In Vitro and In Vivo Effects of Ulvan Polysaccharides from Ulva rigida
Abstract
:1. Introduction
2. Results
2.1. Ulvan Composition and Structure
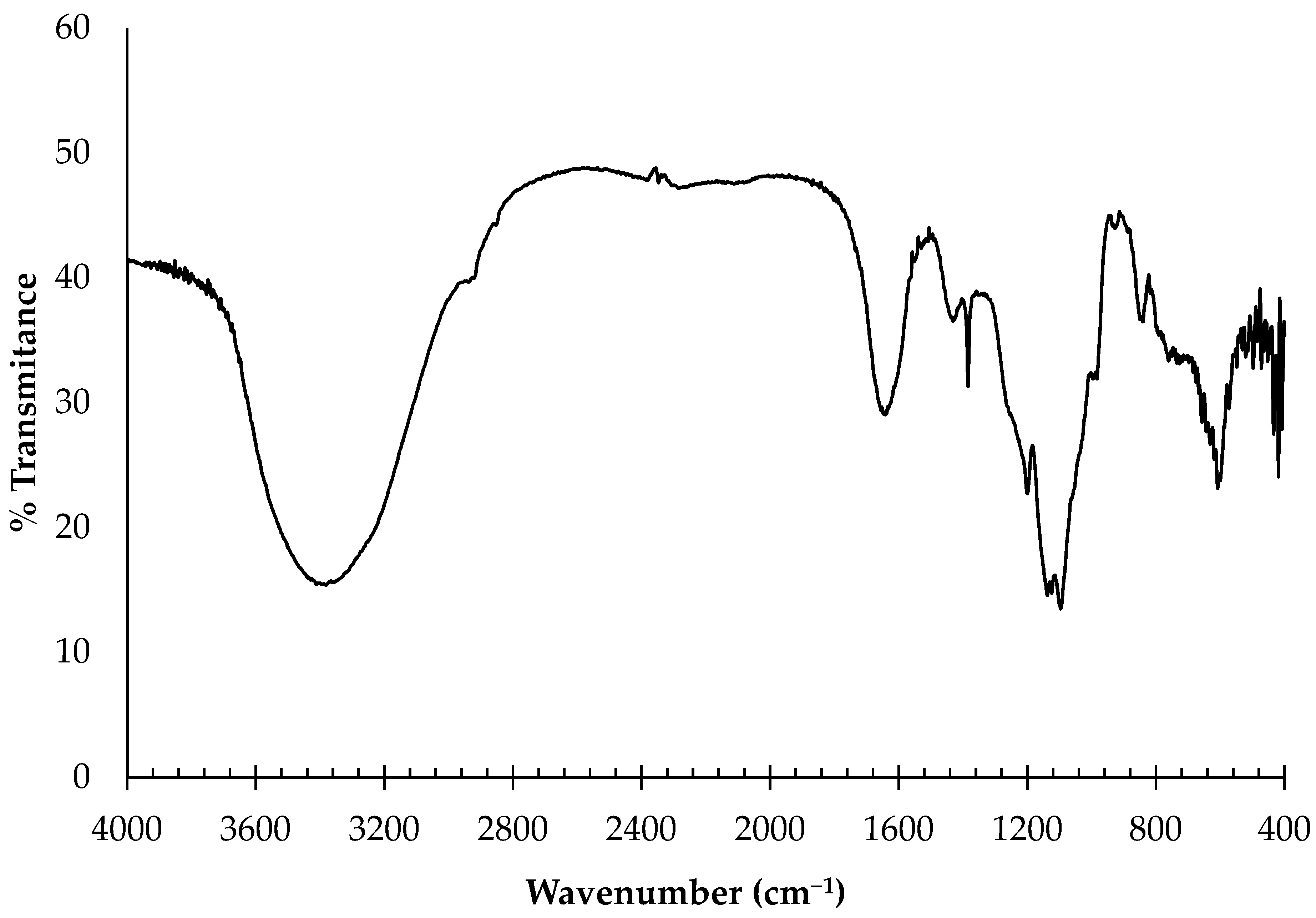
2.1.1. Fourier-Transform Infrared Spectroscopy (FTIR)
2.1.2. Gas Chromatography–Mass Spectrometry (GC–MS)
2.2. Cytotoxic Activity of Ulvan Polysaccharides
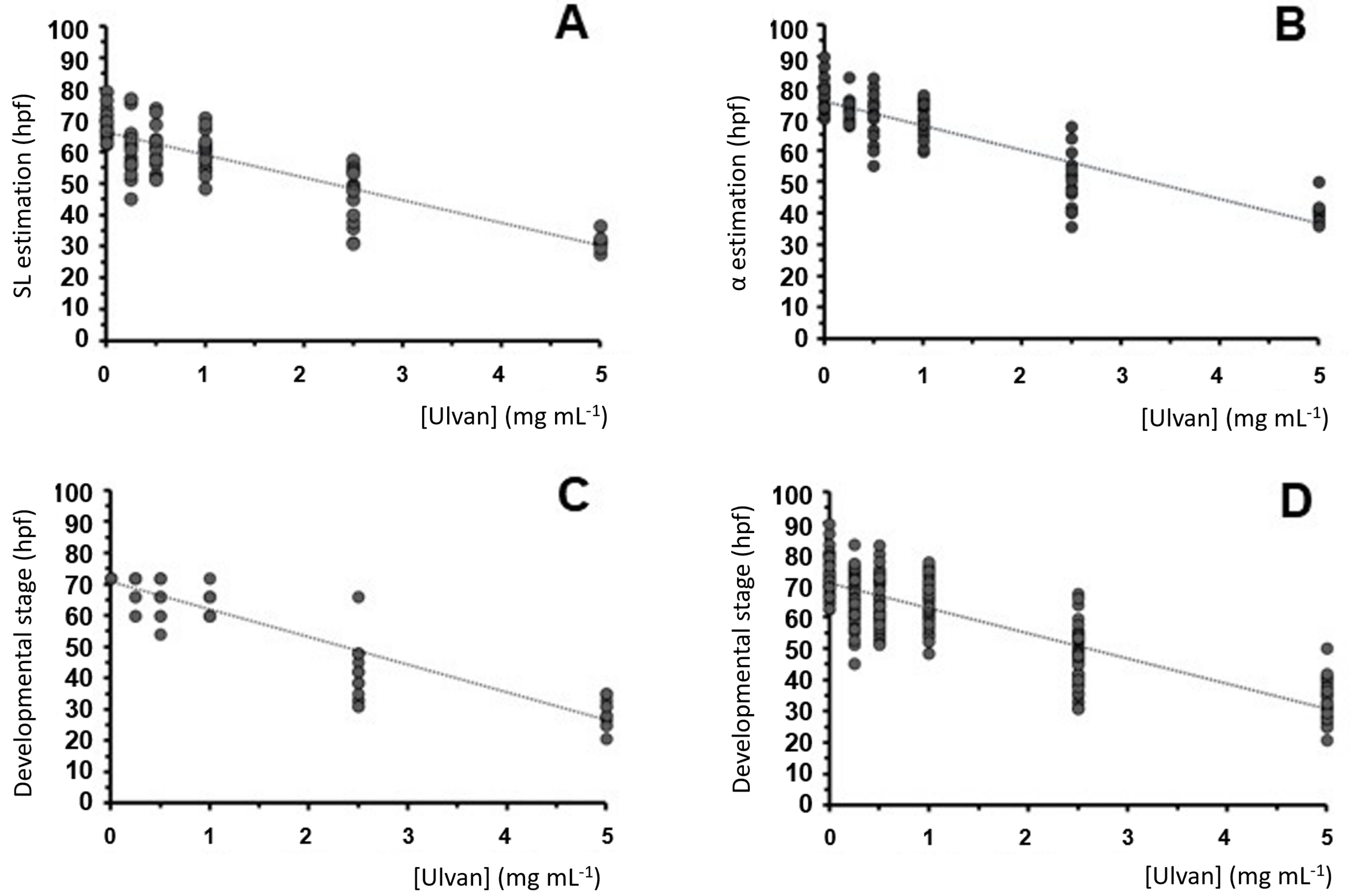
2.3. Zebrafish Exposure to Increasing Concentrations of Ulvan Polysaccharides
3. Discussion
4. Materials and Methods
4.1. Ulvan Preparation
4.2. Chemical Composition and Structure of Ulvans
4.2.1. Fourier-Transform Infrared Spectroscopy (FTIR)
4.2.2. Gas Chromatography–Mass Spectrometry (GC–MS)
4.3. Cytotoxic Effect Assay
4.4. Zebrafish Husbandry and Embryo Collection
4.5. Zebrafish Embryo Toxicity Assay
4.6. Phenotypic Analysis
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Abotaleb, M.; Kubatka, P.; Caprnda, M.; Varghese, E.; Zolakova, B.; Zubor, P.; Opatrilova, R.; Kruzliak, P.; Stefanicka, P.; Büsselberg, D. Chemotherapeutic agents for the treatment of metastatic breast cancer: An update. Biomed. Pharmacother. 2018, 101, 458–477. [Google Scholar] [CrossRef] [PubMed]
- Schirrmacher, V. From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment (Review). Int. J. Oncol. 2019, 54, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Liu, C.; Yao, J.; Wan, H.; Wan, G.; Li, Y.; Chen, N. Breast cancer stem cells, heterogeneity, targeting therapies and therapeutic implications. Pharmacol. Res. 2021, 163, 105320. [Google Scholar] [CrossRef]
- Zhong, L.; Li, Y.; Xiong, L.; Wang, W.; Wu, M.; Yuan, T.; Yang, W.; Tian, C.; Miao, Z.; Wang, T.; et al. Small molecules in targeted cancer therapy: Advances, challenges, and future perspectives. Signal Transduct. Target. Ther. 2021, 6, 201. [Google Scholar] [CrossRef]
- Si, W.; Shen, J.; Zheng, H.; Fan, W. The role and mechanisms of action of microRNAs in cancer drug resistance. Clin. Epigenet. 2019, 11, 25. [Google Scholar] [CrossRef]
- Craig, M.; Jenner, A.L.; Namgung, B.; Lee, L.P.; Goldman, A. Engineering in Medicine to Address the Challenge of Cancer Drug Resistance: From Micro-and Nanotechnologies to Computational and Mathematical Modeling. Chem. Rev. 2021, 121, 3352–3389. [Google Scholar] [CrossRef]
- Gutiérrez-Rodríguez, A.G.; Juárez-Portilla, C.; Olivares-Bañuelos, T.; Zepeda, R.C. Anticancer activity of seaweeds. Drug Discov. Today 2018, 23, 434–447. [Google Scholar] [CrossRef]
- Costa, M.; Cardoso, C.; Afonso, C.; Bandarra, N.M.; Prates, J.A.M. Current knowledge and future perspectives of the use of seaweeds for livestock production and meat quality: A systematic review. J. Anim. Physiol. Anim. Nutr. 2021, 105, 1075–1102. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Tran, G.; Heuzé, V.; Giger-Reverdin, S.; Lessire, M.; Lebas, F.; Ankers, P. Seaweeds for livestock diets: A review. Anim. Feed Sci. Technol. 2016, 212, 1–17. [Google Scholar] [CrossRef]
- Alves, C.; Silva, J.; Pinteus, S.; Gaspar, H.; Alpoim, M.C.; Botana, L.M.; Pedrosa, R. From Marine Origin to Therapeutics: The Antitumor Potential of Marine Algae-Derived Compounds. Front. Pharmacol. 2018, 9, 777. [Google Scholar] [CrossRef]
- Mantri, V.A.; Kazi, M.A.; Balar, N.B.; Gupta, V.; Gajaria, T. Concise review of green algal genus Ulva Linnaeus. J. Appl. Phycol. 2020, 32, 2725–2741. [Google Scholar] [CrossRef]
- Mata, L.; Magnusson, M.; Paul, N.A.; de Nys, R. The intensive land-based production of the green seaweeds Derbesia tenuissima and Ulva ohnoi: Biomass and bioproducts. J. Appl. Phycol. 2016, 28, 365–375. [Google Scholar] [CrossRef]
- Kidgell, J.T.; Magnusson, M.; de Nys, R.; Glasson, C.R.K. Ulvan: A systematic review of extraction, composition and function. Algal Res. 2019, 39, 101422. [Google Scholar] [CrossRef]
- Tran, T.T.V.; Huy, B.T.; Truong, H.B.; Bui, M.L.; Thanh, T.T.T.; Dao, D.Q. Structure analysis of Sulphated polysaccharides extracted from green seaweed Ulva lactuca: Experimental and density functional theory studies. Mon. Chem. 2018, 149, 197–205. [Google Scholar] [CrossRef]
- Robic, A.; Sassi, J.F.; Dion, P.; Lerat, Y.; Lahaye, M. Seasonal variability of physicochemical and rheological properties of ulvan in two Ulva species (Chlorophyta) from the Brittany coast. J. Phycol. 2009, 45, 962–973. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Lowe, B.; Anil, S.; Manivasagan, P.; Kheraif, A.A.A.; Kang, K.H.; Kim, S.K. Seaweed polysaccharides and their potential biomedical applications. Starch—Stärke 2015, 67, 381–390. [Google Scholar] [CrossRef]
- Cunha, L.; Grenha, A. Sulfated Seaweed Polysaccharides as Multifunctional Materials in Drug Delivery Applications. Mar. Drug. 2016, 14, 42. [Google Scholar] [CrossRef]
- Tanna, B.; Mishra, A. Nutraceutical Potential of Seaweed Polysaccharides: Structure, Bioactivity, Safety, and Toxicity. Compr. Rev. Food Sci. Food Saf. 2019, 18, 817–831. [Google Scholar] [CrossRef]
- Tziveleka, L.A.; Ioannou, E.; Roussis, V. Ulvan, a bioactive marine sulphated polysaccharide as a key constituent of hybrid biomaterials: A review. Carbohydr. Polym. 2019, 218, 355–370. [Google Scholar] [CrossRef]
- Ahmed, O.M.; Ahmed, R.R. Anti-Proliferative and Apoptotic Efficacies of Ulvan Polysaccharides against Different Types of Carcinoma Cells In Vitro and In Vivo. J. Cancer Sci. Ther. 2014, 6, 202–208. [Google Scholar] [CrossRef]
- Thanh, T.T.; Quach, T.M.; Nguyen, T.N.; Vu Luong, D.; Bui, M.L.; Tran, T.T. Structure and cytotoxic activity of ulvan extracted from green seaweed Ulva lactuca. Int. J. Biol. Macromol. 2016, 93 Pt A, 695–702. [Google Scholar] [CrossRef]
- Xie, C.; Lu, X.; Han, L.; Xu, J.; Wang, Z.; Jiang, L.; Wang, K.; Zhang, H.; Ren, F.; Tang, Y. Biomimetic Mineralized Hierarchical Graphene Oxide/Chitosan Scaffolds with Adsorbability for Immobilization of Nanoparticles for Biomedical Applications. ACS Appl. Mater. Interfaces 2016, 8, 1707–1717. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Hong, P.; Cheng, Y.; Liao, M.; Li, S. Polysaccharides from Enteromorpha tubulosa: Optimization of extraction and cytotoxicity. J. Food Process. Preserv. 2018, 42, e13373. [Google Scholar] [CrossRef]
- Usuldin, S.R.A.; Wan-Mohtar, W.A.A.Q.I.; Ilham, Z.; Jamaludin, A.A.; Abdullah, N.R.; Rowan, N. In vivo toxicity of bioreactor-grown biomass and exopolysaccharides from Malaysian tiger milk mushroom mycelium for potential future health applications. Sci. Rep. 2021, 11, 23079. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Kong, W.W.; Shao, C.L.; Li, Y.; Liu, Y.Z.; Liu, M.; Guan, F.F.; Wang, C.Y. Zebrafish Embryo Toxicity Microscale Model for Ichthyotoxicity Evaluation of Marine Natural Products. Mar. Biotechnol. 2016, 18, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Cassar, S.; Adatto, I.; Freeman, J.L.; Gamse, J.T.; Iturria, I.; Lawrence, C.; Muriana, A.; Peterson, R.T.; Van Cruchten, S.; Zon, L.I. Use of Zebrafish in Drug Discovery Toxicology. Chem. Res. Toxicol. 2020, 33, 95–118. [Google Scholar] [CrossRef]
- Achenbach, J.C.; Leggiadro, C.; Sperker, S.A.; Woodland, C.; Ellis, L.D. Comparison of the Zebrafish Embryo Toxicity Assay and the General and Behavioral Embryo Toxicity Assay as New Approach Methods for Chemical Screening. Toxics 2020, 8, 126. [Google Scholar] [CrossRef]
- Dooley, K.; Zon, L.I. Zebrafish: A model system for the study of human disease. Curr. Opin. Genet. Dev. 2000, 10, 252–256. [Google Scholar] [CrossRef]
- Kari, G.; Rodeck, U.; Dicker, A.P. Zebrafish: An emerging model system for human disease and drug discovery. Clin. Pharmacol. Ther. 2007, 82, 70–80. [Google Scholar] [CrossRef]
- Marí-Beffa, M.; Mesa-Román, A.B.; Duran, I. Zebrafish Models for Human Skeletal Disorders. Front. Genet. 2021, 12, 675331. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.C.; Sneed, B.; Haider, J.; Blavo, D.; White, A.; Aiyejorun, T.; Baranowski, T.C.; Rubinstein, A.L.; Doan, T.N.; Dingledine, R.; et al. Automated, quantitative screening assay for antiangiogenic compounds using transgenic zebrafish. Cancer Res. 2007, 67, 11386–11392. [Google Scholar] [CrossRef] [PubMed]
- Tobia, C.; De Sena, G.; Presta, M. Zebrafish embryo, a tool to study tumor angiogenesis. Int. J. Dev. Biol. 2011, 55, 505–509. [Google Scholar] [CrossRef]
- Wilkinson, R.N.; van Eeden, F.J. The zebrafish as a model of vascular development and disease. Prog. Mol. Biol. Transl. Sci. 2014, 124, 93–122. [Google Scholar] [CrossRef]
- García-Caballero, M.; Quesada, A.R.; Medina, M.A.; Marí-Beffa, M. Fishing anti(lymph)angiogenic drugs with zebrafish. Drug Discov. Today 2018, 23, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.H.; Chen, C.C.; Lee, C.H.; Chen, X.A.; Chang, T.Y.; Cheng, Y.C.; Young, J.J.; Lu, J.J. Fungicidal and anti-biofilm activities of trimethylchitosan-stabilized silver nanoparticles against Candida species in zebrafish embryos. Int. J. Biol. Macromol. 2020, 143, 724–731. [Google Scholar] [CrossRef]
- Kang, M.C.; Kim, S.Y.; Kim, Y.T.; Kim, E.A.; Lee, S.H.; Ko, S.C.; Wijesinghe, W.A.; Samarakoon, K.W.; Kim, Y.S.; Cho, J.H.; et al. In vitro and in vivo antioxidant activities of polysaccharide purified from aloe vera (Aloe barbadensis) gel. Carbohydr. Polym. 2014, 99, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.N.; Li, W.; Mehmood, S.; Pan, W.J.; Wang, Y.; Meng, F.J.; Wang, X.F.; Lu, Y.M.; Chen, Y. Structural characterization, in vitro and in vivo antioxidant activities of a heteropolysaccharide from the fruiting bodies of Morchella esculenta. Carbohydr. Polym. 2018, 195, 29–38. [Google Scholar] [CrossRef]
- Raguraman, V.; Abraham, L., S.; Jyotsna, J.; Palaniappan, S.; Gopal, S.; Thirugnanasambandam, R.; Kirubagaran, R. Sulfated polysaccharide from Sargassum tenerrimum attenuates oxidative stress induced reactive oxygen species production in in vitro and in zebrafish model. Carbohydr. Polym. 2019, 203, 441–449. [Google Scholar] [CrossRef]
- Wang, W.; Liu, J. Efficient extraction, antioxidant activities and anti-inflammation of polysaccharides from Notopterygium franchetii Boiss. Carbohydr. Polym. 2020, 248, 116783. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Qiu, Z.; Li, L.; Vidyarthi, S.K.; Zheng, Z.; Zhang, R. Structural characterization and antioxidant activities of one neutral polysaccharide and three acid polysaccharides from Ziziphus jujuba cv. Hamidazao: A comparison. Carbohydr. Polym. 2021, 261, 117879. [Google Scholar] [CrossRef]
- Iman, V.; Mohan, S.; Abdelwahab, S.I.; Karimian, H.; Nordin, N.; Fadaeinasab, M.; Noordin, M.I.; Noor, S.M. Anticancer and anti-inflammatory activities of girinimbine isolated from Murraya koenigii. Drug Des. Dev. Ther. 2016, 11, 103–121. [Google Scholar] [CrossRef] [PubMed]
- Fernando, I.P.S.; Sanjeewa, K.K.A.; Samarakoon, K.W.; Lee, W.W.; Kim, H.S.; Kang, N.; Ranasinghe, P.; Lee, H.S.; Jeon, Y.J. A fucoidan fraction purified from Chnoospora minima; a potential inhibitor of LPS-induced inflammatory responses. Int. J. Biol. Macromol. 2017, 104 Pt A, 1185–1193. [Google Scholar] [CrossRef]
- Jayawardena, T.U.; Fernando, I.P.S.; Lee, W.W.; Sanjeewa, K.K.A.; Kim, H.S.; Lee, D.S.; Jeon, Y.J. Isolation and purification of fucoidan fraction in Turbinaria ornata from the Maldives; Inflammation inhibitory potential under LPS stimulated conditions in in-vitro and in-vivo models. Int. J. Biol. Macromol. 2019, 131, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ni, L.; Fu, X.; Duan, D.; Xu, J.; Gao, X. A Sulfated Polysaccharide from Saccharina japonica Suppresses LPS-Induced Inflammation Both in a Macrophage Cell Model via Blocking MAPK/NF-κB Signal Pathways In Vitro and a Zebrafish Model of Embryos and Larvae In Vivo. Mar. Drugs 2020, 18, 593. [Google Scholar] [CrossRef] [PubMed]
- Siddhu, N.S.S.; Guru, A.; Satish Kumar, R.C.; Almutairi, B.O.; Almutairi, M.H.; Juliet, A.; Vijayakumar, T.M.; Arockiaraj, J. Pro-inflammatory cytokine molecules from Boswellia serrate suppresses lipopolysaccharides induced inflammation demonstrated in an in-vivo zebrafish larval model. Mol. Biol. Rep. 2022, 49, 7425–7435. [Google Scholar] [CrossRef]
- Watzke, J.; Schirmer, K.; Scholz, S. Bacterial lipopolysaccharides induce genes involved in the innate immune response in embryos of the zebrafish (Danio rerio). Fish Shellfish Immunol. 2007, 23, 901–905. [Google Scholar] [CrossRef]
- Shi, Z.; An, L.; Zhang, S.; Li, Z.; Li, Y.; Cui, J.; Zhang, J.; Jin, D.Q.; Tuerhong, M.; Abudukeremu, M.; et al. A heteropolysaccharide purified from leaves of Ilex latifolia displaying immunomodulatory activity in vitro and in vivo. Carbohydr. Polym. 2020, 245, 116469. [Google Scholar] [CrossRef]
- Rajapaksha, D.C.; Edirisinghe, S.L.; Nikapitiya, C.; Dananjaya, S.; Kwun, H.J.; Kim, C.H.; Oh, C.; Kang, D.H.; De Zoysa, M. Spirulina maxima Derived Pectin Nanoparticles Enhance the Immunomodulation, Stress Tolerance, and Wound Healing in Zebrafish. Mar. Drugs 2020, 18, 556. [Google Scholar] [CrossRef]
- Zhang, S.; An, L.; Li, Z.; Wang, H.; Shi, L.; Zhang, J.; Li, Y.; Jin, D.Q.; Tuerhong, M.; Ohizumi, Y.; et al. An active heteropolysaccharide from the rinds of Garcinia mangostana Linn.: Structural characterization and immunomodulation activity evaluation. Carbohydr. Polym. 2020, 235, 115929. [Google Scholar] [CrossRef]
- Eid, J.I.; Al-Tuwaijri, M.M.; Mohanty, S.; Das, B. Chaga mushroom (Inonotus obliquus) polysaccharides exhibit genoprotective effects in UVB-exposed embryonic zebrafish (Danio rerio) through coordinated expression of DNA repair genes. Heliyon 2021, 7, e06003. [Google Scholar] [CrossRef] [PubMed]
- Xiong, G.; Deng, Y.; Cao, Z.; Liao, X.; Zhang, J.; Lu, H. The hepatoprotective effects of Salvia plebeia R. Br. extract in zebrafish (Danio rerio). Fish Shellfish Immunol. 2019, 95, 399–410. [Google Scholar] [CrossRef]
- Zhang, S.; Song, Z.; Shi, L.; Zhou, L.; Zhang, J.; Cui, J.; Li, Y.; Jin, D.Q.; Ohizumi, Y.; Xu, J.; et al. A dandelion polysaccharide and its selenium nanoparticles: Structure features and evaluation of antitumor activity in zebrafish models. Carbohydr. Polym. 2021, 270, 118365. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.L.; Qi, W.; Han, F.; Shao, J.Z.; Gao, J.Q. Toxicity evaluation of biodegradable chitosan nanoparticles using a zebrafish embryo model. Int. J. Nanomed. 2011, 6, 3351–3359. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Fernando, I.P.S.; Kim, S.Y.; Kim, H.S.; Ahn, G.; Jee, Y.; Jeon, Y.J. In vitro and in vivo anti-inflammatory activities of high molecular weight sulfated polysaccharide; containing fucose separated from Sargassum horneri: Short communication. Int. J. Biol. Macromol. 2018, 107 Pt A, 803–807. [Google Scholar] [CrossRef]
- Wan-Mohtar, W.A.A.Q.I.; Ilham, Z.; Jamaludin, A.A.; Rowan, N. Use of Zebrafish Embryo Assay to Evaluate Toxicity and Safety of Bioreactor-Grown Exopolysaccharides and Endopolysaccharides from European Ganoderma applanatum Mycelium for Future Aquaculture Applications. Int. J. Mol. Sci. 2021, 22, 1675. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Chen, J.; Kalaiselvi, V.; Tungare, K.; Bhori, M.; González-Sánchez, Z.I.; Durán-Lara, E.F. Marine polysaccharide laminarin embedded ZnO nanoparticles and their based chitosan capped ZnO nanocomposites: Synthesis, characterization and in vitro and in vivo toxicity assessment. Environ. Res. 2022, 213, 113655. [Google Scholar] [CrossRef]
- Edirisinghe, S.L.; Rajapaksha, D.C.; Nikapitiya, C.; Oh, C.; Lee, K.A.; Kang, D.H.; De Zoysa, M. Spirulina maxima derived marine pectin promotes the in vitro and in vivo regeneration and wound healing in zebrafish. Fish Shellfish Immunol. 2020, 107 Pt A, 414–425. [Google Scholar] [CrossRef]
- Yaich, H.; Amira, A.B.; Abbes, F.; Bouaziz, M.; Besbes, S.; Richel, A.; Blecker, C.; Attia, H.; Garna, H. Effect of extraction procedures on structural, thermal and antioxidant properties of ulvan from Ulva lactuca collected in Monastir coast. Int. J. Biol. Macromol. 2017, 105, 1430–1439. [Google Scholar] [CrossRef]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef]
- Ocaña, M.C.; Martínez-Poveda, B.; Marí-Beffa, M.; Quesada, A.R.; Medina, M.A. Fasentin diminishes endothelial cell proliferation, differentiation and invasion in a glucose metabolism-independent manner. Sci. Rep. 2020, 10, 6132. [Google Scholar] [CrossRef] [PubMed]
- Isogai, S.; Horiguchi, M.; Weinstein, B.M. The vascular anatomy of the developing zebrafish: An atlas of embryonic and early larval development. Dev. Biol. 2001, 230, 278–301. [Google Scholar] [CrossRef] [PubMed]
- Priyanka, K.R.; Rajaram, R.; Sivakumar, S.R. A critical review on pharmacological properties of marine macroalgae. Biomass Convers. Biorefinery 2022, 1, 1–25. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Abd-Ellatef, G.F.; Ahmed, O.M.; Abdel-Reheim, E.S.; Abdel-Hamid, A.Z. Ulva lactuca polysaccharides prevent Wistar rat breast carcinogenesis through the augmentation of apoptosis, enhancement of antioxidant defense system, and suppression of inflammation. Breast Cancer (Dove Med. Press) 2017, 9, 67–83. [Google Scholar] [CrossRef] [PubMed]
- Hussein, U.K.; Mahmoud, H.M.; Farrag, A.G.; Bishayee, A. Chemoprevention of Diethylnitrosamine-Initiated and Phenobarbital-Promoted Hepatocarcinogenesis in Rats by Sulfated Polysaccharides and Aqueous Extract of Ulva lactuca. Integr. Cancer Ther. 2015, 14, 525–545. [Google Scholar] [CrossRef] [PubMed]
- Majee, S.B.; Avlani, D.; Biswas, G.R. Pharmacological, pharmaceutical, cosmetic and diagnostic applications of sulphated polysaccharides from marine algae and bacteria. Afr. J. Pharm. Pharmacol. 2017, 11, 68–77. [Google Scholar] [CrossRef]
- Liao, D.-w.; Cheng, C.; Liu, J.-p.; Zhao, L.-y.; Huang, D.-c.; Chen, G.-t. Characterization and antitumor activities of polysaccharides obtained from ginger (Zingiber officinale) by different extraction methods. Int. J. Biol. Macromol. 2020, 152, 894–903. [Google Scholar] [CrossRef]
- Miao, J.; Regenstein, J.M.; Qiu, J.; Zhang, J.; Zhang, X.; Li, H.; Zhang, H.; Wang, Z. Isolation, structural characterization and bioactivities of polysaccharides and its derivatives from Auricularia-A review. Int. J. Biol. Macromol. 2020, 150, 102–113. [Google Scholar] [CrossRef]
- Weerapreeyakul, N.; Nonpunya, A.; Barusrux, S.; Thitimetharoch, T.; Sripanidkulchai, B. Evaluation of the anticancer potential of six herbs against a hepatoma cell line. Chin. Med. 2012, 7, 15. [Google Scholar] [CrossRef]
- Shao, P.; Chen, X.; Sun, P. In vitro antioxidant and antitumor activities of different sulfated polysaccharides isolated from three algae. Int. J. Biol. Macromol. 2013, 62, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Matloub, A.A.; Aglan, H.A.; Mohamed El Souda, S.S.; Aboutabl, M.E.; Maghraby, A.S.; Ahmed, H.H. Influence of bioactive sulfated polysaccharide-protein complexes on hepatocarcinogenesis, angiogenesis and immunomodulatory activities. Asian Pac. J. Trop. Med. 2016, 9, 1200–1211. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Li, X.; Li, T.; Jiang, P.; Zhang, L.; Wu, M.; Zhang, L. Characterization and antitumor activity of alkali-extracted polysaccharide from Enteromorpha intestinalis. Int. Immunopharmacol. 2009, 9, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Rusdi, N.A.; Ku, C.S.; Yu, K.-X.; Lau, B.F.; Chung, L.Y.; Kiew, L.V. Assessment of Potential Anticancer Activity of Brown Seaweed Compounds Using Zebrafish Phenotypic Assay. Nat. Prod. Commun. 2019, 14, 1–5. [Google Scholar] [CrossRef]
- Colanesi, S.; Taylor, K.L.; Temperley, N.D.; Lundegaard, P.R.; Liu, D.; North, T.E.; Ishizaki, H.; Kelsh, R.N.; Patton, E.E. Small molecule screening identifies targetable zebrafish pigmentation pathways. Pigment Cell Melanoma Res. 2012, 25, 131–143. [Google Scholar] [CrossRef]
- Duan, Z.; Duan, X.; Zhao, S.; Wang, X.; Wang, J.; Liu, Y.; Peng, Y.; Gong, Z.; Wang, L. Barrier function of zebrafish embryonic chorions against microplastics and nanoplastics and its impact on embryo development. J. Hazard. Mater. 2020, 395, 122621. [Google Scholar] [CrossRef]
- Ko, E.Y.; Heo, S.J.; Cho, S.H.; Lee, W.; Kim, S.Y.; Yang, H.W.; Ahn, G.; Cha, S.H.; Kwon, S.H.; Jeong, M.S.; et al. 3 Bromo 5 (ethoxymethyl) 1,2 benzenediol inhibits LPS-induced pro-inflammatory responses by preventing ROS production and downregulating NF-κB in vitro and in a zebrafish model. Int. Immunopharmacol. 2019, 67, 98–105. [Google Scholar] [CrossRef]
- Brown, H.K.; Schiavone, K.; Tazzyman, S.; Heymann, D.; Chico, T.J.A. Zebrafish xenograft models of cancer and metastasis for drug discovery. Expert Opin Drug Discov. 2017, 12, 379–389. [Google Scholar] [CrossRef]
- Truong, L.; Tanguay, R.L. Evaluation of Embryotoxicity Using the Zebrafish Model. Methods Mol. Biol. 2017, 1641, 325–333. [Google Scholar] [CrossRef]
- Béress, A.; Wassermann, O.; Tahhan, S.; Bruhn, T.; Béress, L.; Kraiselburd, E.N.; Gonzalez, L.V.; de Motta, G.E.; Chavez, P.I. A new procedure for the isolation of anti-HIV compounds (polysaccharides and polyphenols) from the marine alga Fucus vesiculosus. J. Nat. Prod. 1993, 56, 478–488. [Google Scholar] [CrossRef]
- Parra-Riofrío, G.; García-Márquez, J.; Casas-Arrojo, V.; Uribe-Tapia, E.; Abdala-Díaz, R.T. Antioxidant and Cytotoxic Effects on Tumor Cells of Exopolysaccharides from Tetraselmis suecica (Kylin) Butcher Grown Under Autotrophic and Heterotrophic Conditions. Mar. Drugs 2020, 18, 534. [Google Scholar] [CrossRef] [PubMed]
- Parra-Riofrío, G.; Casas-Arrojo, V.; Pino-Selles, R.; García-Márquez, J.; Abdala-Díaz, R.T.; Uribe-Tapia, E. Adaptation of autotrophic to heterotrophic culture of Porphyridium purpureum (Bory) K.M. Drew & R.Ross: Characterization of biomass and production of exopolysaccharides. J. Appl. Phycol. 2021, 33, 3603–3615. [Google Scholar] [CrossRef]
- Abdala Díaz, R.T.; Casas Arrojo, V.; Arrojo Agudo, M.A.; Cárdenas, C.; Dobretsov, S.; Figueroa, F.L. Immunomodulatory and Antioxidant Activities of Sulfated Polysaccharides from Laminaria ochroleuca, Porphyra umbilicalis, and Gelidium corneum. Mar. Biotechnol. 2019, 21, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Nguyen, T.T.H.; Kim, C.; Kim, D. Antimelanogenesis Effects of Fungal Exopolysaccharides Prepared from Submerged Culture of Fomitopsis castanea Mycelia. J. Microbiol. Biotechnol. 2019, 29, 1204–1211. [Google Scholar] [CrossRef] [PubMed]
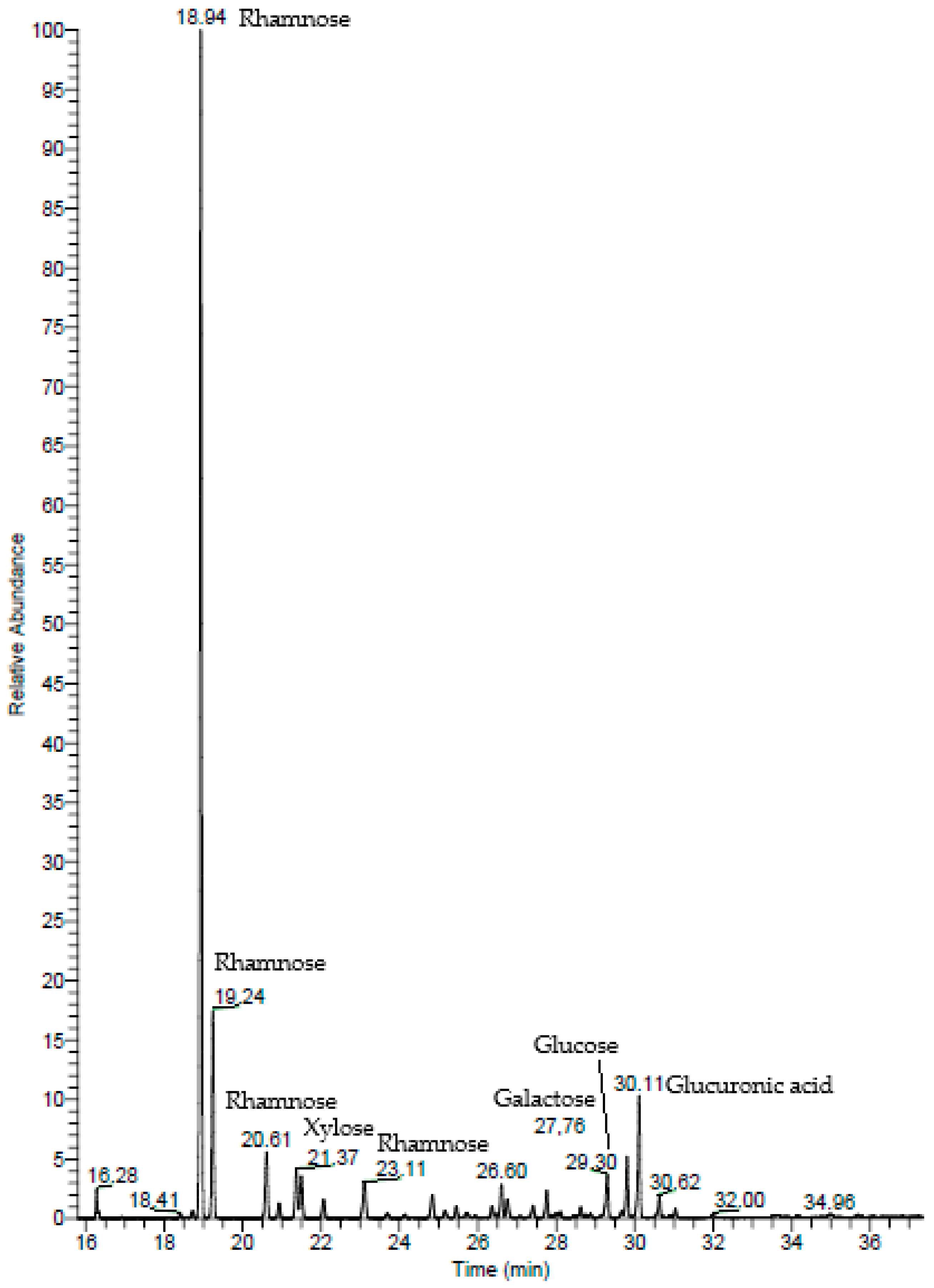





| Monosaccharide | % |
|---|---|
| Rhamnose | 80.60 |
| Glucuronic acid | 9.14 |
| Xylose | 4.01 |
| Glucose | 3.78 |
| Galactose | 2.48 |
| Selectivity Index | |
|---|---|
| HACAT/HCT-116 | 40.9 |
| 1064sk/HCT-116 | 11.5 |
| HACAT/U-937 | 1.8 |
| 1064sk/U-937 | 0.5 |
| HACAT/G-361 | 0.9 |
| 1064sk/G-361 | 0.3 |
| Ulvan (mg mL−1) | Head–Trunk Angle (hpf) | Standard Length (hpf) | Developmental Stage (hpf) | Mean Estimation (hpf) |
|---|---|---|---|---|
| 5 | 40.25 ± 3.55 *** | 31.56 ± 2.15 *** | 29.90 ± 4.23 *** | 33.91 ± 5.56 (13) *** |
| 2.5 | 49.73 ± 8.94 *** | 47.03 ± 8.50 *** | 41.64 ± 8.46 *** | 46.13 ± 4.12 (16) *** |
| 1 | 70.10 ± 5.34 *** | 59.98 ± 5.56 *** | 63.14 ± 3.61 *** | 64.41 ± 5.18 (19) *** |
| 0.5 | 70.62 ± 7.78 ** | 61.24 ± 6.92 *** | 66.80 ± 5.49 ** | 66.22 ± 4.72 (15) ** |
| 0.25 | 73.06 ± 3.86 ** | 61.70 ± 8.91 ** | 69.88 ± 4.21 * | 68.21 ± 5.86 (16) ** |
| 0 | 77.61 ± 5.28 | 69.60 ± 5.02 | 72.00 ± 0.00 | 73.07 ± 4.11 (18) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Márquez, J.; Moreira, B.R.; Valverde-Guillén, P.; Latorre-Redoli, S.; Caneda-Santiago, C.T.; Acién, G.; Martínez-Manzanares, E.; Marí-Beffa, M.; Abdala-Díaz, R.T. In Vitro and In Vivo Effects of Ulvan Polysaccharides from Ulva rigida. Pharmaceuticals 2023, 16, 660. https://doi.org/10.3390/ph16050660
García-Márquez J, Moreira BR, Valverde-Guillén P, Latorre-Redoli S, Caneda-Santiago CT, Acién G, Martínez-Manzanares E, Marí-Beffa M, Abdala-Díaz RT. In Vitro and In Vivo Effects of Ulvan Polysaccharides from Ulva rigida. Pharmaceuticals. 2023; 16(5):660. https://doi.org/10.3390/ph16050660
Chicago/Turabian StyleGarcía-Márquez, Jorge, Bruna Rodrigues Moreira, Piedad Valverde-Guillén, Sofía Latorre-Redoli, Candela T. Caneda-Santiago, Gabriel Acién, Eduardo Martínez-Manzanares, Manuel Marí-Beffa, and Roberto T. Abdala-Díaz. 2023. "In Vitro and In Vivo Effects of Ulvan Polysaccharides from Ulva rigida" Pharmaceuticals 16, no. 5: 660. https://doi.org/10.3390/ph16050660
APA StyleGarcía-Márquez, J., Moreira, B. R., Valverde-Guillén, P., Latorre-Redoli, S., Caneda-Santiago, C. T., Acién, G., Martínez-Manzanares, E., Marí-Beffa, M., & Abdala-Díaz, R. T. (2023). In Vitro and In Vivo Effects of Ulvan Polysaccharides from Ulva rigida. Pharmaceuticals, 16(5), 660. https://doi.org/10.3390/ph16050660







