The First Anti-Snakebite and Hepatoprotective Characterization of a Trypsin Kunitz-like Inhibitor (EcTI) from the Plant Enterolobium contortisiliquum; A Case of Two Soul Mates Meeting
Abstract
1. Introduction
2. Results
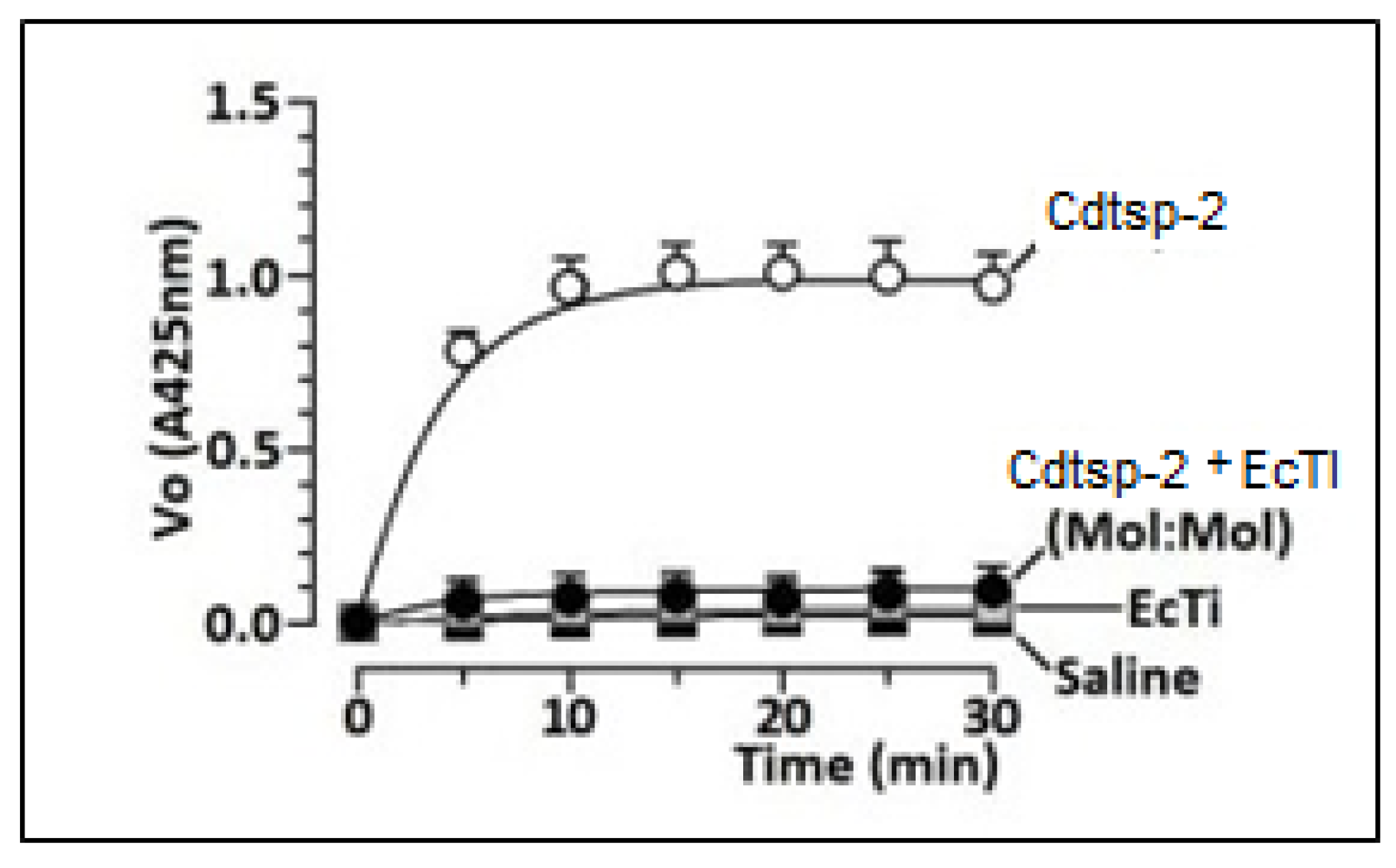
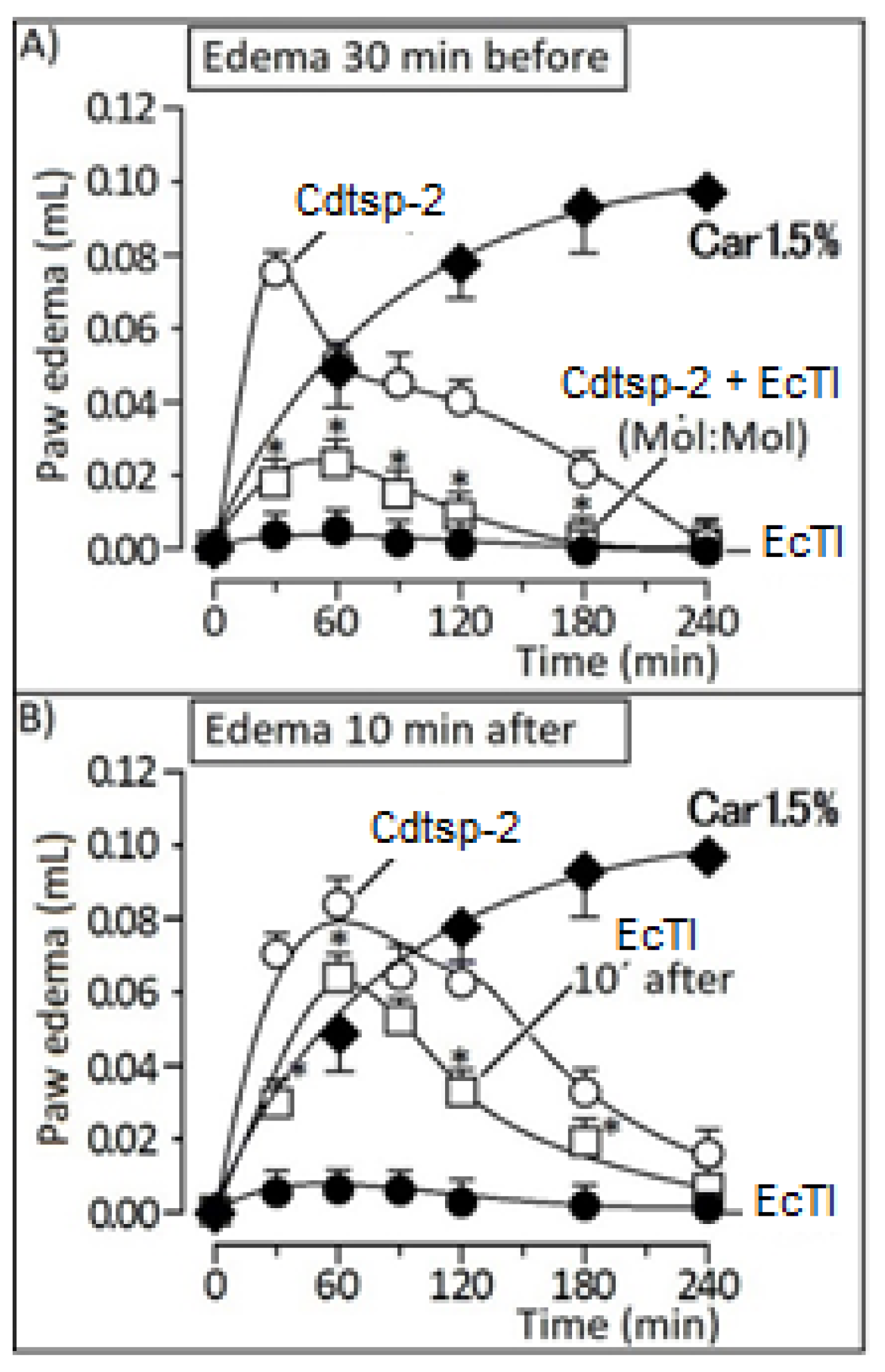
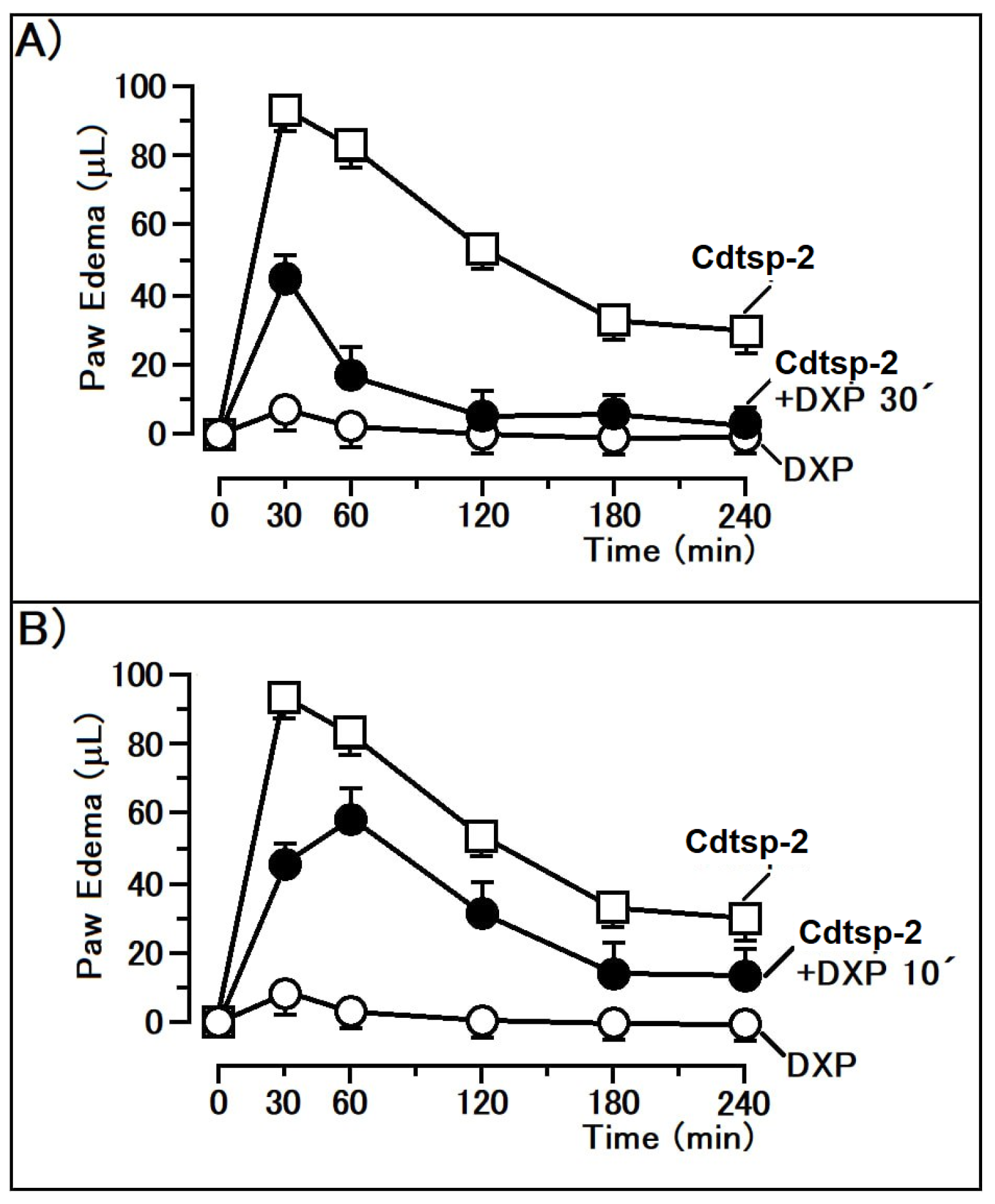
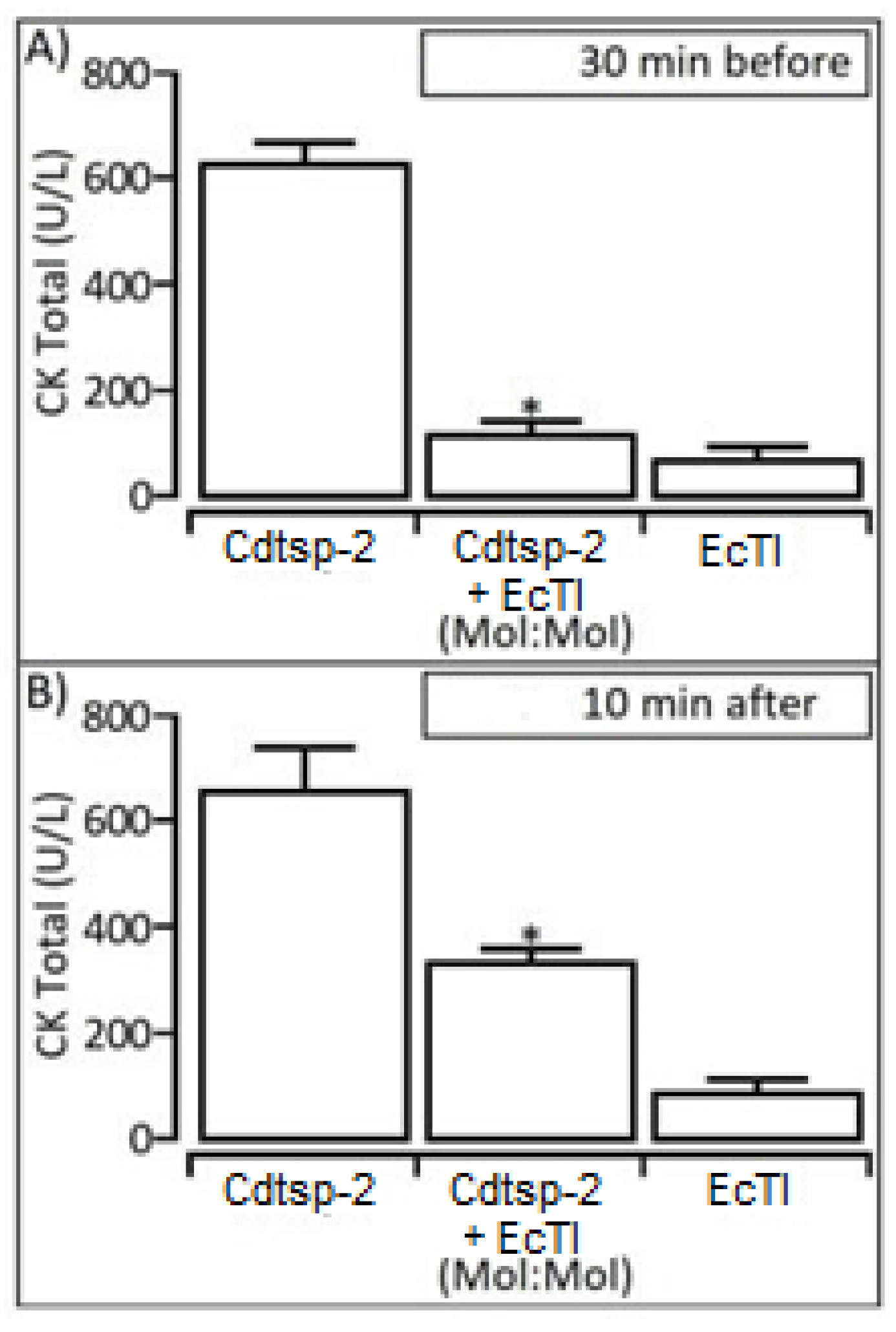
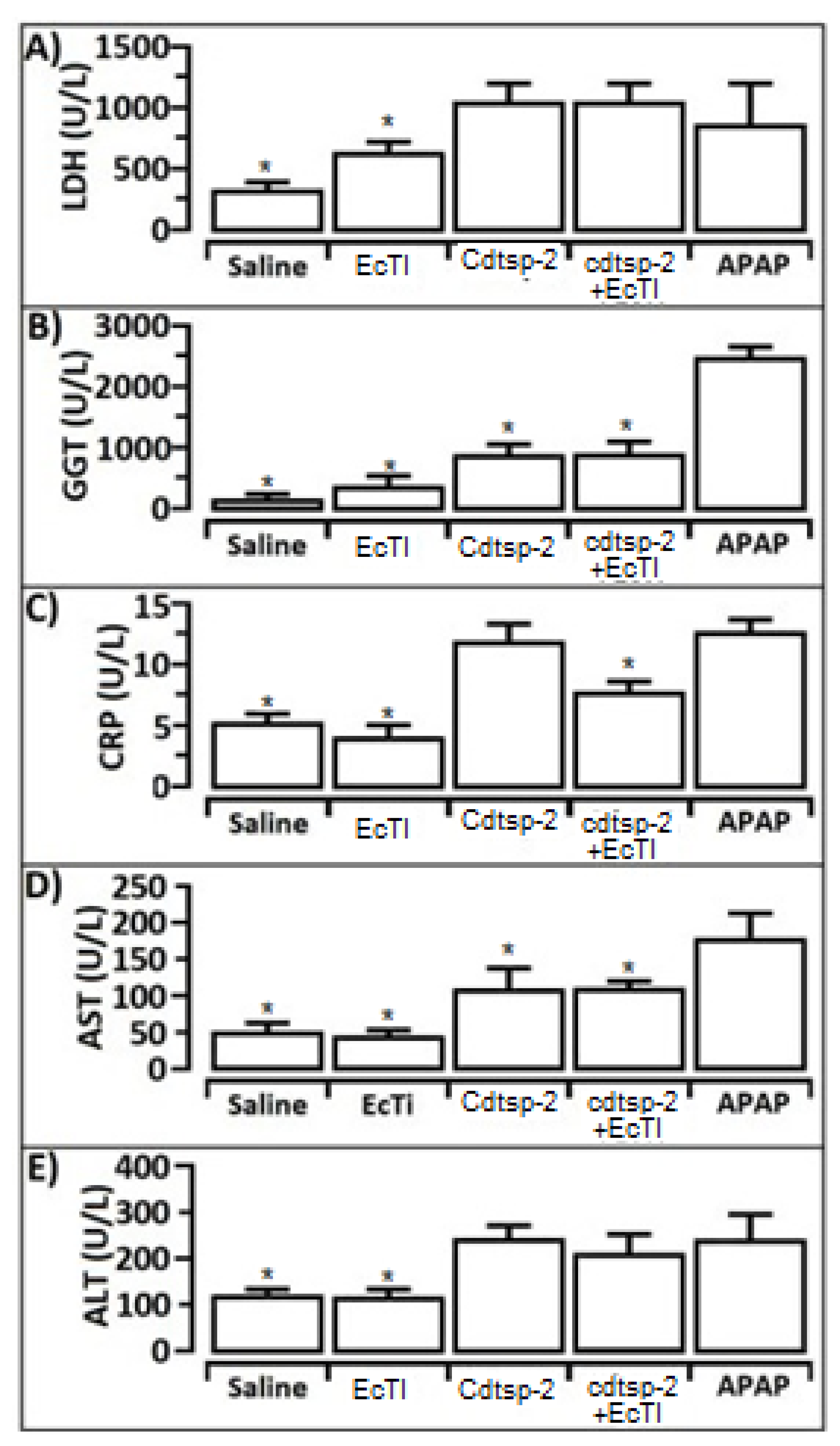
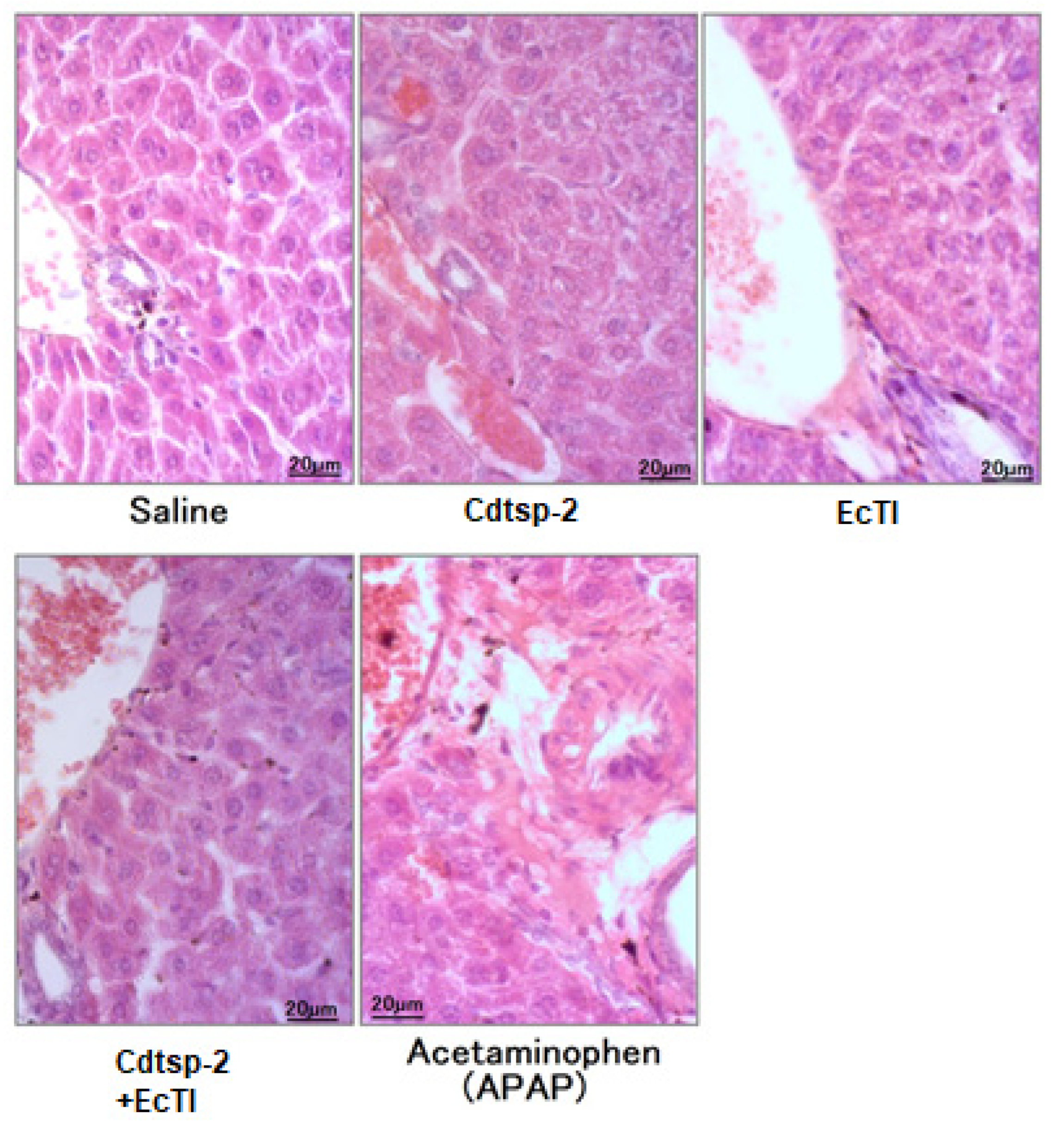
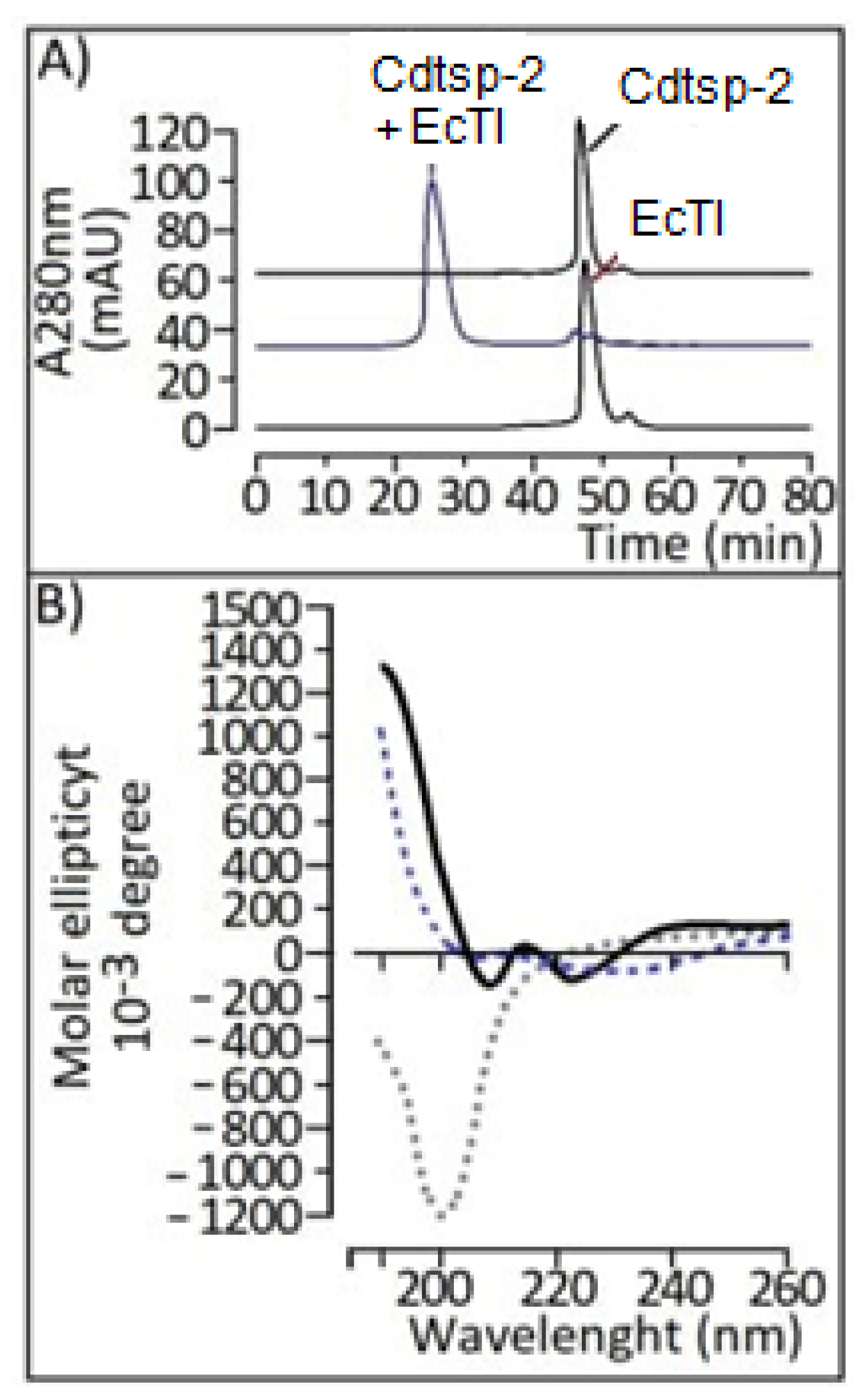
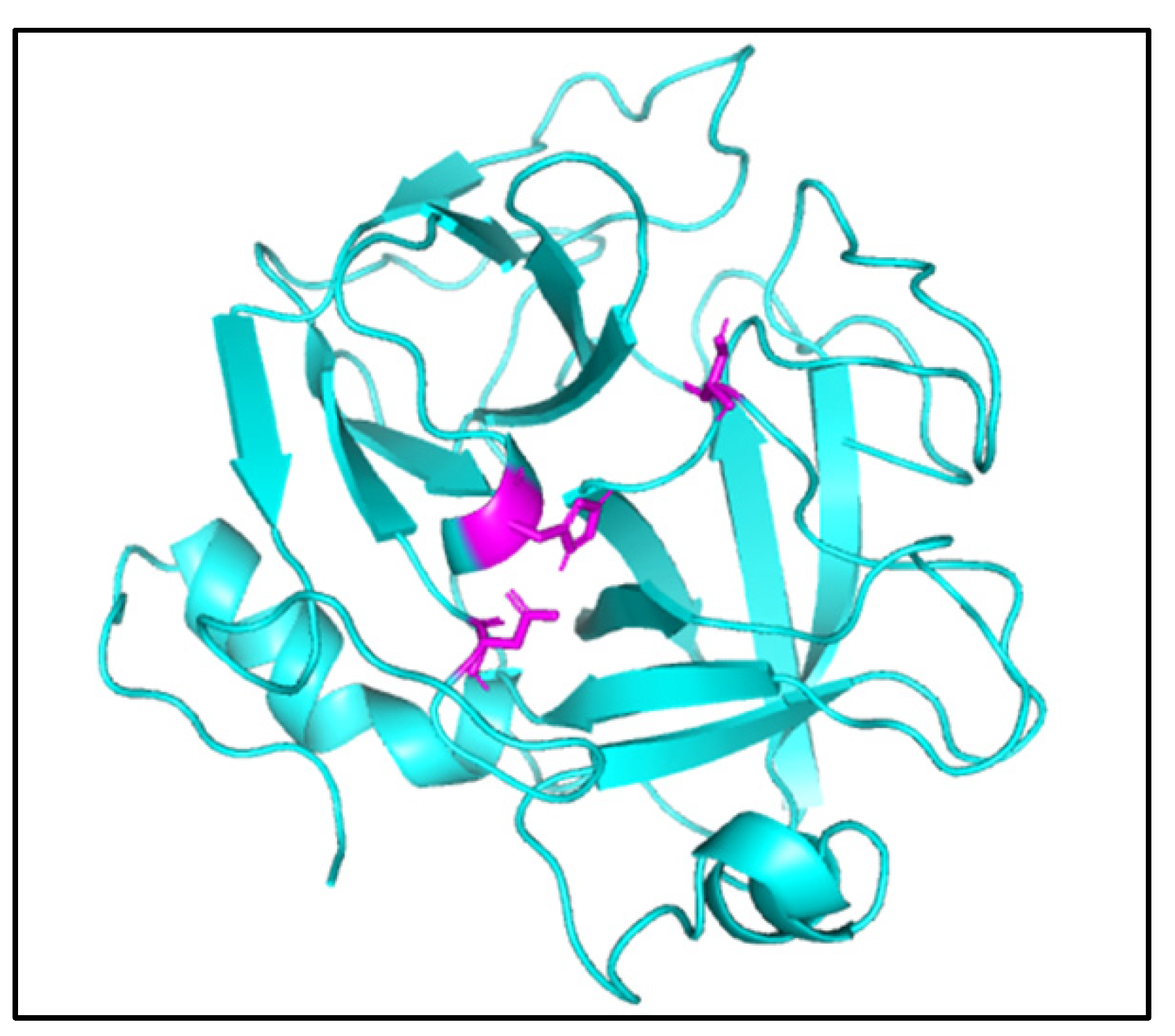
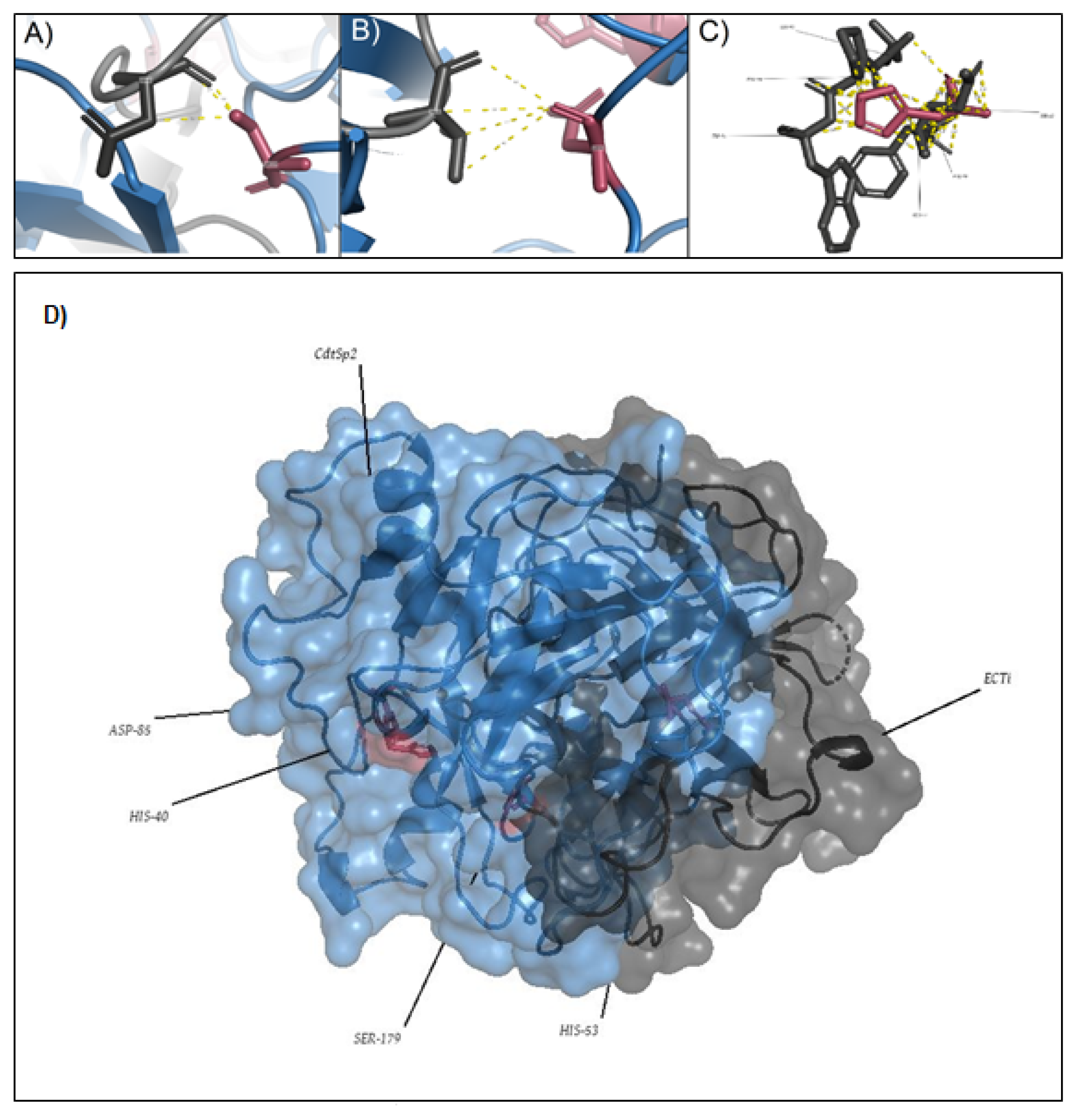
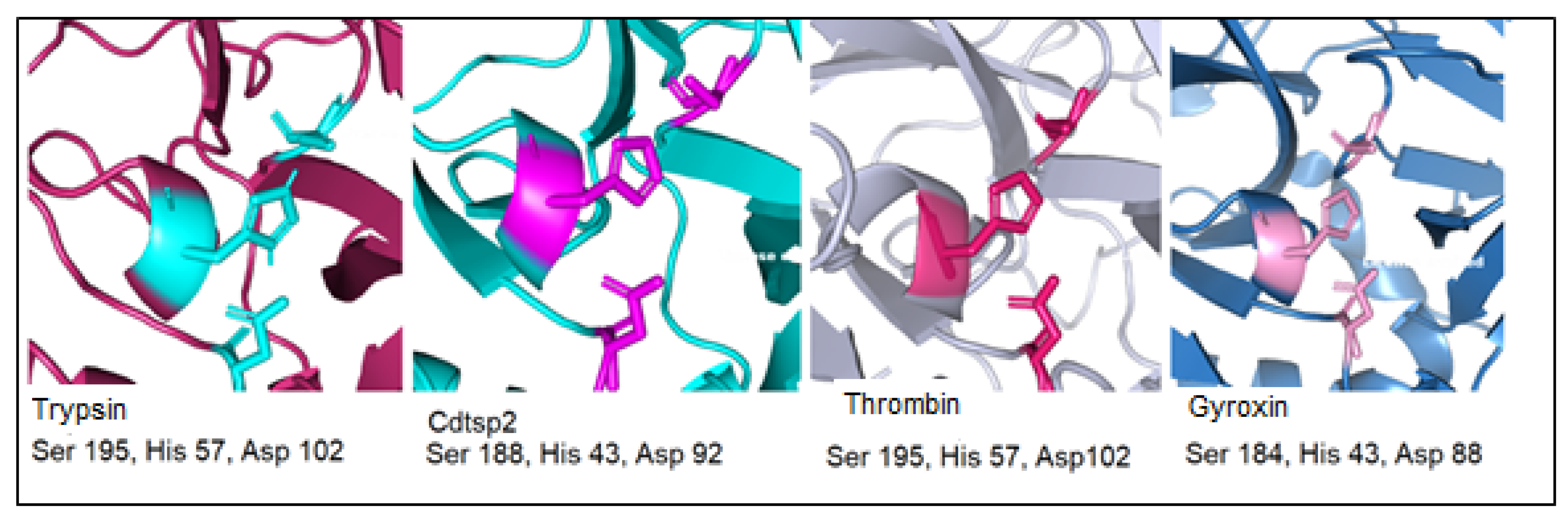
3. Discussion
4. Materials and Methods
4.1. Purification of Cdtsp-2
4.2. Reagentes
4.3. Enzyme Assay of Cdtsp-2 Inhibition
4.4. Circular Dichroism
4.5. Evaluation of Paw Edema
4.6. Hepatotoxicity
Histopathology
4.7. CK Level Measurement
4.8. Statistical Analysis
4.9. Preparation of Ligands
4.10. Molecular Docking
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Da Silva, G.M.; De Souza, D.H.B.; Waitman, K.B.; Ebram, M.C.; Fessel, M.R.; Zainescu, I.C.; Portaro, F.C.; Heras, M.; De Andrade, S.A. Design, synthesis, and evaluation of Bothrops venom serine protease peptidic inhibitors. J. Venom. Anim. Toxins Incl. Trop. Dis. 2021, 27, e20200066. [Google Scholar] [CrossRef]
- Moio Da Cunha, E.; Martins, O.A. Principais Compostos Químicos Presente nos Venenos de Cobras dos Gêneros Bothrops e Crotalus-Uma Revisão. 2012, 2, 21–26. Available online: www.scielo.br (accessed on 12 January 2023).
- Kang, D.H.; Lee, D.J.; Lee, K.W.; Park, Y.S.; Lee, J.Y.; Lee, S.-H.; Koh, Y.J.; Koh, G.-Y.; Choi, C.; Yu, D.-Y.; et al. Peroxiredoxin II Is an Essential Antioxidant Enzyme that Prevents the Oxidative Inactivation of VEGF Receptor-2 in Vascular Endothelial Cells. Mol. Cell 2011, 44, 545–558. [Google Scholar] [CrossRef]
- Waheed, H.F.; Moin, S.I.; Choudhary, M. Snake Venom: From Deadly Toxins to Life-saving Therapeutics. Curr. Med. Chem. 2017, 24, 1874–1891. [Google Scholar] [CrossRef]
- Picolo, G.; Chacur, M.; Gutiérrez, J.M.; Teixeira, C.F.P.; Cury, Y. Evaluation of Antivenoms in the Neutralization of Hyperalgesia and Edema Induced by Bothrops Jararaca and Bothrops Asper Snake Venoms. Braz. J. Med. Biol. Res. 2002, 35, 1221–1228. Available online: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0100-879X2002001000016&lng=en&tlng=en (accessed on 15 March 2023). [CrossRef]
- Miyagui, C.; Brando Prieto da Silva lvaro R de Santana, G. Serine proteases—Cloning, Expression and Potential Applications. In An Integrated View of the Molecular Recognition and Toxinology—From Analytical Procedures to Biomedical Applications; IntechOpen: London, UK, 2013. [Google Scholar]
- Barraviera, B.; Júnior, J.C.B.; Arakaki, D.; Domingues, M.A.C.; Pereira, P.C.M.; Mendes, R.P.; Machado, J.M.; Meira, D.A.; Arkaki, D. A Retrospective Study of 40 Victims of Crotalus Snake Bites: Analysis of the Hepatic Necrosis Observed in One Patient. Rev Soc Bras Med. Trop 1989, 22, 5–12. Available online: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0037-86821989000100002&lng=en&tlng=en (accessed on 15 March 2023). [CrossRef]
- Vital Brazil, O.; Fontana, M.D.; Vital Brazil, O.; Fontana Toxins, M.D. Toxins as tools in the study of sodium channel distribution in the muscle fibre membrane. Toxicon Off. J. Int. Soc. Toxinology 1993, 31, 1085–1098. [Google Scholar] [CrossRef]
- Barraviera, B. Acute-phase response in snakebite. Rev. Inst. Med. Trop. São Paulo 1994, 36, 479. [Google Scholar] [CrossRef]
- Calvete, J.J.; Sanz, L.; Angulo, Y.; Lomonte, B.; Gutiérrez, J.M. Venoms, venomics, antivenomics. FEBS Lett. 2009, 583, 1736–1743. [Google Scholar] [CrossRef]
- Ral, O.; Acg, P.J. Acute hepatotoxicity of Crotalus durissus terrificus (South American rattlesnake) venom in rats. J. Venom. Anim.Toxins Incl. Trop. Dis. V 2008, 13, 61–78. [Google Scholar]
- Costa, C.R.C.; Belchor, M.N.; Rodrigues, C.F.B.; Toyama, D.D.O.; De Oliveira, M.A.; Novaes, D.P.; Toyama, M.H. Edema Induced by a Crotalus durissus terrificus Venom Serine Protease (Cdtsp 2) Involves the PAR Pathway and PKC and PLC Activation. Int. J. Mol. Sci. 2018, 19, 2405. [Google Scholar] [CrossRef]
- Pant, A.; Kopec, A.K.; Luyendyk, J.P. Role of the blood coagulation cascade in hepatic fibrosis. Am. J. Physiol. Gastrointest. Liver. Physiol. 2018, 315, G171–G176. [Google Scholar] [CrossRef]
- Costa, C.R.C. Avaliação de Compostos Polifenólicos da Laguncularia Racemosa Sobre a Atividade Enzimática e Farmacológica de Serino Proteases de Crotalus durissus terrificus. 22 February 2018. Available online: https://repositorio.unesp.br/handle/11449/153268 (accessed on 14 March 2023).
- Batista, I.F.C.; Nonato, M.C.; Bonfadini, M.R.; Beltramini, L.M.; Oliva, M.L.V.; Sampaio, M.U.; Sampaio, C.A.; Garratt, R.C. Preliminary crystallographic studies of EcTI, a serine proteinase inhibitor from Enterolobium contortisiliquum seeds. Acta Crystallogr. D Biol. Crystallogr. 2001, 57, 602–604. [Google Scholar] [CrossRef]
- Lobo, Y.A.; Bonazza, C.; Batista, F.P.; Castro, R.A.; Bonturi, C.R.; Salu, B.R.; Sinigaglia, R.D.C.; Toma, L.; Vicente, C.M.; Pidde, G.; et al. EcTI impairs survival and proliferation pathways in triple-negative breast cancer by modulating cell-glycosaminoglycans and inflammatory cytokines. Cancer Lett. 2020, 491, 108–120. [Google Scholar] [CrossRef]
- Zhou, D.; Lobo, Y.A.; Batista, I.F.C.; Marques-Porto, R.; Gustchina, A.; Oliva, M.L.V.; Wlodawer, A. Crystal Structures of a Plant Trypsin Inhibitor from Enterolobium contortisiliquum (EcTI) and of Its Complex with Bovine Trypsin. PLoS ONE 2013, 4, e62252. [Google Scholar] [CrossRef]
- Prasa, D.; Svendsen, L.; Stürzebecher, J. Inhibition of thrombin generation in plasma by inhibitors of factor Xa. Thromb Haemost 1997, 78, 1215–1220. [Google Scholar] [CrossRef]
- da Cunha de Medeiros, P.; Samelo, R.R.; Silva, A.P.G.; da Silva Araujo Santiago, M.; Duarte, F.A.; Perobelli, J.E. Prepubertal exposure to low doses of sodium arsenite impairs spermatogenesis and epididymal histophysiology in rats. Environ Toxicol. 2019, 34, 83–91. [Google Scholar] [CrossRef]
- Society of Toxicology Pathology. Standardized System of Nomenclature and Diagnostic Criteria (SSNDC) Guides. Available online: https://www.toxpath.org/ssndc.asp (accessed on 10 December 2018).
- National Toxicology Program Health and Human Services. Guides. Available online: https://ntp.niehs.nih.gov/nnl/hepatobiliary/liver/fatty_change/index.htm#group-5 (accessed on 10 December 2018).
- Mitchell, J.R.; Jollow, D.J.; Potter, W.Z.; Davis, D.C.; Gillette, J.R.; Brodie, B.B. Acetaminophen-induced hepatic necrosis. I. Role of drug metabolism. J. Pharmacol. Exp. Ther. 1973, 187, 185–194. [Google Scholar]
- Rodrigues, C.F.B.; Gaeta, H.H.; Belchor, M.N.; Ferreira, M.J.P.; Pinho, M.V.T.; Toyama, D.D.O.; Toyama, M.H. Evaluation of Potential Thrombin Inhibitors from the White Mangrove (Laguncularia racemosa (L.) C.F. Gaertn). Mar. Drugs 2015, 13, 4505–4519. [Google Scholar] [CrossRef]
- DI ROSA, M. Biological Properties of Carrageenan. J. Pharm. Pharmacol. 2011, 24, 89–102. Available online: https://academic.oup.com/jpp/article/24/2/89/6200741 (accessed on 14 March 2023). [CrossRef]
- Menaldo, D.L.; Bernardes, C.P.; Pereira, J.C.; Silveira, D.S.; Mamede, C.C.; Stanziola, L.; de Oliveira, F.; Pereira-Crott, L.S.; Faccioli, L.H.; Sampaio, S.V. Effects of two serine proteases from Bothrops pirajai snake venom on the complement system and the inflammatory response. Int. Immunopharmacol. 2013, 15, 764–771. [Google Scholar] [CrossRef]
- Yadav, R.; Liu, Y.; Kwok, S.; Hama, S.; France, M.; Eatough, R.; Pemberton, P.; Schofield, J.; Siahmansur, T.J.; Malik, R.; et al. Effect of extended-release niacin on high-density lipoprotein (HDL) functionality, lipoprotein metabolism, and mediators of vascular inflammation in statin-treated patients. J Am Heart Assoc 2015, 4, e001508. [Google Scholar] [CrossRef]
- Schumann, K.; Mauch, C.; Klespe, K.; Loquai, C.; Nikfarjam, U.; Schlaak, M.; Akçetin, L.; Kölblinger, P.; Hoellwerth, M.; Meissner, M.; et al. Real-World Outcomes Using PD-1 Antibodies and BRAF + MEK Inhibitors for Adjuvant Melanoma Treatment from 39 Skin Cancer Centers in Germany, Austria and Switzerland. J. Eur. Acad. Dermatol. Venereol. 2022, 37, 894–906. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/jdv.18779 (accessed on 14 March 2023). [CrossRef] [PubMed]
- Drent, M.; Cobben, N.A.M.; Henderson, R.F.; Wouters, E.F.M.; Van Dieijen-Visser, M. Usefulness of Lactate Dehydrogenase and Its Isoenzymes as Indicators of Lung Damage or Inflammation. Eur. Respir. J. 1996, 9, 1736–1742. Available online: https://erj.ersjournals.com/content/9/8/1736 (accessed on 14 March 2023). [CrossRef] [PubMed]
- Farhana, A.; Lappin, S.L. Biochemistry, Lactate Dehydrogenase. StatPearls 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557536/ (accessed on 14 March 2023).
- Al-Quraishy, S.; Dkhil, M.A.; Abdel Moneim, A.E. Hepatotoxicity and oxidative stress induced by Naja hajecrude venom. J. Venom. Anim. Toxins Incl. Trop. Dis. 2014, 20, 42. [Google Scholar] [CrossRef]
- Naftalin, L.; Sexton, M.; Whitaker, J.F.; Tracey, D. Clinica chimica acta 293 a routine procedure for estimating serum y-glutamyl-transpeptidase activity. Clin. Chim. Acta 1997, 258, 21–30. [Google Scholar]
- Aronson, S.J.; Junge, N.; Trabelsi, M.; Kelmemi, W.; Hubert, A.; Brigatti, K.W.; Fox, M.D.; de Knegt, R.J.; Escher, J.C.; Ginocchio, V.M.; et al. Disease burden and management of Crigler-Najjar syndrome: Report of a world registry. Liver Int. 2022, 42, 1593–1604. [Google Scholar] [CrossRef] [PubMed]
- Yigit, M.; Sogut, O.; Tataroglu, Ö.; Yamanoglu, A.; Yigit, E.; Guler, E.M.; Ozer, O.F.; Kocyigit, A. Oxidative/antioxidative status, lymphocyte DNA damage, and urotensin-2 receptor level in patients with migraine attacks. Neuropsychiatr. Dis. Treat 2018, 14, 367–374. [Google Scholar] [CrossRef]
- Gwaltney-Brant, S.M. Veterinary Forensic Toxicology. Vet Pathol. 2016, 53, 1067–1077. [Google Scholar] [CrossRef]
- Yang, Y.; Pham, T.X.; Wegner, C.J.; Kim, B.; Ku, C.S.; Park, Y.K.; Lee, J.Y. Astaxanthin Lowers Plasma TAG Concentrations and Increases Hepatic Antioxidant Gene Expression in Diet-Induced Obesity Mice. Br. J. Nutr. 2014, 112, 1797–1804. Available online: https://www.cambridge.org/core/journals/british-journal-of-nutrition/article/astaxanthin-lowers-plasma-tag-concentrations-and-increases-hepatic-antioxidant-gene-expression-in-dietinduced-obesity-mice/49F447C0D80B03FB07FAD952E805E990 (accessed on 15 March 2023). [CrossRef]
- McGill, M.R. The Past and Present of Serum Aminotransferases and the Future of Liver Injury Biomarkers. EXCLI J. 2016, 15, 817–828. Available online: http://www.ncbi.nlm.nih.gov/pubmed/28337112 (accessed on 12 March 2023).
- Thomas Francis, J. Biographical Memoirs: Volume 44-National Academy of Sciences-Google Livros; Rock & Read Books Publishing Company: Shanghai, China, 1936. [Google Scholar]
- Nehring, S.M.; Goyal, A.; Bansal, P.; Patel, B.C. C Reactive Protein. StatPearls 2017, 65, 237–244. Available online: http://europepmc.org/books/NBK441843 (accessed on 15 March 2023).
- Shimomura, T.; Denda, K.; Kitamura, A.; Kawaguchi, T.; Kito, M.; Kondo, J.; Kagaya, S.; Qin, L.; Takata, H.; Miyazawa, K.; et al. Hepatocyte Growth Factor Activator Inhibitor, a Novel Kunitz-Type Serine Protease Inhibitor. J. Biol. Chem. 1997, 272, 6370–6376. Available online: http://www.jbc.org/article/S0021925818411520/fulltext (accessed on 15 March 2023). [CrossRef]
- Shearer, A.M.; Rana, R.; Austin, K.; Baleja, J.D.; Nguyen, N.; Bohm, A.; Covic, L.; Kuliopulos, A. Targeting Liver Fibrosis with a Cell-Penetrating Protease-Activated Receptor-2 (PAR2) Pepducin. J. Biol. Chem. 2016, 291, 23188–23198. [Google Scholar] [CrossRef]
- Oliva, M.L.V.; Silva, M.C.C.; Sallai, R.C.; Brito, M.V.; Sampaio, M.U. A novel subclassification for Kunitz proteinase inhibitors from leguminous seeds. Biochimie 2010, 92, 1667–1673. [Google Scholar] [CrossRef]
- Kuniyoshi, A.K.; Rocha, M.; Carvalho, D.C.; Juliano, M.A.; Neto, L.J.; Tambourgi, D.V.; Portaro, F.C.V. Angiotensin-degrading serine peptidase: A new chymotrypsin-like activity in the venom of Bothrops jararaca partially blocked by the commercial antivenom. Toxicon 2012, 59, 124–131. [Google Scholar] [CrossRef]
- Otlewski, J.; Jelen, F.; Zakrzewska, M.; Oleksy, A. The Many Faces of Protease–Protein Inhibitor Interaction. EMBO J. 2005, 24, 1303–1310. Available online: https://onlinelibrary.wiley.com/doi/full/10.1038/sj.emboj.7600611 (accessed on 15 March 2023). [CrossRef]
- Grütter, M.G.; Priestle, J.P.; Rahuel, J.; Grossenbacher, H.; Bode, W.; Hofsteenge, J.; Stone, S.R. Crystal Structure of the Thrombin-Hirudin Complex: A Novel Mode of Serine Protease Inhibition. EMBO J. 1990, 9, 2361–2365. Available online: https://onlinelibrary.wiley.com/doi/full/10.1002/j.1460-2075.1990.tb07410.x (accessed on 15 March 2023). [CrossRef]
- Richardson, L. Turning Points in Qualitative Research: Tying Knots in a Handkerchief-Google Livros. Available online: https://books.google.com.br/books?hl=pt-BR&lr=&id=8aXWAQAAQBAJ&oi=fnd&pg=PA379&dq=Writing+a+method+of+inquiry&ots=lhcYI-FNKx&sig=XXfeR0Yi77HNo2OuMuzujUSvKas&redir_esc=y#v=onepage&q=Writing%20a%20method%20of%20inquiry&f=false (accessed on 15 March 2023).
- Xu, Y.; Wang, S.; Hu, Q.; Gao, S.; Ma, X.; Zhang, W.; Shen, Y.; Chen, F.; Lai, L.; Pei, J. CavityPlus: A web server for protein cavity detection with pharmacophore modelling, allosteric site identification and covalent ligand binding ability prediction. Nucleic Acids Res. 2018, 46, W374–W379. [Google Scholar] [CrossRef]
- Hewedy, W.A. Effects of treatment with sitagliptin on hepatotoxicity induced by acetaminophen in mice. Braz. J. Pharm. Sci. 2021, 56, e18482. Available online: https://www.scielo.br/j/bjps/a/RjzXGxphxnzrZ3MxxwKdCWD/?lang=en# (accessed on 16 March 2023). [CrossRef]
- Goodsell, D.S.; Morris, G.M.; Olson, A.J. Automated Docking of Flexible Ligands: Applications of AutoDock. J. Mol. Recognit. 1996, 9, 2–6. [Google Scholar] [CrossRef]
- Fonseca, F.V.; Antunes, E.; Morganti, R.P.; Monteiro, H.S.A.; Martins, A.M.C.; Toyama, D.O.; Marangoni, S.; Toyama, M.H. Charcterization of a new platelet aggregating factor from crotoxin Crotalus durissus cascavella venom. Protein J. 2006, 25, 183–192. [Google Scholar] [CrossRef]
- Halgren, T.A. Identifying and characterizing binding sites and assessing druggability. J. Chem. Inf. Model. 2009, 49, 377–389. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2009, 31, 455–461. [Google Scholar] [CrossRef]
- Wallace, A.C.; Laskowski, R.A.; Thornton, J.M. LIGPLOT: A Program to Generate Schematic Diagrams of Protein-Ligand Interactions. Protein Eng. Des. Sel. 1995, 8, 127–134. Available online: https://academic.oup.com/peds/article/8/2/127/1561050 (accessed on 15 March 2023). [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, C.R.C.; Belchor, M.N.; Roggero, A.; Moraes, L.L.; Samelo, R.; Annunciato, I.; Bonturi, C.R.; Oliva, M.L.V.; Sousa, S.F.; de Oliveira, M.A.; et al. The First Anti-Snakebite and Hepatoprotective Characterization of a Trypsin Kunitz-like Inhibitor (EcTI) from the Plant Enterolobium contortisiliquum; A Case of Two Soul Mates Meeting. Pharmaceuticals 2023, 16, 632. https://doi.org/10.3390/ph16040632
Costa CRC, Belchor MN, Roggero A, Moraes LL, Samelo R, Annunciato I, Bonturi CR, Oliva MLV, Sousa SF, de Oliveira MA, et al. The First Anti-Snakebite and Hepatoprotective Characterization of a Trypsin Kunitz-like Inhibitor (EcTI) from the Plant Enterolobium contortisiliquum; A Case of Two Soul Mates Meeting. Pharmaceuticals. 2023; 16(4):632. https://doi.org/10.3390/ph16040632
Chicago/Turabian StyleCosta, Caroline R. C., Mariana N. Belchor, Airam Roggero, Laila L. Moraes, Ricardo Samelo, Isabelly Annunciato, Camila R. Bonturi, Maria L. V. Oliva, Sergio F. Sousa, Marcos A. de Oliveira, and et al. 2023. "The First Anti-Snakebite and Hepatoprotective Characterization of a Trypsin Kunitz-like Inhibitor (EcTI) from the Plant Enterolobium contortisiliquum; A Case of Two Soul Mates Meeting" Pharmaceuticals 16, no. 4: 632. https://doi.org/10.3390/ph16040632
APA StyleCosta, C. R. C., Belchor, M. N., Roggero, A., Moraes, L. L., Samelo, R., Annunciato, I., Bonturi, C. R., Oliva, M. L. V., Sousa, S. F., de Oliveira, M. A., & Toyama, M. H. (2023). The First Anti-Snakebite and Hepatoprotective Characterization of a Trypsin Kunitz-like Inhibitor (EcTI) from the Plant Enterolobium contortisiliquum; A Case of Two Soul Mates Meeting. Pharmaceuticals, 16(4), 632. https://doi.org/10.3390/ph16040632







