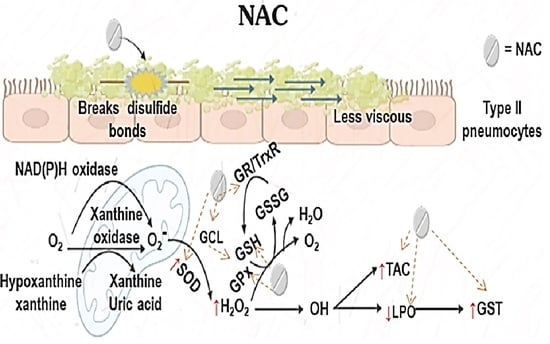
N-Acetyl Cysteine Restores the Diminished Activity of the Antioxidant Enzymatic System Caused by SARS-CoV-2 Infection: Preliminary Findings
Abstract
:1. Introduction
2. Results
2.1. Demographic Characteristics of the Patients Infected by SARS-CoV-2
2.2. Demographic Characteristics of the Healthy Subjects
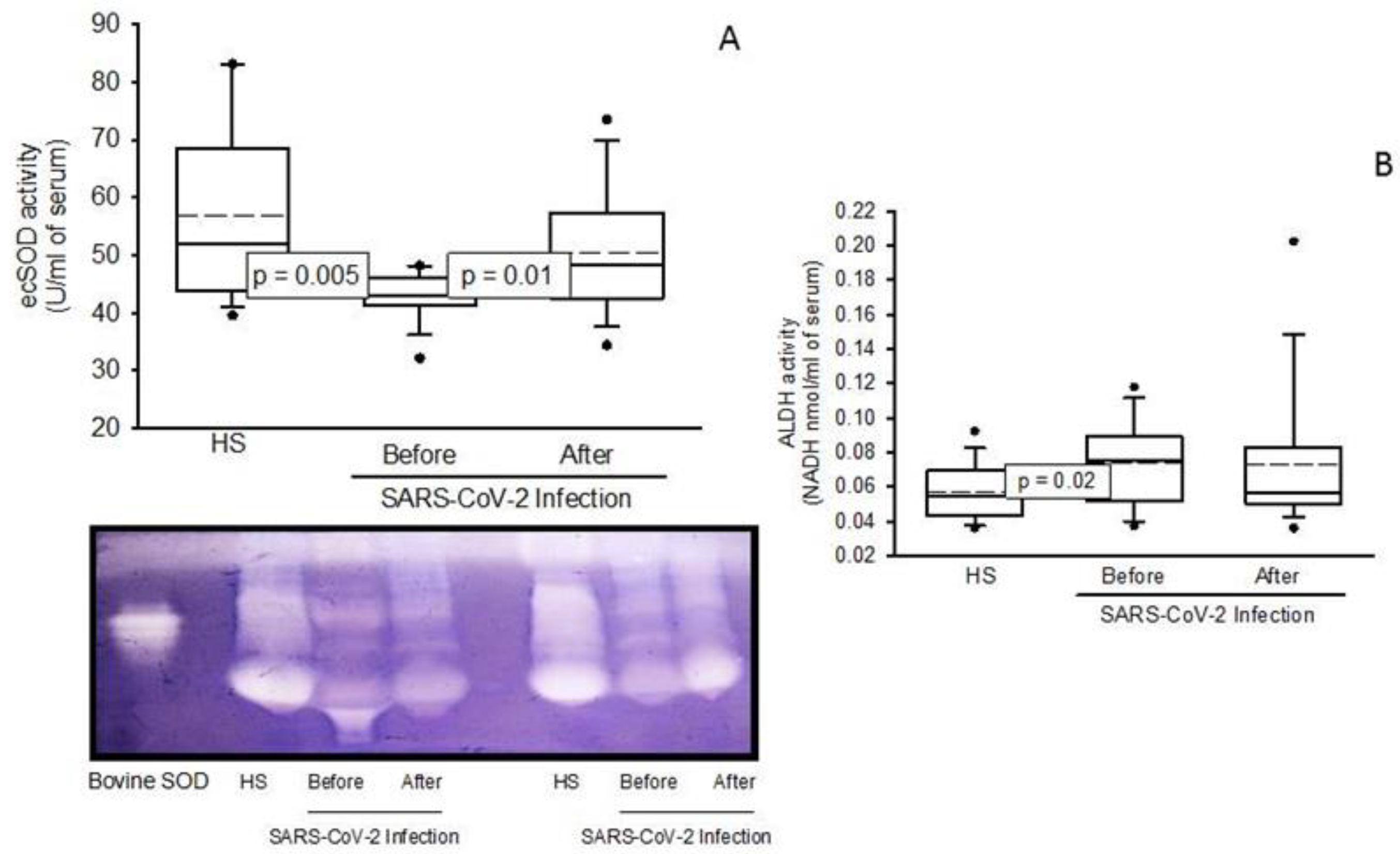
2.3. Oxidative Stress Markers
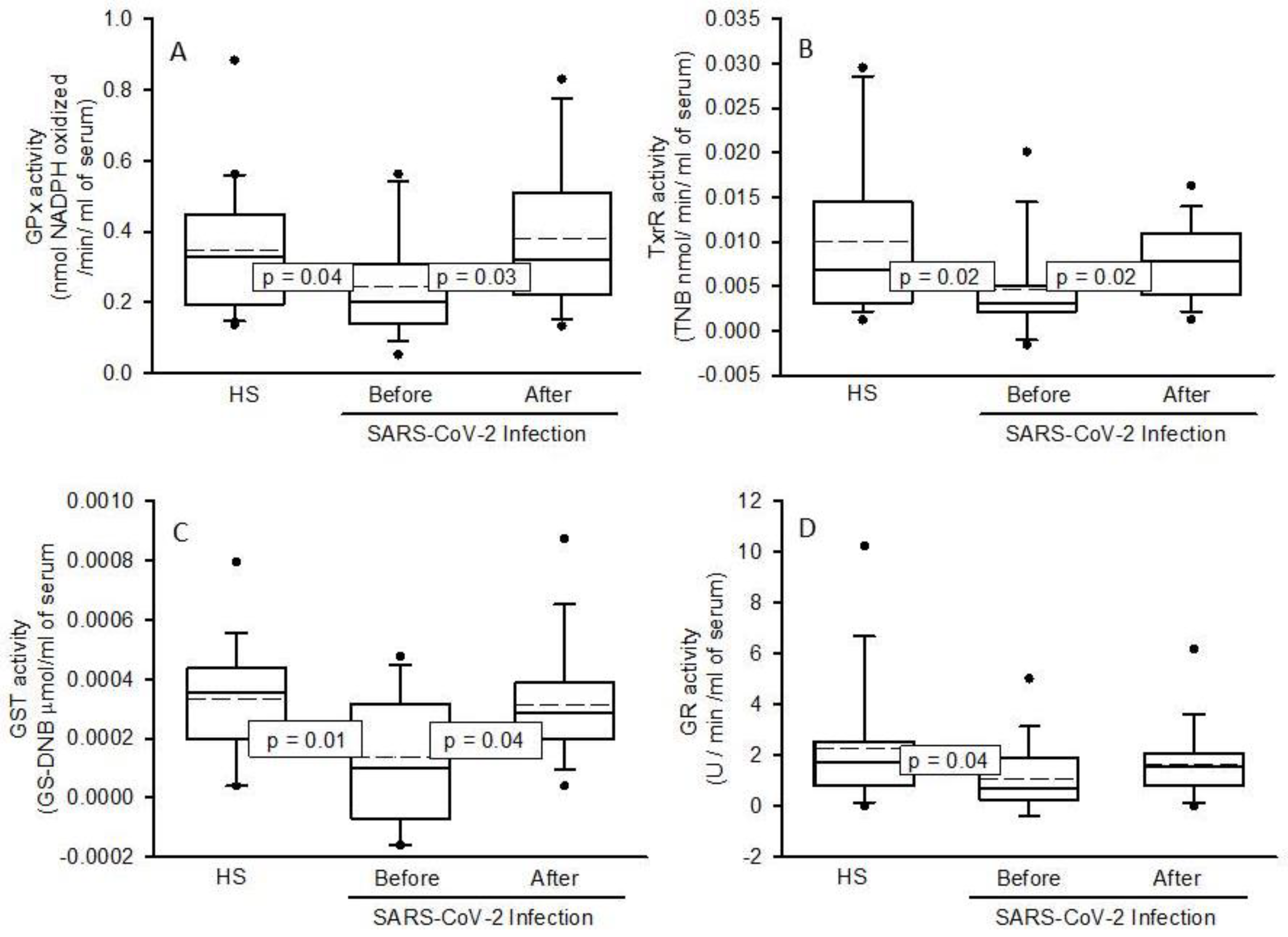
2.4. Enzymatic Activities the ecSOD, GPx, TxrR, GST, GR, and ALDH
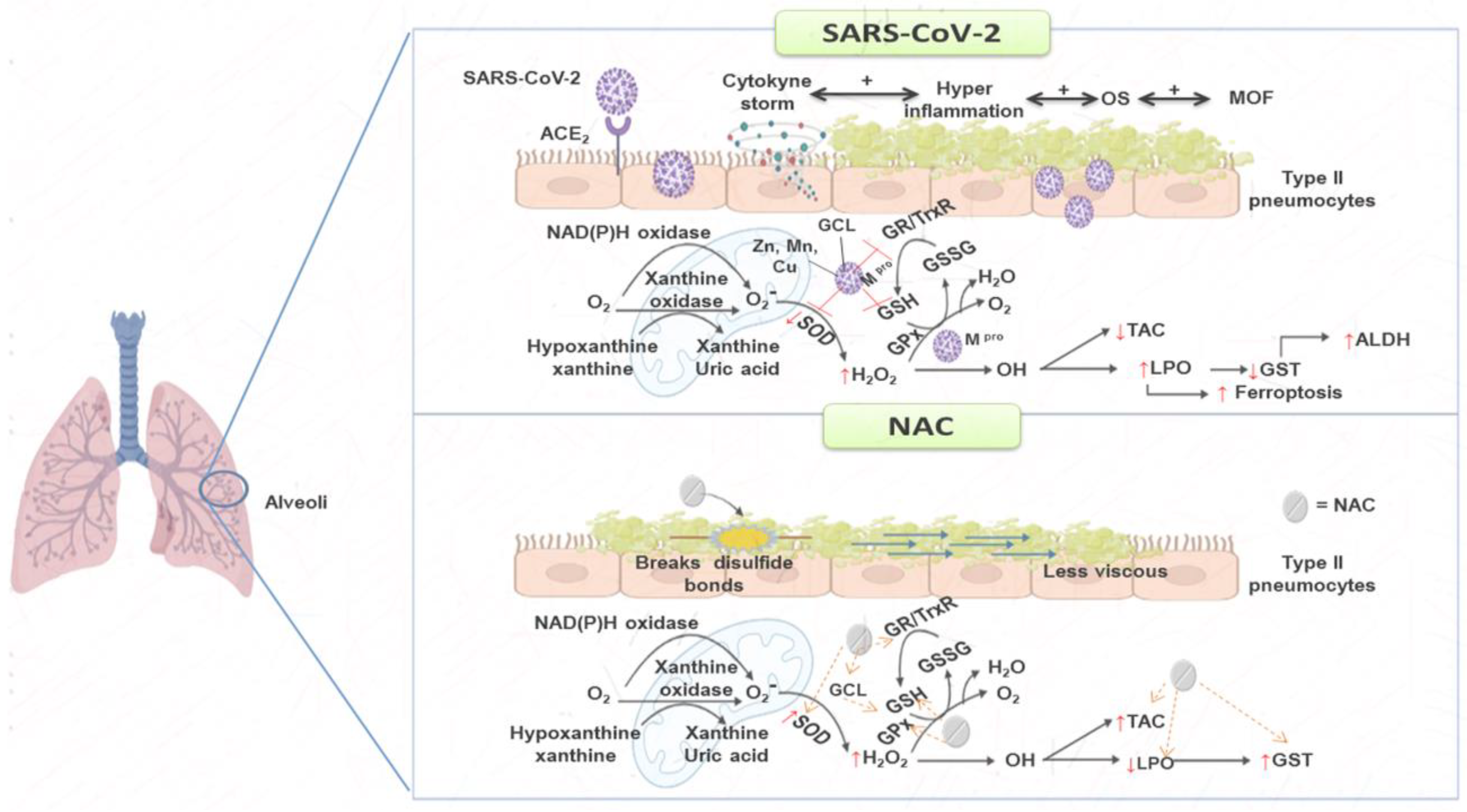
3. Discussion
Study Limitations
4. Materials and Methods
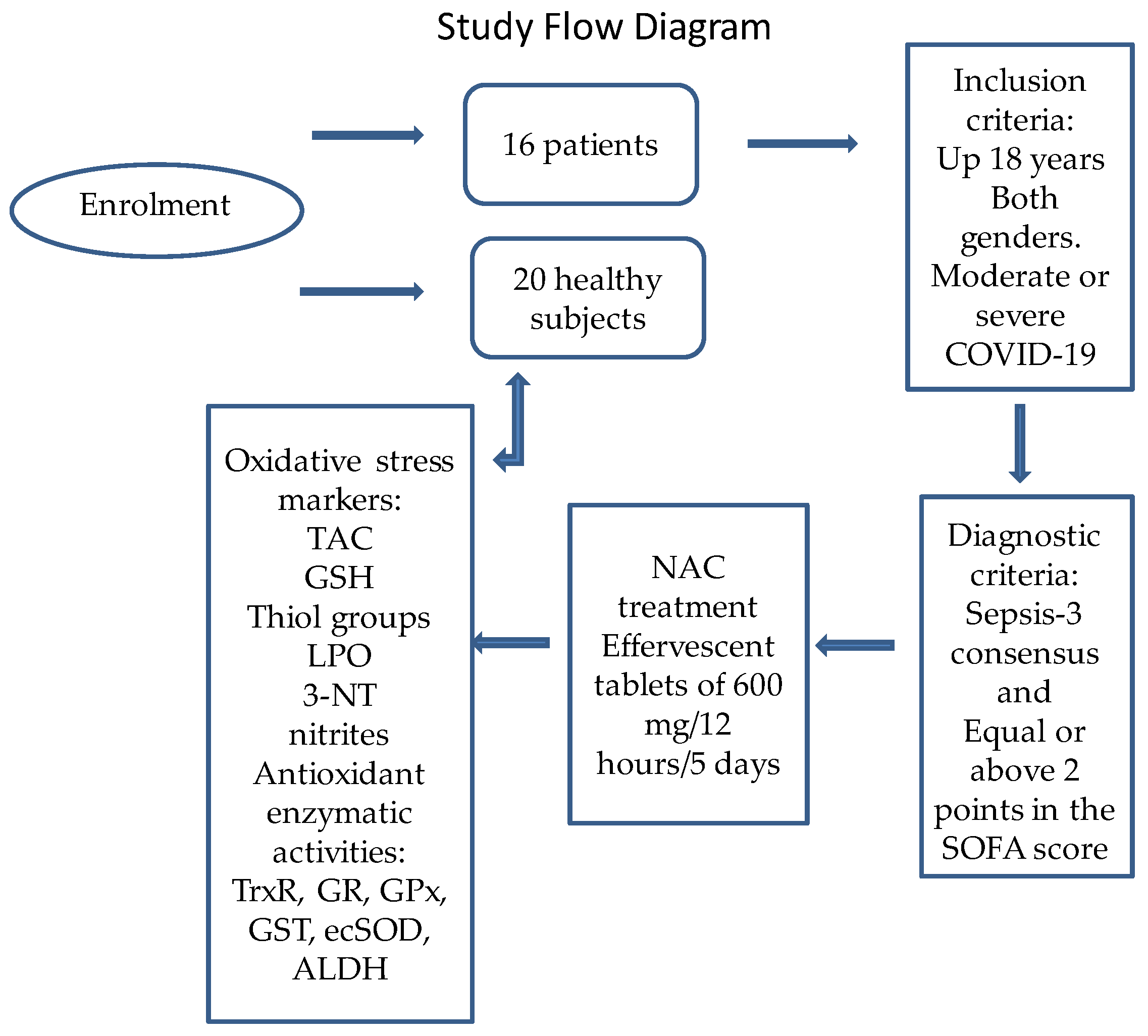
4.1. Description of the Population Studied
4.2. Detection of SARS-CoV-2 by qRT-PCR Technique
4.3. Healthy Subject (Control Group)
4.4. Therapeutic Management
4.5. Doses of the NAC Therapy
4.6. Blood Sample Obtainment and Storage
4.7. Oxidative Stress Markers
Evaluation of Total Antioxidant Capacity (TAC)
4.8. Glutathione Levels (GSH)
4.8.1. Determination of Total Thiol Groups
4.8.2. Determination of Lipid Peroxidation (LPO) Levels, Aldehyde Deshydrogenase Activity (ALDH) and 3-Nitrotyrosine (3-NT)
4.8.3. Nitrites (NO2–)
4.9. Determinations of TrxR, GPx, GST and GR Activities
4.10. Interleukin-6 Concentration
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pérez-Torres, I.; Guarner-Lans, V.; Soria-Castro, E.; Manzano-Pech, L.; Palacios-Chavarría, A.; Valdez-Vázquez, R.R.; Domínguez-Cherit, J.G.; Herrera-Bello, H.; Castillejos-Suastegui, H.; Moreno-Castañeda, L.; et al. Alteration in the lipid profile and the desaturases activity in patients with severe pneumonia by SARS-CoV-2. Front. Physiol. 2021, 12, 667024. [Google Scholar] [CrossRef] [PubMed]
- Minkoff, J.M.; TenOever, B. Innate immune evasion strategies of SARS-CoV-2. Nat. Rev. Microbiol. 2023, 21, 178–194. [Google Scholar] [CrossRef] [PubMed]
- Soto, M.E.; Guarner-Lans, V.; Soria-Castro, E.; Manzano-Pech, L.; Pérez-Torres, I. Is antioxidant therapy a useful complementary measure for COVID-19 treatment? An algorithm for tts application. Medicina 2020, 56, 386. [Google Scholar] [CrossRef] [PubMed]
- Fitero, A.; Bungau, S.G.; Tit, D.M.; Endres, L.; Khan, S.A.; Bungau, A.F.; Romanul, I.; Vesa, C.M.; Radu, A.F.; Tarce, A.G.; et al. Comorbidities, associated diseases, and risk assessment in COVID-19. A systematic review. Int. J. Clin. Pract. 2022, 2022, 1571826. [Google Scholar] [CrossRef]
- Tavassolifar, M.J.; Aghdaei, H.A.; Sadatpour, O.; Maleknia, S.; Fayazzadeh, S.; Mohebbi, S.S.; Montazer, F.; Rabbani, A.; Zali, M.R.; Izad, M.; et al. New insights into extracellular and intracellular redox status in COVID-19 patients. Redox. Biol. 2023, 59, 102563. [Google Scholar] [CrossRef]
- Santus, P.; Danzo, F.; Zuffi, A.; Pini, S.; Saad, M.; Visconti, A.; Radovanovic, D. Oxidative stress and viral infections: Rationale, experiences, and perspectives on N-acetylcysteine. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 8582–8590. [Google Scholar]
- Ho, W.Z.; Douglas, S.D. Glutathione and N-acetylcysteine suppression of human immunodeficiency virus replication in human monocyte/macrophages in vitro AIDS. Res. Hum. Retrovir. 1992, 8, 1249–1253. [Google Scholar]
- Beckman, J.S.; Ischiropoulos, H.; Zhu, L.; van der Woerd, M.; Smith, C.; Chen, J.; Harrison, J.; Martin, J.C.; Tsai, M. Kinetics of superoxide dismutase- and iron-catalyzed nitration of phenolics by peroxynitrite. Arch. Biochem. Biophys. 1992, 298, 438–445. [Google Scholar] [CrossRef]
- Semenova, N.V.; Rychkova, L.V.; Darenskaya, M.A.; Kolesnikov, S.I.; Nikitina, O.A.; Petrova, A.G.; Vyrupaeva, E.V.; Kolesnikova, V.I. Superoxide dismutase activity in male and female patients of different age with moderate COVID-19. Bull. Exp. Biol. Med. 2022, 173, 51–53. [Google Scholar] [CrossRef]
- Sentman, M.L.; Granström, M.; Jakobson, H.; Reaume, A.; Basu, S.; Marklund, S.L. Phenotypes of mice lacking extracellular superoxide dismutase and copper- and zinc-containing superoxide dismutase. J. Biol. Chem. 2006, 281, 6904–6909. [Google Scholar] [CrossRef] [Green Version]
- Negru, P.A.; Radu, A.F.; Vesa, C.M.; Behl, T.; Abdel-Daim, M.M.; Nechifor, A.C.; Endres, L.; Stoicescu, M.; Pasca, B.; Tit, D.M.; et al. Therapeutic dilemmas in addressing SARS-CoV-2 infection: Favipiravir versus Remdesivir. Biomed. Pharmacother. 2022, 147, 112700. [Google Scholar] [CrossRef]
- Heitmann, J.S.; Bilich, T.; Tandler, C.; Nelde, A.; Maringer, Y.; Marconato, M.; Reusch, J.; Jäger, S.; Denk, M.; Richter, M.; et al. A COVID-19 peptide vaccine for the induction of SARS-CoV-2 T cell immunity. Nature 2022, 601, 617–622. [Google Scholar] [CrossRef]
- De Flora, S.; Balansky, R.; La Maestra, S. Rationale for the use of N -acetylcysteine in both prevention and adjuvant therapy of COVID-19. FASEB J. 2020, 34, 13185–13193. [Google Scholar] [CrossRef]
- Aldini, G.; Altomare, A.; Baron, G.; Vistoli, G.; Carini, M.; Borsani, L.; Sergio, S. Acetylcysteine as an antioxidant and disulphide breaking agent: The reasons why. Free. Radic. Res. 2018, 52, 751–762. [Google Scholar] [CrossRef] [Green Version]
- Soto, M.E.; Guarner-Lans, V.; Díaz-Díaz, E.; Manzano-Pech, L.; Palacios-Chavarría, A.; Valdez-Vázquez, R.R.; Aisa-Álvarez, A.; Saucedo-Orozco, H.; Pérez-Torres, I. Hyperglycemia and Loss of Redox Homeostasis in COVID-19 Patients. Cells 2022, 11, 932. [Google Scholar] [CrossRef]
- Stipanuk, M.H. Sulfur amino acid metabolism: Pathways for production and removal of homocysteine and cysteine. Annu. Rev. Nutr. 2004, 24, 539–577. [Google Scholar] [CrossRef]
- Aisa-Alvarez, A.; Soto, M.E.; Guarner-Lans, V.; Camarena-Alejo, G.; Franco-Granillo, J.; Martínez-Rodríguez, E.A.; Gamboa-Ávila, R.; Manzano-Pech, L.; Israel Pérez-Torres, I. Usefulness of antioxidants as adjuvant therapy for septic shock: A randomized clinical trial. Medicina 2020, 56, 619. [Google Scholar] [CrossRef]
- Shi, Z.; Puyo, C.A. N-Acetylcysteine to combat COVID-19: An evidence review. Ther. Clin. Risk. Manag. 2020, 16, 1047–1055. [Google Scholar] [CrossRef]
- Muhammad, Y.; Kani, Y.A.; Iliya, S.; Muhammad, J.B.; Binji, A.; El-Fulaty, A.A.; Kabir, M.B.; Bindawa, K.U.; Ahmed, A. Deficiency of antioxidants and increased oxidative stress in COVID-19 patients: A cross-sectional comparative study in Jigawa, Northwestern Nigeria. SAGE Open Med. 2021, 9, 2050312121991246. [Google Scholar] [CrossRef]
- Engin, A.B.; Engin, E.D.; Engin, A. Can iron, zinc, copper and selenium status be a prognostic determinant in COVID-19 patients? Environ. Toxicol. Pharmacol. 2022, 5, 103937. [Google Scholar] [CrossRef]
- Goud, P.T.; Bai, D.; Abu-Soud, H.M. A Multiple-hit hypothesis involving reactive oxygen species and myeloperoxidase explains clinical deterioration and fatality in COVID-19. Int. J. Biol. Sci. 2021, 17, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.W.; Radding, W. Understanding Selenium and Glutathione as Antiviral Factors in COVID-19: Does the viral Mpro protease target host selenoproteins and glutathione synthesis? Front. Nutr. 2020, 7, 143. [Google Scholar] [CrossRef]
- Singh, J.; Dhindsa, R.S.; Misra, V.; Singh, B. SARS-CoV2 infectivity is potentially modulated by host redox status. Comput. Struct. Biotechnol. J. 2020, 18, 3705–3711. [Google Scholar] [CrossRef] [PubMed]
- Hiffler, L.; Rakotoambinina, B. Selenium and RNA Virus interactions: Potential implications for SARS-CoV-2 infection (COVID-19). Front. Nutr. 2020, 7, 164. [Google Scholar] [CrossRef]
- Naghashpour, M.; Ghiassian, H.; Mobarak, S.; Adelipour, M.; Piri, M.; Seyedtabib, M.; Golabi, S. Profiling serum levels of glutathione reductase and interleukin-10 in positive and negative-PCR COVID-19 outpatients: A comparative study from southwestern Iran. Med. Virol. 2022, 94, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Couto, N.; Wood, J.; Barber, J. The role of glutathione reductase and related enzymes on cellular redox homoeostasis network. Free. Radic. Biol. Med. 2016, 95, 27–42. [Google Scholar] [CrossRef]
- Brücksken, K.A.; Palacio, P.L.; Hanschmann, E.M. Thiol modifications in the extracellular space-key proteins in inflammation and viral infection. Front. Immunol. 2022, 13, 932525. [Google Scholar] [CrossRef]
- NaveenKumar, S.K.; Hemshekhar, M.; Jagadish, S.; Manikanta, K.; Vishalakshi, G.J.; Kemparaju, K.; Girish, K.S. Melatonin restores neutrophil functions and prevents apoptosis amid dysfunctional glutathione redox system. J. Pineal. Res. 2020, 69, e12676. [Google Scholar] [CrossRef]
- Raza, H. Dual localization of glutathione S-transferasa in the cytosol and mitochondria: Implications in oxidative stress, toxicity and disease. FEBS. Lett. 2011, 278, 4243–4251. [Google Scholar] [CrossRef] [Green Version]
- Alsayed, B.A.; Mir, R. Severe COVID-19 Pneumonia and genetic susceptibility: A case report and literature review. Cureus 2022, 14, e23636. [Google Scholar] [CrossRef]
- Hanumanram, G.; Suthakaran, P.K.; Mohanan, J.; Nair, L.D.V.; Rajendran, K. Extracellular oxidative stress markers in COVID-19 Patients with diabetes as co-morbidity. Clin. Pract. 2022, 12, 168–176. [Google Scholar]
- Abbas, M.; Verma, S.; Verma, S.; Siddiqui, S.; Khan, F.H.; Raza, S.T.; Siddiqi, Z.; Eba, A.; Mahdi, F. Association of GSTM1 and GSTT1 gene polymorphisms with COVID-19 susceptibility and its outcome. J. Med. Virol. 2021, 93, 5446–5451. [Google Scholar] [CrossRef]
- Soria-Castro, E.; Soto, M.E.; Guarner-Lans, V.; Rojas, G.; Perezpeña-Diazconti, M.; Críales-Vera, S.A.; Manzano-Pech, L.; Pérez-Torres, I. The kidnapping of mitochondrial function associated with the SARS-CoV-2 infection. Histol. Histopathol. 2021, 36, 947–965. [Google Scholar]
- Martín-Fernández, M.; Aller, R.; Heredia-Rodríguez, M.; Gómez-Sánchez, E.; Martínez-Paz, P.; Gonzalo-Benito, H.; Sánchez-de Prada, L.; Gorgojo, O.; Carnicero-Frutos, I.; Tamayo, E.; et al. Lipid peroxidation as a hallmark of severity in COVID-19 patients. Redox. Biol. 2021, 48, 102181. [Google Scholar] [CrossRef]
- Castelli, G.; Pelosi, E.; Testa, U. Liver cancer: Molecular characterization, clonal evolution and cancer stem cells. Cancers 2017, 9, 127. [Google Scholar] [CrossRef] [Green Version]
- Cho, N.E.; Bang, B.R.; Gurung, P.; Meng Li Clemens, D.L.; Underhill, T.M.; James, L.P.; Chase, J.R.; Saito, T. Retinoid regulation of antiviral innate immunity in hepatocytes. Hepatology 2016, 63, 1783–1795. [Google Scholar] [CrossRef] [Green Version]
- Yaghoubi, N.; Youssefi, M.; Azad, F.J.; Farzad, F.; Yavari, Z.; Avval, F.A. Total antioxidant capacity as a marker of severity of COVID-19 infection: Possible prognostic and therapeutic clinical application. J. Med. Virol. 2022, 94, 1558–1565. [Google Scholar] [CrossRef]
- Dairou, J.; Pluvinage, B.; Noiran, J.; Petit, E.; Vinh, J.; Haddad, I.; Mary, J.; Dupret, J.M.; Rodrigues-Lima, F. Nitration of a critical tyrosine residue in the allosteric inhibitor site of muscle glycogen phosphorylase impairs its catalytic activity. J. Mol. Biol. 2007, 372, 1009–1021. [Google Scholar] [CrossRef]
- Pérez-Torres, I.; Manzano-Pech, L.; Rubio-Ruíz, M.E.; Soto, M.E.; Guarner-Lans, V. Nitrosative stress and its association with cardiometabolic disorders. Molecules 2020, 25, 2555. [Google Scholar] [CrossRef]
- Scialo, F.; Daniele, A.; Amato, F.; Pastore, L.; Gabriella Matera, M.; Mario Cazzola, M.; Castaldo, G.; Bianco, A. ACE2: The major cell entry receptor for SARS-CoV-2. Lung 2020, 198, 867–877. [Google Scholar] [CrossRef]
- Busch, M.H.; Timmermans, S.A.; Van Kuijk, S.M.J.; Aendekerk, J.P.; Ysermans, R.; Van Doorn, D.P.C.; Potjewijd, J.; Van de Poll, M.C.G.; Van der Horst, I.C.C.; Damoiseaux, J.G.M.; et al. Thrombin formation via the intrinsic coagulation pathway and von Willebrand factor reflect disease severity in COVID-19. Haematologica, 2022; online ahead of print. [Google Scholar] [CrossRef]
- Izquierdo-Alonso, J.L.; Pérez-Rial, S.; Rivera, C.G.; Peces-Barba, G. N-acetylcysteine for prevention and treatment of COVID-19: Current state of evidence and future directions. J. Infect. Public Health 2022, 15, 1477–1483. [Google Scholar] [CrossRef] [PubMed]
- Maradi, R.; Joshi, V.; Balamurugan, V.; Thomas, D.S.; Goud, M.B. Importance of microminerals for maintaining antioxidant function after COVID-19-induced oxidative stress. Rep. Biochem. Mol. Biol. 2022, 11, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Manz, X.D.; Bogaard, H.J.; Aman, J. Regulation of VWF (Von Willebrand Factor) in inflammatory thrombosis. Arterioscler. Thromb. Vasc. Biol. 2022, 42, 1307–1320. [Google Scholar] [CrossRef] [PubMed]
- Olson, G.; Andrew, M.D. Diagnosis and treatment of adults with community-acquired pneumonia. JAMA 2020, 323, 885–886. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. Declaration of Helsinki. Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [Green Version]
- Lambden, S.; Laterre, P.F.; Levy, M.M.; Francois, B. The SOFA score-development, utility, and challenges of accurate assessment in clinical trials. Crit. Care 2019, 23, 374. [Google Scholar] [CrossRef] [Green Version]
- Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar]
- Chavarría, A.P.; Vázquez, R.R.V.; Cherit, J.G.D.; Bello, H.H.; Suastegui, H.C.; Moreno-Castañeda, L.; Alanís-Estra, G.; Hernández, F.; González-Marcos, O.; Saucedo-Orozco, H.; et al. Antioxidants and pentoxifylline as coadjuvant measures to standard therapy to improve prognosis of patients with pneumonia by COVID-19. Comput. Struct. Biotechnol. J. 2021, 19, 1379–1390. [Google Scholar] [CrossRef]
- Brower, R.G.; Lanken, P.N.; MacIntyre, N.; Matthay, M.A.; Morris, A.; Marek, A. National heart, lung, and blood institute ARDS clinical trials network higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N. Engl. J. Med. 2004, 351, 327–336. [Google Scholar]
- Thachil, J.; Tang, N.; Gando, S.; Falanga, A.; Cattaneo, M.; Levi, M.; Clark, C.; Iba, T. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J. Thromb. Haemost. 2020, 18, 1023–1026. [Google Scholar] [CrossRef] [Green Version]
- Guimarães, H.P.; Timerman, S.; Rodrigues, R.D.R.; Corrêa, T.D.; Schubert, D.U.C.; Freitas, A.P. Position statement: Cardiopulmonary resuscitation of patients with confirmed or suspected COVID-19—2020. Arq. Bras. Cardiol. 2020, 114, 1078–1087. [Google Scholar] [CrossRef]
- Erel, O.; Neselioglu, S. A novel and automated assay for thiol/disulphide homeostasis. Clin. Biochem. 2014, 49, 326–332. [Google Scholar] [CrossRef]
- Pérez-Torres, I.; Manzano-Pech, L.; Guarner-Lans, V.; Soto, M.E.; Castrejón-Téllez, V.; Márquez-Velasco, R.; Vargas-González, Á.; Martínez-Memije, R.; Del Valle-Mondragón, L.; Díaz-Juárez, J.A.; et al. Deodorized garlic decreases oxidative stress caused by lipopolysaccharide in rat heart through hydrogen sulfide: Preliminary Findings. Int. J. Mol. Sci. 2022, 23, 12529. [Google Scholar] [CrossRef]
- Rodríguez-Fierros, F.L.; Guarner-Lans, V.; Soto, M.E.; Manzano-Pech, L.; Díaz-Díaz, E.; Soria-Castro, E.; Rubio-Ruiz, M.E.; Jiménez-Trejo, F.; Israel Pérez-Torres, I. Modulation of renal function in a metabolic syndrome rat model by Antioxidants in Hibiscus sabdariffa L. Molecules 2021, 26, 2074. [Google Scholar] [CrossRef]
| Variables | Median and (Min–Max) |
|---|---|
| Age | 55 (27–84) |
| BMI | 28 (23–38) |
| Comorbidities | N (%) |
| Diabetes Mellitus | 8 (47) |
| Hypertension | 8 (47) |
| Dyslipidemia | 7 (41) |
| Normal Weight | 6 (35) |
| Overweight | 7 (41) |
| Obesity | 3 (24) |
| Signs | Median (Min–Max) |
| PaO2 | 70 (59–179) |
| PCO2 | 31.7 (12–39) |
| PaO2/FIO2 (mmHg) | 131.5 (45–282) |
| SPO2/FIO2 (mmHg) | 119 (61–290) |
| HR lpm | 74 (20–116) |
| MAP (mmHg) | 78 (71–102) |
| Temperature °C | 36 (36.5–37.2) |
| Glucose | 141 (100–359) |
| Creatinine in serum mg/dL | 0.85 (0.50–1.30) |
| BUN | 17 (5.6–36.6) |
| TC mg/dL | 133 (89–210) |
| TG mg/dL | 186.5 (80–726) |
| HDL-Cholesterol mg/dL | 26 (14–40) |
| LDL mg/dL | 62 (40–1109) |
| Leukocytes 10^3/µL | 8.8 (3.5–14.9) |
| Lymphocytes 10^3/µL | 8.3 (0.14–1.54) |
| Platelets 10^3/µL | 253 (164–408) |
| Ferritin ng/mL | 435.4 (146–2592) |
| D-dimer ng/dL | 605 (200–2100) |
| IL-6 pg/mL | 16.2 (7.8–324) |
| N/L | 8.4 (3–67) |
| C reactive protein mg/dL | 143.15 (44.7–221) |
| Procalcitonin ng/dL | 0.23 (0.08–11.4) |
| SOFA | 0 (0–3) |
| APACHE | 5.5 (4–8) |
| SAPS | 27 (18–32) |
| Patients in moderate condition | 8 (47%) |
| Patients with severe condition | 9 (53%) |
| Days With Mechanic ventilation | 13 (5–28) |
| Days in ICU | 17 (3–30) |
| Variable | Median and (Min–Max) |
|---|---|
| Gender | 10 men and 10 female |
| Age | 53 (30–63) |
| Glucose (mg/dL) | 83.0 (62.0–120.0) |
| Insulin (ng/mL) | 0.3 (0.15–0.47) |
| Cholesterol (mg/dL) | 169.0 (143.0–209.0) |
| Triglycerides (mg/dL) | 93.5 (45.0–223.0) |
| HDL (mg/dL) | 41.5 (32.0–80.0) |
| LDL (mg/dL) | 94.5 (65.0–121.0) |
| Creatinine (mg/dL) | 0.8 (0.69–0.9) |
| Variables (mL/Serum) | HS Median (Min–Max) (Q1, Q3, IQR) | Before the NAC Treatment SARS-CoV-2 Infection Median and (Min–Max) (Q1, Q3, IQR) | After the NAC Treatment SARS-CoV-2 Infection Median and (Min–Max) (Q1, Q3, IQR) |
|---|---|---|---|
| TAC (nM Trolox) | 12,783.5 (8111.7–33,063.9) (9598.1, 26,719.6, 17,121.4) | 5727.1 (4710.5–7034.6) *** (5143.2, 6527.7, 1384.4) | 7143.3 (6193.6–10,021.6) *** (6564.9, 7730.4, 1165.5) |
| GSH (µM) | 0.07 (0.05–0.22) | 0.03 (0.01–0.07) *** | 0.07 (0.03–0.43) *** |
| (0.06, 0.10, 0.03) | (0.03, 0.04, 0.01) | (0.04, 0.27, 0.23) | |
| Thiols (µM) | 9.1 (3.3–16.4) | 3.1 (0.4–6.6) *** | 4.7 (0.7–9.8)* |
| (6.4, 11.1, 4.6) | (1.6, 4.5, 2.9) | (3.1, 6.8, 3.6) | |
| LPO (nM MDA) | 1.2 (0.3–2.7) | 2.1 (0.6–3.6) ** | 1.1 (0.2–2.6) * |
| (0.7, 1.8, 1.1) | (1.2, 2.7, 1.5) | (0.3, 2.3, 1.9) | |
| NO2 (nM) | 22.0 (10.7–67.5) | 3.5 (2.5–11.1) *** | 24.1 (9.5–45.7) *** |
| (19.9, 28.6, 8.7) | (2.9, 6.5, 3.6) | (15.0, 30.7, 15.7) | |
| 3-NT (nM) | 7.0 × 10−7 (1.3 × 10−8–8.0 × 10−3) (4.4 × 10−7, 5.6 × 10−3, 5.6 × 10−3) | 7.1 × 10−3 (5.3 × 10−4 – 2.1 × 10−2) *** (2.9 × 10−3, 9.8 × 10−3, 6.9 × 10−3) | 1.2 × 10−2 (2.8 × 10−3–4.4 × 10−2) * (6.2 × 10−3, 1.6 × 10−2, 1.0 × 10−2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soto, M.E.; Manzano-Pech, L.; Palacios-Chavarría, A.; Valdez-Vázquez, R.R.; Guarner-Lans, V.; Pérez-Torres, I. N-Acetyl Cysteine Restores the Diminished Activity of the Antioxidant Enzymatic System Caused by SARS-CoV-2 Infection: Preliminary Findings. Pharmaceuticals 2023, 16, 591. https://doi.org/10.3390/ph16040591
Soto ME, Manzano-Pech L, Palacios-Chavarría A, Valdez-Vázquez RR, Guarner-Lans V, Pérez-Torres I. N-Acetyl Cysteine Restores the Diminished Activity of the Antioxidant Enzymatic System Caused by SARS-CoV-2 Infection: Preliminary Findings. Pharmaceuticals. 2023; 16(4):591. https://doi.org/10.3390/ph16040591
Chicago/Turabian StyleSoto, María Elena, Linaloe Manzano-Pech, Adrían Palacios-Chavarría, Rafael Ricardo Valdez-Vázquez, Verónica Guarner-Lans, and Israel Pérez-Torres. 2023. "N-Acetyl Cysteine Restores the Diminished Activity of the Antioxidant Enzymatic System Caused by SARS-CoV-2 Infection: Preliminary Findings" Pharmaceuticals 16, no. 4: 591. https://doi.org/10.3390/ph16040591
APA StyleSoto, M. E., Manzano-Pech, L., Palacios-Chavarría, A., Valdez-Vázquez, R. R., Guarner-Lans, V., & Pérez-Torres, I. (2023). N-Acetyl Cysteine Restores the Diminished Activity of the Antioxidant Enzymatic System Caused by SARS-CoV-2 Infection: Preliminary Findings. Pharmaceuticals, 16(4), 591. https://doi.org/10.3390/ph16040591