Synthesis, In Silico and In Vitro Characterization of Novel N,N-Substituted Pyrazolopyrimidine Acetamide Derivatives for the 18KDa Translocator Protein (TSPO)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
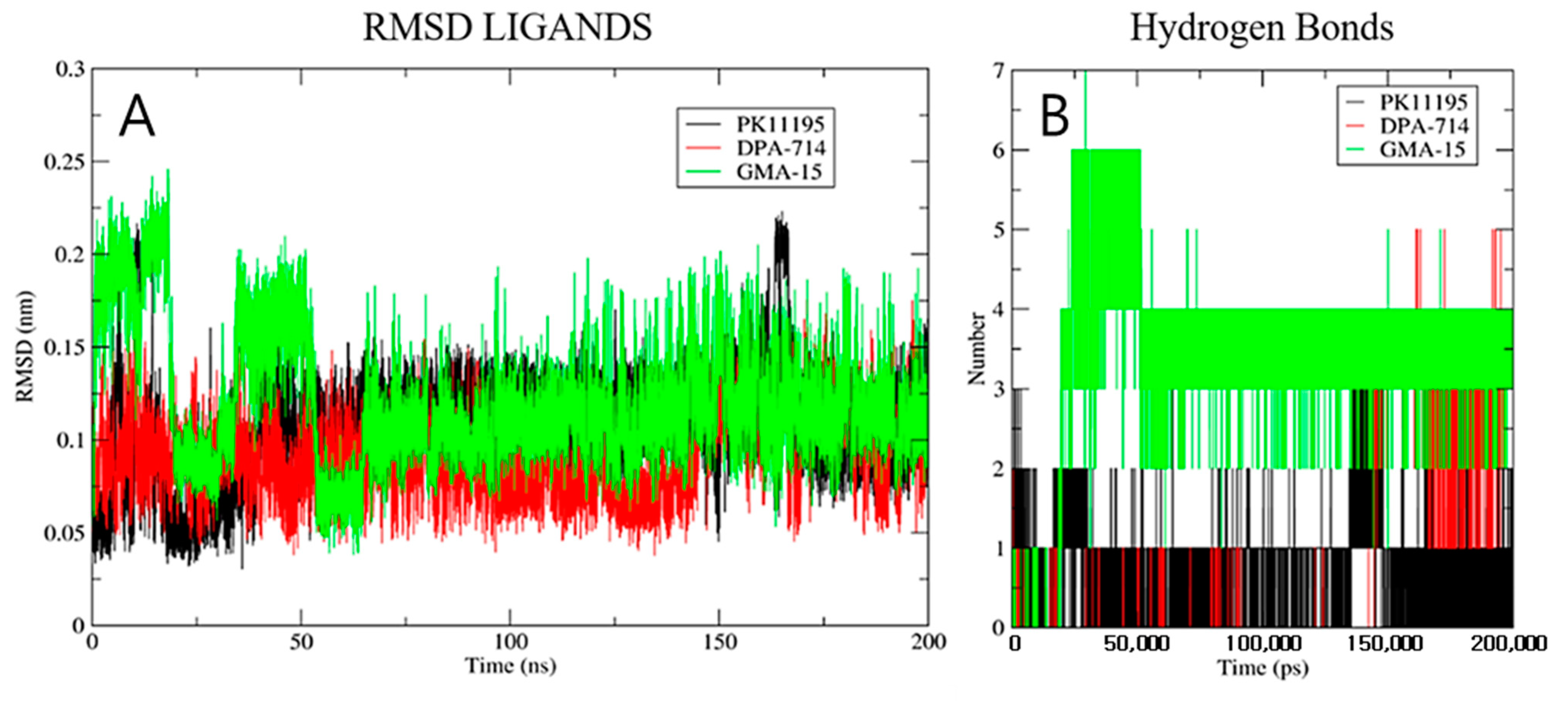
2.2. Molecular Docking, Molecular Dynamic and In Silico ADMET Studies
3. Materials and Methods
3.1. Chemistry
General Experimental Information
3.2. Molecular Docking, Molecular Dynamic and In Silico ADMET Studies
3.2.1. Molecular Docking
3.2.2. Molecular Dynamics Studies
3.2.3. In Silico Physicochemical and ADMET Studies
3.2.4. In Vitro Cytotoxicity Studies
3.3. In Vitro TSPO Binding Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kempuraj, D.; Thangavel, R.; Natteru, P.A.; Selvakumar, G.P.; Saeed, D.; Zahoor, H.; Zaheer, S.; Iyer, S.S.; Zaheer, A. Neuroinflammation Induces Neurodegeneration. J. Neurol. Neurosurg. Spine. 2016, 1, 1003. [Google Scholar] [PubMed]
- Casellas, P.; Galiegue, S.; Basile, A.S. Peripheral benzodiazepine receptors and mitochondrial function. Neurochem. Int. 2002, 40, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, V.; Baraldi, M.; Guilarte, T.R.; Knudsen, T.B.; Lacapère, J.J.; Lindemann, P.; Norenberg, M.D.; Nutt, D.; Weizman, A.; Zhang, M.R.; et al. Translocator protein (18 kDa): New nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol. Sci. 2006, 27, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, V.; Brown, A.S. Role of the peripheral-type benzodiazepine receptor and the polypeptide diazepam binding inhibitor in steroidogenesis. J. Steroid Biochem. Mol. Biol. 1995, 53, 103–110. [Google Scholar] [CrossRef]
- Bhoola, N.H.; Mbita, Z.; Hull, R.; Dlamini, Z. Translocator Protein (TSPO) as a Potential Biomarker in Human Cancers. Int. J. Mol. Sci. 2018, 19, 2176. [Google Scholar] [CrossRef] [Green Version]
- Ammer, L.M.; Vollmann-Zwerenz, A.; Ruf, V.; Wetzel, C.H.; Riemenschneider, M.J.; Albert, N.L.; Beckhove, P.; Hau, P. The Role of Translocator Protein TSPO in Hallmarks of Glioblastoma. Cancers 2020, 12, 2973. [Google Scholar] [CrossRef]
- Mendonça-Torres, M.C.; Roberts, S.S. The translocator protein (TSPO) ligand PK11195 induces apoptosis and cell cycle arrest and sensitizes to chemotherapy treatment in pre- and post-relapse neuroblastoma cell lines. Cancer Biol. Ther. 2013, 14, 319–326. [Google Scholar] [CrossRef] [Green Version]
- Rupprecht, R.; Papadopoulos, V.; Rammes, G.; Baghai, T.C.; Fan, J.; Akula, N.; Groyer, G.; Adams, D.; Schumacher, M. Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nat. Rev. Drug Discov. 2010, 9, 971–988. [Google Scholar] [CrossRef]
- Nothdurfter, C.; Baghai, T.C.; Schüle, C.; Rupprecht, R. Translocator protein (18 kDa) (TSPO) as a therapeutic target for anxiety and neurologic disorders. Eur. Arch. Psychiatry. Clin. Neurosci. 2012, 262 (Suppl. S2), S107–S112. [Google Scholar] [CrossRef]
- Dimitrova-Shumkovska, J.; Krstanoski, L.; Veenman, L. Diagnostic and Therapeutic Potential of TSPO Studies Regarding Neurodegenerative Diseases, Psychiatric Disorders, Alcohol Use Disorders, Traumatic Brain Injury, and Stroke: An Update. Cells 2020, 9, 870. [Google Scholar] [CrossRef] [Green Version]
- Papadopoulos, V.; Lecanu, L. Translocator protein (18 kDa) TSPO: An emerging therapeutic target in neurotrauma. Exp. Neurol. 2009, 219, 53–57. [Google Scholar] [CrossRef] [Green Version]
- Luu, T.G.; Kim, H.-K. 18F-Radiolabeled Translocator Protein (TSPO) PET Tracers: Recent Development of TSPO Radioligands and Their Application to PET Study. Pharmaceutics 2022, 14, 2545. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Schulte, M.L.; Nickels, M.L.; Manning, H.C. New structure-activity relationships of N-acetamide substituted pyrazolopyrimidines as pharmacological ligands of TSPO. Bioorg. Med. Chem. Lett. 2016, 26, 3472–3477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reynolds, A.; Hanani, R.; Hibbs, D.; Damont, A.; Da Pozzo, E.; Selleri, S.; Dollé, F.; Martini, C.; Kassiou, M. Pyrazolo [1,5-a]pyrimidine acetamides: 4-Phenyl alkyl ether derivatives as potent ligands for the 18 kDa translocator protein (TSPO). Bioorg. Med. Chem. Lett. 2010, 20, 5799–5802. [Google Scholar] [CrossRef] [PubMed]
- Damont, A.; Marguet, F.; Puech, F.; Dollé, F. Synthesis and in vitro characterization of novel fluorinated derivatives of the TSPO 18 kDa ligand SSR180575. Eur. J. Med. Chem. 2015, 101, 736–745. [Google Scholar] [CrossRef] [Green Version]
- Tang, D.; Fujinaga, M.; Hatori, A.; Zhang, Y.; Yamasaki, T.; Xie, L.; Mori, W.; Kumata, K.; Liu, J.; Manning, H.C.; et al. Evaluation of the novel TSPO radiotracer 2-(7-butyl-2-(4-(2-([18F]fluoroethoxy)phenyl)-5-methylpyrazolo [1,5-a]pyrimidin-3-yl)-N,N-diethylacetamide in a preclinical model of neuroinflammation. Eur. J. Med. Chem. 2018, 150, 1–8. [Google Scholar] [CrossRef]
- Banister, S.D.; Beinat, C.; Wilkinson, S.M.; Shen, B.; Bartoli, C.; Selleri, S.; Da Pozzo, E.; Martini, C.; Chin, F.T.; Kassiou, M. Ether analogues of DPA-714 with subnanomolar affinity for the translocator protein (TSPO). Eur. J. Med. Chem. 2015, 93, 392–400. [Google Scholar] [CrossRef] [Green Version]
- Alam, M.M.; Lee, J.; Lee, S.Y. Recent Progress in the Development of TSPO PET Ligands for Neuroinflammation Imaging in Neurological Diseases. Nucl. Med. Mol. Imaging. 2017, 51, 283–296. [Google Scholar] [CrossRef]
- Betlazar, C.; Middleton, R.J.; Banati, R.; Liu, G.J. The Translocator Protein (TSPO) in Mitochondrial Bioenergetics and Immune Processes. Cells 2020, 9, 512. [Google Scholar] [CrossRef] [Green Version]
- Chauveau, F.; Becker, G.; Boutin, H. Have (R)-[11C]PK11195 challengers fulfilled the promise? A scoping review of clinical TSPO PET studies. Eur. J. Nucl. Med. Mol. Imaging. 2021, 49, 201–220. [Google Scholar] [CrossRef]
- Tang, D.; Hight, M.R.; McKinley, E.T.; Fu, A.; Buck, J.R.; Smith, R.A.; Tantawy, M.N.; Peterson, T.E.; Colvin, D.C.; Ansari, M.S.; et al. Quantitative preclinical imaging of TSPO expression in glioma using N,N-diethyl-2-(2-(4-(2-18F-fluoroethoxy)phenyl)-5,7-dimethylpyrazolo [1,5-a]pyrimidin-3-yl)acetamide. J. Nucl. Med. 2012, 53, 287–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golla, S.S.; Boellaard, R.; Oikonen, V.; Hoffmann, A.; van Berckel, B.N.; Windhorst, A.D.; Virta, J.; Haaparanta-Solin, M.; Luoto, P.; Savisto, N.; et al. Quantification of [18F]DPA-714 binding in the human brain: Initial studies in healthy controls and Alzheimer’s disease patients. J. Cereb. Blood. Flow. Metab. 2015, 35, 766–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vo, S.V.; Banister, S.D.; Freelander, I.; Werry, E.L.; Reekie, T.A.; Ittner, L.M.; Kassiou, M. Reversing binding sensitivity to A147T translocator protein. RSC Med. Chem. 2020, 11, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Pulagam, K.R.; Colás, L.; Padro, D.; Plaza-García, S.; Gómez-Vallejo, V.; Higuchi, M.; Llop, J.; Martín, A. Evaluation of the novel TSPO radiotracer [18F] VUIIS1008 in a preclinical model of cerebral ischemia in rats. EJNMMI Res. 2017, 7, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, D.; Li, J.; Buck, J.R.; Tantawy, M.N.; Xia, Y.; Harp, J.M.; Nickels, M.L.; Meiler, J.; Manning, H.C. Evaluation of TSPO PET Ligands [18F]VUIIS1009A and [18F]VUIIS1009B: Tracers for Cancer Imaging. Mol. Imaging. Biol. 2017, 19, 578–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Médran-Navarrete, V.; Bernards, N.; Kuhnast, B.; Damont, A.; Pottier, G.; Peyronneau, M.A.; Kassiou, M.; Marguet, F.; Puech, F.; Boisgard, R.; et al. [18F]DPA-C5yne, a novel fluorine-18-labelled analogue of DPA-714: Radiosynthesis and preliminary evaluation as a radiotracer for imaging neuroinflammation with PET. J. Labelled. Comp. Radiopharm. 2014, 57, 410–418. [Google Scholar] [CrossRef]
- Lee, J.; Jung, J.H.; Lee, B.C.; Lee, S.-Y. Design and Synthesis of Phenoxypyridyl Acetamide or Aryl-oxodihydropurine Derivatives for the Development of Novel PET Ligands Targeting the Translocator Protein 18 kDa (TSPO). Bull. Korean Chem. Soc. 2016, 37, 1874–1877. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Alam, M.M.; Lee, J.; Ido, T. Amino group and Fluorine Introduced Proton-emitting Tomographic Radioactive Compounds. Patent KR102180603B1, 19 November 2020. Available online: https://patents.google.com/patent/KR102180603B1 (accessed on 7 February 2023).
- Médran-Navarrete, V.; Damont, A.; Peyronneau, M.A.; Kuhnast, B.; Bernards, N.; Pottier, G.; Marguet, F.; Puech, F.; Boisgard, R.; Dollé, F. Preparation and evaluation of novel pyrazolo [1,5-a]pyrimidine acetamides, closely related to DPA-714, as potent ligands for imaging the TSPO 18kDa with PET. Bioorg. Med. Chem. Lett. 2014, 24, 1550–1556. [Google Scholar] [CrossRef]
- Damont, A.; Médran-Navarrete, V.; Cacheux, F.; Kuhnast, B.; Pottier, G.; Bernards, N.; Marguet, F.; Puech, F.; Boisgard, R.; Dollé, F. Novel Pyrazolo [1,5-a]pyrimidines as Translocator Protein 18 kDa (TSPO) Ligands: Synthesis, in Vitro Biological Evaluation, [(18)F]-Labeling, and in Vivo Neuroinflammation PET Images. J. Med. Chem. 2015, 58, 7449–7464. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Kalathur, R.C.; Liu, Q.; Kloss, B.; Bruni, R.; Ginter, C.; Kloppmann, E.; Rost, B.; Hendrickson, W.A. Protein structure. Structure and activity of tryptophan-rich TSPO proteins. Science 2015, 347, 551–555. [Google Scholar] [CrossRef] [Green Version]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra precision glide: Docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Páll, S.; Zhmurov, A.; Bauer, P.; Abraham, M.; Lundborg, M.; Gray, A.; Hess, B.; Lindahl, E. Heterogeneous parallelization and acceleration of molecular dynamics simulations in GROMACS. J. Chem. Phys. 2020, 153, 134110. [Google Scholar] [CrossRef] [PubMed]
- Sharma, T.; Harioudh, M.K.; Kuldeep, J.; Kumar, S.; Banerjee, D.; Ghosh, J.K.; Siddiqi, M.I. Identification of Potential Inhibitors of Cathepsin-B using Shape & Pharmacophore-based Virtual Screening, Molecular Docking and Explicit Water Thermodynamics. Mol. Inform. 2020, 39, e1900023. [Google Scholar] [CrossRef] [PubMed]
- Sharma, T.; Siddiqi, M.I. In silico identification and design of potent peptide inhibitors against PDZ-3 domain of Postsynaptic Density Protein (PSD-95). J. Biomol. Struct. Dyn. 2019, 37, 1241–1253. [Google Scholar] [CrossRef] [PubMed]
- The Physicochemical and Absorption, Distribution, Metabolism, Excretion, and Toxicity (ADMET) Properties of the Lead Compound Were Predicted Using Online Tools SwisssADME. 2017. Available online: http://www.swissadme.ch (accessed on 15 January 2023).
- The Physicochemical and Absorption, Distribution, Metabolism, Excretion, and Toxicity (ADMET) Properties of the Lead Compound Were Predicted Using Online Tools admetsar1. Available online: http://lmmd.ecust.edu.cn/admetsar1 (accessed on 15 January 2023).
- Yadav, D.K.; Kumar, S.; Saloni Misra, S.; Yadav, L.; Teli, M.; Sharma, P.; Chaudhary, S.; Kumar, N.; Choi, E.H.; Kim, H.S.; et al. Molecular Insights into the Interaction of RONS and Thieno [3,2-c]pyran Analogs with SIRT6/COX-2: A Molecular Dynamics Study. Sci. Rep. 2018, 8, 4777. [Google Scholar] [CrossRef] [Green Version]
- Zhong, H.; Tran, L.M.; Stang, J.L. Induced-fit docking studies of the active and inactive states of protein tyrosine kinases. J. Mol. Graph. Model. 2009, 28, 336–346. [Google Scholar] [CrossRef]
- Moon, B.S.; Kim, B.S.; Park, C.; Jung, J.H.; Lee, Y.W.; Lee, H.Y.; Chi, D.Y.; Lee, B.C.; Kim, S.E. [18F]Fluoromethyl-PBR28 as a potential radiotracer for TSPO: Preclinical comparison with [11C]PBR28 in a rat model of neuroinflammation. Bioconjug. Chem. 2014, 25, 442–450. [Google Scholar] [CrossRef]
- Kreisl, W.C.; Fujita, M.; Fujimura, Y.; Kimura, N.; Jenko, K.J.; Kannan, P.; Hong, J.; Morse, C.L.; Zoghbi, S.S.; Gladding, R.L.; et al. Comparison of [11C]-(R)-PK 11195 and [11C]PBR28, two radioligands for translocator protein (18 kDa) in human and monkey: Implications for positron emission tomographic imaging of this inflammation biomarker. Neuroimage 2010, 49, 2924–2932. [Google Scholar] [CrossRef] [Green Version]

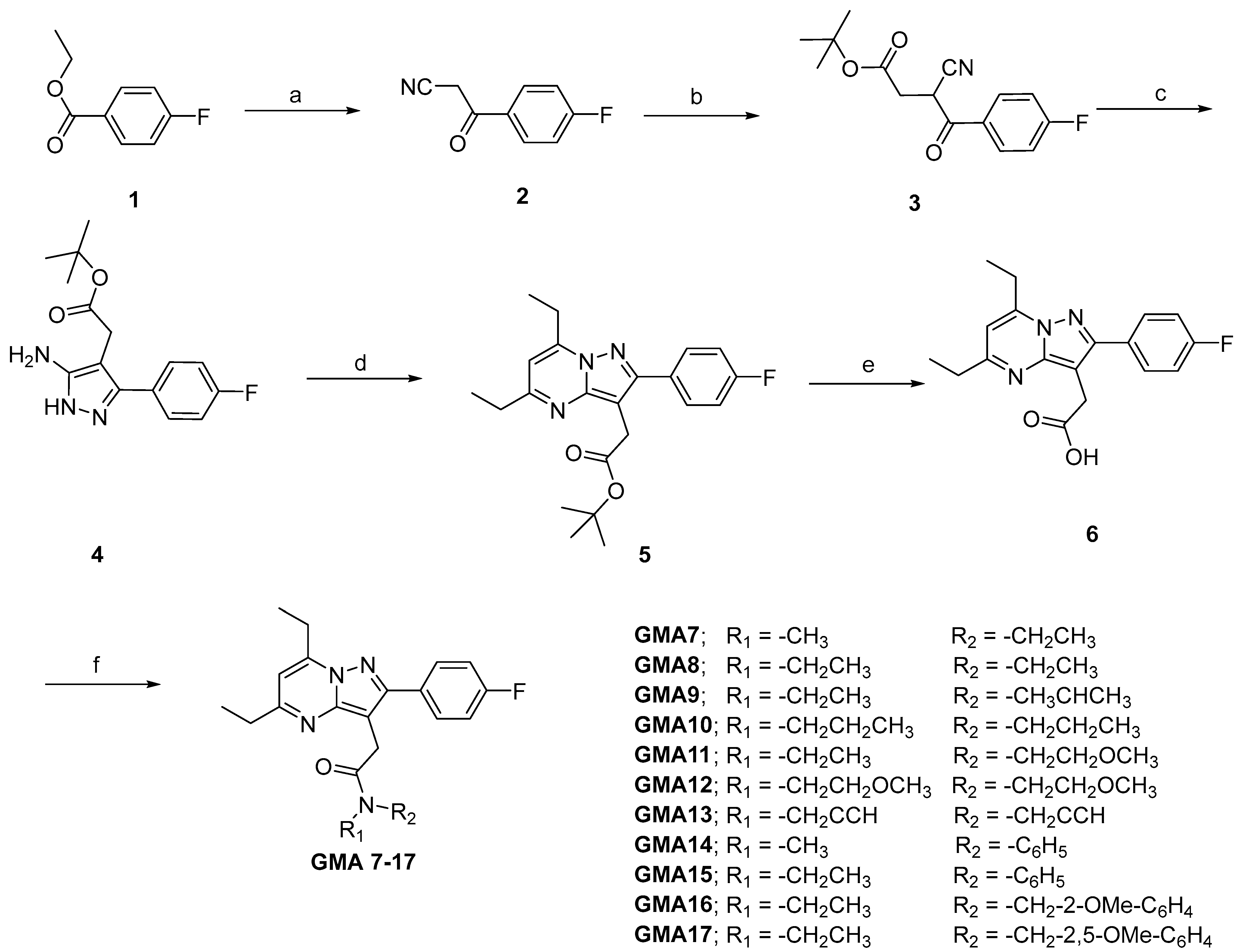

| Compound | R1 | R2 | Ki (nM) 1 | cLogP 2 |
|---|---|---|---|---|
| GMA 7 | -Me | -CH2CH3 | 17.10 | 3.83 |
| GMA 8 | -CH2CH3 | -CH2CH3 | 8.79 | 4.36 |
| GMA 9 | -CH2CH3 | -CH(CH3)2 | 13.27 | 4.66 |
| GMA 10 | -CH2 CH2CH3 | -CH2CH2CH3 | 0.18 | 5.41 |
| GMA 11 | -CH2CH3 | -CH2CH2OCH3 | 0.19 | 4.34 |
| GMA 12 | -CH2CH2OCH3 | -CH2CH2OCH3 | 25.37 | 4.32 |
| GMA 13 | -CH2CCH | -CH2CCH | 0.90 | 4.50 |
| GMA 14 | -CH3 | -C6H5 | N.D. 3 | 5.21 |
| GMA 15 | -CH2CH3 | -C6H5 | 0.06 | 5.74 |
| GMA 16 | -CH2CH3 | -CH2-2-OMe-C6H4 | 0.49 | 5.43 |
| GMA 17 | -CH2CH3 | -CH2-2,5-OMe-C6H4 | 0.54 | 5.52 |
| PK11195 | 1.34 | 4.6 | ||
| DPA-714 | 3.66 | 3.33 |
| Compound | Total Score | Amino Acid Involved in Active Pocket in 3 Å | Involved Group of Amino Acid | Length of H-Bond Å | No. of H-Bond |
|---|---|---|---|---|---|
| GMA 10 | −9.206 | Phe-55, Cys-107, Val-110, Ile-47, Trp-51, Pro-42, Phe-141, Ala-142, Ile-145, Ser-146, Ile-149, Ile-27, Tyr-32, Ser-91, Phe-90, Ser-21, Ser-22, Asn-87 | Trp-51 | 2.6 | 1 |
| GMA 11 | −8.988 | Phe-55, Cys-107, Val-110, Ile-47, Trp-51, Pro-42, Phe-141, Ala-142, Ile-145, Ser-146, Ile-149, Ile-27, Tyr-32, Ser-91, Phe-90, Ser-21, Ser-22, Asn-87, Phe-95, Tyr-32, Gln-94, Tyr-135, Trp-138 | Trp-138 Trp-51 | 2.5 2.2 | 2 |
| GMA 15 | −12.443 | Phe-55, Asn-87, Ser-22, Ser-21, Phe-90, Ser-91, Cys-107, Val-110, Tyr-135, Trp-135, Trp-138, Ser-139, Pro-42, Ile-47, Phe-141, Ala-142, Ile-145, Leu-145, Ser-146, Ile-149, Trp-51, Ile-27, Tyr-32 | Trp-138 Trp-51 | 2.5 2.1 | 2 |
| PK11195 | −10.083 | Phe-55, Ser-22, Val-110, Asn-87, Cys-107, Ile-47, Phe-90, Ser-91, Trp-138, Trp-51, Phe-141, Pro-42, Ala-142, Leu-145 | Trp-138 Trp-51 | 2.7 2.1 | 2 |
| DPA-714 | −8.252 | Ser-21, Ser-91, Asn-87, Phe-90, Cys-107, Val-110, Ser-146, Leu-145, Ala-142, Phe-142, Phe-141, Trp-138, Pro-42, Tyr-32, Gly-44, Ile-27, Ile-47 | Trp-138 Trp-51 | 2.4 2.0 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Wasim, S.; Jung, J.H.; Kim, M.-h.; Lee, B.C.; Alam, M.M.; Lee, S.-Y. Synthesis, In Silico and In Vitro Characterization of Novel N,N-Substituted Pyrazolopyrimidine Acetamide Derivatives for the 18KDa Translocator Protein (TSPO). Pharmaceuticals 2023, 16, 576. https://doi.org/10.3390/ph16040576
Park J, Wasim S, Jung JH, Kim M-h, Lee BC, Alam MM, Lee S-Y. Synthesis, In Silico and In Vitro Characterization of Novel N,N-Substituted Pyrazolopyrimidine Acetamide Derivatives for the 18KDa Translocator Protein (TSPO). Pharmaceuticals. 2023; 16(4):576. https://doi.org/10.3390/ph16040576
Chicago/Turabian StylePark, Jaekyung, Sobia Wasim, Jae Ho Jung, Mi-hyun Kim, Byung Chul Lee, Mohammad Maqusood Alam, and Sang-Yoon Lee. 2023. "Synthesis, In Silico and In Vitro Characterization of Novel N,N-Substituted Pyrazolopyrimidine Acetamide Derivatives for the 18KDa Translocator Protein (TSPO)" Pharmaceuticals 16, no. 4: 576. https://doi.org/10.3390/ph16040576






