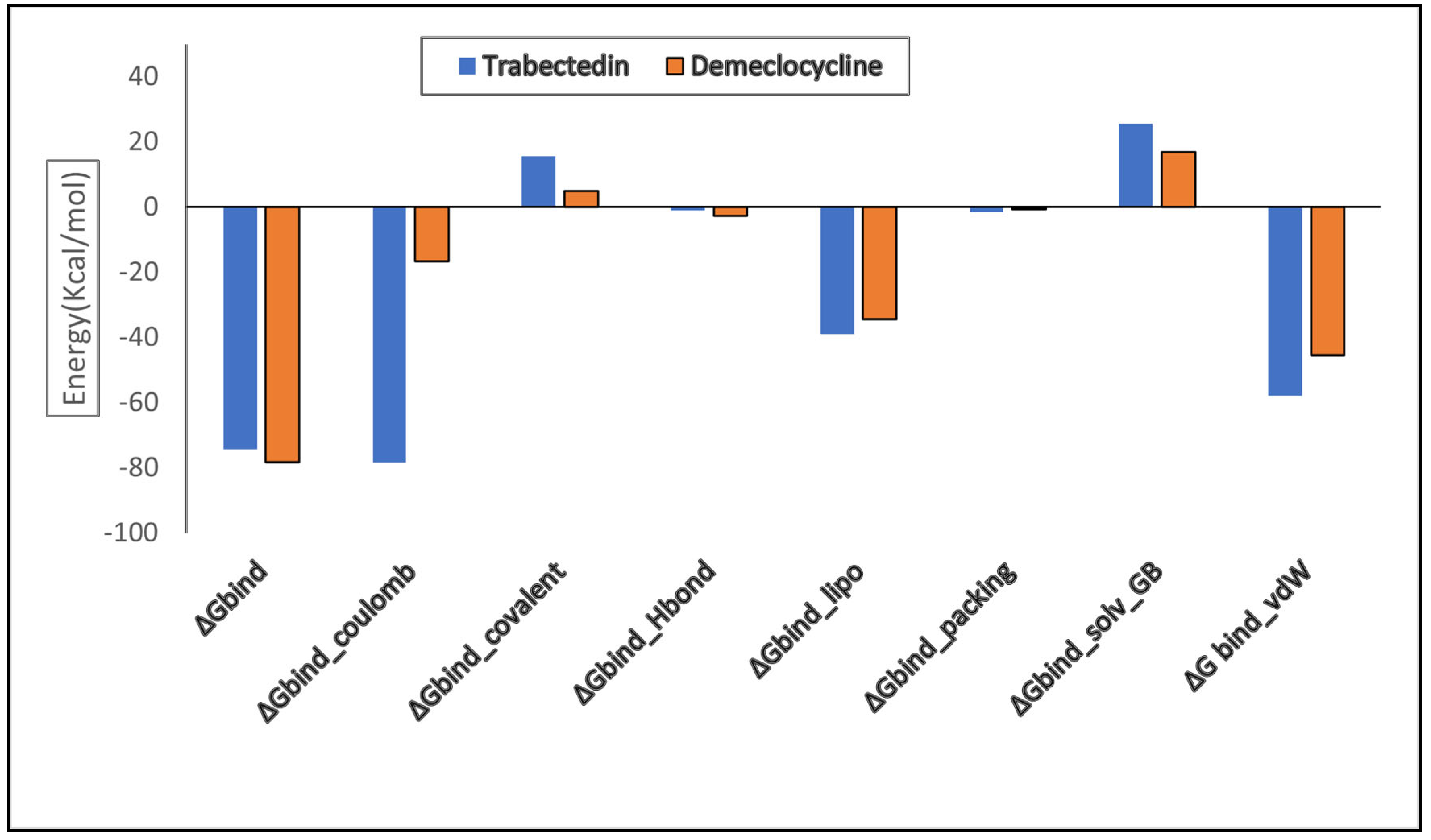
2.8. Binding Free Energy (MMGBSA) Calculation
2.9. Alpha-Glucosidase Assay
3. Material and Methods
3.1. Target Protein and Its Properties
3.2. Protein Structure Refinement, Optimization, and Minimization
3.3. Retrieval of Reported Inhibitors and Docking
3.4. Pharmacophore Query, Database Preparation, and Virtual Screening
3.5. Molecular Docking, Validation, and Lead Identification
3.6. MD Simulations and Binding-Free Energy (MMGBSA) Calculation
3.7. Chemicals and Reagents
3.8. α-glucosidase Inhibition Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aamir, A.H.; Ul-Haq, Z.; Mahar, S.A.; Qureshi, F.M.; Ahmad, I.; Jawa, A.; Sheikh, A.; Raza, A.; Fazid, S.; Jadoon, Z.; et al. Diabetes Prevalence Survey of Pakistan (DPS-PAK): Prevalence of type 2 diabetes mellitus and prediabetes using HbA1c: A population-based survey from Pakistan. BMJ Open 2019, 9, e025300. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of Type 2 Diabetes—Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health 2020, 10, 107–111. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation. 2022. Available online: https://diabetesatlas.org/2022-reports/ (accessed on 13 April 2022).
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Astrup, A. Healthy lifestyles in Europe: Prevention of obesity and type II diabetes by diet and physical activity. Public Health Nutr. 2001, 4, 499–515. [Google Scholar] [CrossRef]
- Shah, M.; Bashir, S.; Jaan, S.; Nawaz, H.; Nishan, U.; Abbasi, S.W.; Jamal, S.B.; Khan, A.; Afridi, S.G.; Iqbal, A. Computational Analysis of Plant-Derived Terpenes as α -glucosidase Inhibitors for the Discovery of Therapeutic Agents against Type 2 Diabetes Mellitus. S. Afr. J. Bot. 2021, 143, 462–473. [Google Scholar] [CrossRef]
- Zabidi, N.A.; Ishak, N.A.; Hamid, M.; Ashari, S.E.; Mohammad Latif, M.A. Inhibitory evaluation of Curculigo latifolia on α-glucosidase, DPP (IV) and in vitro studies in antidiabetic with molecular docking relevance to type 2 diabetes mellitus. J. Enzym. Inhib. Med. Chem. 2021, 36, 109–121. [Google Scholar] [CrossRef]
- Scheen, A.J. Is There a Role for α-Glucosidase Inhibitors in the Prevention of Type 2 Diabetes Mellitus? Drugs 2003, 63, 933–951. [Google Scholar] [CrossRef]
- Reuser, A.J.J.; Wisselaar, H.A. An evaluation of the potential side-effects of α-glucosidase inhibitors used for the management of diabetes mellitus. Eur. J. Clin. Investig. 1994, 24, 19–24. [Google Scholar] [CrossRef]
- Jiang, M.; Yan, H.; He, R.; Ma, Y. Purification and a molecular docking study of α-glucosidase-inhibitory peptides from a soybean protein hydrolysate with ultrasonic pretreatment. Eur. Food Res. Technol. 2018, 244, 1995–2005. [Google Scholar] [CrossRef]
- Luna, B.; Feinglos, M.N. Oral agents in the management of type 2 diabetes mellitus. Am. Fam. Physician 2001, 63, 1747–1756. [Google Scholar]
- Yen, F.-S.; Wei, J.C.-C.; Lin, M.-C.; Hsu, C.-C.; Hwu, C.-M. Long-term outcomes of adding alpha-glucosidase inhibitors in insulin-treated patients with type 2 diabetes. BMC Endocr. Disord. 2021, 21, 25. [Google Scholar] [CrossRef]
- Dirir, A.M.; Daou, M.; Yousef, A.F.; Yousef, L.F. A review of alpha-glucosidase inhibitors from plants as potential candidates for the treatment of type-2 diabetes. Phytochem. Rev. Proc. Phytochem. Soc. Eur. 2022, 21, 1049–1079. [Google Scholar] [CrossRef] [PubMed]
- Trang Nguyen, N.D.; Le, L.T. Targeted proteins for diabetes drug design. Adv. Nat. Sci. Nanosci. Nanotechnol. 2012, 3, 013001. [Google Scholar] [CrossRef]
- Alqahtani, A.S.; Hidayathulla, S.; Rehman, T.; ElGamal, A.A.; Al-Massarani, S.; Razmovski-Naumovski, V.; Alqahtani, M.S.; El Dib, R.A.; AlAjmi, M.F. Alpha-Amylase and Alpha-Glucosidase Enzyme Inhibition and Antioxidant Potential of 3-Oxolupenal and Katononic Acid Isolated from Nuxia oppositifolia. Biomolecules 2020, 10, 61. [Google Scholar] [CrossRef] [PubMed]
- Roig-Zamboni, V.; Cobucci-Ponzano, B.; Iacono, R.; Ferrara, M.C.; Germany, S.; Bourne, Y.; Parenti, G.; Moracci, M.; Sulzenbacher, G. Structure of human lysosomal acid α-glucosidase—A guide for the treatment of Pompe disease. Nat. Commun. 2017, 8, 1111. [Google Scholar] [CrossRef]
- Muzammil, S.; Andleeb, R.; Hayat, S.; Ijaz, M.U.; Ashraf, A.; Zafar, N.; Naz, S.; Shaheen, M. Integrating In Silico and In Vitro Approaches to Screen the Antidiabetic Properties from Tabernaemontana divaricata (Jasmine) Flowers. Evid.-Based Complement. Altern. Med. 2022, 2022, 4616815. [Google Scholar] [CrossRef]
- Wairata, J.; Sukandar, E.R.; Fadlan, A.; Purnomo, A.S.; Taher, M.; Ersam, T. Evaluation of the Antioxidant, Antidiabetic, and Antiplasmodial Activities of Xanthones Isolated from Garcinia forbesii and Their In Silico Studies. Biomedicines 2021, 9, 1380. [Google Scholar] [CrossRef]
- Hossain, U.; Das, A.K.; Ghosh, S.; Sil, P.C. An overview on the role of bioactive α-glucosidase inhibitors in ameliorating diabetic complications. Food Chem. Toxicol. 2020, 145, 111738. [Google Scholar] [CrossRef]
- Le, T.K.D.; Danova, A.; Aree, T.; Duong, T.H.; Koketsu, M.; Ninomiya, M.; Sawada, Y.; Kamsri, P.; Pungpo, P.; Chavasiri, W. α-Glucosidase Inhibitors from the Stems of Knema globularia. J. Nat. Prod. 2022, 85, 776–786. [Google Scholar] [CrossRef]
- Carter, N.J.; Keam, S.J. Trabectedin: A review of its use in the management of soft tissue sarcoma and ovarian cancer. Drugs 2007, 67, 2257–2276. [Google Scholar] [CrossRef] [PubMed]
- Shahid, R.K.; Ahmed, S.; Le, D.; Yadav, S. Diabetes and Cancer: Risk, Challenges, Management and Outcomes. Cancers 2021, 13, 5735. [Google Scholar] [CrossRef] [PubMed]
- Deming, D.T.; Garman, S.C. The molecular basis of Pompe disease: Crystal structure of acid alpha glucosidase. Mol. Genet. Metab. 2016, 117, S40–S41. [Google Scholar] [CrossRef]
- Ramachandran, S.; Kota, P.; Ding, F.; Dokholyan, N.V. Automated minimization of steric clashes in protein structures. Proteins: Struct. Funct. Bioinform. 2011, 79, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Chen, C.; Lei, X.; Zhao, J.; Liang, J. CASTp 3.0: Computed atlas of surface topography of proteins. Nucleic Acids Res. 2018, 46, W363–W367. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An open chemical toolbox. J. Cheminformatics 2011, 3, 33. [Google Scholar] [CrossRef]
- Bowers, K.J.; Chow, E.; Xu, H.; Dror, R.O.; Eastwood, M.P.; Gregersen, B.A.; Klepeis, J.L.; Kolossvary, I.; Moraes, M.A.; Sacerdoti, F.D.; et al. Scalable algorithms for molecular dynamics simulations on commodity clusters. In Proceedings of the 2006 ACM/IEEE Conference on Supercomputing, Association for Computing Machinery, Tampa, FL, USA, 11 November 2006. [Google Scholar]
- Shivakumar, D.; Williams, J.; Wu, Y.; Damm, W.; Shelley, J.; Sherman, W. Prediction of Absolute Solvation Free Energies using Molecular Dynamics Free Energy Perturbation and the OPLS Force Field. J. Chem. Theory Comput. 2010, 6, 1509–1519. [Google Scholar] [CrossRef]
- Jacobson, M.P.; Pincus, D.L.; Rapp, C.S.; Day, T.J.F.; Honig, B.; Shaw, D.E.; Friesner, R.A. A hierarchical approach to all-atom protein loop prediction. Proteins: Struct. Funct. Bioinform. 2004, 55, 351–367. [Google Scholar] [CrossRef]
- Flores-Bocanegra, L.; Pérez-Vásquez, A.; Torres-Piedra, M.; Bye, R.; Linares, E.; Mata, R. α-Glucosidase Inhibitors from Vauquelinia corymbosa. Molecules 2015, 20, 15330–15342. [Google Scholar] [CrossRef]
- Hao, Q.; Huang, Z.; Li, Q.; Liu, D.; Wang, P.; Wang, K.; Li, J.; Cao, W.; Deng, W.; Wu, K.; et al. A Novel Metabolic Reprogramming Strategy for the Treatment of Diabetes-Associated Breast Cancer. Adv. Sci. 2022, 9, 2102303. [Google Scholar] [CrossRef]
- Joharatnam-Hogan, N.; Chambers, P.; Dhatariya, K.; Board, R.; Joint British Diabetes Society for Inpatient Care (JBDS); UK Chemotherapy Board (UKCB). A guideline for the outpatient management of glycaemic control in people with cancer. Diabet. Med. 2022, 39, e14636. [Google Scholar] [CrossRef]
| Small Molecules | IC50, and Kd | Structure | HBA | HBD | MW (KDa) | RB | LOG P | Lipinski Violation | Publications |
|---|---|---|---|---|---|---|---|---|---|
| Celgosivir | 15.95 mM | 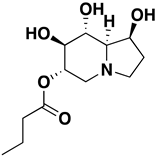 | 6 | 3 | 259.40 | 4 | −0.8 | NO | https://journals.sagepub.com/doi/pdf/10.1177/095632020401500304 (accessed on 12 April 2022) |
| 4-(4-methylbenzenesulfonyl)-N,N-diphenylpiperazine-1-carboxamide | 25.1189 µM | 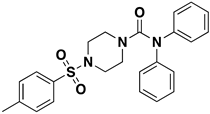 | 4 | 0 | 435.4 | 4 | 3.8 | NO | https://pubchem.ncbi.nlm.nih.gov/compound/1322817 (accessed on 12 April 2022) |
| Voglibose | 23.4 µM | 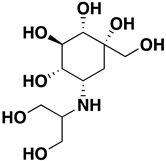 | 8 | 8 | 267.28 | 5 | 3.1 | NO | https://pubmed.ncbi.nlm.nih.gov/15558946/ (accessed on 12 April 2022) |
| IDs | Drug/ Molecule Name | Structures | Binding Score | Ph4 Score |
|---|---|---|---|---|
| ZINC000150338708 | Trabectedin | 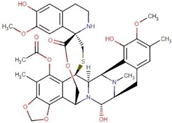 | −8.8 | 0.490963817 |
| ZINC000100036924 | Demeclocycline | 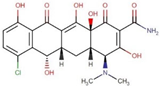 | −8.6 | 0.64888829 |
| ZINC000085537053 | Docetaxel | 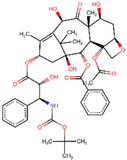 | −8.6 | 0.780447185 |
| ZINC000028232750 | Valstar | 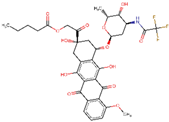 | −8 | 0.726529598 |
| ZINC000096006020 | Paclitaxel | 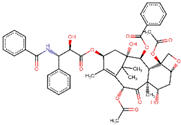 | −7.9 | 0.727842093 |
| ZINC000003927198 | E-Cefdinir | 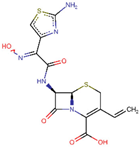 | −7.8 | 0.501275837 |
| ZINC000085536932 | Cabazitaxel | 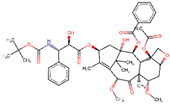 | −7.7 | 0.719660044 |
| ZINC000003830215 | Amoxicillin | 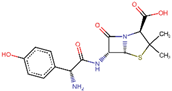 | −7.5 | 0.82327193 |
| ZINC000004474682 | Travoprost | 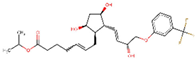 | −7.4 | 0.78199023 |
| ZINC000003794794 | Mitoxantrone | 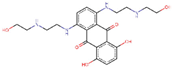 | −7.2 | 0.807539582 |
| ZINC000004474405 | Latisse | 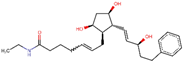 | −6.7 | 0.685938478 |
| Parameters | Trabectedin (Kcal/mol) | Demeclocycline (Kcal/mol) |
|---|---|---|
| ΔGbind | −74.36 | −78.31 |
| ΔGbind_Coulomb | −16.15 | −16.73 |
| ΔGbind_covalent | 15.66 | 4.85 |
| ΔGbind_Hbond | −0.98 | −2.78 |
| ΔGbind_lipo | −39.11 | −34.51 |
| ΔGbind_packing | −1.42 | −0.60 |
| ΔGbind_solv_GB | 25.53 | 16.96 |
| ΔG bind_vdW | −57.89 | −45.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rashid, R.S.M.; Temurlu, S.; Abourajab, A.; Karsili, P.; Dinleyici, M.; Al-Khateeb, B.; Icil, H. Drug Repurposing of FDA Compounds against α-Glucosidase for the Treatment of Type 2 Diabetes: Insights from Molecular Docking and Molecular Dynamics Simulations. Pharmaceuticals 2023, 16, 555. https://doi.org/10.3390/ph16040555
Rashid RSM, Temurlu S, Abourajab A, Karsili P, Dinleyici M, Al-Khateeb B, Icil H. Drug Repurposing of FDA Compounds against α-Glucosidase for the Treatment of Type 2 Diabetes: Insights from Molecular Docking and Molecular Dynamics Simulations. Pharmaceuticals. 2023; 16(4):555. https://doi.org/10.3390/ph16040555
Chicago/Turabian StyleRashid, Rebwar Saeed M., Selin Temurlu, Arwa Abourajab, Pelin Karsili, Meltem Dinleyici, Basma Al-Khateeb, and Huriye Icil. 2023. "Drug Repurposing of FDA Compounds against α-Glucosidase for the Treatment of Type 2 Diabetes: Insights from Molecular Docking and Molecular Dynamics Simulations" Pharmaceuticals 16, no. 4: 555. https://doi.org/10.3390/ph16040555
APA StyleRashid, R. S. M., Temurlu, S., Abourajab, A., Karsili, P., Dinleyici, M., Al-Khateeb, B., & Icil, H. (2023). Drug Repurposing of FDA Compounds against α-Glucosidase for the Treatment of Type 2 Diabetes: Insights from Molecular Docking and Molecular Dynamics Simulations. Pharmaceuticals, 16(4), 555. https://doi.org/10.3390/ph16040555






