Application of the Population Pharmacokinetics Model-Based Approach to the Prediction of Drug–Drug Interaction between Rivaroxaban and Carbamazepine in Humans
Abstract
:1. Introduction
2. Results
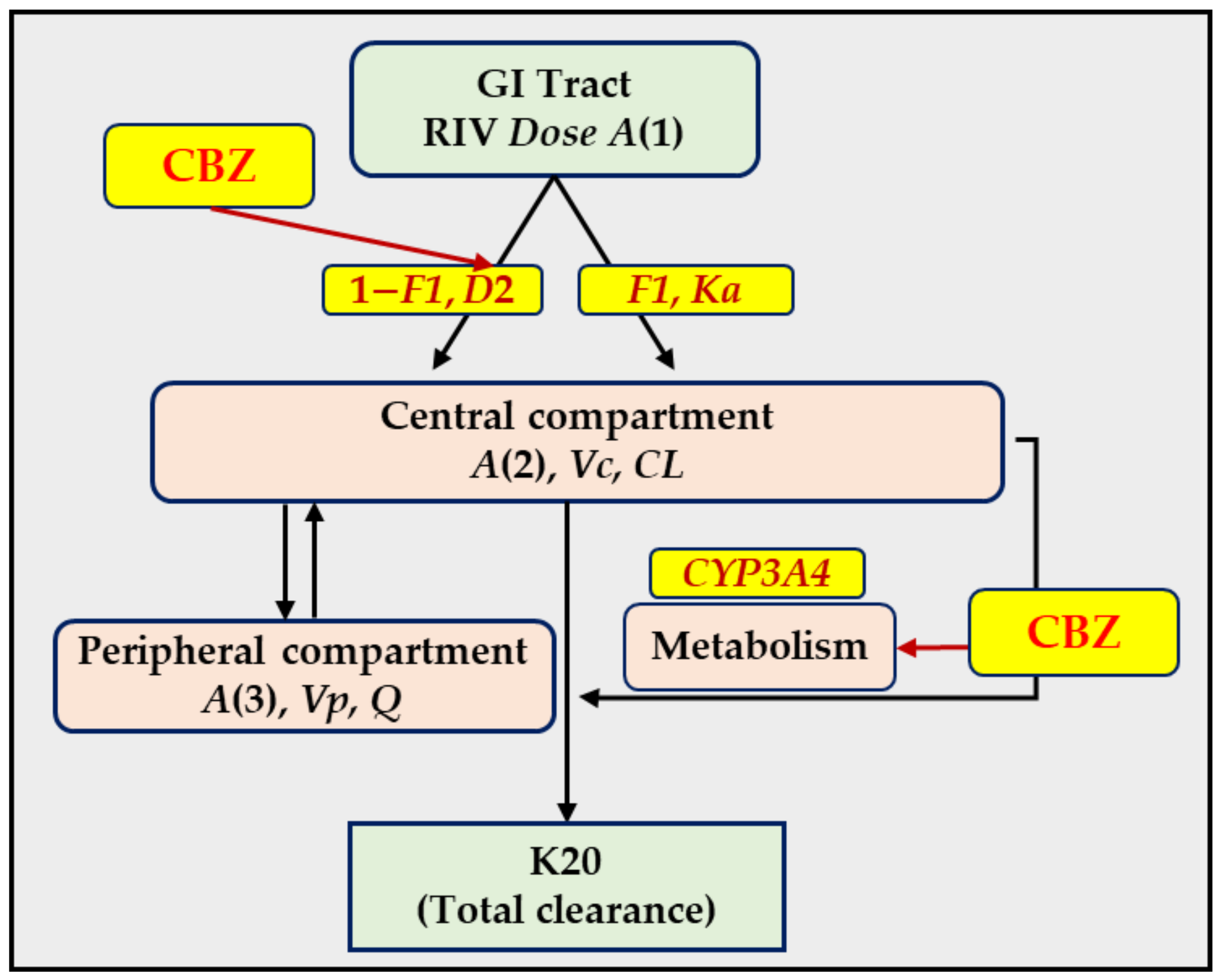
2.1. PopPK Model of RIV in Rats
2.2. Extrapolation of RIV PK Parameters from Rats to Humans
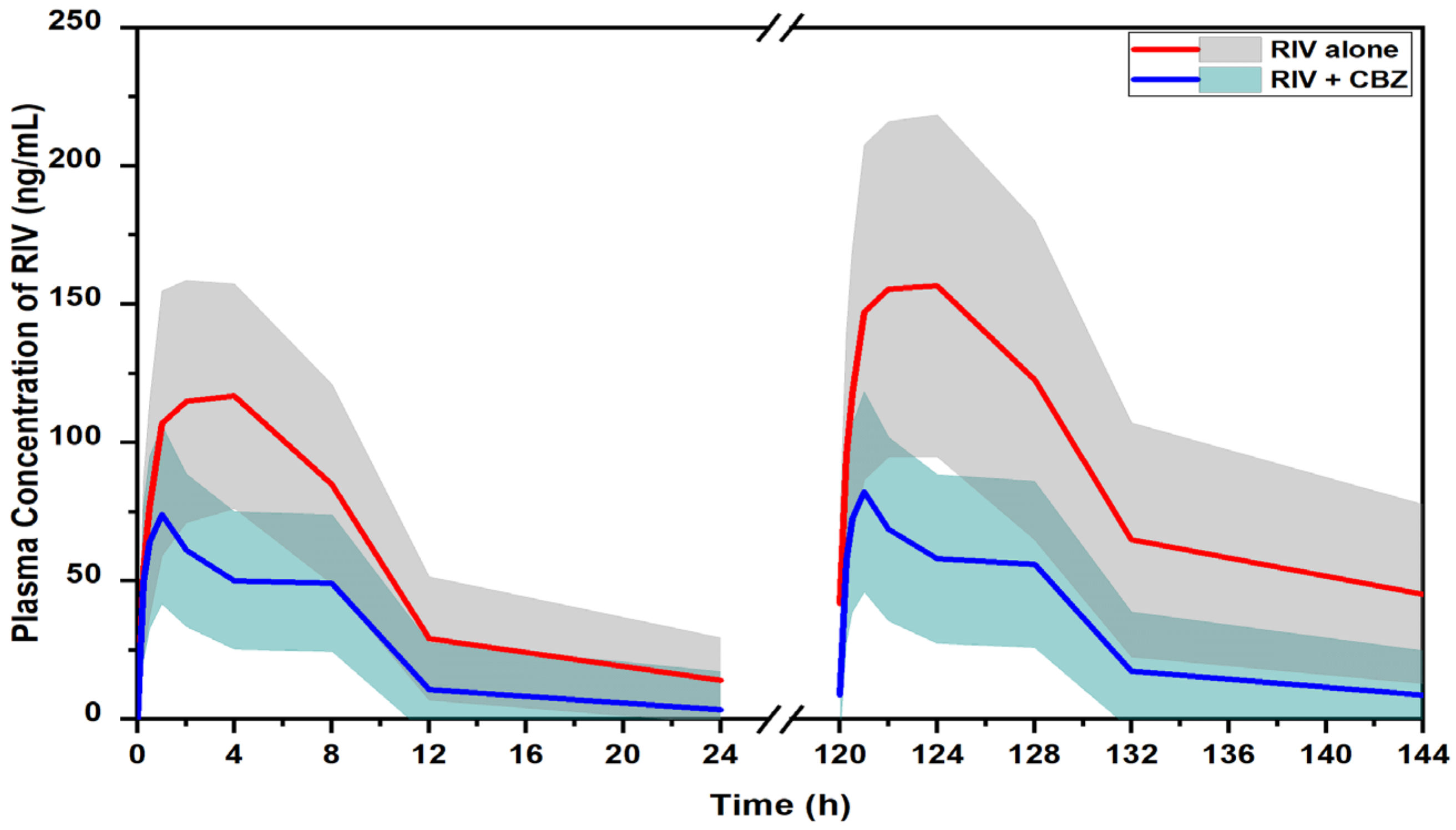
2.3. Prediction of DDI Profile of RIV and CBZ in Humans
2.4. Comparison of PopPK and PBPK Model-Based Approaches
3. Discussion
4. Materials and Methods
4.1. PK Study in Rats
4.2. PopPK Model of RIV in Rats
4.3. Extrapolation of RIV PK Parameters from Rats to Humans
4.4. Evaluation of the Extrapolation Results in Humans
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Samama, M.M. The Mechanism of Action of Rivaroxaban—An Oral, Direct Factor Xa Inhibitor—Compared with Other Anticoagulants. Thromb. Res. 2011, 127, 497–504. [Google Scholar] [CrossRef]
- Bayer Pharma AG. Xarelto (Rivaroxaban) Summary of Product Characteristics; Bayer Pharma AG: Wuppertal, Germany, 2013. [Google Scholar]
- European Medicines Agency. CHMP Assessment Report for Xarelto; European Medicines Agency: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Mueck, W.; Stampfuss, J.; Kubitza, D.; Becka, M. Clinical Pharmacokinetic and Pharmacodynamic Profile of Rivaroxaban. Clin. Pharmacokinet. 2014, 53, 1–16. [Google Scholar] [CrossRef]
- Mueck, W.; Kubitza, D.; Becka, M. Co-Administration of Rivaroxaban with Drugs That Share Its Elimination Pathways: Pharmacokinetic Effects in Healthy Subjects. Br. J. Clin. Pharmacol. 2013, 76, 455–466. [Google Scholar] [CrossRef]
- Gnoth, M.J.; Buetehorn, U.; Muenster, U.; Schwarz, T.; Sandmann, S. In Vitro and In Vivo P-Glycoprotein Transport Characteristics of Rivaroxaban. J. Pharmacol. Exp. Ther. 2011, 338, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Hodin, S.; Basset, T.; Jacqueroux, E.; Delezay, O.; Clotagatide, A.; Perek, N.; Mismetti, P.; Delavenne, X. In Vitro Comparison of the Role of P-Glycoprotein and Breast Cancer Resistance Protein on Direct Oral Anticoagulants Disposition. Eur. J. Drug Metab. Pharmacokinet. 2018, 43, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Gong, I.Y.; Mansell, S.E.; Kim, R.B. Absence of Both MDR1 (ABCB1) and Breast Cancer Resistance Protein (ABCG2) Transporters Significantly Alters Rivaroxaban Disposition and Central Nervous System Entry. Basic Clin. Pharmacol. Toxicol. 2013, 112, 164–170. [Google Scholar] [CrossRef]
- Weinz, C.; Schwarz, T.; Kubitza, D.; Mueck, W.; Lang, D. Metabolism and Excretion of Rivaroxaban, an Oral, Direct Factor Xa Inhibitor, in Rats, Dogs, and Humans. Drug Metab. Dispos. 2009, 37, 1056–1064. [Google Scholar] [CrossRef]
- Chang, S.-H.; Chou, I.-J.; Yeh, Y.-H.; Chiou, M.-J.; Wen, M.-S.; Kuo, C.-T.; See, L.-C.; Kuo, C.-F. Association Between Use of Non-Vitamin K Oral Anticoagulants With and Without Concurrent Medications and Risk of Major Bleeding in Nonvalvular Atrial Fibrillation. JAMA 2017, 318, 1250–1259. [Google Scholar] [CrossRef]
- Risselada, A.J.; Visser, M.J.; van Roon, E.N. Pulmonary embolism due to interaction between rivaroxaban and carbamazepine. Ned. Tijdschr. Geneeskd. 2013, 157, A6568. [Google Scholar]
- Stöllberger, C.; Finsterer, J. Recurrent Venous Thrombosis under Rivaroxaban and Carbamazepine for Symptomatic Epilepsy. Neurol. Neurochir. Pol. 2017, 51, 194–196. [Google Scholar] [CrossRef] [PubMed]
- Becerra, A.F.; Amuchastegui, T.; Tabares, A.H. Decreased Rivaroxaban Levels in a Patient with Cerebral Vein Thrombosis Receiving Phenytoin. Case Rep. Hematol. 2017, 2017, 4760612. [Google Scholar] [CrossRef]
- Emoto, C.; McPhail, B.T.; Fukuda, T. Clinical Applications of Physiologically Based Pharmacokinetic Modeling: Perspectives on the Advantages and Challenges. Ther. Drug Monit. 2020, 42, 157–158. [Google Scholar] [CrossRef] [PubMed]
- Einolf, H.J. Comparison of Different Approaches to Predict Metabolic Drug-Drug Interactions. Xenobiotica 2007, 37, 1257–1294. [Google Scholar] [CrossRef] [PubMed]
- Yeo, K.R.; Jamei, M.; Rostami-Hodjegan, A. Predicting Drug–Drug Interactions: Application of Physiologically Based Pharmacokinetic Models under a Systems Biology Approach. Expert Rev. Clin. Pharmacol. 2013, 6, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Ngo, T.L.; Yang, S.; Shin, S.; Cao, D.T.; Nguyen, V.H.; Jung, S.; Lee, J.-Y.; Lee, J.-H.; Yun, H.; Chae, J. Application of Physiologically-Based Pharmacokinetic Model Approach to Predict Pharmacokinetics and Drug–Drug Interaction of Rivaroxaban: A Case Study of Rivaroxaban and Carbamazepine. CPT Pharmacometrics Syst. Pharmacol. 2022. [Google Scholar] [CrossRef]
- Johannessen, S.I.; Landmark, C.J. Antiepileptic Drug Interactions—Principles and Clinical Implications. Curr. Neuropharmacol. 2010, 8, 254–267. [Google Scholar] [CrossRef]
- Patsalos, P.N.; Fröscher, W.; Pisani, F.; Van Rijn, C.M. The Importance of Drug Interactions in Epilepsy Therapy. Epilepsia 2002, 43, 365–385. [Google Scholar] [CrossRef]
- Owen, A.; Goldring, C.; Morgan, P.; Park, B.K.; Pirmohamed, M. Induction of P-Glycoprotein in Lymphocytes by Carbamazepine and Rifampicin: The Role of Nuclear Hormone Response Elements. Br. J. Clin. Pharmacol. 2006, 62, 237–242. [Google Scholar] [CrossRef]
- Giessmann, T.; May, K.; Modess, C.; Wegner, D.; Hecker, U.; Zschiesche, M.; Dazert, P.; Grube, M.; Schroeder, E.; Warzok, R. Carbamazepine Regulates Intestinal P-Glycoprotein and Multidrug Resistance Protein MRP2 and Influences Disposition of Talinolol in Humans. Clin. Pharmacol. Ther. 2004, 76, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Vlase, L.; Neag, M.; Popa, A.; Muntean, D.; Bâldea, I.; Leucuta, S.E. Pharmacokinetic Interaction between Ivabradine and Carbamazepine in Healthy Volunteers. J. Clin. Pharm. Ther. 2011, 36, 225–229. [Google Scholar] [CrossRef]
- Yamada, S.; Yasui-Furukori, N.; Akamine, Y.; Kaneko, S.; Uno, T. Effects of the P-Glycoprotein Inducer Carbamazepine on Fexofenadine Pharmacokinetics. Ther. Drug Monit. 2009, 31, 764–768. [Google Scholar] [CrossRef]
- Ngo, L.T.; Yang, S.; Tran, Q.T.; Kim, S.K.; Yun, H.; Chae, J. Effects of Carbamazepine and Phenytoin on Pharmacokinetics and Pharmacodynamics of Rivaroxaban. Pharmaceutics 2020, 12, 1040. [Google Scholar] [CrossRef] [PubMed]
- Mueck, W.; Becka, M.; Kubitza, D.; Voith, B.; Zuehlsdorf, M. Population Model of the Pharmacokinetics and Pharmacodynamics of Rivaroxaban—An Oral, Direct Factor Xa Inhibitor—In Healthy Subjects. Int. J. Clin. Pharmacol. Ther. 2007, 45, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Open Systems Pharmacology. Carbamazepine Model. Available online: https://github.com/Open-Systems-Pharmacology/Carbamazepine-Model (accessed on 30 April 2023).
- Fuhr, L.M.; Marok, F.Z.; Hanke, N.; Selzer, D.; Lehr, T. Pharmacokinetics of the CYP3A4 and CYP2B6 Inducer Carbamazepine and Its Drug–Drug Interaction Potential: A Physiologically Based Pharmacokinetic Modeling Approach. Pharmaceutics 2021, 13, 270. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. XARELTO® (Rivaroxaban): Prescribing Information; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2011. [Google Scholar]
- U.S. Food and Drug Administration. Tegretol® (Carbamazepine USP): Prescribing Information; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2007. [Google Scholar]
- U.S. Department of Health and Human Services; Food and Drug Administration; Center for Drug Evaluation and Research. Guidance for Industry Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers; Center for Drug Evaluation and Research: Silver Spring, MD, USA, 2005. [Google Scholar]
- Mahmood, I. Integration of in Vitro Data and Brain Weight in Allometric Scaling to Predict Clearance in Humans: Some Suggestions†. J. Pharm. Sci. 1998, 87, 527–529. [Google Scholar] [CrossRef] [PubMed]
- Lavé, T.; Coassolo, P.; Reigner, B. Prediction of Hepatic Metabolic Clearance Based on Interspecies Allometric Scaling Techniques and in Vitro-in Vivo Correlations. Clin. Pharmacokinet. 1999, 36, 211–231. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Riviere, J.E. The Application of Allometric Scaling Principles to Predict Pharmacokinetic Parameters across Species. Expert Opin. Drug Metab. Toxicol. 2014, 10, 1241–1253. [Google Scholar] [CrossRef]
- Moore, K.T.; Krook, M.A.; Vaidyanathan, S.; Sarich, T.C.; Damaraju, C.V.; Fields, L.E. Rivaroxaban Crushed Tablet Suspension Characteristics and Relative Bioavailability in Healthy Adults When Administered Orally or via Nasogastric Tube. Clin. Pharmacol. Drug Dev. 2014, 3, 321–327. [Google Scholar] [CrossRef]
- Stampfuss, J.; Kubitza, D.; Becka, M.; Mueck, W. The Effect of Food on the Absorption and Pharmacokinetics of Rivaroxaban. Int. J. Clin. Pharmacol. Ther. 2013, 51, 549–561. [Google Scholar] [CrossRef]
- Kubitza, D.; Becka, M.; Roth, A.; Mueck, W. Absence of Clinically Relevant Interactions between Rivaroxaban—An Oral, Direct Factor Xa Inhibitor—And Digoxin or Atorvastatin in Healthy Subjects. J. Int. Med. Res. 2012, 40, 1688–1707. [Google Scholar] [CrossRef]
- Chen, M.L.; Yu, L.; Grillo, J.A.; Zhao, P.; Bullock, J.; Booth, B.P.; Lu, M.; Robie-Suh, K.; Berglund, E.G.; Pang, K.S.; et al. Safety, Pharmacokinetics and Pharmacodynamics of Single/Multiple Doses of the Oral, Direct Factor Xa Inhibitor Rivaroxaban in Healthy Chinese Subjects. Br. J. Clin. Pharmacol. 2009, 68, 77–88. [Google Scholar] [CrossRef]
- Kubitza, D.; Becka, M.; Wensing, G.; Voith, B.; Zuehlsdorf, M. Safety, Pharmacodynamics, and Pharmacokinetics of BAY 59-7939—An Oral, Direct Factor Xa Inhibitor—After Multiple Dosing in Healthy Male Subjects. Eur. J. Clin. Pharmacol. 2005, 61, 873–880. [Google Scholar] [CrossRef]
- Alvariza, S.; Fagiolino, P.; Vázquez, M.; Feria-Romero, I.; Orozco-Suárez, S. Chronic Administration of Phenytoin Induces Efflux Transporter Overexpression in Rats. Pharmacol. Reports 2014, 66, 946–951. [Google Scholar] [CrossRef]
- Sinha, V.K.; De Buck, S.S.; Fenu, L.A.; Smit, J.W.; Nijsen, M.; Gilissen, R.A.H.J.; Van Peer, A.; Lavrijsen, K.; Mackie, C.E. Predicting Oral Clearance in Humans. Clin. Pharmacokinet. 2008, 47, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Chiou, W.L.; Hsu, F.H. Correlation of Unbound Plasma Clearances of Fifteen Extensively Metabolized Drugs between Humans and Rats. Pharm. Res. 1988, 5, 668–672. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, I. Interspecies Scaling: Role of Protein Binding in the Prediction of Clearance from Animals to Humans. J. Clin. Pharmacol. 2000, 40, 1439–1446. [Google Scholar] [CrossRef]
- Keizer, R.J.; Karlsson, M.O.; Hooker, A. Modeling and Simulation Workbench for NONMEM: Tutorial on Pirana, PsN, and Xpose. CPT Pharmacometrics Syst. Pharmacol. 2013, 2, e50. [Google Scholar] [CrossRef]
- Boxenbaum, H. Interspecies Scaling, Allometry, Physiological Time, and the Ground Plan of Pharmacokinetics. J. Pharmacokinet. Biopharm. 1982, 10, 201–227. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Hussain, A.; Leal, M.; Mayersohn, M.; Fluhler, E. Interspecies Prediction of Human Drug Clearance Based on Scaling Data from One or Two Animal Species. Drug Metab. Dispos. 2007, 35, 1886–1893. [Google Scholar] [CrossRef]
- Caldwell, G.W.; Masucci, J.A.; Yan, Z.; Hageman, W. Allometric Scaling of Pharmacokinetic Parameters in Drug Discovery: Can Human CL, Vss and T1/2 Be Predicted Fromin-Vivo Rat Data? Eur. J. Drug Metab. Pharmacokinet. 2004, 29, 133–143. [Google Scholar] [CrossRef]
- Dedrick, R.L. Animal Scale-Up. J. Pharmacokinet. Biopharm. 1973, 1, 435–461. [Google Scholar] [CrossRef]
- Ward, K.W.; Smith, B.R. A Comprehensive Quantitative and Qualitative Evaluation of Extrapolation of Intravenous Pharmacokinetic Parameters from Rat, Dog, and Monkey to Humans. I. Clearance. Drug Metab. Dispos. 2004, 32, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Jolivette, L.J.; Ward, K.W. Extrapolation of Human Pharmacokinetic Parameters from Rat, Dog, and Monkey Data: Molecular Properties Associated with Extrapolative Success or Failure. J. Pharm. Sci. 2005, 94, 1467–1483. [Google Scholar] [CrossRef]
- Evans, C.A.; Jolivette, L.J.; Nagilla, R.; Ward, K.W. Extrapolation of Preclinical Pharmacokinetics and Molecular Feature Analysis of “Discovery-like” Molecules to Predict Human Pharmacokinetics. Drug Metab. Dispos. 2006, 34, 1255–1265. [Google Scholar] [CrossRef] [PubMed]
- Nagilla, R.; Ward, K.W. A Comprehensive Analysis of the Role of Correction Factors in the Allometric Predictivity of Clearance from Rat, Dog, and Monkey to Humans. J. Pharm. Sci. 2004, 93, 2522–2534. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.R.; Lou, X.; Brown, R.R.; Hutchaleelaha, A. Allometric Pharmacokinetic Scaling: Towards the Prediction of Human Oral Pharmacokinetics. Pharm. Res. 2000, 17, 410–418. [Google Scholar] [CrossRef]

| Description | Estimated in Rats [24] | Extrapolated in Humans | RSE (%) |
|---|---|---|---|
| PK parameters | |||
| CL/F in control group | 0.609 (L/h/kg) | 9.03 (L/h) | 36.9 |
| CL/F in test group | 1.894 (L/h/kg) | 28.1 (L/h) | 69.7 |
| /F | 0.701 (L/kg) | 42.06 (L) | 44.2 |
| Q/F | 0.665 (L/h/kg) | 9.86 (L/h) | 21.4 |
| /F | 5.60 (L/kg) | 336 (L) | 38.1 |
| D2 in control group | 6.62 (h) | Same | |
| D2 in test group | 8.84 (h) | Same | 17.2 |
| Ka | 2.31 (1/h) | 0.97 [25] | 33.6 |
| F1 | 0.260 | Same | 9.10 |
| Alag2 | 0.501 (h) | Same | |
| Inter-individual variability (IIV) | |||
| /F | 47.0 (%) | Same | 33.0 |
| IIV for CL/F | 49.0 (%) | Same | 35.0 |
| Residual error | |||
| Additive error | 13.6 (ng/mL) | Same | 35.8 |
| Proportional error | 23.2 (%) | Same | 17.2 |
| Parameters | Unit | RIV alone | RIV + CBZ | Relative Change (%) | |
|---|---|---|---|---|---|
| PopPK model-based approach | AUC_first | ng · h/mL | 1291.7 (770.9–1837.0) | 615.7 (296.8–1025.4) | 52.3 |
| AUC_SS | ng · h/mL | 2157.5 (983.6–3756.9) | 775.2 (323.8–1463.1) | 68.5 | |
| Cmax_first | ng/mL | 133.2 (88.1–185.4) | 78.6 (45.8–121.0) | 41.0 | |
| Cmax_SS | ng/mL | 172.2 (101.5–259.9) | 86.5 (48.3–136.1) | 49.8 | |
| t1/2 | h | 6.65 | 5.01 | - | |
| PBPK model-based approach ¥ | AUC_first | ng · h/mL | 2221.3 (488.3–4087.9 | 1438.7 (338.8–3002.0) | 35.2 |
| AUC_SS | ng · h/mL | 2467.3 (544.6–4667.7) | 1838.4 (463.8–3684.4) | 25.5 | |
| Cmax_first | ng/mL | 266.3 (80.7–452.3) | 166.1 (54.1–299.4) | 37.7 | |
| Cmax_SS | ng/mL | 282.3 (86.2–473.6) | 179.5 (59.0–321.5) | 36.4 | |
| t1/2 | h | 12.9 | 15.5 | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngo, L.T.; Yun, H.-y.; Chae, J.-w. Application of the Population Pharmacokinetics Model-Based Approach to the Prediction of Drug–Drug Interaction between Rivaroxaban and Carbamazepine in Humans. Pharmaceuticals 2023, 16, 684. https://doi.org/10.3390/ph16050684
Ngo LT, Yun H-y, Chae J-w. Application of the Population Pharmacokinetics Model-Based Approach to the Prediction of Drug–Drug Interaction between Rivaroxaban and Carbamazepine in Humans. Pharmaceuticals. 2023; 16(5):684. https://doi.org/10.3390/ph16050684
Chicago/Turabian StyleNgo, Lien Thi, Hwi-yeol Yun, and Jung-woo Chae. 2023. "Application of the Population Pharmacokinetics Model-Based Approach to the Prediction of Drug–Drug Interaction between Rivaroxaban and Carbamazepine in Humans" Pharmaceuticals 16, no. 5: 684. https://doi.org/10.3390/ph16050684
APA StyleNgo, L. T., Yun, H.-y., & Chae, J.-w. (2023). Application of the Population Pharmacokinetics Model-Based Approach to the Prediction of Drug–Drug Interaction between Rivaroxaban and Carbamazepine in Humans. Pharmaceuticals, 16(5), 684. https://doi.org/10.3390/ph16050684







