Ethnobotanical, Phytochemical, Toxicological, and Pharmacological Properties of Ziziphus lotus (L.) Lam.: A Comprehensive Review
Abstract
1. Introduction
2. Methodology
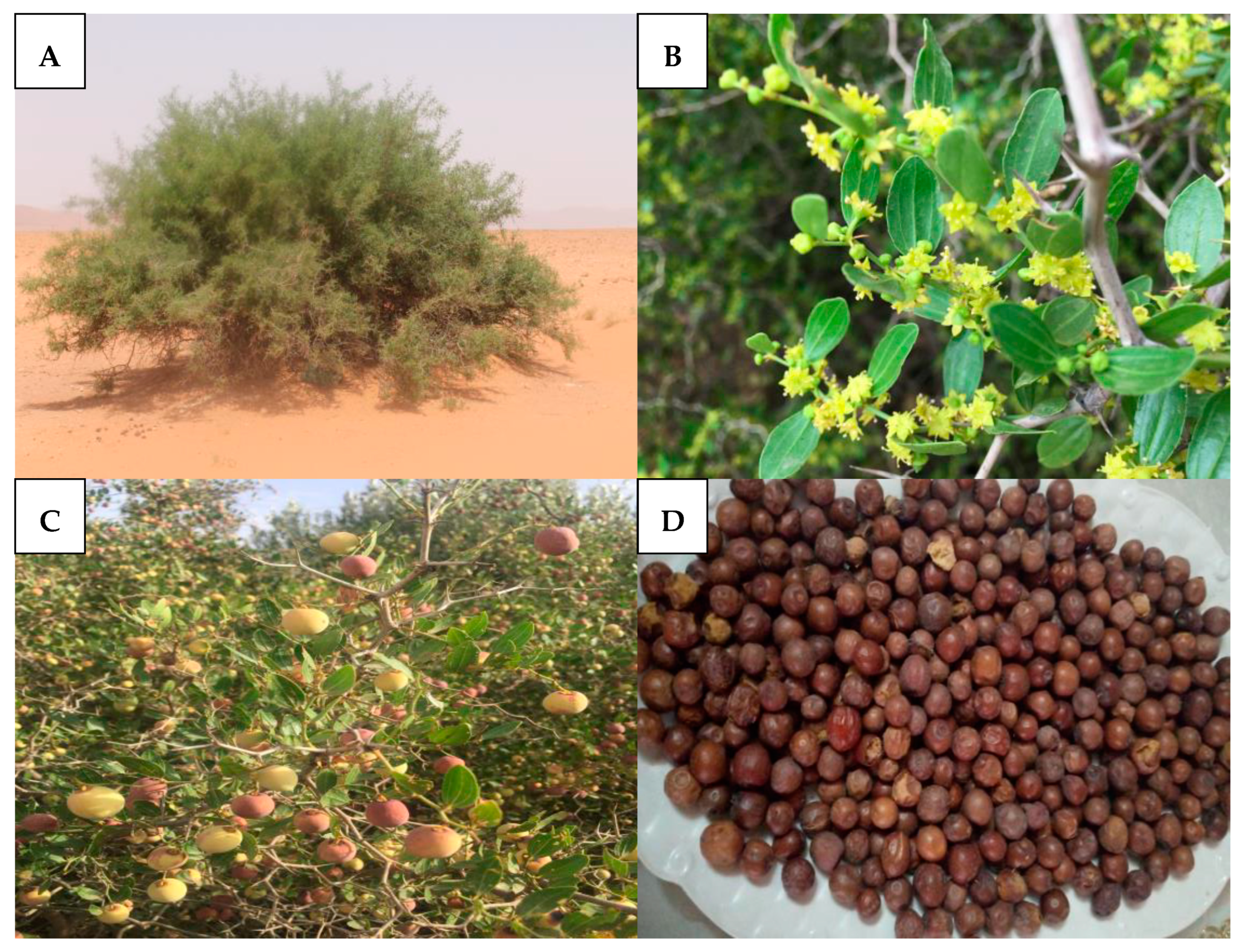
3. Botanical Description
4. Eco-Geographical Features
5. Ethnomedicinal Uses
6. Phytoconstituents of Z. lotus
7. Pharmacological Activities
7.1. Antidiabetic Activity
7.2. Anti-Obesity and Dyslipidemic Activity
7.3. Antiulcerogenic and Anti-Spasmodic Activities
7.4. Anti-Inflammatory and Immunomodulatory Activities
7.5. Analgesic Activity
7.6. Anti-Cancer and Anti-Proliferative Activities
7.7. Hepato-Renoprotective Effects
7.8. Antimicrobial Activity
7.9. Antioxidant Activity
7.10. Others Activities
8. Toxicology
9. Concluding Remarks and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
References
- Ouassou, H.; Bouhrim, M.; Kharchoufa, L.; Imtara, H.; Daoudi, N.E.; Benoutman, A.; Bencheikh, N.; Ouahhoud, S.; Elbouzidi, A.; Bnouham, M. Caralluma europaea (Guss) N.E.Br.: A review on ethnomedicinal uses, phytochemistry, pharmacological activities, and toxicology. J. Ethnopharmacol. 2021, 273, 113769. [Google Scholar] [CrossRef]
- Bencheikh, N.; Elachouri, M.; Subhash, C.M. Ethnobotanical, pharmacological, phytochemical, and clinical investigations on Moroccan medicinal plants traditionally used for the management of renal dysfunctions. J. Ethnopharmacol. 2022, 292, 115178. [Google Scholar] [CrossRef]
- Singh, J.; Singh, J.; Sharma, D. Traditional wisdom to treat the most common ailments in chopal region of Shimla district, himachal pradesh, India. Plant Arch. 2018, 18, 2759–2769. [Google Scholar]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef]
- Taib, M.; Rezzak, Y.; Bouyazza, L.; Lyoussi, B. Medicinal Uses, Phytochemistry, and Pharmacological Activities of Quercus Species. Evidence-Based Complement. Altern. Med. 2020, 2020, 20. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef]
- Li, J.W.-H.; Vederas, J.C. Drug Discovery and Natural Products: End of an Era or an Endless Frontier? Science 2009, 325, 161–165. [Google Scholar] [CrossRef]
- Mohammed, A.; Jamila, F.; Mostafa, E. First insight on ethnobotanical appraisal of plants used traditionally as medicine by berber community (Amazigh-speaking), living in driouch province (north-eastern morocco). Ethnobot. Res. Appl. 2021, 22, 2–70. [Google Scholar] [CrossRef]
- Alami Merrouni, I.; Kharchoufa, L.; Bencheikh, N.; Elachouri, M. Ethnobotanical profile of medicinal plants used by people of North-eastern Morocco: Cross-cultural and historical approach (part I). Ethnobot. Res. Appl. 2021, 21, 1–45. [Google Scholar] [CrossRef]
- Fakchich, J.; Elachouri, M. An overview on ethnobotanico-pharmacological studies carried out in Morocco, from 1991 to 2015: Systematic review (part 1). J. Ethnopharmacol. 2021, 267, 113–200. [Google Scholar] [CrossRef]
- Gorai, M.; Maraghni, M.; Neffati, M. TPED Relationship between phenological traits and water potential patterns of the wild jujube Ziziphus lotus (L.) Lam. in southern Tunisia. Plant Ecol. Divers. 2010, 3, 273–280. [Google Scholar] [CrossRef]
- Richardson, J.E.; Chatrou, L.W.; Mols, J.B.; Erkens, R.H.J.; Pirie, M.D. Historical biogeography of two cosmopolitan families of flowering plants: Annonaceae and Rhamnaceae. Philos. Trans. R. Soc. B Biol. Sci. 2004, 359, 1495–1508. [Google Scholar] [CrossRef]
- Adeli, M.; Samavati, V. Studies on the steady shear flow behavior and chemical properties of water-soluble polysaccharide from Ziziphus lotus fruit. Int. J. Biol. Macromol. 2014, 72, 580–587. [Google Scholar] [CrossRef]
- Berkani, F.; Serralheiro, M.L.; Dahmoune, F.; Mahdjoub, M.; Kadri, N.; Dairi, S.; Achat, S.; Remini, H.; Abbou, A.; Adel, K.; et al. Ziziphus lotus (L.) Lam. plant treatment by ultrasounds and microwaves to improve antioxidants yield and quality: An overview. N. Afr. J. Food Nutr. Res. 2021, 5, 53–68. [Google Scholar] [CrossRef]
- Benammar, C.; Baghdad, C.; Belarbi, M.; Subramaniam, S.; Hichami, A.; Khan, N.A. Antidiabetic and Antioxidant Activities of Zizyphus lotus L Aqueous Extracts in Wistar Rats. J. Nutr. Food Sci. 2014, s8, 8–13. [Google Scholar] [CrossRef]
- Abdoul-Azize, S.; Bendahmane, M.; Hichami, A.; Dramane, G.; Simonin, A.-M.; Benammar, C.; Sadou, H.; Akpona, S.; EI Boustani, E.-S.; Khan, N.A. Effects of Zizyphus lotus L. (Desf.) polyphenols on Jurkat cell signaling and proliferation. Int. Immunopharmacol. 2013, 15, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Dhibi, M.; Amri, Z.; Bhouri, A.M.; Hammami, S.; Hammami, M. Comparative study of the phenolic profile and antioxidant activities of Moringa (Moringa oleifera Lam.) and Jujube (Ziziphus lotus Linn.) leaf extracts and their protective effects in frying stability of corn oil. Meas. Food 2022, 7, 100045. [Google Scholar] [CrossRef]
- Chaimae, R.; Meryem, B.; Chaimae, S.; Bouchamma, E.-O.; Hamza, E.; Laila, E.; Lahsen, E.G.; Meryem, B. Antimicrobial and radical scavenging activities of Moroccan Ziziphus lotus L. seeds. J. Phytopharm. 2019, 8, 155–160. [Google Scholar] [CrossRef]
- Yahia, Y.; Benabderrahim, M.A.; Tlili, N.; Bagues, M.; Nagaz, K. Bioactive compounds, antioxidant and antimicrobial activities of extracts from different plant parts of two Ziziphus Mill. species. PLoS ONE 2020, 15, e0232599. [Google Scholar] [CrossRef]
- Marmouzi, I.; Kharbach, M.; El Jemli, M.; Bouyahya, A.; Cherrah, Y.; Bouklouze, A.; Vander Heyden, Y.; Faouzi, M.E.A. Antidiabetic, dermatoprotective, antioxidant and chemical functionalities in Zizyphus lotus leaves and fruits. Ind. Crops Prod. 2019, 132, 134–139. [Google Scholar] [CrossRef]
- Bencheikh, N.; Bouhrim, M.; Kharchoufa, L.; Al Kamaly, O.M.; Mechchate, H.; Es-Safi, I.; Dahmani, A.; Ouahhoud, S.; El Assri, S.; Eto, B.; et al. The nephroprotective effect of Zizyphus lotus L. (Desf.) fruits in a gentamicin-induced acute kidney injury model in rats: A biochemical and histopathological investigation. Molecules 2021, 26, 4806. [Google Scholar] [CrossRef] [PubMed]
- Bencheikh, N.; Bouhrim, M.; Kharchoufa, L.; Choukri, M.; Bnouham, M.; Elachouri, M. Protective Effect of Zizyphus lotus L. (Desf.) Fruit against CCl4-Induced Acute Liver Injury in Rat. Evid.-Based Complement. Altern. Med. 2019, 2019, 2–9. [Google Scholar] [CrossRef]
- Bekkar, N.E.H.; Meddah, B.; Keskin, B.; Sonnet, P. Oral acute toxicity, influence on the gastrointestinal microbiota and in vivo anti-salmonellosis effect of Zizyphus lotus (L.) and Ruta chalepensis (L.) essential oils. J. Appl. Biotechnol. Rep. 2021, 8, 13–26. [Google Scholar] [CrossRef]
- El Cadi, H.; EL Bouzidi, H.; Selama, G.; El Cadi, A.; Ramdan, B.; El Majdoub, Y.O.; Alibrando, F.; Dugo, P.; Mondello, L.; Fakih Lanjri, A.; et al. Physico-Chemical and Phytochemical Characterization of Moroccan Wild Jujube “Zizyphus lotus (L.)” Fruit Crude Extract and Fractions. Molecules 2020, 25, 5237. [Google Scholar] [CrossRef] [PubMed]
- Letaief, T.; Garzoli, S.; Masci, V.L.; Tiezzi, A.; Ovidi, E. Chemical composition and biological activities of tunisian Ziziphus lotus extracts: Evaluation of drying effect, solvent extraction, and extracted plant parts. Plants 2021, 10, 2651. [Google Scholar] [CrossRef] [PubMed]
- Rached, W.; Barros, L.; Ziani, B.E.C.; Bennaceur, M.; Calhelha, R.C.; Heleno, S.A.; Alves, M.J.; Marouf, A.; Ferreira, I.C.F.R. HPLC-DAD-ESI-MS/MS screening of phytochemical compounds and the bioactive properties of different plant parts of: Zizyphus lotus (L.) Desf. Food Funct. 2019, 10, 5898–5909. [Google Scholar] [CrossRef]
- Ghazghazi, H.; Aouadhi, C.; Riahi, L.; Maaroufi, A.; Hasnaoui, B. Fatty acids composition of Tunisian Ziziphus lotus L. (Desf.) fruits and variation in biological activities between leaf and fruit extracts. Nat. Prod. Res. 2014, 28, 1106–1110. [Google Scholar] [CrossRef]
- Abdoul-Azize, S. Potential Benefits of Jujube (Zizyphus lotus L.) Bioactive Compounds for Nutrition and Health. J. Nutr. Metab. 2016, 2016, 2867470. [Google Scholar] [CrossRef]
- Borgi, W.; Ghedira, K.; Chouchane, N. Antiinflammatory and analgesic activities of Zizyphus lotus root barks. Fitoterapia 2007, 78, 16–19. [Google Scholar] [CrossRef]
- Dahlia, F.; Benita, C.; Boussaid, M. Genetic diversity of fruits in wild jujube (Ziziphus lotus L. Desf.) natural populations from Algeria. Agric. For. 2019, 65, 165–183. [Google Scholar] [CrossRef]
- De Cortes Sánchez-Mata, M.; Tardío, J. Mediterranean Wild Edible Plants: Ethnobotany and Food Composition Tables; Springer: New York, NY, USA, 2016; ISBN 9781493933297. [Google Scholar]
- Ghedira, K. Zizyphus lotus (L.) Desf. (Rhamnaceae): Jujubier sauvage. Phytotherapie 2013, 11, 149–153. [Google Scholar] [CrossRef]
- Asatryan, A.; Tel-Zur, N. Intraspecific and interspecific crossability in three Ziziphus species (Rhamnaceae). Genet. Resour. Crop Evol. 2014, 61, 215–233. [Google Scholar] [CrossRef]
- Maraghni, M.; Gorai, M.; Neffati, M. Seed germination at different temperatures and water stress levels, and seedling emergence from different depths of Ziziphus lotus. S. Afr. J. Bot. 2010, 76, 453–459. [Google Scholar] [CrossRef]
- Bencheikh, N.; Bouhrim, M.; Merrouni, I.A.; Boutahiri, S.; Legssyer, A.; Elachouri, M. Antihyperlipidemic and Antioxidant Activities of Flavonoid-Rich Extract of Ziziphus lotus (L.) Lam. Fruits. Appl. Sci. 2021, 11, 7788. [Google Scholar] [CrossRef]
- Bencheikh, N.; Elbouzidi, A.; Kharchoufa, L.; Ouassou, H.; Merrouni, I.A.; Mechchate, H.; Es-Safi, I.; Hano, C.; Addi, M.; Bouhrim, M.; et al. Inventory of medicinal plants used traditionally to manage kidney diseases in north-eastern Morocco: Ethnobotanical fieldwork and pharmacological evidence. Plants 2021, 10, 1966. [Google Scholar] [CrossRef] [PubMed]
- Ammor, K.; Mahjoubi, F.; Bousta, D.; Chaqroune, A. Ethnobotanical survey of medicinal plants used in the treatment of kidney stones in Region of Fez-Meknes, Morocco. Ethnobot. Res. Appl. 2020, 19, 1–12. [Google Scholar] [CrossRef]
- Belhaj, S.; Dahmani, J.; Belahbib, N.; Zidane, L. Ethnopharmacological and ethnobotanical study of medicinal plants in the high atlas central, Morocco. Ethnobot. Res. Appl. 2020, 20, 1–40. [Google Scholar] [CrossRef]
- Ouhaddou, H.; Alaoui, A.; Laaribya, S.; Ayan, S. An ethnobotanical study on medicinal plants used for curing diabetes in Agadir Ida Outanane Region, Southwest Morocco. Biol. Divers. Conserv. 2020, 13, 80–87. [Google Scholar] [CrossRef]
- Cheriti, A.; Rouissat, A.; Sekkoum, K.; Balansard, G. Plantes de la pharmacopée traditionelle dans la région d’El-Bayadh (Algérie). Fitoter 1995, 66, 525–538. [Google Scholar]
- Allali, H.; Benmehdi, H.; Dib, M.A.; Tabti, B.; Ghalem, S.; Benabadji, N. Phytotherapy of diabetes in West Algeria. Asian J. Chem. 2008, 20, 2701–2710. [Google Scholar]
- Zatout, F.; Benarba, B.; Bouazza, A.; Babali, B.; Bey, N.N.; Morsli, A. Ethnobotanical investigation on medicinal plants used by local populations in tlemcen national park (extreme North West Algeria). Mediterr. Bot. 2021, 42, e69396. [Google Scholar] [CrossRef]
- Benderradji, L.; Rebbas, K.; Ghadbane, M.; Bounar, R.; Brini, F.; Bouzerzour, H. Ethnobotanical Study of Medicinal Plants in Djebel messaad region (M’Sila, Algeria). Glob. J. Res. Med. Plants Indig. Med. 2014, 3, 445–459. [Google Scholar]
- Madani, S.; Hendel, N.; Boudjelal, A.; Sarri, D. Inventory of medicinal plants used for traditional treatment of eczema in the region of Honda (M’Sila-Algeria). Glob. J. Res. Med. Plants Indig. Med. 2012, 1, 97–100. [Google Scholar]
- Madani, S.; Djamel, S.; Noui, H.; Amel, B. Ethnobotanical study of therapeutic plants used to treat arterial hypertension in the Hodna region of Algeria. Glob. J. Res. Med. Plants Indig. Med. 2012, 1, 411–417. [Google Scholar]
- Lakhdari, W.; Dehliz, A.; Acheuk, F.; Mlik, R.; Hammi, H.; Doumandji-Mitiche, B.; Gheriani, S.; Berrekbia, M.; Guermit, K.; Chergui, S. Ethnobotanical study of some plants used in traditional medicine in the region of Oued Righ (Algerian Sahara). J. Med. Plants Stud. 2016, 4, 204–211. [Google Scholar]
- EL Ould, M.D.; Hadj-Mahammed, M.; Zabeirou, H. Place des plantes spontanees dans la medicine traditionnelle de la region de Ouargla (sahara septentrional Est). Courr. Du Savoir 2003, 3, 47–51. [Google Scholar]
- Yebouk, C.; Redouan, F.Z.; Benítez, G.; Bouhbal, M.; Kadiri, M.; Boumediana, A.I.; Molero-Mesa, J.; Merzouki, A. Ethnobotanical study of medicinal plants in the Adrar Province, Mauritania. J. Ethnopharmacol. 2020, 246, 112217. [Google Scholar] [CrossRef] [PubMed]
- El-Mokasabi, F. Survey of Wild Trees and Shrubs in Eastern Region of Libya and Their Economical Value. AlQalam J. Med. Appl. Sci. 2022, 5, 48–55. [Google Scholar]
- El-Mokasabi, F.M. Floristic Composition and Traditional Uses of Plant Species at Wadi Alkuf, Al-Jabal Al-Akhdar, Libya. Am. Agric. Environ. Sci. 2014, 14, 685–697. [Google Scholar]
- El-Mokasabi, F.M.; Al-Sanousi, M.F.; El-Mabrouk, R.M. Taxonomy and Ethnobotany of Medicinal Plants in Eastern Region of Libya. J. Environ. Sci. Toxicol. Food Technol. 2018, 12, 14–23. [Google Scholar]
- Oran, S.A.; Al-Eisawi, D.M. Ethnobotanical survey of the medicinal plants in the central mountains (North–South) in Jordan. J. Biodivers. Environ. Sci. J. Bio. Env. Sci. 2015, 6, 2220–6663. [Google Scholar]
- Al-Khalil, S. A survey of plants used in jordanian traditional medicine. Pharm. Biol. 1995, 33, 317–323. [Google Scholar] [CrossRef]
- Jaradat, N.A.; Ayesh, O.I.; Anderson, C. Ethnopharmacological survey about medicinal plants utilized by herbalists and traditional practitioner healers for treatments of diarrhea in the West Bank/Palestine. J. Ethnopharmacol. 2016, 182, 57–66. [Google Scholar] [CrossRef]
- Belhaj, S.; Chaachouay, N.; Zidane, L. Ethnobotanical and toxicology study of medicinal plants used for the treatment of diabetes in the High Atlas Central of Morocco. J. Pharm. Pharmacogn. Res. 2021, 9, 619–662. [Google Scholar] [CrossRef]
- Fatiha, B.A.; Souad, S.; Ouafae, B.; Jamila, D.; Allal, D.; Lahcen, Z. Ethnobotanical study of medicinal plants used in the region of middle oum Rbia (Morocco). Plant Arch. 2019, 19, 2005–2017. [Google Scholar]
- Benali, T.; Ennabili, A.; Hammani, K. Ethnopharmacological prospecting of medicinal plants from the Province of Guercif (NE of Morocco). Moroc. J. Biol. 2017, 14, 1114–8756. [Google Scholar] [CrossRef]
- Chaachouay, N.; Douira, A.; Zidane, L. COVID-19, prevention and treatment with herbal medicine in the herbal markets of Salé Prefecture, North-Western Morocco. Eur. J. Integr. Med. 2021, 42, 101285. [Google Scholar] [CrossRef] [PubMed]
- Chaachouay, N.; Douira, A.; Zidane, L. Herbal Medicine Used in the Treatment of Human Diseases in the Rif, Northern Morocco. Arab. J. Sci. Eng. 2022, 47, 131–153. [Google Scholar] [CrossRef]
- Fatiha, E.; Nazha, A.; Fouad, Z.; Ouafae, B.; Maryama, H.; Lahcen, Z. Ethnomedicinal Evaluation of Medicinal Plants Used against Gastrointestinal Disorders in the Western Middle Atlas Region (Morocco). Annu. Res. Rev. Biol. 2018, 28, 1–11. [Google Scholar] [CrossRef]
- El Hachlafi, N.; Chebat, A.; Bencheikh, R.S.; Fikri-Benbrahim, K. Ethnopharmacological study of medicinal plants used for chronic diseases treatment in Rabat-Sale-Kenitra region (Morocco). Ethnobot. Res. Appl. 2020, 20, 1–23. [Google Scholar] [CrossRef]
- El Haouari, M.; El Makaoui, S.; Jnah, M.; Haddaouy, A. A survey of medicinal plants used by herbalists in Taza (Northern Morocco) to manage various ailments. J. Mater. Environ. Sci. 2018, 9, 1875–1888. [Google Scholar]
- El Khomsi, M.; Dandani, Y.; Chaachouay, N.; Hmouni, D. Ethnobotanical study of plants used for medicinal, cosmetic, and food purposes in the region of Moulay Yacoub, Northeast of Morocco. J. Pharm. Pharmacogn. Res. 2022, 10, 13–29. [Google Scholar] [CrossRef]
- Cherifi, K.; Idm’, E.; Msanda, F. Étude ethnobotanique des plantes médicinales utilisées dans le traitement de la lithiase urinaire dans la province de Tarfaya (Maroc) [Ethnobotanical survey of medicinal plants used in treatment of kidney stones in Tarfaya province (Morocco)]. Int. J. Innov. Appl. Stud. 2019, 26, 711–719. [Google Scholar]
- Idm’Hand, E.; Msanda, F.; Cherifi, K. Ethnobotanical study and biodiversity of medicinal plants used in the Tarfaya Province, Morocco. Acta Ecol. Sin. 2020, 40, 134–144. [Google Scholar] [CrossRef]
- Fatima-Zahra, E.; Fouzia, R.F.; Abdelilah, R. Ethnobotanical study of medicinal plants used in traditional medicine in the province of Sidi Kacem, Morocco. Asian J. Pharm. Clin. Res. 2017, 10, 121–130. [Google Scholar] [CrossRef]
- Es-Safi, I.; Mechchate, H.; Amaghnouje, A.; Jawhari, F.Z.; Bari, A.; Cerruti, P.; Avella, M.; Grafov, A.; Bousta, D. Medicinal plants used to treat acute digestive system problems in the region of fez-meknes in Morocco: An ethnopharmacological survey. Ethnobot. Res. Appl. 2020, 20, 1–14. [Google Scholar] [CrossRef]
- Fadili, K.; Sekkate, C.; Alistiqsa, F.; Haloui, Z.; Chakir, S.; Zair, T. Ethnobotanical study of medicinal plants from Er-Rich region (Moroccan High Atlas). Adv. Environ. Biol. 2017, 11, 27–40. [Google Scholar]
- Jaadan, H.; Akodad, M.; Moumen, A.; Baghour, M.; Skalli, A.; Ezrari, S.; Belmalha, S. Ethnobotanical survey of medicinal plants growing in the region of “Oulad daoud zkhanine” (Nador province), in Northeastern Morocco. Ethnobot. Res. Appl. 2020, 19, 1–12. [Google Scholar] [CrossRef]
- Kachmar, M.R.; Naceiri Mrabti, H.; Bellahmar, M.; Ouahbi, A.; Haloui, Z.; El Badaoui, K.; Bouyahya, A.; Chakir, S. Traditional Knowledge of Medicinal Plants Used in the Northeastern Part of Morocco. Evid.-based Complement. Altern. Med. 2021, 2021, 6002949. [Google Scholar] [CrossRef]
- Katiri, A.; Barkaoui, M.; Msanda, F.; Boubaker, H. Ethnobotanical Survey of Medicinal Plants Used for the Treatment of Diabetes in the Tizi n’ Test Region (Taroudant Province, Morocco). J. Pharmacogn. Nat. Prod. 2017, 3, 2–10. [Google Scholar] [CrossRef]
- Kharchoufa, L.; Bouhrim, M.; Bencheikh, N.; Addi, M.; Hano, C.; Mechchate, H.; Elachouri, M. Potential Toxicity of Medicinal Plants Inventoried in Northeastern Morocco: An Ethnobotanical Approach. Plants 2021, 10, 1108. [Google Scholar] [CrossRef]
- Khouchlaa, A.; Tijane, M.; Chebat, A.; Hseini, S.; Kahouadji, A. Ethnopharmacology study of medicinal plants used in the treatment of urolithiasis (Morocco). Phytotherapie 2017, 15, 274–287. [Google Scholar] [CrossRef]
- Mechchate, H.; Es-Safi, I.; Jawhari, F.Z.; Bari, A.; Grafov, A.; Bousta, D. Ethnobotanical survey about the management of diabetes with medicinal plants used by diabetic patients in region of fez- meknes, Morocco. Ethnobot. Res. Appl. 2020, 19, 1–28. [Google Scholar] [CrossRef]
- Naceiri Mrabti, H.; Bouyahya, A.; Naceiri Mrabti, N.; Jaradat, N.; Doudach, L.; Faouzi, M.E.A. Ethnobotanical Survey of Medicinal Plants Used by Traditional Healers to Treat Diabetes in the Taza Region of Morocco. Evid.-based Complement. Altern. Med. 2021, 2021, 16. [Google Scholar] [CrossRef]
- Azzi, R.; Djaziri, R.; Lahfa, F.; Sekkal, F.; Benmehdi, H.; Belkacem, N. Ethnopharmacological survey of medicinal plants used in the traditional treatment of diabetes mellitus in the North Western and South Western Algeria. J. Med. Plants Res. 2012, 6, 2041–2050. [Google Scholar] [CrossRef]
- Miara, M.D.; Hammou, M.A.; Aoul, S.H. Phytothérapie et taxonomie des plantes médicinales spontanées dans la région de Tiaret (Algérie). Phytotherapie 2013, 11, 206–218. [Google Scholar] [CrossRef]
- Boudjelal, A.; Henchiri, C.; Sari, M.; Sarri, D.; Hendel, N.; Benkhaled, A.; Ruberto, G. Herbalists and wild medicinal plants in M’Sila (North Algeria): An ethnopharmacology survey. J. Ethnopharmacol. 2013, 148, 395–402. [Google Scholar] [CrossRef]
- Sarria, M.; Mouyet, F.Z.; Benziane, M.; Cheriet, A. Traditional use of medicinal plants in a city at steppic character (M’sila, Algeria). J. Pharm. Pharmacogn. Res. 2014, 2, 31–35. [Google Scholar]
- Chermat, S.; Gharzouli, R. Ethnobotanical Study of Medicinal Flora in the North East of Algeria—An Empirical Knowledge in Djebel Zdimm (Setif). J. Mater. Sci. Eng. A 2015, 5, 50–59. [Google Scholar] [CrossRef]
- Farah, R.; Mahfoud, H.M.; Mohamed, D.O.H.; Amoura, C.; Roukia, H.; Naima, H.; Houria, M.; Imane, B.; Chaima, B. Ethnobotanical study of some medicinal plants from Hoggar, Algeria. J. Med. Plants Res. 2015, 9, 820–827. [Google Scholar] [CrossRef]
- Sarri, M.; Boudjelal, A.; Hendel, N.; Sarri, D.; Benkhaled, A. Flora and ethnobotany of medicinal plants in the southeast of the capital of Hodna (Algeria). Arab. J. Med. Aromat. Plants 2015, 1, 24–30. [Google Scholar]
- Benarba, B. Medicinal plants used by traditional healers from South-West Algeria: An ethnobotanical study. J. Intercult. Ethnopharmacol. 2016, 5, 320–330. [Google Scholar] [CrossRef]
- Bendif, H.; Miara, M.D.; Harir, M.; Merabti, K.; Souilah, N.; Guerroudj, S.; Labza, R. An Ethnobotanical Survey of Medicinal Plants in El Mansourah (West of Bordj Bou Arreridj, Algeria). J. Soil Plant Biol. 2018, 1, 45–60. [Google Scholar] [CrossRef]
- Kefifa, A.; Saidi, A.; Hachem, K.; Mehalhal, O. An ethnobotanical survey and quantitative study of indigenous medicinal plants used in the algerian semi-arid region. Phytotherapie 2020, 18, 204–219. [Google Scholar] [CrossRef]
- Chohra, D.; Ferchichi, L. Ethnobotanical study of Belezma National Park (BNP) plants in Batna: East of Algeria. Acta Sci. Nat. 2019, 6, 40–54. [Google Scholar] [CrossRef]
- Senouci, F.; Ababou, A.; Chouieb, M. Ethnobotanical Survey of the Medicinal Plants used in the Southern Mediterranean. Case study: The region of Bissa (northeastern Dahra Mountains, Algeria). Pharmacogn. J. 2019, 11, 647–659. [Google Scholar] [CrossRef]
- Miara, M.D.; Teixidor-Toneu, I.; Sahnoun, T.; Bendif, H.; Ait Hammou, M. Herbal remedies and traditional knowledge of the Tuareg community in the region of Illizi (Algerian Sahara). J. Arid Environ. 2019, 167, 65–73. [Google Scholar] [CrossRef]
- Bouzabata, A.; Mahomoodally, M.F. A quantitative documentation of traditionally-used medicinal plants from Northeastern Algeria: Interactions of beliefs among healers and diabetic patients. J. Herb. Med. 2020, 22, 100318. [Google Scholar] [CrossRef]
- Baziz, K.; Maougal, R.T.; Amroune, A. An ethnobotanical survey of spontaneous plants used in traditional medicine in the region of aures, algeria. Eur. J. Ecol. 2020, 6, 49–69. [Google Scholar] [CrossRef]
- Hamdi, B.; Souilah, N.; Djamel, M.M.; Daoud, N. Medicinal plants popularly used in the rural communities of Ben Srour (Southeast of M’sila, Algeria). AgroLife Sci. J. 2020, 9, 45–55. [Google Scholar]
- Mechaala, S.; Bouatrous, Y.; Adouane, S. Traditional knowledge and diversity of wild medicinal plants in El Kantara’s area (Algerian Sahara gate): An ethnobotany survey. Acta Ecol. Sin. 2022, 42, 33–45. [Google Scholar] [CrossRef]
- Bouhaous, L.; Miara, M.D.; Bendif, H.; Souilah, N. Medicinal plants used by patients to fight cancer in northwestern Algeria. Bull. Cancer 2022, 109, 296–306. [Google Scholar] [CrossRef]
- Adli, B.; Touati, M.; Yabrir, B.; Bezini, E.; Mohamed, H.; Yousfi, I.; Dahia, M. Consensus level and knowledge of spontaneous medicinal plants used in Algerian central steppe region (Djefla). Agric. Conspec. Sci. 2021, 86, 139–152. [Google Scholar]
- Djahafi, A.; Taïbi, K.; Abderrahim, L.A. Aromatic and medicinal plants used in traditional medicine in the region of Tiaret, North West of Algeria. Mediterr. Bot. 2021, 42, 71465. [Google Scholar] [CrossRef]
- Al-Traboulsi, M.; Alaib, M.A. A Survey of Medicinal Plants of Wadi Al-Kouf in Al-Jabal Al-Akhdar, Libya. Nat. Croat. 2021, 30, 389–404. [Google Scholar] [CrossRef]
- Qasem, J.R. Prospects of wild medicinal and industrial plants of saline habitats in the Jordan Valley. Pak. J. Bot. 2015, 47, 551–570. [Google Scholar]
- Jaradat, N.A.; Shawahna, R.; Eid, A.M.; Al-Ramahi, R.; Asma, M.K.; Zaid, A.N. Herbal remedies use by breast cancer patients in the West Bank of Palestine. J. Ethnopharmacol. 2016, 178, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ciftcioglu, G.C. Sustainable wild-collection of medicinal and edible plants in Lefke region of North Cyprus. Agrofor. Syst. 2015, 89, 917–931. [Google Scholar] [CrossRef]
- Abubakar, I.B.; Ukwuani-Kwaja, A.N.; Garba, A.D.; Singh, D.; Malami, I.; Salihu, T.S.; Muhammad, A.; Yahaya, Y.; Sule, S.M.; Ahmed, S.J. Ethnobotanical study of medicinal plants used for cancer treatment in Kebbi state, North-west Nigeria. Acta Ecol. Sin. 2020, 40, 306–314. [Google Scholar] [CrossRef]
- Aya, K.; M’Hamed, T. Chemical Compounds, Antioxidant Activity, and in Vitro and in Silico Litholytic Effects of Zizyphus Lotus Extracts. J. Basic Clin. Physiol. Pharmacol. 2020, 31, 1–12. [Google Scholar] [CrossRef]
- Zazouli, S.; Chigr, M.; Ramos, P.A.B.; Rosa, D.; Castro, M.M.; Jouaiti, A.; Duarte, M.F.; Santos, S.A.O.; Silvestre, A.J.D. Chemical Profile of Lipophilic Fractions of Different Parts of Zizyphus lotus L. by GC-MS and Evaluation of Their Antiproliferative and Antibacterial Activities. Molecules 2022, 27, 483. [Google Scholar] [CrossRef] [PubMed]
- Abcha, I.; Ben Haj Said, L.; Salmieri, S.; Criado, P.; Neffati, M.; Lacroix, M. Optimization of extraction parameters, characterization and assessment of bioactive properties of Ziziphus lotus fruit pulp for nutraceutical potential. Eur. Food Res. Technol. 2021, 247, 2193–2209. [Google Scholar] [CrossRef]
- Tlili, H.; Hanen, N.; Arfa, A.B.; Neffati, M.; Boubakri, A.; Buonocore, D.; Dossena, M.; Verri, M.; Id, E.D. Biochemical profile and in vitro biological activities of extracts from seven folk medicinal plants growing wild in southern Tunisia. PLoS ONE 2019, 14, e0213049. [Google Scholar] [CrossRef] [PubMed]
- Tlili, H.; Marino, A.; Ginestra, G.; Cacciola, F.; Mondello, L.; Miceli, N.; Taviano, M.F.; Najjaa, H.; Nostro, A. Polyphenolic profile, antibacterial activity and brine shrimp toxicity of leaf extracts from six Tunisian spontaneous species. Nat. Prod. Res. 2021, 35, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Rais, C.; Rais, C.; Slimani, C.; Benidir, M.; Elhanafi, L.; Elhanafi, L.; Zeouk, I.; Errachidi, F.; El Ghadraoui, L.; Louahlia, S. Seeds of Zizyphus lotus: In Vivo Healing Properties of the Vegetable Oil. Sci. World J. 2020, 2020, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Letaief, T.; Garzoli, S.; Ovidi, E.; Tiezzi, A.; Jeribi, C. Organ dependency variation of the chemical composition of Ziziphus lotus volatile fractions. Eur. J. Biol. Res. 2021, 11, 501–508. [Google Scholar]
- Le Croueour, G.; Thepenier, P.; Richard, B.; Petermann, C.; Ghedira, K.; Zeches-Hanrot, M. Lotusine G: A new cyclopeptide alkaloid from Zizyphus lotus. Fitoterapia 2002, 73, 63–68. [Google Scholar] [CrossRef]
- Ghedira, K.; Chemli, R.; Caron, C.; Nuzillard, J.; Zeches, M.; Men-Olivler, L.L.E. Four cyclopeptide alkaloids from Zizyphus lotus. Phytochemistry 1995, 38, 767–772. [Google Scholar] [CrossRef]
- Ghedira, K.; Chemli, R.; Richard, B.; Nwllard, J.; Zeches, M.; Men-Olivier, L.L.E. Two cyclopeptide alkaloids from Zizyphus lotus. Phytochemisry 1993, 32, 1591–1594. [Google Scholar] [CrossRef]
- Fidan, H.; Stefanova, G.; Kostova, I.; Stankov, S.; Damyanova, S.; Stoyanova, A.; Zheljazkov, V.D. Chemical Composition and Antimicrobial Activity of Laurus nobilis L. Essential oils from Bulgaria. Molecules 2019, 24, 804. [Google Scholar] [CrossRef]
- Ardalani, H.; Hejazi Amiri, F.; Hadipanah, A.; Kongstad, K.T. Potential antidiabetic phytochemicals in plant roots: A review of in vivo studies. J. Diabetes Metab. Disord. 2021, 20, 1837–1854. [Google Scholar] [CrossRef]
- Bouyahya, A.; El Omari, N.; Elmenyiy, N.; Guaouguaou, F.E.; Balahbib, A.; Belmehdi, O.; Salhi, N.; Imtara, H.; Mrabti, H.N.; El-Shazly, M.; et al. Moroccan antidiabetic medicinal plants: Ethnobotanical studies, phytochemical bioactive compounds, preclinical investigations, toxicological validations and clinical evidences; challenges, guidance and perspectives for future management of diabetes worldw. Trends Food Sci. Technol. 2021, 115, 147–254. [Google Scholar] [CrossRef]
- Dahlia, F.; Barouagui, S.; Hemida, H.; Bousaadia, D.; Rahmoune, B. Influence of environment variations on anti-glycaemic, anti-cholesterolemic, antioxidant and antimicrobial activities of natural wild fruits of Ziziphus lotus (L.). S. Afr. J. Bot. 2020, 132, 215–225. [Google Scholar] [CrossRef]
- Touiss, I.; Harnafi, M.; Khatib, S.; Bekkouch, O.; Ouguerram, K.; Amrani, S.; Harnafi, H. Rosmarinic acid-rich extract from Ocimum basilicum L. decreases hyperlipidemia in high fat diet-induced hyperlipidemic mice and prevents plasma lipid oxidation. Physiol. Pharmacol. 2019, 23, 197–207. [Google Scholar]
- Berrichi, M.; Benammar, C.; Murtaza, B.; Hichami, A.; Belarbi, M.; Khan, N.A. Zizyphus lotus L. fruit attenuates obesity-associated alterations: In vivo mechanisms. Arch. Physiol. Biochem. 2021, 127, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Bakhtaoui, F.-Z.Z.; Lakmichi, H.; Megraud, F.; Chait, A.; Gadhi, C.-E.A. Gastroprotective, Anti-Helicobacter pylori and, Antioxidant Properties of Moroccan Zizyphus lotus L. J. Appl. Pharm. Sci. 2014, 4, 81–87. [Google Scholar] [CrossRef]
- Wahida, B.; Abderrahman, B.; Nabil, C. Antiulcerogenic activity of Zizyphus lotus (L.) extracts. J. Ethnopharmacol. 2007, 112, 228–231. [Google Scholar] [CrossRef]
- Borgi, W.; Chouchane, N. Anti-spasmodic effects of Zizyphus lotus (L.) Desf. extracts on isolated rat duodenum. J. Ethnopharmacol. 2009, 126, 571–573. [Google Scholar] [CrossRef]
- Okin, D.; Medzhitov, R. Evolution of Inflammatory Diseases. Curr. Biol. 2012, 22, 733–740. [Google Scholar] [CrossRef]
- Libby, P. Inflammation and cardiovascular disease mechanisms. Am. J. Clin. Nutr. 2006, 83, 456–460. [Google Scholar] [CrossRef]
- Wyss-Coray, T.; Mucke, L. Inflammation in Neurodegenerative Disease—A Double-Edged Sword. Neuron 2002, 35, 419–432. [Google Scholar] [CrossRef]
- Trinchieri, G. Cancer and Inflammation: An Old Intuition with Rapidly Evolving New Concepts. Annu. Rev. Ofimmunol. 2012, 30, 677–706. [Google Scholar] [CrossRef] [PubMed]
- Vodovotz, Y.; Constantine, G.; Rubin, J.; Csete, M.; Voit, E.O.; An, G. Mechanistic simulations of inflammation: Current state and future prospects. Math. Biosci. 2009, 217, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Benammar, C.; Hichami, A.; Yessoufou, A.; Simonin, A.; Belarbi, M.; Allali, H. Zizyphus lotus L. (Desf.) modulates antioxidant activity and human T-cell proliferation. Complement. Altern. Med. 2010, 10, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Almeida, R.N.; Navarro, D.S.; Barbosa-Filho, J.M. Plants with central analgesic activity. Phytomedicine 2001, 8, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Kitic, D.; Miladinovic, B.; Randjelovic, M.; Szopa, A.; Sharifi-Rad, J.; Calina, D.; Seidel, V. Anticancer Potential and Other Pharmacological Properties of Prunus armeniaca L.: An Updated Overview. Plants 2022, 11, 1885. [Google Scholar] [CrossRef]
- Jain, D.; Chaudhary, P.; Varshney, N.; Bin Razzak, K.S.; Verma, D.; Khan Zahra, T.R.; Janmeda, P.; Sharifi-Rad, J.; Daştan, S.D.; Mahmud, S.; et al. Tobacco Smoking and Liver Cancer Risk: Potential Avenues for Carcinogenesis. J. Oncol. 2021, 2021, 5905357. [Google Scholar] [CrossRef]
- Khouchlaa, A.; Talbaoui, A.; El Yahyaoui El Idrissi, A.; Bouyahya, A.; Ait Lahsen, S.; Kahouadji, A.; Tijane, M. Détermination des composés phénoliques et évaluation de l’activité litholytique in vitro sur la lithiase urinaire d’extrait de Zizyphus lotus L. d’origine marocaine. Phytotherapie 2017, 17, 1–6. [Google Scholar] [CrossRef]
- Chakit, M.; Boussekkour, R.; El Hessni, A.; Bahbiti, Y.; Nakache, R.; El Mustaphi, H.; Mesfioui, A. Antiurolithiatic Activity of Aqueous Extract of Ziziphus lotus on Ethylene Glycol-Induced Lithiasis in Rats. Pharmacogn. J. 2022, 14, 596–602. [Google Scholar] [CrossRef]
- Asadi, A.; Razavi, S.; Talebi, M.; Gholami, M. A review on anti-adhesion therapies of bacterial diseases. Infection 2019, 47, 13–23. [Google Scholar] [CrossRef]
- Billing, J.; Sherman, P.W. Antimicrobial function of species: Why some like it hot. Q. Rev. Biol. 1999, 51, 3–47. [Google Scholar]
- Ait, L.; Khaled, A. Assessment of the Antimicrobial and Antioxidant Activities of Ziziphus lotus and Peganum harmala. Iran. J. Sci. Technol. Trans. A 2017, 25, 19–26. [Google Scholar] [CrossRef]
- Belmaghraoui, W.; El Madani, N.; Manni, A.; Harir, M.; Filali-Maltouf, A.; El Hajjaji, S.; El Fatni, O.K. Total phenolic and flavonoid content, antioxidant and antibacterial activity of Ziziphus lotus from morocco. Pharmacologyonline 2018, 3, 176–183. [Google Scholar]
- Naili, M.B.; Alghazeer, R.O.; Saleh, N.A.; Al-Najjar, A.Y. Evaluation of antibacterial and antioxidant activities of Artemisia campestris (Astraceae) and Ziziphus lotus (Rhamnacea). Arab. J. Chem. 2010, 3, 79–84. [Google Scholar] [CrossRef]
- Rais, C.; Driouch, A.; Slimani, C.; Bessi, A.; Balouiri, M.; El Ghadraoui, L.; Lazraq, A.; Al Figuigui, J. Antimicrobial and antioxidant activity of pulp extracts from three populations of Ziziphus lotus L. Nutr. Food Sci. 2018, 49, 1014–1028. [Google Scholar] [CrossRef]
- Painuli, S.; Quispe, C.; Herrera-Bravo, J.; Semwal, P.; Martorell, M.; Almarhoon, Z.M.; Seilkhan, A.; Ydyrys, A.; Rad, J.S.; Alshehri, M.M.; et al. Nutraceutical Profiling, Bioactive Composition, and Biological Applications of Lepidium sativum L. Oxid. Med. Cell. Longev. 2022, 2022, 2910411. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, M.M.; Quispe, C.; Herrera-Bravo, J.; Sharifi-Rad, J.; Tutuncu, S.; Aydar, E.F.; Topkaya, C.; Mertdinc, Z.; Ozcelik, B.; Aital, M.; et al. A Review of Recent Studies on the Antioxidant and Anti-Infectious Properties of Senna Plants. Oxid. Med. Cell. Longev. 2022, 2022, 6025900. [Google Scholar] [CrossRef]
- Hossain, R.; Quispe, C.; Herrera-Bravo, J.; Islam, M.S.; Sarkar, C.; Islam, M.T.; Martorell, M.; Cruz-Martins, N.; Al-Harrasi, A.; Al-Rawahi, A.; et al. Lasia spinosa Chemical Composition and Therapeutic Potential: A Literature-Based Review. Oxid. Med. Cell. Longev. 2021, 2021, 1602437. [Google Scholar] [CrossRef]
- Cacciola, A.; D’Angelo, V.; Raimondo, F.M.; Germanò, M.P.; Braca, A.; De Leo, M. Ziziphus lotus (L.) Lam. as a Source of Health Promoting Products: Metabolomic Profile, Antioxidant and Tyrosinase Inhibitory Activities. Chem. Biodivers. 2022, 19, e202200237. [Google Scholar] [CrossRef]
- Yoon, J.I.; Al-Reza, S.M.; Kang, S.C. Hair growth promoting effect of Zizyphus jujuba essential oil. Food Chem. Toxicol. 2010, 48, 1350–1354. [Google Scholar] [CrossRef] [PubMed]
- Dorman, H.J.D.; Koşar, M.; Kahlos, K.; Holm, Y.; Hiltunen, R. Antioxidant properties and composition of aqueous extracts from Mentha species, hybrids, varieties, and cultivars. J. Agric. Food Chem. 2003, 51, 4563–4569. [Google Scholar] [CrossRef] [PubMed]
- Elaloui, M.; Ennajah, A.; Ghazghazi, H.; Youssef, I.B.; Othman, N.B.; Hajlaoui, M.R.; Khouja, A.; Laamouri, A. Quantification of total phenols, flavonoides and tannins from Ziziphus jujuba (mill.) and Ziziphus lotus (L.) (Desf). Leaf extracts and their effects on antioxidant and antibacterial activities. Int. J. Second. Metab. 2016, 4, 18–26. [Google Scholar] [CrossRef]
- Karioti, A.; Protopappa, A.; Skaltsa, H. Identification of tyrosinase inhibitors from Marrubium velutinum and Marrubium cylleneum. Bioorg. Med. Chem. 2007, 15, 2708–2714. [Google Scholar] [CrossRef] [PubMed]
- Khazri, A.; Lazher, M.; Ali, M.; Sellami, B.; Hamouda, B.; Ezzeddine, M. Protective effect of Zizyphus lotus jujube fruits against cypermethrin-induced oxidative stress and neurotoxicity in mice. Biomarkers 2017, 35, 167–173. [Google Scholar] [CrossRef]






| Country | Region | Vernacular Name | Parts Used | Mode of Preparation | Mode of Administration | Therapeutic Uses | Reference |
|---|---|---|---|---|---|---|---|
| Morocco | Northeastern | Asadra, Nbeg, Tazakort | Leaves, seeds | Decoction, raw, or fresh | Oral | Digestive problems, skin problems, nervous system disorders, diabetes, urinary tract problems, endocrine and metabolic disorders, and muscles diseases | [8] |
| Northeastern Morocco including eight province districts | - | Flowers, leaves, roots | Decoction, infusion, powder | - | Diabetes, urinary infections, antispasmodic, kidney diseases, hair care, circulatory disorders, and respiratory problems | [9] | |
| Region of Fez-Meknes | Nbeg | Fruits, leaves | Decoction | - | Kidney stones | [37] | |
| High Atlas Central Morocco | Ssedra, Azgour | Fruits, leaves | Decoction powder | - | Antiulcer, antidiarrheal, anorexia | [55] | |
| High Atlas Central of Morocco | Ssedra, Azgour | Fruits, leaves | Decoction, infusion, powder | - | Antidiarrheal, promotes the healing of wounds, antiulcer, aperitif, antidiabetic, | [38] | |
| High Atlas Central of Morocco | Ssedra, Azougar | Fruits, leaves, roots | Decoction, infusion, powder | - | Diabetes | [55] | |
| Middle Oum Rbia | Sdar, Nbeg | Fruits, leaves, seeds | - | - | Digestive, dermatological, genitourinary, cardiovascular, metabolic | [56] | |
| Guercif Province | Sadra | Roots | Maceration | - | Diabetes, intestinal pain | [57] | |
| Northeastern Morocco | Asadra, Nbeg | Leaves, fruits, roots | Decoction, infusion, powder | Oral | Urine retention, diuretic, renal colic, pyelonephritis, polycystic kidney disease, and kidney stones | [36] | |
| Markets of Salé Prefecture, Northwestern Morocco | Sedr | - | Decoction | - | COVID-19 | [58] | |
| Rif, Northern Morocco | Nbeg, Tazart | Seeds | - | - | Digestive system disorders | [59] | |
| Region of Tadla Azilal | Nbeg | Fruits, leaves | Powder | - | Gastrointestinal disorder | [60] | |
| Rabat-Sale-Kenitr | Nbeg | Fruits, leaves | Decoction | - | Chronic kidney diseases | [61] | |
| Taza | Sadra | Fruits, leaves | Infusion, powder | Externally, oral | Kidney problems, digestive system, diabetes, antimicrobial, hair care | [62] | |
| Moulay Yacoub Region | Asadra | Fruits | Infusion, powder | Oral | Stomach ache, hair care | [63] | |
| Tarfaya Province | Seder | Leaves | Powder | Oral | Kidney stones | [64] | |
| Province of Tarfaya | Ssder | Fruits, leaves | Powder, poultice | Oral, externally | Kidney stones, stomach pain, hair loss | [65] | |
| Province of Sidi Kacem | Ssedra | Seeds | Raw | - | Digestive infection | [66] | |
| Region of Fez-Meknes | Sidra, Nbeg | Seeds | Decoction, | - | Acute ache, digestion problems, intestinal comfort, bloating | [67] | |
| Er-Rich region | Azouggar | Fruits | Powder | - | Stomach pain, colon pain, anemia | [68] | |
| Nador Province | Thazagorth, Sidra | Fruits, leaves | Decoction; powder | - | Digestive diseases, diabetes | [69] | |
| Northeastern of Morocco | Sedra | Leaves, stems | Infusion | Oral | Headache, joint pain | [70] | |
| Province of Taroudant | Azougar, Sedr, Nbeg | Roots | Infusion | Oral | Diabetes | [71] | |
| Northeastern Morocco | Sedr, Nbeg | Roots | - | - | Digestive disease, diabetes | [72] | |
| - | Nbague | Fruits | Decoction, infusion | Oral | Kidney stones | [73] | |
| Region of Fez-Meknes | Sidra, Nbeg | Seeds | Decoction | Diabetes, kidney problems | [74] | ||
| Taza Region | Nbeg | Leaves | Decoction, powder | - | Diabetes | [75] | |
| Agadir Ida Outanane region | Azegar, Sedra, Nbeg | Seeds | Decoction | - | Diabetes | [39] | |
| Algeria | El-Bayadh | Sedra | Leaves | Decoction | Externally use, internally use | Antitussive, antiseptic | [40] |
| Region of Ouargla | - | Fruits, leaves, roots | Decoction, maceration | - | Anti-inflammatory, moisturizer, sedative, diuretic | [47] | |
| Tlemcen | - | Roots | Decoction | Oral | Diabetes | [41] | |
| The region of Hodna (M’Sila) | Sedra | Leaves | Infusion | - | Eczema | [44] | |
| North and southwestern Algeria | Sadra | Leaves | Decoction | - | Diabetes mellitus | [76] | |
| Region of Tiaret | Sedra | - | - | - | Pulmonary affections | [77] | |
| M’Sila (North Algeria) | Sedra | Leaves | Decoction, infusion, powder | - | Anti-inflammatory, wound-healing, dermal eczema | [78] | |
| Djebel Messaad Region (M’Sila, Algeria) | - | Fruits, leaves, roots | Decoction | - | Anti-inflammatory, emollient, pectoral | [43] | |
| M’Sila | Sedra | Leaves | Bath, infusion, lotion | - | Hair loss | [79] | |
| Djebel Zdimm (Setif) | - | Fruits, leaves, seeds | - | - | Stomach acidity, hypertension | [80] | |
| Hoggar, Algeria | Tabakat | Fruits, leaves | Decoction, powder | - | Digestive diseases, diarrhea, diabetes | [81] | |
| Southeast of the capital of Hodna (Algeria) | Sedra | Leaves | Lotion | - | Fever, eye diseases | [82] | |
| Oued Righ (Algerian Sahara) | Nbak, Sedra | Fruits, leaves, roots | Decoction, maceration | - | Tonic, emollient, sedative, anti-inflammatory, pectoral, diuretic | [46] | |
| Roots | Decoction | - | Gastrointestinal tract diseases, liver diseases | ||||
| Fruits | - | - | Respiratory system | ||||
| Adrar and Bechar | Roots | Infusion | Oral | Diabetes | [83] | ||
| Fruits | Decoction, raw | - | Renal disorders | ||||
| - | Decoction | - | Infections | ||||
| Raw | - | Hair loss | |||||
| West of Bordj Bou Arreridj (El Mansourah), Algeria | Sedra | Fruits, roots | Powder, raw | - | Pectoral, emollient activity, hepatic, chlorosis, lungs diseases, jaundice | [84] | |
| Algerian Semi-Arid Region | E’ssedra | Fruits, leaves, roots | - | - | Measles, constipation, diuretic, hair care, heart diseases, hyperglycemia, renal pain, renal stones, stomach ache, urinary infections | [85] | |
| East of Algeria | Essedra | Leaves | Infusion | - | Skin, digestive | [86] | |
| Region of Bissa | Sedra | Roots | Decoction | White washing | Toothache | [87] | |
| Northeast | Sedra | Roots | Powder, raw | - | Pulmonary affections, jaundice | [88] | |
| Northeastern Algeria | Sedra | Aerial parts, roots | Infusion | - | Diabetes | [89] | |
| Region of Aures | Thazzgarth | Fruits | Decoction used in mixture with honey, and Algerian tea | - | Urinary calculus | [90] | |
| Southeast of M’Sila (rural communities of Ben Srour) | Sadra | Fruits, leaves, roots | Cataplasm, powder, raw | - | Pulmonary affections, jaundice, eczema, emollient, stomach pain, headache | [91] | |
| Tlemcen National Park (extreme northwest of Algeria) | Essadra | Leaves, roots | Decoction | Oral | Stomach ache, colon, body pain, arthritis | [42] | |
| El Hammadia region | Sedra | - | - | - | Lungs diseases, jaundice, emollient | [91] | |
| El Kantara | Sedra, Nebag | Fruits, leaves | Chewing, decoction | - | Heartburn, constipation, weakness of heart, diuretic, breastfeeding | [92] | |
| Northwestern of Algeria | Sedra | Leaves | Powder with honey | - | Breast cancer | [93] | |
| Algerian Central Steppe Region (Djelfa) | Sedra | Leaves, roots | Decoction, lotion, maceration | - | Insomnia, renal diseases, hydatid cysts | [94] | |
| Region of Tiaret, Northwest of Algeria | Sedra | Fruits, leaves, roots | Decoction, infusion | Oral | All reported ailments | [95] | |
| Mauritania | Adrar Province | Sdar hreytek | Aerial parts, fruits | Chewing, infusion, powder | Oral | Abdominal pain, epigastric | [48] |
| Leaves | Macerated white water | Fever | |||||
| Powder, maceration | Oral | Hypertension | |||||
| Infusion | Oral | Non-insulin dependent diabetes | |||||
| Fruits | Powder | Oral | Kidney symptoms | ||||
| Libya | Al-Jabal Al-Akhder, Wadi Alkuf, Libya | Sidr, Nabq | - | - | - | Hair parasites, sciatica, abscess, piles, hepatitis, gastritis, constipation | [50] |
| Northeastern region | Sidr, Nabq | Barks fruits, leaves, roots | - | - | Hair parasites, gastritis, sciatica, abscess, reinforcement and activation of piles hepatitis, psychiatric and spiritual counseling abdominal issues, constipation | [51,96] | |
| Jordan | The central mountains (North–South) in Jordan | Sader, Orkod | Fruits | Edible | - | Cough and measles | [52] |
| Jordan (countryside, and desert) | Ceder | Fruits, seeds | Decoction | - | Vermifuge and antispasmodic | [53] | |
| - | - | Bark, branches, gum, leaves, root | Powder | - | Toothache | [97] | |
| Leaves | Powder | - | Cleaning dead bodies. Women utilize leaf powder, which works extremely well as shampoo, in combination with hot water to wash their hair. | ||||
| Decoction | - | Head lice | |||||
| - | - | Treat dandruff and counter obesity | |||||
| - | - | - | Anti-diabetic, antibacterial, anti-cancer, anti-hypertensive, anti-nociceptive, anti-diarrheal, intestinal ailments, colds, and skin. | ||||
| Palestine | West Bank | Lotus jujube, zyzafun | Leaves | Decoction | - | Diarrhea | [98] |
| Cyprus | North Cyprus | Gonnara | Fruits | Raw | - | - | [99] |
| Nigeria | Kebbi State (northwest) | Tsada | Bark, root | - | - | Cancer | [100] |
| Country | Used Part | Used Extract | Chemical Compounds | References |
|---|---|---|---|---|
| Tunisia | Roots | Petroleum ether extract | Ethyl tridecanoate; 2-pentadecanone; pentadecanoic acid, ethyl ester; 13-epimanool; tetradecanoic acid; n-hexadecanoic acid | [25] |
| Dichloromethane extract | Ethyl tridecanoate; tetradecanoic acid, ethyl ester; 13-epimanool | |||
| Leaves | Methanol extract | Luteolin; trans cinnamic acid; quercetin; rutin; gallic acid; syringic acid; 4-O-caffeoylquinic acid; epicatechin; trans-ferulic acid; hyperoside; p-coumaric acid; quercitrin; naringin; kaempferol; naringenin; apigenin; acacetin; quinic acid; (+)-catechin | [19] | |
| Fruits | Quinic acid; luteolin-7-O-glucoside; rutin; epicatechin; p-coumaric acid; apigenin-7-O-glucoside; quercitrin; naringin; chlorogenic acid; 1,3-di-O-caffeoylquinic acid; cirsilineol | |||
| Seeds | Quinic acid; catechin (+); 4,5-di-O-caffeoylquinic acid; chlorogenic acid; syringic acid; hyperoside; rutin; 3,4-di-O-caffeoylquinic acid; quercitrin; naringin; apigenin-7-O-glucoside; 4-O-caffeoylquinic acid; trans cinnamic acid; quercetin; p-coumaric acid; luteolin; naringenin; apigenin | |||
| Leaves, and flowers | Essential oil | Nonanal; decanal; linalool; α-cadinol; azulol; farnesyl acetone; 2-undecanone; 2-pentadecanone; tetradecanoic acid, ethyl ester; α-farnesene; carvone; γ-cadinene; tridecanal; trans-β-ionone; D-nerolidol; E-nerolidol; hexyl-benzoate; hexahydrofarnesyl acetone; cis-hexenyl-3-benzoate; cadalene; dodecanoic acid; tetradecanoic acid; decanoic acid, ethyl ester; undecanoic acid, ethyl ester; 2-tridecanone; β-cyclocitral; L-α-terpineol; dodecanoic acid, ethyl ester; ethyl tridecanoate; ledol; pentadecanoic acid, ethyl ester; hexadecanoic acid, ethyl ester; damascenone; geranylacetone; α-calacorene | [107] | |
| Fruits | Methanolic extract | Lauric acid; myristic acid; pentadecyclic acid; palmitic acid; heptaguaric acid; oleic acid; elaidic acid; linoleic acid; α-linolenic; stearidonic acid; arachidic acid; behenic acid; erucic acid | [27] | |
| Leaves | Methanolic extract | Fumaric acid; catechin; tyrosol; gallic acid; syringic acid; p-coumaric acid; vanillin; ferulic acid; caffeic acid; cinnamic acid | [17] | |
| Leaves | Acetonic extract | Rutin; luteolin-7-O-glucoside; naringin; luteolin; kaempferol | [105] | |
| Leaves | Acetonic extract | Quinic acid; gallic acid; protocatchuic acid; catechin (+); quercetin-3-O-galactoside; 4-O-caffeoylquinic acid; syringic acid; epicatechin; p-coumaric acid; rutin; quercetin-3-O-rhamonoside; quercetin; kaempherol; naringenin; apegenin; luteolin | [104] | |
| Root bark | Classical acid–base method (Alkaloid extract) | Lotusine A; lotusine D | [110] | |
| Root bark | Classical acid–base method (Alkaloid extract) | Lotusine B; lotusine C; lotusine E; lotusine F | [109] | |
| Root bark | Classical acid–base method (Alkaloid extract) | Lotusine G | [108] | |
| Morocco | Seeds | Essential oil | Oleic acid; palmitic acid; linoleic acid; stearic acid | [106] |
| Fruits | Ethanol extract | Synapic acid; benzoic acid; p-coumaric acid; p-hydroxybenzoic acid; p-coumaroyl glucose; cinnamic acid derivative | [24] | |
| Methanol extract | Malic acid; (-)-catechin 3-O-gallate; quercetin; galloyl shikimic acid; quercetin di-glucoside; rhamnosyl-rhamnosylglucoside; eriodictyol | |||
| Leaves | Aqueous extract | Gallic acid; catechin; rutin; p-hydroxybenzoic acid; caffeic acid; vanillic acid; epicatechin; resveratrol; syringic acid; p-coumaric acid; 3-hydroxycinnamic acid; pyrogallol; salicylic acid; naringin; ferulic acid; chlorogenic acid; sinapic acid; rosmarinic acid; quercetin | [20] | |
| Fruits | Gallic acid; chlorogenic acid; catechin; rutin; p-hydroxybenzoic acid; 3-hydroxycinnamic acid; vanillic acid; epicatechin; caffeic acid; syringic acid; p-coumaric acid; ferulic acid; pyrogallol; sinapic acid; naringin; salicylic acid; rosmarinic acid; resveratrol; catechol | |||
| Fruits | Methanolic extract | Macrocarpon C; isovitexin-2″-O-rhamnoside; amorfrutin A; hyperin; astragalin | [101] | |
| Leaves | 7,8-Dihydrobiopterin; quercetin-3-galactoside; kaempferol-3-diglucoside | |||
| Pulp | Dichloromethane extract | Decanoic acid; tridecanoic acid; tetradecanoic acid;; heptadecanoic acid; octadecanoic acid; nonadecanoic acid; eicosanoic acid; heneicosanoic acid; hexacosanoic acid; heptacosanoic acid; octacosanoic acid; triacontanoic acid; tetradecenoic acid; hexadecenoic acid; heptadecenoic acid; (9Z,12Z)-octadeca-9,12-dienoic acid; (9Z,12Z,15Z)-octadeca-9,12,15-trienoic acid; (9Z)-octadec-9-enoic acid; (9E)-octadec-9-enoic acid; nonadecenoic acid; pentadecanoic acid; hexadecanoic acid; undecanoic acid; dodecanoic acid; eicos-11-enoic acid; hexadecanedioic acid; 22-hydroxydocosanoic acid; 2-hydroxytetracosanoic acid; ethyl decanoate; ethyl tetradecanoate; ethyl pentadecanoate; ethyl hexadec-9-enoate; docosanoic acid; pentacosanoic acid; ethyl hexadecanoate; ethyl (9Z)-octadec-9-enoate; ethyl (9E)-octadec-9-enoate; ethyl octadecanoate; ethyl eicosanoate; methyl hexadecanoate; 1-palmitoylglycerol; 1-oleoylglycerol; hexadecan-1-ol; (9Z)-octadec-9-en-1-ol; oleanolic acid; betulinic acid; ursolic acid; stigmasterol; β-sitosterol; benzoic acid; vanillic acid; p-coumaric acid; solerol; glycerol; octacosanal; nonacosan-10-one; triacontanal | [102] | |
| Seeds | Decanoic acid; dodecanoic acid; tetradecanoic acid; pentadecanoic acid; hexadecanoic acid; heptadecanoic acid; octadecanoic acid; eicosanoic acid; docosanoic acid; hexadecenoic acid; heptadecenoic acid; (9Z,12Z)-octadeca-9,12-dienoic acid; (9Z)-octadec-9-enoic acid; (9E)-octadec-9-enoic acid; eicos-11-enoic acid; ethyl hexadecanoate; ethyl (9Z)-octadec-9-enoate; methyl (9Z)-octadec-9-enoate; 2-palmitoylglycerol; 1-palmitoylglycerol; 1-linoleoylglycerol; 1-oleoylglycerol; 1-stearoylglycerol; hexadecan-1-ol; oleanolic acid; betulinic acid; ursolic acid; stigmasterol; β-sitosterol; benzoic acid; vanillin; vanillyl alcohol; E-ferulic acid; glycerol; squalene | |||
| Leaves | Decanoic acid; dodecanoic acid; hexadecanoic acid; heptadecanoic acid; octadecanoic acid; eicosanoic acid; heneicosanoic acid; docosanoic acid; tetracosanoic acid; pentacosanoic acid; octacosanoic acid; hexadecenoic acid; (9Z,12Z)-octadeca-9,12-dienoic acid; tetradecanoic acid; pentadecanoic acid; (9Z,12Z,15Z)-octadeca-9,12,15-trienoic acid; (9Z)-octadec-9-enoic acid; (9E)-octadec-9-enoic acid; eicos-11-enoic acid; 22-hydroxydocosanoic acid; 2-palmitoylglycerol; 1-palmitoylglycerol; 1-linoleoylglycerol; 1-linolenoylglycerol; 1-stearoylglycerol; hexadecan-1-ol; (9Z)-octadec-9-en-1-ol; octadecan-1-ol; lupeol; oleanolic acid; betulinic acid; campesterol; β-Sitosterol; benzoic acid; salicylic acid; vanillic acid; p-coumaric acid; glycerol; loliolide; neophytadiene; inositol; phytol; squalene; γ-tocopherol; tetracosyl acetate; α-tocopherol | |||
| Root Bark | Decanoic acid; dodecanoic acid; heptadecanoic acid; nonadecanoic acid; eicosanoic acid; docosanoic acid; tetracosanoic acid; pentacosanoic acid; hexadecenoic acid; heptadecenoic acid; (9Z,12Z)-octadeca-9,12-dienoic acid; (9Z,12Z,15Z)-octadeca-9,12,15-trienoic acid; (9Z)-octadec-9-enoic acid; octadecanoic acid; (9E)-octadec-9-enoic acid; eicos-11-enoic acid; 22-hydroxydocosanoic acid; tricosanoic acid; heneicosanoic acid; methyl (9Z)-octadec-9-enoate; 1-palmitoylglycerol; 1-linoleoylglycerol; pentadecanoic acid; hexadecanoic acid; 1-oleoylglycerol; 1-stearoylglycerol; tetradecan-1-ol; hexadecan-1-ol; octadecan-1-ol; lupeol; oleanolic acid; betulinic acid; campesterol; stigmasterol; β-Sitosterol; vanillin; vanillyl alcohol; syringaldehyde; homovanillyl alcohol; vanillic acid; hydroxytyrosol; protocatechuic acid; syringic acid; glycerol | |||
| Fruits | Aqueous extract | 3-hydroxycinnamic acid; catechin; hydroxytyrosol; naringenin; p-coumaric acid; quercetin; rutin; vanillic acid; ferulic acid; gallic acid | [21] | |
| Algeria | Branches | Decoction | Catechin; quercetin-3-O-rutinoside; apigenin-O-hexoside-O-deoxyhexoside; eriodictyol-O-deoxyhexoside; oleuropein; quercetin-O-deoxyhexoside; oleuropein hexoside | [26] |
| Infusion | Catechin; quercetin-3-O-rutinoside; apigenin-O-hexoside-O-deoxyhexoside; eriodictyol-O-deoxyhexoside; oleuropein; quercetin-O-deoxyhexoside; oleuropein hexoside | |||
| Hydroethanolic extract | Catechin; quercetin-3-O-rutinoside; oleuropein hexoside; apigenin-O-hexoside-O-deoxyhexoside; eriodictyol-O-deoxyhexoside; oleuropein; quercetin-O-deoxyhexoside | |||
| Leaves | Decoction | Quercetin-3-O-(2,6-di-O-rhamnosylglucoside)-7-O-rhamnoside; myricetin-3-O-rutinoside; quercetin-3-O-(2,6-di-orhamnosylglucoside-7-O-glucuronide; kaempferol-3-O-(2,6-di-o rhamnosylglucoside); phloretin-di-c-hexoside; kaempferol-O-hexoside; kaempferol-3-O-(2,6-di-O-rhamnosylglucoside); oleuropein hexoside; kaempferol-3-O-rutinoside; kaempferol-3-O-(6-O-rhamnosyl-glucoside); apigenin-O-hexoside-O-deoxyhexoside; quercetin-3-O-rutinoside; oleuropein; quercetin-3-O-(2,6-di-O-rhamnosylglucoside) | ||
| Infusion | Quercetin-3-O-(2,6-di-O-rhamnosylglucoside)-7-O-rhamnoside; apigenin-O-hexoside-O-deoxyhexoside; myricetin-3-O-rutinoside; quercetin-3-O-(2,6-di-orhamnosylglucoside-7-O-glucuronide; kaempferol-3-O-(2,6-di-O-rhamnosylglucoside); phloretin-di-c-hexoside; quercetin-3-O-rutinoside; quercetin-3-O-(2,6-di-O-rhamnosylglucoside); kaempferol-O-hexoside; kaempferol-3-O-(2,6-di-O-rhamnosylglucoside); oleuropein hexoside; kaempferol-3-O-rutinoside; kaempferol-3-O-(6-O-rhamnosyl-glucoside); oleuropein | |||
| Hydroethanolic extract | Quercetin-3-O-(2,6-di-O-rhamnosylglucoside)-7-O-rhamnoside; myricetin-3-O-rutinoside; quercetin-3-O-(2,6-di-orhamnosylglucoside-7-O-glucuronide; kaempferol-3-O-(2,6-di-O-rhamnosylglucoside); phloretin-di-c-hexoside; quercetin-3-O-rutinoside; kaempferol-O-hexoside; kaempferol-3-O-(2,6-di-O-rhamnosylglucoside); oleuropein hexoside; kaempferol-3-O-rutinoside; kaempferol-3-O-(6-O-rhamnosyl-glucoside); apigenin-O-hexoside-O-deoxyhexoside; oleuropein; quercetin-3-O-(2,6-di-O-rhamnosylglucoside) | |||
| Root barks | Decoction | (Epi)catechin-(epi)gallocatechin; (+)-catechin; (-)-epicatechin; myricetin-3-O-rutinoside | ||
| Infusion | (Epi)catechin-(epi)gallocatechin; (-)-epicatechin; myricetin-3-O-rutinoside | |||
| Hydroethanolic | (Epi)catechin-(epi)gallocatechin; (+)-Catechin; (-)-Epicatechin; Myricetin-3-O-rutinoside | |||
| Stem barks | Decoction | Oleoside; eriodictyol-O-hexoside; quercetin-O-deoxyhexoside; eriodictyol-O-pentoside; eriodictyol-O-deoxyhexoside; eriodictyol-O-deoxyhexoside | ||
| Infusion | Oleoside; quercetin-O-deoxyhexoside; eriodictyol-O-pentoside; eriodictyol-O-deoxyhexoside; eriodictyol-O-deoxyhexoside | |||
| Hydroethanolic | Oleoside; eriodictyol-O-hexoside; quercetin-O-deoxyhexoside; eriodictyol-O-pentoside; eriodictyol-O-deoxyhexoside; eriodictyol-O-deoxyhexoside |
| Used Parts | Extracts | Bacteria or Fungi (Concentration) | References |
|---|---|---|---|
| Leaves | Acetonic extract | S. aureus (MIC = 1000 µg/mL; MBC = 2000 µg/mL), S. aureus methicillin-resistant (MIC = 250 µg/mL; MBC = 2000 µg/mL), S. epidermidis (MIC = 250 µg/mL; MBC = 500 µg/mL), S. epidermidis methicillin-resistant (MIC = 500 µg/mL; MBC = 1000 µg/mL), L. monocytogenes (MIC = 500 µg/mL; MBC = 2000 µg/mL) | [105] |
| Pulps | Lipophilic extract | E. coli (MIC >2048 µg/mL), S. aureus (MIC >2048 µg/mL), S. epidermidis (MIC > 2048 µg/mL) | [102] |
| Seeds | E. coli (MIC >2048 µg/mL), S. aureus (MIC >2048 µg/mL), S. epidermidis (MIC = 1024 µg/mL) | ||
| Leaves | E. coli (MIC = 1024 µg/mL), S. aureus (MIC = 2048 µg/mL), S. epidermidis (MIC = 1024 µg/mL) | ||
| Root bark | E. coli (MIC >2048 µg/mL), S. aureus (MIC = 2048 µg/mL), S. epidermidis (MIC = 2048 µg/mL) | ||
| Leaves | Methanolic extract | S. aureus (10 mg/mL; IZ = 12–13 mm), L. monocytogenes (10 mg/mL; IZ = 10–12.2 mm), S. typhimurium (10 mg/mL; IZ = 11–12.2 mm), E. coli (10 mg/mL; IZ = 10.6–11.8 mm) | [19] |
| Seeds | Ethanolic extract | E. coli (MIC = 50 mg/mL), P. aeruginosa (MIC = 50 mg/mL), S. aureus (MIC = 100 mg/mL), E. faecalis (MIC = 50 mg/mL) | [18] |
| Methanolic extract | E. coli (MIC = 100 mg/mL), P. aeruginosa (MIC = 50 mg/mL), S. aureus (MIC = 100 mg/mL), E. faecalis (MIC = 50 mg/mL) | ||
| Aqueous extract | E. coli (MIC = 200 mg/mL), P. aeruginosa (MIC = 100 mg/mL), S. aureus (MIC = 200 mg/mL), E. faecalis (MIC = 100 mg/mL) | ||
| Stems | Methanolic extract | S. aureus (MIC = 7 mg/mL), E. coli (MIC = 6 mg/mL), P. aeruginosa (MIC = 6 mg/mL) | [133] |
| Fruits | Methanol extract | E. coli (MIC = 400 μg/mL), Agrobacterium sp (MIC = 400 μg/mL), Rhizobium sp (MIC = 3.2 μg/mL), B. pumilus (MIC = 320 μg/mL), B. subtilis (MIC = 340 μg/mL) | [134] |
| Leaves | Methanolic extract | B. subtilis (MIC = 12.5 μg/mL), S. aureus (MIC = 25.0 μg/mL), E. coli (MIC = 1000 μg/mL), P. aeruginosa (MIC = 1000 μg/mL), S. Typhimurium (MIC = 1000 μg/mL) | [135] |
| Fruits | Ethanolic extract | E. coli (MIC = 50 mg/mL), P. aeruginosa (MIC = 25 mg/mL), S. aureus (MIC = 25 mg/mL), S. epidermidis (MIC = 25 mg/mL), E. faecalis (MIC = 25 mg/mL), B. subtilis (MIC = 25 mg/mL), M. luteus (MIC = 25 mg/mL) | [136] |
| Methanolic extract | E. coli (MIC = 50 mg/mL), P. aeruginosa (MIC = 25 mg/mL), S. aureus (MIC = 25 mg/mL), S. epidermidis (MIC = 25 mg/mL), E. faecalis (MIC = 25 mg/mL), B. subtilis (MIC = 25 mg/mL), M. luteus (MIC = 25 mg/mL), C. tropicalis (MIC = 50 mg/mL) | ||
| Aqueous extract | E. coli (MIC = 100 mg/mL), P. aeruginosa (MIC = 200 mg/mL), S. aureus (MIC = 12.5 mg/mL), S. epidermidis (MIC = 200 mg/mL), E. faecalis (MIC = 200 mg/mL), B. subtilis (MIC = 100 mg/mL), M. luteus (MIC = 100 mg/mL), C. tropicalis (MIC = 100 mg/mL) | ||
| Fruits | Methanolic extract | H. pylori (MIC = 128 μg/mL) | [117] |
| Country | Region | Used Part | Extract | Method | Results | References |
|---|---|---|---|---|---|---|
| Morocco | Northeastern | Fruits | AqE | DPPH | IC50 = 116 ± 0.02 µg/mL | [35] |
| β-carotene bleaching test | 12.5 µg/mL (42.24% of oxidation), 25 µg/mL (31.68% of oxidation), 50 µg/mL (26.92% of oxidation), and 100 µg/mL (21.11% of oxidation) | |||||
| Region of Ihahen (southern region) | Fruits | HxE | DPPH | IC50 = 8 mg/mL | [101] | |
| MtOH | IC50 = 5 mg/mL | |||||
| DiMtn | IC50 > 10 mg/mL | |||||
| Leaves | HxE | IC50 > 40 mg/mL | ||||
| MtOH | IC50 = 0.7 mg/mL | |||||
| DiMtn | IC50 > 40 mg/mL | |||||
| Fez (Zouagha-Moulay Yaâcoub) | Seeds | MtOH | DPPH | IC50 = 1.33 ± 0.01 mg/mL | [18] | |
| EtOH | IC50 = 1.32 ± 0.09 mg/mL | |||||
| AqE | IC50 = 3.11 ± 0.05 mg/mL | |||||
| Region of Sidi Sliman | Fruits | AqE | DPPH | 74.87 ± 16.74 mg TE/g EDW | [20] | |
| ABTS | 46.31 ± 11.02 mg TE/g EDW | |||||
| FRAP | 55.30 ± 2.30 mg AAE/g EDW | |||||
| Leaves | DPPH | 241.75 ± 17.37 mg TE/g EDW | ||||
| ABTS | 301.34 ± 8.26 mg TE/g EDW | |||||
| FRAP | 160.10 ± 2.30 mg AAE/g EDW | |||||
| Zaouiat Cheikh Area, Oued Zem City | Fruits | MtOH | DPPH | IC50 = 131.01 µg/mL | [134] | |
| ABTS | IC50 = 52.42 µg/mL | |||||
| Tunisia | Oudhref-Gabes Region (South of Tunisia) | Roots | PeE | ABTS | IC50 = 14.76 ± 0.02 mg/L | [25] |
| DiMtn | ABTS | IC50 = 136.58 ± 0.41 mg/L | ||||
| MtOH | IC50 = 14.31 ± 0.13 mg/L | |||||
| EtOH | IC50 = 27.42 ± 0.32 mg/L | |||||
| AqE | IC50 = 8.96 ± 0.38 mg/L | |||||
| PeE | DPPH | IC50 = 101.06 ± 0.40 mg/L | ||||
| DiMtn | IC50 = 192.33 ± 0.60 mg/L | |||||
| MtOH | IC50 = 18.03 ± 0.61 mg/L | |||||
| EtOH | IC50 = 39.50 ± 0.49 mg/L | |||||
| AqE | IC50 = 16.46 ± 0.60 mg/L | |||||
| PeE | TAC | 105.56 ± 0.37 mg AAE/mg EDW | ||||
| DiMtn | 91.11 ± 2.20 mg AAE/mg EDW | |||||
| MtOH | 304.07 ± 1.11 mg AAE/mg EDW | |||||
| EtOH | 167.41 ± 7.40 mg AAE/mg EDW | |||||
| AqE | 191.85 ± 0.00 mg AAE/mg EDW | |||||
| Leaves | PeE | ABTS | IC50 = 28.98 ± 0.06 mg/L | |||
| DiMtn | IC50 = 29.51 ± 1.23 mg/L | |||||
| MtOH | IC50 = 23.48 ± 0.63 mg/L | |||||
| EtOH | IC50 = 249.37 ± 1.26 mg/L | |||||
| AqE | IC50 = 29.01 ± 0.44 mg/L | |||||
| PeE | DPPH | Not active | ||||
| DiMtn | Not active | |||||
| MtOH | IC50 = 33.66 ± 0.11 mg/L | |||||
| EtOH | IC50 = 375.50 ± 1.50 mg/L | |||||
| AqE | IC50 = 64.80 ± 0.36 mg/L | |||||
| PeE | TAC | Not active | ||||
| DiMtn | 154.44 ± 6.20 mg AAE/mg EDW | |||||
| MtOH | 142.47 ± 0.85 mg AAE/mg EDW | |||||
| EtOH | 173.09 ± 2.99 mg AAE/mg EDW | |||||
| AqE | 99.26 ± 4.62 mg AAE/mg EDW | |||||
| Fruits | MtOH | ABTS | IC50 = 173.93 ± 0.88 mg/L | |||
| AqE | IC50 = 342.25 ± 1.25 mg/L | |||||
| MtOH | DPPH | IC50 = 343.00 ± 1.32 mg/L | ||||
| AqE | IC50 = 383.33 ± 0.29 mg/L | |||||
| MtOH | TAC | 26.42 ± 2.26 mg AAE/mg EDW | ||||
| AqE | 40.74 ± 3.39 mg AAE/mg EDW | |||||
| Tozeur (South of Tunisia) | Fruits | EtOH | TAC | 75.981 mg GAE/g EDW | [141] | |
| DPPH | 0.289 mg/mL | |||||
| Region Sidi Aich in the south | Leaves | MtOH | DPPH | IC50 = 1.28 ± 0.13 mg/mL | [142] | |
| FRAP | IC50 = 2.18 ± 0.05 mg/mL | |||||
| Kairouan | Leaves | AqE | DPPH | IC50 = 0.4 µg/mL | [143] | |
| EtOH | IC50 = 0.1 µg/mL | |||||
| Rouhia | AqE | IC50 = 0.6 µg/mL | ||||
| EtOH | IC50 = 0.03 µg/mL | |||||
| Mahres | AqE | IC50 = 0.55 µg/mL | ||||
| EtOH | IC50 = 0.46 µg/mL | |||||
| Mahdia | AqE | IC50 = 0.64 µg/mL | ||||
| EtOH | IC50 = 0.35 µg/mL | |||||
| Bengardane | Leaves | MtOH | TAC | 30.95 ± 0.01 mg GAE/g EDW | [19] | |
| DPPH | IC50 = 18.27 ± 0.28 µg/mL | |||||
| Fruits | TAC | 23.87 ± 0.34 mg GAE/g EDW | ||||
| DPPH | IC50 = 12.16 ± 0.31µg/mL | |||||
| Seeds | TAC | 22.03 ± 3.08 mg GAE/g EDW | ||||
| DPPH | IC50 = 18.57 ± 6.67µg/mL | |||||
| Oued Esseder | Leaves | MtOH | TAC | 30.91 ± 0.06 mg GAE/g EDW | ||
| DPPH | IC50 = 16.60 ± 1.58 µg/mL | |||||
| Fruits | TAC | 25.02 ± 0.55 mg GAE/g EDW | ||||
| DPPH | IC50 = 15.15 ± 0.90 µg/mL | |||||
| Seeds | TAC | 22.80 ± 0.15 mg GAE/g EDW | ||||
| DPPH | IC50 = 11.41 ± 0.35 µg/mL | |||||
| Algeria | Not defined | Fruits | AqE | DPPH | IC50 = 11–30 µg/mL | [114] |
| Steppic Region of Tiaret | Stems | MtOH | DPPH | 480.20 ± 40.64 mg AAE/g EDW | [133] | |
| Italy | Addaura (the northern slopes of Monte Pellegrino, Palermo, Italy) | Stem bark | MtOH | DPPH | 304.02 ± 4.80 mg AAE/g EDW | [140] |
| Metal chelating | 39.01 ± 4.30 mg EDTAE/g EDW | |||||
| FRAP | 296.68 ± 1.81 mg TE/g EDW |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bencheikh, N.; Radi, F.Z.; Fakchich, J.; Elbouzidi, A.; Ouahhoud, S.; Ouasti, M.; Bouhrim, M.; Ouasti, I.; Hano, C.; Elachouri, M. Ethnobotanical, Phytochemical, Toxicological, and Pharmacological Properties of Ziziphus lotus (L.) Lam.: A Comprehensive Review. Pharmaceuticals 2023, 16, 575. https://doi.org/10.3390/ph16040575
Bencheikh N, Radi FZ, Fakchich J, Elbouzidi A, Ouahhoud S, Ouasti M, Bouhrim M, Ouasti I, Hano C, Elachouri M. Ethnobotanical, Phytochemical, Toxicological, and Pharmacological Properties of Ziziphus lotus (L.) Lam.: A Comprehensive Review. Pharmaceuticals. 2023; 16(4):575. https://doi.org/10.3390/ph16040575
Chicago/Turabian StyleBencheikh, Noureddine, Fatima Zahrae Radi, Jamila Fakchich, Amine Elbouzidi, Sabir Ouahhoud, Mohammed Ouasti, Mohamed Bouhrim, Imane Ouasti, Christophe Hano, and Mostafa Elachouri. 2023. "Ethnobotanical, Phytochemical, Toxicological, and Pharmacological Properties of Ziziphus lotus (L.) Lam.: A Comprehensive Review" Pharmaceuticals 16, no. 4: 575. https://doi.org/10.3390/ph16040575
APA StyleBencheikh, N., Radi, F. Z., Fakchich, J., Elbouzidi, A., Ouahhoud, S., Ouasti, M., Bouhrim, M., Ouasti, I., Hano, C., & Elachouri, M. (2023). Ethnobotanical, Phytochemical, Toxicological, and Pharmacological Properties of Ziziphus lotus (L.) Lam.: A Comprehensive Review. Pharmaceuticals, 16(4), 575. https://doi.org/10.3390/ph16040575









