Extensive CArdioVAscular Characterization and Follow-Up of Patients Receiving Immune Checkpoint Inhibitors: A Prospective Multicenter Study
Abstract
1. Introduction
- How should hs-TnI/hs-TnT levels be graded? The Common Terminology Criteria for Adverse Events (CTCAE, Version 5) is widely accepted as the standard classification and severity grading scale for adverse events in cancer therapy, clinical trials, and other oncology settings [38]. However, the current grading system makes it challenging to accurately document and report the severity and incidence of increased cardiac troponins, leading to ICI-induced CV toxicity. Furthermore, BNP and NT-proBNP are not even listed in the current CTCAE criteria.
- What are the cut-off values for cardiac markers for clinically meaningful changes in ICI-treated patients? When do further investigations need to be performed? (The upper limit of normal (ULN) is determined on a healthy patient population).
- What is the appropriate time interval between the testing of cardiac markers?
- What is the appropriate monitoring duration of cardiac markers? Three months or longer?
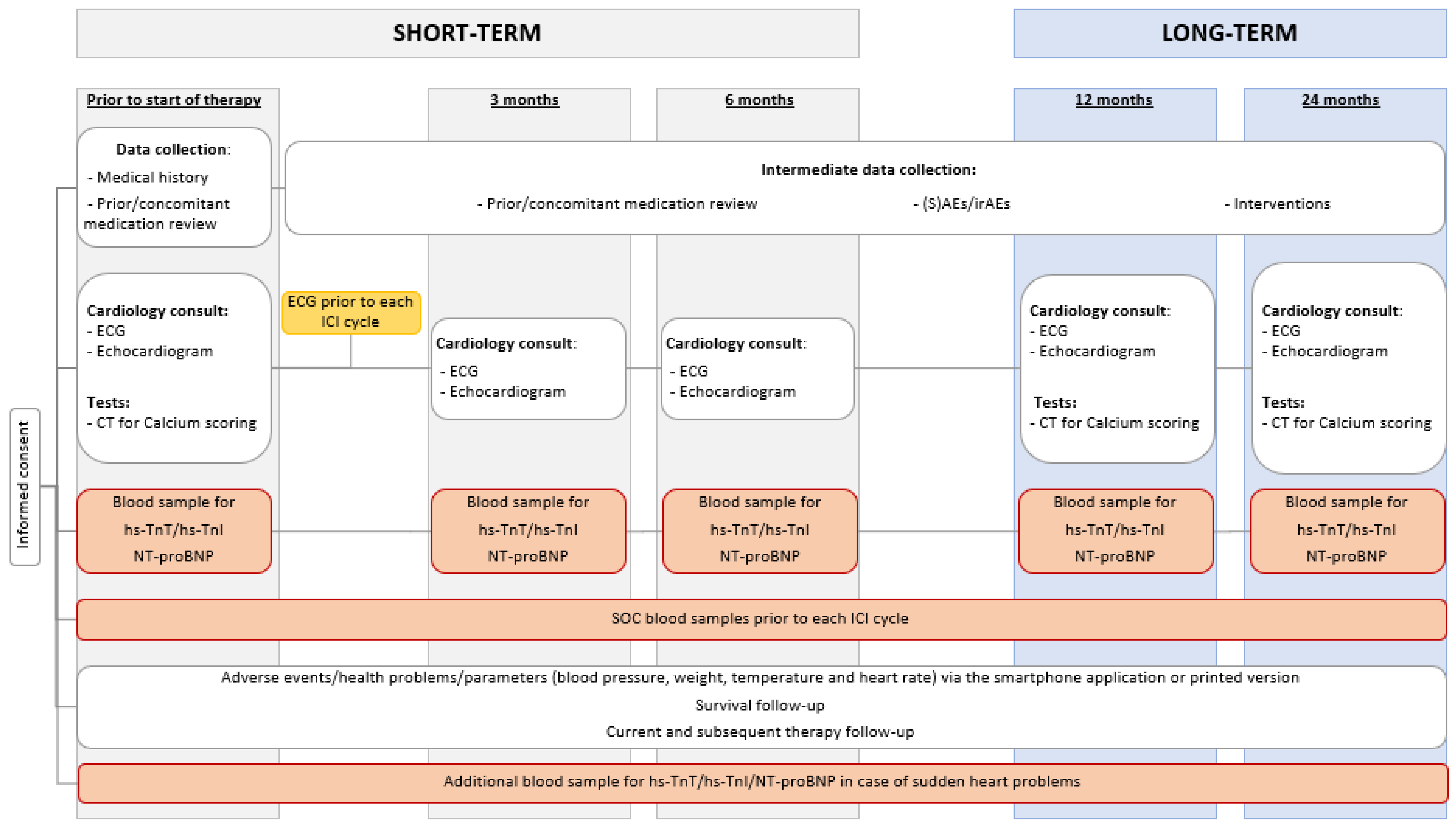
2. Methods and Analysis
2.1. Study Flow
2.2. Participants
2.2.1. Inclusion Criteria
- At least 18 years of age, at the time of giving informed consent.
- Able to provide informed consent.
- Have a solid tumor and will receive one of the following FDA-approved therapies, i.e., anti-PD-1, anti-PD-L1, and/or anti-CTLA-4 therapy in mono- or combination therapy.
- Have to be literate in Dutch or English.
2.2.2. Exclusion Criteria
- Prior treatment with immunotherapy (ICIs, T-cell transfer therapy, cancer treatment vaccines, or immune system modulators).
- Receive a regimen where ICIs will be administered together with other systemic anti-cancer agents (chemotherapy, tyrosine kinase inhibitors (TKI), etc.).
- History of human immunodeficiency virus infection.
- History of hepatitis B (defined as hepatitis B surface antigen [HBsAg] reactive) or known hepatitis C virus (HCV) (defined as detectable HCV RNA via qualitative nucleic acid testing) infection.
- Diagnosis of immunodeficiency or receiving chronic systemic steroid therapy (in daily doses exceeding 10 mg of prednisone equivalent).
2.3. Schedule of Examinations
2.3.1. Baseline Patient/Disease Characteristics
- Informed consent.
- Demographics (Eastern Cooperative Oncology Group performance status, age at start of treatment, sex, body mass index).
- Medical history: CV risk, COVID-19, auto-immune diseases, and other medical conditions.
- Current oncological disease (clinical and pathological TNM classification, current disease status, prior treatment, and molecular screening profile).
- Prior cancer history.
- Prior/concomitant medication review, especially antibiotic and oral steroid use.
- Other relevant parameters.
2.3.2. Electrocardiogram (ECG)
2.3.3. Transthoracic Echocardiogram (TTE)
2.3.4. Computed Tomography (CT) Scan for Calcium Scoring
2.3.5. Blood Sampling
2.4. Endpoints
2.4.1. Primary Endpoint
2.4.2. Secondary Endpoints
- The incidence of hs-TnT/NT-proBNP elevations at 6, 12, and 24 months.
- The incidence of hs-TnT/NT-proBNP above the ULN at baseline, 3, 6, 12, and 24 months *.
- Evolution of hs-TnT/NT-proBNP in 24 months compared to baseline.
- Evolution of transthoracic echocardiography parameters at baseline, 3, 6, 12, and 24 months *.
- Evolution of electrocardiography parameters at baseline, 3, 6, 12, and 24 months.*
- Association between the evolution of troponin/NT-proBNP and transthoracic echocardiography and electrocardiography parameters at baseline, 3, 6, 12, and 24 months *.
| Normal | Patients with Normal Biomarkers and LV Function Parameters |
|---|---|
| Mild | Asymptomatic patients with LVEF ≥ 50% with elevated biomarkers Asymptomatic patients with LVEF ≥ 50% with at least one additional abnormal echo parameter: (1) Increased LVESV (2) LA area > 30 cm2 (3) 10% decrease of LVEF to an LVEF < 53% (4) Average E/e’ > 14 (5) GLS > −18% (6) 15% relative reduction of GLS from baseline |
| Moderate | Asymptomatic patients with LVEF ≥ 40% and <50% with or without biomarker increase or other LV function abnormalities |
| Severe | Patients with asymptomatic LVEF < 40% Clinical HF: - HFrEF: HF symptoms/signs and LVEF < 40% - HFmrEF: symptoms/signs of HF with elevated NT-proBNP, LVEF 40–49%, and at least one additional criteria (enlarged LA, LV hypertrophy, or other relevant diastolic function parameters) - HFpEF: in presence of symptoms/signs of HF, elevated NT-proBNP, LVEF ≥ 50%, and at least one additional criteria (enlarged LA, LV hypertrophy, or other diastolic dysfunction parameters) |
- Association between the evolution of troponin/NT-proBNP and CV abnormalities.
- Cumulative incidence of MACEs at 3, 6, 12, and 24 months. MACEs were defined as the composite outcomes of nonfatal stroke, nonfatal myocardial infarction, hospital admission for heart failure (HF), cardiac revascularization, and CV death.
- Overall survival *.
- Association between the evolution of troponin/NT-proBNP and MACEs.
- The difference in the evolution of hs-TnT/NT-proBNP/transthoracic echocardiography and electrocardiography parameters between combination therapy and monotherapy.
- Association between patient characteristics and troponin.
- Association between patient characteristics and NT-proBNP.
- Agreement between hs-TnT and hs-TnI levels at baseline, 3, 6, 12, and 24 months *.
- The proportion of severe immune-related non-CV toxicities (grades 3–5).
- Association between the evolution of troponin/NT-proBNP and severe immune-related non-CV toxicities (grade 3–5).
- Association between the evolution of troponin/NT-proBNP and overall survival.
- Association between the evolution of troponin and diastolic function (based on the recommendations of Nagueh et al. [42]).
- Association between the evolution of troponin and calcium score.
2.5. Sample Size
2.6. Data Analysis
3. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, D.Y.; Okoye, G.D.; Neilan, T.G.; Johnson, D.B.; Moslehi, J.J. Cardiovascular Toxicities Associated with Cancer Immunotherapies. Curr. Cardiol. Rep. 2017, 19, 21. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.; Bahia, J.; Mohebtash, M.; Barac, A. Cardiovascular Complications Associated with Novel Cancer Immunotherapies. Curr. Treat. Options Cardiovasc. Med. 2017, 19, 36. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Y.; Salem, J.-E.; Cohen, J.V.; Chandra, S.; Menzer, C.; Ye, F.; Zhao, S.; Das, S.; Beckermann, K.E.; Ha, L.; et al. Fatal Toxic Effects Associated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol. 2018, 4, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Michel, L.; Rassaf, T.; Totzeck, M. Cardiotoxicity from immune checkpoint inhibitors. IJC Heart Vasc. 2019, 25, 100420. [Google Scholar] [CrossRef]
- Escudier, M.; Cautela, J.; Malissen, N.; Ancedy, Y.; Orabona, M.; Pinto, J.; Monestier, S.; Grob, J.J.; Scemama, U.; Jacquier, A.; et al. Clinical features, management, and outcomes of immune checkpoint inhibitor-related cardiotoxicity. Circulation 2017, 136, 2085–2087. [Google Scholar] [CrossRef]
- Lyon, A.R.; Yousaf, N.; Battisti, N.M.L.; Moslehi, J.; Larkin, J. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol. 2018, 19, e447–e458. [Google Scholar] [CrossRef]
- Pirozzi, F.; Poto, R.; Aran, L.; Cuomo, A.; Galdiero, M.R.; Spadaro, G.; Abete, P.; Bonaduce, D.; Marone, G.; Tocchetti, C.G.; et al. Cardiovascular Toxicity of Immune Checkpoint Inhibitors: Clinical Risk Factors. Curr. Oncol. Rep. 2021, 23, 1–8. [Google Scholar] [CrossRef]
- Salem, J.E.; Manouchehri, A.; Moey, M.; Lebrun-Vignes, B.; Bastarache, L.; Pariente, A.; Gobert, A.; Spano, J.P.; Balko, J.M.; Bonaca, M.P.; et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: An observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018, 19, 1579–1589. [Google Scholar] [CrossRef]
- Hu, J.R.; Florido, R.; Lipson, E.J.; Naidoo, J.; Ardehali, R.; Tocchetti, C.G.; Lyon, A.R.; Padera, R.F.; Johnson, D.B.; Moslehi, J. Cardiovascular toxicities associated with immune checkpoint inhibitors. Cardiovasc. Res. 2019, 115, 854–868. [Google Scholar] [CrossRef]
- Daxini, A.; Cronin, K.; Sreih, A.G. Vasculitis associated with immune checkpoint inhibitors—A systematic review. Clin. Rheumatol. 2018, 37, 2579–2584. [Google Scholar] [CrossRef]
- Chen, D.Y.; Huang, W.K.; Chien-Chia Wu, V.; Chang, W.C.; Chen, J.S.; Chuang, C.K.; Chu, P.H. Cardiovascular toxicity of immune checkpoint inhibitors in cancer patients: A review when cardiology meets immuno-oncology. J. Formos. Med. Assoc. 2020, 119, 1461–1475. [Google Scholar] [CrossRef]
- Spallarossa, P.; Tini, G.; Sarocchi, M.; Arboscello, E.; Grossi, F.; Queirolo, P.; Zoppoli, G.; Ameri, P. Identification and management of immune checkpoint inhibitor–related myocarditis: Use troponin wisely. J. Clin. Oncol. 2019, 37, 2201–2205. [Google Scholar] [CrossRef] [PubMed]
- Laenens, D.; Yu, Y.; Santens, B.; Jacobs, J.; Beuselinck, B.; Bechter, O.; Wauters, E.; Staessen, J.; Janssens, S.; Van Aelst, L. Incidence of Cardiovascular Events in Patients Treated with Immune Checkpoint Inhibitors. J. Clin. Oncol. 2022, 40, 3430–3438. [Google Scholar] [CrossRef] [PubMed]
- Solinas, C.; Saba, L.; Sganzerla, P.; Petrelli, F. Venous and arterial thromboembolic events with immune checkpoint inhibitors: A systematic review. Thromb. Res. 2020, 196, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Oren, O.; Yang, E.H.; Molina, J.R.; Bailey, K.R.; Blumenthal, R.S.; Kopecky, S.L. Cardiovascular Health and Outcomes in Cancer Patients Receiving Immune Checkpoint Inhibitors. Am. J. Cardiol. 2020, 125, 1920–1926. [Google Scholar] [CrossRef]
- D’Souza, M.; Nielsen, D.; Svane, I.M.; Iversen, K.; Rasmussen, P.V.; Madelaire, C.; Fosbøl, E.; Køber, L.; Gustafsson, F.; Andersson, C.; et al. The risk of cardiac events in patients receiving immune checkpoint inhibitors: A nationwide Danish study. Eur. Heart J. 2021, 42, 1621–1631. [Google Scholar] [CrossRef]
- Dolladille, C.; Akroun, J.; Morice, P.M.; Dompmartin, A.; Ezine, E.; Sassier, M.; Da-Silva, A.; Plane, A.F.; Legallois, D.; L’Orphelin, J.M.; et al. Cardiovascular immunotoxicities associated with immune checkpoint inhibitors: A safety meta-analysis. Eur. Heart J. 2021, 42, 4964–4977. [Google Scholar] [CrossRef]
- Suero-Abreu, G.A.; Zanni, M.V.; Neilan, T.G. Atherosclerosis with Immune Checkpoint Inhibitor Therapy Evidence, Diagnosis, and Management: JACC: CardioOncology State-of-the-Art Review. Cardio Oncol. 2022, 4, 598–615. [Google Scholar] [CrossRef]
- Drobni, Z.D.; Alvi, R.M.; Taron, J.; Zafar, A.; Murphy, S.P.; Rambarat, P.K.; Mosarla, R.C.; Lee, C.; Zlotoff, D.A.; Raghu, V.K.; et al. Association between Immune Checkpoint Inhibitors with Cardiovascular Events and Atherosclerotic Plaque. Circulation 2020, 142, 2299–2311. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, S.; Yang, F.; Qi, X.; Wang, X.; Guan, X.; Shen, C.; Duma, N.; Aguilera, J.V.; Chintakuntlawar, A.; et al. Treatment-Related Adverse Events of PD-1 and PD-L1 Inhibitors in Clinical Trials: A Systematic Review and Meta-analysis. JAMA Oncol. 2019, 5, 1008. [Google Scholar] [CrossRef]
- Gelsomino, F.; Fiorentino, M.; Zompatori, M.; Poerio, A.; Melotti, B.; Sperandi, F.; Gargiulo, M.; Borghi, C.; Ardizzoni, A. Programmed death-1 inhibition and atherosclerosis: Can nivolumab vanish complicated atheromatous plaques? Focus Liq. Bicopsy 2018, 29, 284–286. [Google Scholar] [CrossRef] [PubMed]
- Chitturi, K.R.; Xu, J.; Araujo-Gutierrez, R.; Bhimaraj, A.; Guha, A.; Hussain, I.; Kassi, M.; Bernicker, E.H.; Trachtenberg, B.H. Immune Checkpoint Inhibitor-Related Adverse Cardiovascular Events in Patients with Lung Cancer. JACC CardioOncol. 2019, 1, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Bar, J.; Markel, G.; Gottfried, T.; Percik, R.; Leibowitz-Amit, R.; Berger, R.; Golan, T.; Daher, S.; Taliansky, A.; Dudnik, E.; et al. Acute vascular events as a possibly related adverse event of immunotherapy: A single-institute retrospective study. Eur. J. Cancer 2019, 120, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Newman, J.L.; Stone, J.R. Immune checkpoint inhibition alters the inflammatory cell composition of human coronary artery atherosclerosis. Cardiovasc. Pathol. 2019, 43, 107148. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.-B.; Zhang, Q.; Li, H.-J.; Michot, J.M.; Liu, H.-B.; Zhan, P.; Lv, T.-F.; Song, Y. Evaluation of rare but severe immune related adverse effects in PD-1 and PD-L1 inhibitors in non-small cell lung cancer: A meta-analysis. Transl. Lung Cancer Res. 2017, 6, 8–20. [Google Scholar] [CrossRef]
- Nso, N.; Antwi-Amoabeng, D.; Ulanja, M.B.; Ghuman, J.; Hanfy, A.; Doshi, R.; Gullapalli, N.; Beutler, B.D.; Nimo-Boampong, J.; Atanga, S.; et al. Cardiac adverse events of immune checkpoint inhibitors in oncology patients: A systematic review and meta-analysis. World J. Cardiol. 2020, 12, 584–5598. [Google Scholar] [CrossRef]
- Thompson, J.A.; Schneider, B.J.; Achufusi, A.; Armand, P.; Berkenstock, M.K.; Bhatia, S.; Budde, L.E.; Davies, M.; Elshoury, A.; Gesthalter, Y.; et al. NCCN Guidelines Version 1.2022 Management of Immunotherapy-Related Toxicities NCCN Guidelines Panel Disclosures Continue NCCN. 2022. Available online: https://www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf (accessed on 9 February 2023).
- Brahmer, J.R.; Lacchetti, C.; Schneider, B.J.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; Ernstoff, M.S.; Gardner, J.M.; Ginex, P.; et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J. Clin. Oncol. 2018, 36, 1714–1768. [Google Scholar] [CrossRef]
- Puzanov, I.; Diab, A.; Abdallah, K.; Bingham, C.O.; Brogdon, C.; Dadu, R.; Hamad, L.; Kim, S.; Lacouture, M.E.; LeBoeuf, N.R.; et al. Managing toxicities associated with immune checkpoint inhibitors: Consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J. Immunother. Cancer 2017, 5, 95. [Google Scholar] [CrossRef]
- Bonaca, M.P.; Olenchock, B.A.; Salem, J.E.; Wiviott, S.D.; Ederhy, S.; Cohen, A.; Stewart, G.C.; Choueiri, T.K.; Di Carli, M.; Allenbach, Y.; et al. Myocarditis in the Setting of Cancer Therapeutics: Proposed Case Definitions for Emerging Clinical Syndromes in Cardio-Oncology. Circulation 2019, 140, 80–91. [Google Scholar] [CrossRef]
- Pudil, R.; Mueller, C.; Čelutkienė, J.; Henriksen, P.A.; Lenihan, D.; Dent, S.; Barac, A.; Stanway, S.; Moslehi, J.; Suter, T.M.; et al. Role of serum biomarkers in cancer patients receiving cardiotoxic cancer therapies: A position statement from the Cardio-Oncology Study Group of the Heart Failure Association and the Cardio-Oncology Council of the European Society of Cardiology. Eur. J. Heart Fail. 2020, 22, 1966–1983. [Google Scholar] [CrossRef]
- Zamorano, J.L.; Lancellotti, P.; Rodriguez Muñoz, D.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.H.; Lyon, A.R.; et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines. Eur. Heart J. 2016, 37, 2768–2801. [Google Scholar] [CrossRef] [PubMed]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS)Developed by the task force on cardio-oncology of the European Society of Cardiology (ESC). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef] [PubMed]
- Ganatra, S.; Neilan, T.G. Immune Checkpoint Inhibitor-Associated Myocarditis. Oncologist 2018, 23, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Müller, O.J.; Spehlmann, M.E.; Frey, N. Cardio-toxicity of checkpoint inhibitors. J. Thorac. Dis. 2018, 10, S4400–S4404. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, R.; Nautiyal, A.; Singh, S. Diagnosis of immune checkpoint inhibitor-associated myocarditis: A systematic review. Int. J. Cardiol. 2019, 296, 113–121. [Google Scholar] [CrossRef]
- Thuny, F.; Bonaca, M.P.; Cautela, J. What Is the Evidence of the Diagnostic Criteria and Screening of Immune Checkpoint Inhibitor–Induced Myocarditis? JACC CardioOncol. 2022, 4, 624–628. [Google Scholar] [CrossRef]
- Cancer Institute, N. Common Terminology Criteria for Adverse Events (CTCAE) Common Terminology Criteria for Adverse Events (CTCAE) v5.0; United States Department of Health and Human Services: Washington, DC, USA, 2017.
- Delombaerde, D.; Vervloet, D.; Franssen, C.; Croes, L.; Gremonprez, F.; Prenen, H.; Peeters, M.; Vulsteke, C. Clinical implications of isolated troponinemia following immune checkpoint inhibitor therapy. ESMO Open 2021, 6, 100216. [Google Scholar] [CrossRef]
- Douglas, P.S.; Garcia, M.J.; Haines, D.E.; Lai, W.W.; Manning, W.J.; Patel, A.R.; Picard, M.H.; Polk, D.M.; Ragosta, M.; Ward, R.P.; et al. ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 Appropriate use criteria for echocardiography. J. Am. Soc. Echocardiogr. 2011, 24, 229–267. [Google Scholar] [CrossRef]
- López-Sendón, J.; Álvarez-Ortega, C.; Zamora Auñon, P.; Buño Soto, A.; Lyon, A.R.; Farmakis, D.; Cardinale, D.; Canales Albendea, M.; Feliu Batlle, J.; Rodríguez Rodríguez, I.; et al. Classification, prevalence, and outcomes of anticancer therapy-induced cardiotoxicity: The CARDIOTOX registry. Eur. Heart J. 2020, 41, 1720–1729. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F.; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, G.A.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. An introduction to multiplicity issues in clinical trials: The what, why, when and how. Int. J. Epidemiol. 2017, 46, 746–755. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Taljaard, M.; Van Den Heuvel, E.R.; Levine, M.A.; Cook, D.J.; Wells, G.A.; Devereaux, P.J.; Thabane, L. An introduction to multiplicity issues in clinical trials: The what, why, when and how. Int. J. Epidemiol. 2017, 46, 746–755. [Google Scholar] [CrossRef] [PubMed]
- Quinaglia, T.; Gongora, C.; Awadalla, M.; Hassan, M.Z.O.; Zafar, A.; Drobni, Z.D.; Mahmood, S.S.; Zhang, L.; Coelho-Filho, O.R.; Suero-Abreu, G.A.; et al. Global Circumferential and Radial Strain Among Patients with Immune Checkpoint Inhibitor Myocarditis. JACC Cardiovasc. Imaging 2022, 15, 1883–1896. [Google Scholar] [CrossRef] [PubMed]
- Kurzhals, J.K.; Graf, T.; Boch, K.; Grzyska, U.; Frydrychowicz, A.; Zillikens, D.; Terheyden, P.; Langan, E.A. Serum Troponin T Concentrations Are Frequently Elevated in Advanced Skin Cancer Patients Prior to Immune Checkpoint Inhibitor Therapy: Experience from a Single Tertiary Referral Center. Front. Med. 2021, 8, 665. [Google Scholar] [CrossRef]
- Waissengein, B.; Abu Ata, B.; Merimsky, O.; Shamai, S.; Wolf, I.; Arnold, J.H.; Bar-On, T.; Banai, S.; Khoury, S.; Laufer-Perl, M. The predictive value of high sensitivity troponin measurements in patients treated with immune checkpoint inhibitors. Clin. Res. Cardiol. 2023, 112, 409–418. [Google Scholar] [CrossRef]
- Tamura, Y.; Tamura, Y.; Takemura, R.; Yamada, K.; Taniguchi, H.; Iwasawa, J.; Yada, H.; Kawamura, A. Longitudinal Strain and Troponin I Elevation in Patients Undergoing Immune Checkpoint Inhibitor Therapy. JACC CardioOncol. 2022, 4, 673. [Google Scholar] [CrossRef]
- Furukawa, A.; Tamura, Y.; Taniguchi, H.; Kawamura, A.; Nagase, S.; Hayashi, A.; Tada, Y.; Sase, K.; Hatake, K. Prospective screening for myocarditis in cancer patients treated with immune checkpoint inhibitors. J. Cardiol. 2023, 81, 63–67. [Google Scholar] [CrossRef]
- Caforio, A.L.P.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Heliö, T.; Heymans, S.; Jahns, R.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–2648. [Google Scholar] [CrossRef] [PubMed]
- Vasbinder, A.; Chen, Y.; Procureur, A.; Gradone, A.; Azam, T.U.; Perry, D.; Shadid, H.; Anderson, E.; Catalan, T.; Blakely, P.; et al. Biomarker Trends, Incidence, and Outcomes of Immune Checkpoint Inhibitor–Induced Myocarditis. JACC CardioOncol. 2022, 4, 689–700. [Google Scholar] [CrossRef]
- Rini, B.I.; Moslehi, J.J.; Bonaca, M.; Schmidinger, M.; Albiges, L.; Choueiri, T.K.; Motzer, R.J.; Atkins, M.B.; Haanen, J.; Mariani, M.; et al. Prospective Cardiovascular Surveillance of Immune Checkpoint Inhibitor-Based Combination Therapy in Patients with Advanced Renal Cell Cancer: Data from the Phase III JAVELIN Renal 101 Trial. J. Clin. Oncol. 2022, 40, 1929–1938. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.; Yuan, M.; Zhan, X.; Zhao, G.; Mu, G.; Wang, T.; Hu, H.; Fu, H. Early detection of immune checkpoint inhibitor-related subclinical cardiotoxicity: A pilot study by using speckle tracking imaging and three-dimensional echocardiography. Front. Cardiovasc. Med. 2022, 9, 1087287. [Google Scholar] [CrossRef]
- Faubry, C.; Faure, M.; Toublanc, A.C.; Veillon, R.; Lemaître, A.I.; Vergnenègre, C.; Cochet, H.; Khan, S.; Raherison, C.; Dos Santos, P.; et al. A Prospective Study to Detect Immune Checkpoint Inhibitors Associated with Myocarditis among Patients Treated for Lung Cancer. Front. Cardiovasc. Med. 2022, 9, 878211. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, S.S.; Fradley, M.G.; Cohen, J.V.; Nohria, A.; Reynolds, K.L.; Heinzerling, L.M.; Sullivan, R.J.; Damrongwatanasuk, R.; Chen, C.L.; Gupta, D.; et al. Myocarditis in Patients Treated with Immune Checkpoint Inhibitors. J. Am. Coll. Cardiol. 2018, 71, 1755–1764. [Google Scholar] [CrossRef] [PubMed]
- Isawa, T.; Toi, Y.; Sugawara, S.; Taguri, M.; Toyoda, S. Incidence, Clinical Characteristics, and Predictors of Cardiovascular Immune-Related Adverse Events Associated with Immune Checkpoint Inhibitors. Oncologist 2022, 27, e410. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, T.; Inoue, T.; Otsuka, T.; Kuno, I.; Kukita, Y.; Nakamura, H.; Ikeda, Y.; Yasui, T.; Shioyama, W.; Oka, T.; et al. Prevalence and characteristics of immune checkpoint inhibitor-related myocardial damage: A prospective observational study. PLoS ONE 2022, 17, e0275865. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delombaerde, D.; De Sutter, J.; Croes, L.; Vervloet, D.; Moerman, V.; Van de Veire, N.; Willems, A.-M.; Wouters, K.; Peeters, M.; Prenen, H.; et al. Extensive CArdioVAscular Characterization and Follow-Up of Patients Receiving Immune Checkpoint Inhibitors: A Prospective Multicenter Study. Pharmaceuticals 2023, 16, 625. https://doi.org/10.3390/ph16040625
Delombaerde D, De Sutter J, Croes L, Vervloet D, Moerman V, Van de Veire N, Willems A-M, Wouters K, Peeters M, Prenen H, et al. Extensive CArdioVAscular Characterization and Follow-Up of Patients Receiving Immune Checkpoint Inhibitors: A Prospective Multicenter Study. Pharmaceuticals. 2023; 16(4):625. https://doi.org/10.3390/ph16040625
Chicago/Turabian StyleDelombaerde, Danielle, Johan De Sutter, Lieselot Croes, Delphine Vervloet, Veronique Moerman, Nico Van de Veire, Anne-Marie Willems, Kristien Wouters, Marc Peeters, Hans Prenen, and et al. 2023. "Extensive CArdioVAscular Characterization and Follow-Up of Patients Receiving Immune Checkpoint Inhibitors: A Prospective Multicenter Study" Pharmaceuticals 16, no. 4: 625. https://doi.org/10.3390/ph16040625
APA StyleDelombaerde, D., De Sutter, J., Croes, L., Vervloet, D., Moerman, V., Van de Veire, N., Willems, A.-M., Wouters, K., Peeters, M., Prenen, H., & Vulsteke, C. (2023). Extensive CArdioVAscular Characterization and Follow-Up of Patients Receiving Immune Checkpoint Inhibitors: A Prospective Multicenter Study. Pharmaceuticals, 16(4), 625. https://doi.org/10.3390/ph16040625







