Comprehensive Characterization of a Streptococcus agalactiae Phage Isolated from a Tilapia Farm in Selangor, Malaysia, and Its Potential for Phage Therapy
Abstract
:1. Introduction
2. Results
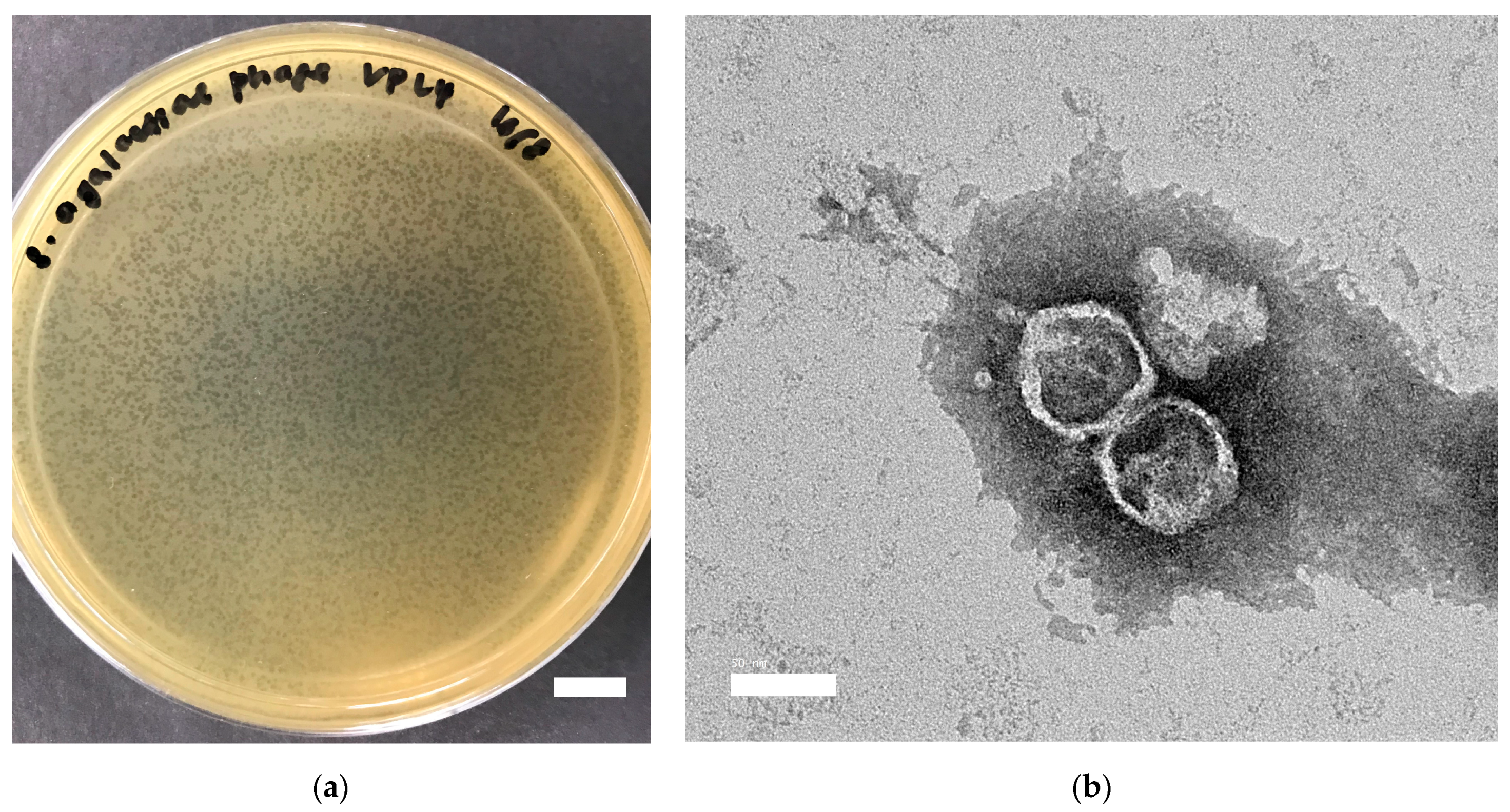
2.1. Characterization of vB_Sags-UPM1 in S. agalactiae smyh01 and smyh02
2.2. Bioinformatics
2.2.1. Overall Genome of vB_Sags-UPM1
2.2.2. DNA Replication/Regulatory Module
2.2.3. Packaging Module
2.2.4. Morphogenesis Module
2.2.5. Lysis Module
2.2.6. Lysogeny Module
2.3. Lytic Activity of vB_Sags-UPM1 Endolysin, Lys60
3. Discussion
4. Materials and Methods
4.1. Bacteria Strains and Culture Condition
4.2. Phage Isolation
4.3. One-Step Growth Curve and Kill Assay
4.4. Lysogeny Efficiency and Host Range Determination
4.5. Transmission Electron Microscopy (TEM)
4.6. Phage DNA Extraction
4.7. Bioinformatics
4.7.1. Illumina Library Preparation and Genome Sequencing
4.7.2. De Novo Assembly—Illumina
4.7.3. Genome Annotation
4.7.4. Bioinformatics Analysis
4.8. Cloning and Expression of Endolysin Gene
4.9. Lys60 Lytic Activity
4.10. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barkham, T.; Zadoks, R.N.; Azmai, M.N.A.; Baker, S.; Bich, V.T.N.; Chalker, V.; Chau, M.L.; Dance, D.; Deepak, R.N.; van Doorn, H.R.; et al. One hypervirulent clone, sequence type 283, accounts for a large proportion of invasive Streptococcus agalactiae isolated from humans and diseased tilapia in Southeast Asia. PLoS Negl. Trop. Dis. 2019, 13, e0007421. [Google Scholar] [CrossRef]
- Cieslewicz, M.J.; Chaffin, D.; Glusman, G.; Kasper, D.; Madan, A.; Rodrigues, S.; Fahey, J.; Wessels, M.R.; Rubens, C.E. Structural and genetic diversity of group B streptococcus capsular polysaccharides. Infect. Immun. 2005, 73, 3096–3103. [Google Scholar] [CrossRef]
- Delannoy, C.M.; Crumlish, M.; Fontaine, M.C.; Pollock, J.; Foster, G.; Dagleish, M.P.; Turnbull, J.F.; Zadoks, R.N. Human Streptococcus agalactiae strains in aquatic mammals and fish. BMC Microbiol. 2013, 13, 41. [Google Scholar] [CrossRef]
- Stringer, J. The development of a phage-typing system for group-B streptococci. J. Med. Microbiol. 1980, 13, 133–143. [Google Scholar] [CrossRef]
- Amal, M.N.A.; Saad, M.Z.; Zahrah, A.S.; Zulkafli, A.R. Water quality influences the presence of Streptococcus agalactiae in cage cultured red hybrid tilapia, Oreochromis niloticus × Oreochromis mossambicus. Aquacul. Res. 2015, 46, 313–323. [Google Scholar] [CrossRef]
- Kalimuddin, S.; Chen, S.L.; Lim, C.T.K.; Koh, T.H.; Tan, T.Y.; Kam, M.; Wong, C.W.; Mehershahi, K.S.; Chau, M.L.; Ng, L.C.; et al. 2015 Epidemic of Severe Streptococcus agalactiae Sequence Type 283 Infections in Singapore Associated with the Consumption of Raw Freshwater Fish: A Detailed Analysis of Clinical, Epidemiological, and Bacterial Sequencing Data. Clin. Infect. Dis. 2017, 64, S145–S152. [Google Scholar] [CrossRef]
- Kayansamruaj, P.; Soontara, C.; Unajak, S.; Dong, H.T.; Rodkhum, C.; Kondo, H.; Hirono, I.; Areechon, N. Comparative genomics inferred two distinct populations of piscine pathogenic Streptococcus agalactiae, serotype Ia ST7 and serotype III ST283, in Thailand and Vietnam. Genomics 2019, 111, 1657–1667. [Google Scholar] [CrossRef]
- Syuhada, R.; Zamri-Saad, M.; Ina-Salwany, M.Y.; Mustafa, M.; Nasruddin, N.N.; Desa, M.N.M.; Nordin, S.A.; Barkham, T.; Amal, M.N.A. Molecular characterization and pathogenicity of Streptococcus agalactiae serotypes Ia ST7 and III ST283 isolated from cultured red hybrid tilapia in Malaysia. Aquaculture 2020, 515, 734543. [Google Scholar] [CrossRef]
- Aisyhah, M.A.; Amal, M.N.; Zamri-Saad, M.; Siti-Zahrah, A.; Shaqinah, N.N. Streptococcus agalactiae isolates from cultured fishes in Malaysia manifesting low resistance pattern towards selected antibiotics. J. Fish. Dis. 2015, 38, 1093–1098. [Google Scholar] [CrossRef]
- Bai, Q.; Zhang, W.; Yang, Y.; Tang, F.; Nguyen, X.; Liu, G.; Lu, C. Characterization and genome sequencing of a novel bacteriophage infecting Streptococcus agalactiae with high similarity to a phage from Streptococcus pyogenes. Arch. Virol. 2013, 158, 1733–1741. [Google Scholar] [CrossRef]
- Russell, H.; Norcross, N.L.; Kahn, D.E. Isolation and characterization of Streptococcus agalactiae bacteriophage. J. Gen. Virol. 1969, 5, 315–317. [Google Scholar] [CrossRef]
- Furfaro, L.L.; Payne, M.S.; Chang, B.J. Host range, morphological and genomic characterisation of bacteriophages with activity against clinical Streptococcus agalactiae isolates. PLoS ONE 2020, 15, e0235002. [Google Scholar] [CrossRef]
- Domelier, A.S.; van der Mee-Marquet, N.; Sizaret, P.Y.; Hery-Arnaud, G.; Lartigue, M.F.; Mereghetti, L.; Quentin, R. Molecular characterization and lytic activities of Streptococcus agalactiae bacteriophages and determination of lysogenic-strain features. J. Bacteriol. 2009, 191, 4776–4785. [Google Scholar] [CrossRef]
- Pritchard, D.G.; Dong, S.; Baker, J.R.; Engler, J.A. The bifunctional peptidoglycan lysin of Streptococcus agalactiae bacteriophage B30. Microbiology 2004, 150, 2079–2087. [Google Scholar] [CrossRef]
- Luo, X.; Liao, G.; Liu, C.; Jiang, X.; Lin, M.; Zhao, C.; Tao, J.; Huang, Z. Characterization of bacteriophage HN48 and its protective effects in Nile tilapia Oreochromis niloticus against Streptococcus agalactiae infections. J. Fish. Dis. 2018, 41, 1477–1484. [Google Scholar] [CrossRef]
- Matsuoka, S.; Hashizume, T.; Kanzaki, H.; Iwamoto, E.; Park, S.C.; Yoshida, T.; Nakai, T. Phage therapy against β-hemolytic streptococcicosis of Japanese flounder Paralichthys olivaceus. Fish. Pathol. 2007, 42, 181–189. [Google Scholar] [CrossRef]
- Kwon, A.S.; Kang, B.J.; Jun, S.Y.; Yoon, S.J.; Lee, J.H.; Kang, S.H. Evaluating the effectiveness of Streptococcus parauberis bacteriophage Str-PAP-1 as an environmentally friendly alternative to antibiotics for aquaculture. Aquaculture 2017, 468, 464–470. [Google Scholar] [CrossRef]
- Preenanka, R.; Safeena, M.P. Morphological, biological and genomic characterization of lytic phages against Streptococcus agalactiae causing streptococcosis in tilapia. Microb. Pathog. 2022, 174, 105919. [Google Scholar] [CrossRef]
- Cheng, Q.; Nelson, D.; Zhu, S.; Fischetti, V.A. Removal of group B streptococci colonizing the vagina and oropharynx of mice with a bacteriophage lytic enzyme. Antimicrob. Agents Chemother. 2005, 49, 111–117. [Google Scholar] [CrossRef]
- Pritchard, D.G.; Dong, S.; Kirk, M.C.; Cartee, R.T.; Baker, J.R. LambdaSa1 and LambdaSa2 prophage lysins of Streptococcus agalactiae. Appl. Environ. Microbiol. 2007, 73, 7150–7154. [Google Scholar] [CrossRef]
- Rezaei Javan, R.; Ramos-Sevillano, E.; Akter, A.; Brown, J.; Brueggemann, A.B. Prophages and satellite prophages are widespread in Streptococcus and may play a role in pneumococcal pathogenesis. Nat. Commun. 2019, 10, 4852. [Google Scholar] [CrossRef]
- Lucchini, S.; Desiere, F.; Brussow, H. Similarly organized lysogeny modules in temperate Siphoviridae from low GC content gram-positive bacteria. Virology 1999, 263, 427–435. [Google Scholar] [CrossRef]
- Brady, A.; Felipe-Ruiz, A.; Gallego Del Sol, F.; Marina, A.; Quiles-Puchalt, N.; Penades, J.R. Molecular Basis of Lysis-Lysogeny Decisions in Gram-Positive Phages. Annu. Rev. Microbiol. 2021, 75, 563–581. [Google Scholar] [CrossRef]
- Azulay, G.; Pasechnek, A.; Stadnyuk, O.; Ran-Sapir, S.; Fleisacher, A.M.; Borovok, I.; Sigal, N.; Herskovits, A.A. A dual-function phage regulator controls the response of cohabiting phage elements via regulation of the bacterial SOS response. Cell Rep. 2022, 39, 110723. [Google Scholar] [CrossRef]
- Sun, S.; Gao, S.; Kondabagil, K.; Xiang, Y.; Rossmann, M.G.; Rao, V.B. Structure and function of the small terminase component of the DNA packaging machine in T4-like bacteriophages. Proc. Natl. Acad. Sci. USA 2012, 109, 817–822. [Google Scholar] [CrossRef]
- Borodovich, T.; Shkoporov, A.N.; Ross, R.P.; Hill, C. Phage-mediated horizontal gene transfer and its implications for the human gut microbiome. Gastroenterol. Rep. 2022, 10, goac012. [Google Scholar] [CrossRef]
- Madsen, P.L.; Hammer, K. Temporal transcription of the lactococcal temperate phage TP901-1 and DNA sequence of the early promoter region. Microbiology 1998, 144 Pt 8, 2203–2215. [Google Scholar] [CrossRef]
- Wong, K.Y.; Megat Mazhar Khair, M.H.; Song, A.A.; Masarudin, M.J.; Chong, C.M.; In, L.L.A.; Teo, M.Y.M. Endolysins against Streptococci as an antibiotic alternative. Front. Microbiol. 2022, 13, 935145. [Google Scholar] [CrossRef]
- Wang, I.N.; Smith, D.L.; Young, R. Holins: The protein clocks of bacteriophage infections. Annu. Rev. Microbiol. 2000, 54, 799–825. [Google Scholar] [CrossRef]
- Grundling, A.; Manson, M.D.; Young, R. Holins kill without warning. Proc. Natl. Acad. Sci. USA 2001, 98, 9348–9352. [Google Scholar] [CrossRef]
- Donovan, D.M.; Foster-Frey, J.; Dong, S.; Rousseau, G.M.; Moineau, S.; Pritchard, D.G. The cell lysis activity of the Streptococcus agalactiae bacteriophage B30 endolysin relies on the cysteine, histidine-dependent amidohydrolase/peptidase domain. Appl. Environ. Microbiol. 2006, 72, 5108–5112. [Google Scholar] [CrossRef]
- Cheng, Q.; Fischetti, V.A. Mutagenesis of a bacteriophage lytic enzyme PlyGBS significantly increases its antibacterial activity against group B streptococci. Appl Microbiol. Biotechnol. 2007, 74, 1284–1291. [Google Scholar] [CrossRef]
- Loeffler, J.M.; Djurkovic, S.; Fischetti, V.A. Phage lytic enzyme Cpl-1 as a novel antimicrobial for pneumococcal bacteremia. Infect. Immun. 2003, 71, 6199–6204. [Google Scholar] [CrossRef]
- McShan, W.M.; McCullor, K.A.; Nguyen, S.V. The Bacteriophages of Streptococcus pyogenes. Microbiol. Spectr. 2019, 7, GPP3–GPP0059. [Google Scholar] [CrossRef]
- Lichvarikova, A.; Soltys, K.; Szemes, T.; Slobodnikova, L.; Bukovska, G.; Turna, J.; Drahovska, H. Characterization of Clinical and Carrier Streptococcus agalactiae and Prophage Contribution to the Strain Variability. Viruses 2020, 12, 1323. [Google Scholar] [CrossRef]
- van der Mee-Marquet, N.; Diene, S.M.; Barbera, L.; Courtier-Martinez, L.; Lafont, L.; Ouachee, A.; Valentin, A.S.; Santos, S.D.; Quentin, R.; Francois, P. Analysis of the prophages carried by human infecting isolates provides new insight into the evolution of Group B Streptococcus species. Clin. Microbiol. Infect. 2018, 24, 514–521. [Google Scholar] [CrossRef]
- Jariah, R.O.A.; Hakim, M.S. Interaction of phages, bacteria, and the human immune system: Evolutionary changes in phage therapy. Rev. Med. Virol. 2019, 29, e2055. [Google Scholar] [CrossRef]
- Lucchini, S.; Desiere, F.; Brussow, H. The genetic relationship between virulent and temperate Streptococcus thermophilus bacteriophages: Whole genome comparison of cos-site phages Sfi19 and Sfi21. Virology 1999, 260, 232–243. [Google Scholar] [CrossRef]
- van der Ploeg, J.R. Genome sequence of the temperate bacteriophage PH10 from Streptococcus oralis. Virus Genes 2010, 41, 450–458. [Google Scholar] [CrossRef]
- Romero, P.; Lopez, R.; Garcia, E. Genomic organization and molecular analysis of the inducible prophage EJ-1, a mosaic myovirus from an atypical pneumococcus. Virology 2004, 322, 239–252. [Google Scholar] [CrossRef]
- McCullor, K.; Postoak, B.; Rahman, M.; King, C.; McShan, W.M. Genomic Sequencing of High-Efficiency Transducing Streptococcal Bacteriophage A25: Consequences of Escape from Lysogeny. J. Bacteriol. 2018, 200, 23. [Google Scholar] [CrossRef]
- Brussow, H.; Hendrix, R.W. Phage genomics: Small is beautiful. Cell 2002, 108, 13–16. [Google Scholar] [CrossRef]
- Chau, M.L.; Chen, S.L.; Yap, M.; Hartantyo, S.H.P.; Chiew, P.K.T.; Fernandez, C.J.; Wong, W.K.; Fong, R.K.; Tan, W.L.; Tan, B.Z.Y.; et al. Group B Streptococcus Infections Caused by Improper Sourcing and Handling of Fish for Raw Consumption, Singapore, 2015–2016. Emerg. Infect. Dis. 2017, 23, 2002–2010. [Google Scholar] [CrossRef]
- Chideroli, R.T.; Amoroso, N.; Mainardi, R.M.; Suphoronski, S.A.; de Padua, S.B.; Alfieri, A.F.; Alfieri, A.A.; Mosela, M.; Moralez, A.T.P.; de Oliveira, A.G.; et al. Emergence of a new multidrug-resistant and highly virulent serotype of Streptococcus agalactiae in fish farms from Brazil. Aquaculture 2017, 479, 45–51. [Google Scholar] [CrossRef]
- Nasr Azadani, D.; Zhang, D.; Hatherill, J.R.; Silva, D.; Turner, J.W. Isolation, characterization, and comparative genomic analysis of a phage infecting high-level aminoglycoside-resistant (HLAR) Enterococcus faecalis. PeerJ 2020, 8, e9171. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Arndt, D.; Grant, J.R.; Marcu, A.; Sajed, T.; Pon, A.; Liang, Y.; Wishart, D.S. PHASTER: A better, faster version of the PHAST phage search tool. Nucleic Acids Res. 2016, 44, W16–W21. [Google Scholar] [CrossRef]
- Salzberg, S.L.; Delcher, A.L.; Kasif, S.; White, O. Microbial gene identification using interpolated Markov models. Nucleic Acids Res. 1998, 26, 544–548. [Google Scholar] [CrossRef]
- Hyatt, D.; Chen, G.L.; Locascio, P.F.; Land, M.L.; Larimer, F.W.; Hauser, L.J. Prodigal: Prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics 2010, 11, 119. [Google Scholar] [CrossRef]
- Richter, M.; Rossello-Mora, R.; Oliver Glockner, F.; Peplies, J. JSpeciesWS: A web server for prokaryotic species circumscription based on pairwise genome comparison. Bioinformatics 2016, 32, 929–931. [Google Scholar] [CrossRef]
- Cantalapiedra, C.P.; Hernandez-Plaza, A.; Letunic, I.; Bork, P.; Huerta-Cepas, J. eggNOG-mapper v2: Functional Annotation, Orthology Assignments, and Domain Prediction at the Metagenomic Scale. Mol. Biol. Evol. 2021, 38, 5825–5829. [Google Scholar] [CrossRef]
- Paysan-Lafosse, T.; Blum, M.; Chuguransky, S.; Grego, T.; Pinto, B.L.; Salazar, G.A.; Bileschi, M.L.; Bork, P.; Bridge, A.; Colwell, L.; et al. InterPro in 2022. Nucleic Acids Res. 2023, 51, D418–D427. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef]
- Darling, A.C.; Mau, B.; Blattner, F.R.; Perna, N.T. Mauve: Multiple alignment of conserved genomic sequence with rearrangements. Genome Res. 2004, 14, 1394–1403. [Google Scholar] [CrossRef]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Soding, J.; et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef]










| Gene | Start–End | Strand | Accession No. | BLASTp Description [Organism] | AA Identity | Accession AA Length | Family/Domain (InterPro ID) | Function |
|---|---|---|---|---|---|---|---|---|
| 1 | 1–651 | − | WP_224208974 | DUF859 family phage minor structural protein [S. agalactiae] | 215/217 (99%) | 504 | Putative viral structural protein, DUF859 (IPR008577) | − |
| 2 a | 652–5619 | − | WP_053515050 | Glucosaminidase domain-containing protein [S. agalactiae] | 1653/1655 (99%) | 1655 | Mannosyl-glycoprotein endo-beta-N-acetylglucosaminidase (IPR002901), CHAP (IPR007921) | Tail protein |
| 3 b | 5610–7172 | − | PPQ23787 | Phage tail protein [S. agalactiae] | 519/520 (99%) | 520 | Siphovirus-type tail component (IPR008841) | Tail protein |
| 4 | 7173–9821 | − | WP_017647846 | Phage tail tape measure protein [S. agalactiae] | 882/882 (100%) | 882 | Phage tail tape measure protein (IPR010090), Rhodanese-like domain (IPR001763) | Tail protein |
| 5 a | 9814–10,119 | − | EPU00268 | Hypothetical protein SAG0108_10345 [S. agalactiae BSU92] | 101/101 (100%) | 103 | − | − |
| 6 | 10,194–10,553 | − | MCK6349393 | Tail assembly chaperone [S. agalactiae] | 118/119 (99%) | 121 | Phage tail assembly chaperone (TAC) proteins (IPR024410) | Tail protein |
| 7 | 10,669–11,154 | − | WP_017647848 | Phage major tail protein, TP901-1 family [S. agalactiae] | 161/161 (100%) | 161 | Phage major tail protein TP901-1 (IPR011855) | Major Tail |
| 8 | 11,163–11,549 | − | WP_017647849 | DUF3168 domain-containing protein [S. agalactiae] | 128/128 (100%) | 128 | Tail completion protein (IPR021508) | Tail protein |
| 9 | 11,546–11,929 | − | WP_000609260 | HK97 gp10 family phage protein [S. agalactiae] | 104/126 (83%) | 126 | Bacteriophage HK97-gp10, putative tail-component (IPR010064) | Tail protein |
| 10 | 11,922–12,221 | − | WP_017647851 | Hypothetical protein [S. agalactiae] | 99/99 (100%) | 99 | − | − |
| 11 | 12,218–12,571 | − | WP_017647852 | Phage head-tail connector protein [S. agalactiae] | 117/117 (100%) | 117 | Phage gp6-like head-tail connector protein (IPR021146) | Head-tail connector |
| 12 a | 12,587–12,841 | − | WP_001229661 | HeH/LEM domain-containing protein [Streptococcus] | 83/84 (99%) | 84 | HeH/LEM domain (IPR025856), Rho termination factor (IPR036269), SAP domain (IPR036361) | DNA/RNA-binding protein |
| 13 | 12,854–13,906 | − | WP_001185213 | Major capsid protein [S. agalactiae] | 350/350 (100%) | 350 | Major capsid protein GpE (IPR005564) | Major capsid |
| 14 | 13906–14289 | − | WP_001042771 | Head decoration protein [Streptococcus] | 127/127 (100%) | 127 | Head decoration protein D (IPR004195) | Head protein |
| 15 | 14,299–14,877 | − | WP_017647853 | DUF4355 domain-containing protein [S. agalactiae] | 192/192 (100%) | 192 | Protein of unknown function DUF4355 (IPR025580) | − |
| 16 | 15,094–16,065 | − | WP_017647854 | Minor capsid protein [S. agalactiae] | 323/323 (100%) | 323 | Phage head morphogenesis domain (IPR006528) | Head protein |
| 17 | 16,049–17,542 | − | WP_017647855 | Phage portal protein [S. agalactiae] | 497/497 (100%) | 497 | Portal protein (IPR021145) | Portal protein |
| 18 | 17,555–18,796 | − | WP_017647856 | PBSX family phage terminase large subunit [S. agalactiae] | 413/413 (100%) | 413 | Bacteriophage terminase, large subunit (IPR006437) | Terminase |
| 19 b | 18,783–19,154 | − | MCC9692304 | Terminase small subunit [S. agalactiae] | 123/123 (100%) | 150 | Terminase small subunit (IPR005335) | Terminase |
| 20 | 19,388–19,573 | + | KXA53940 | Toxin-antitoxin system, toxin component, HicA family [S. agalactiae] | 61/61 (100%) | 84 | HicA mRNA interferase family (IPR012933) | RNA-binding protein |
| 21 | 19,625–20,002 | + | WP_017647857 | Type II toxin-antitoxin system HicB family antitoxin [S. agalactiae] | 125/125 (100%) | 125 | HicB-like antitoxin of toxin-antitoxin system (IPR031807) | RNA-binding protein |
| 22 | 21,203–21,637 | − | WP_000142570 | ArpU family transcriptional regulator [Streptococcus] | 144/144 (100%) | 144 | Putative autolysin regulatory protein ArpU-like (IPR006524) | Transcriptional regulator |
| 23 | 22,023–22,163 | − | AFQ95908 | Hypothetical protein [Streptococcus phage LYGO9] | 46/46 (100%) | 46 | − | − |
| 24 | 22,163–22,330 | − | AFQ95925 | Hypothetical protein [Streptococcus phage LYGO9] | 55/55 (100%) | 55 | − | − |
| 25 | 22,327–22,545 | − | AFQ95980 | Hypothetical protein [Streptococcus phage JX01] | 72/72 (100%) | 81 | − | − |
| 26 | 22,542–23,078 | − | PPQ23766 | Hypothetical protein C4888_10100 [S. agalactiae] | 178/178 (100%) | 179 | HNH nuclease (IPR003615), NUMOD4 (IPR010902) | DNA-binding protein |
| 27 | 23,083–23,643 | − | PPQ23765 | Hypothetical protein C4888_10095 [S. agalactiae] | 185/186 (99%) | 186 | Protein of unknown function DUF1642 (IPR012865) | − |
| 28 | 23,633–23,875 | − | PPQ23764 | Hypothetical protein C4888_10090 [S. agalactiae] | 80/80 (100%) | 80 | − | − |
| 29 | 23,865–24,104 | − | PPQ23763 | Hypothetical protein C4888_10085 [S. agalactiae] | 79/79 (100%) | 79 | − | − |
| 30 | 24,171–24,332 | − | WP_196755760 | Hypothetical protein [S. parauberis] | 51/53 (96%) | 57 | − | − |
| 31 | 24,380–25,129 | − | PPQ23762 | Site-specific DNA-methyltransferase [S. agalactiae] | 249/249 (100%) | 249 | Restriction/modification DNA-methyltransferase (IPR001091) | DNA methylase |
| 32 | 25,132–25,401 | − | WP_053515031 | Hypothetical protein [S. agalactiae] | 89/89 (100%) | 89 | − | − |
| 33 | 25,398–25,562 | − | WP_000159367 | Hypothetical protein [S. agalactiae] | 54/54 (100%) | 54 | − | − |
| 34 | 25,559–25,939 | − | WP_053515030 | YopX family protein [S. agalactiae] | 126/126 (100%) | 126 | YopX protein (IPR019096) | Bacterial type III secretion system |
| 35 | 25,936–26,094 | − | WP_017646174 | Hypothetical protein [Streptococcus] | 52/52 (100%) | 52 | − | − |
| 36 | 26,078–26,374 | − | WP_000763916 | Nucleotide modification associated domain-containing protein [S. agalactiae] | 98/98 (100%) | 98 | Nucleotide modification associated domain 1 (IPR011630) | − |
| 37 b | 26,453–26,803 | − | ASA80945 | VRR-NUC domain-containing protein [S. agalactiae] | 115/116 (99%) | 116 | VRR-NUC domain (IPR014883) | Nuclease |
| 38 | 26,763–27,629 | − | WP_053515029 | Bifunctional DNA primase/polymerase [S. agalactiae] | 288/288 (100%) | 288 | DNA primase/polymerase, bifunctional, N-terminal (IPR015330), Primase, C-terminal 1 (IPR014820) | DNA polymerase/primase |
| 39 | 27,874–29,427 | − | ALB15147 | Phage resistance protein [S. agalactiae] | 517/517 (100%) | 517 | − | − |
| 40 | 29,445–29,927 | − | WP_053515027 | DUF669 domain-containing protein [S. agalactiae] | 160/160 (100%) | 160 | Protein of unknown function DUF669 (IPR007731) | − |
| 41 | 29,940–31,307 | − | ASA80941 | Helicase [S. agalactiae] | 455/455 (100%) | 468 | Helicase, C-terminal (IPR001650), Helicase/UvrB, N-terminal (IPR006935), Helicase superfamily 1/2, ATP-binding domain (IPR014001) | Helicase |
| 42 | 31,382–32,065 | − | WP_000704951 | AAA family ATPase [S. agalactiae] | 227/227 (100%) | 227 | Phage nucleotide-binding protein (IPR006505) | DNA/RNA-binding protein |
| 43 | 32,052–32,342 | − | WP_000650504 | Hypothetical protein [Streptococcus] | 96/96 (100%) | 96 | − | − |
| 44 | 32,339–32,539 | − | WP_001058282 | Hypothetical protein [Streptococcus] | 66/66 (100%) | 66 | − | − |
| 45 | 32,532–32,621 | − | AYJ74898 | Hypothetical protein [Streptococcus phage LF1] | 29/29 (100%) | 29 | − | − |
| 46 | 32,615–32,758 | − | WP_172798516 | Hypothetical protein [S. agalactiae] | 47/47 (100%) | 47 | − | − |
| 47 | 32,788–33,045 | − | WP_001191791 | Hypothetical protein [Streptococcus] | 85/85 (100%) | 85 | − | − |
| 48 | 33,174–33,467 | + | WP_001104373 | Hypothetical protein [S. agalactiae] | 97/97 (100%) | 97 | − | − |
| 49 | 33,464–33,622 | − | WP_017647876 | Hypothetical protein [S. agalactiae] | 52/52 (100%) | 52 | − | − |
| 50 | 33,655–34,380 | − | WP_053515025 | Phage anti-repressor KilAC domain-containing protein [S. agalactiae] | 241/241 (100%) | 241 | AntA/AntB anti-repressor (IPR013557), Anti-repressor protein, C-terminal (IPR005039) | − |
| 51 | 34,409–34,621 | − | WP_053515024 | Helix-turn-helix transcriptional regulator [S. agalactiae] | 70/70 (100%) | 70 | Cro/C1-type helix-turn-helix domain (IPR001387) | Cro/CI repressor |
| 52 | 34,817–35,158 | + | WP_053515023 | Helix-turn-helix transcriptional regulator [S. agalactiae] | 113/113 (100%) | 113 | Cro/C1-type helix-turn-helix domain (IPR001387) | Cro/CI repressor |
| 53 | 35,151–35,519 | + | WP_053515022 | ImmA/IrrE family metallo-endopeptidase [S. agalactiae] | 122/122 (100%) | 125 | IrrE N-terminal-like domain (IPR010359) | Metallopeptidase |
| 54 | 35,528–36,214 | + | WP_053515021 | Hypothetical protein [S. agalactiae] | 228/228 (100%) | 228 | − | − |
| 55 | 36,388–37,485 | + | MBY5048373 | Site-specific integrase [S. agalactiae] | 365/365 (100%) | 384 | AP2-like integrase, N-terminal domain (IPR028259), Integrase, catalytic domain (IPR002104), Integrase, SAM-like, N-terminal (IPR004107) | Integrase |
| 56 | 37,755–37,937 | − | WP_017647839 | Hypothetical protein [S. agalactiae] | 60/60 (100%) | 60 | − | − |
| 57 | 38,060–38,269 | − | WP_000424774 | Helix-turn-helix transcriptional regulator [S. agalactiae] | 69/69 (100%) | 69 | Cro/C1-type helix-turn-helix domain (IPR001387) | Cro/CI repressor |
| 58 | 38,419–38,565 | + | WP_001030869 | Hypothetical protein [S. agalactiae] | 48/48 (100%) | 48 | − | − |
| 59 | 38,706–38,957 | + | WP_000455662 | Hypothetical protein [S. agalactiae] | 83/83 (100%) | 83 | − | − |
| 60 | 39,101–40,435 | − | WP_017647840 | GH25 family lysozyme [S. agalactiae] | 444/444 (100%) | 444 | CHAP domain (IPR007921), Glycoside hydrolase, family 25 (IPR002053), putative SH3 domains | Endolysin |
| 61 | 40,561–40,788 | − | WP_000609111 | Phage holin [Streptococcus] | 75/75 (100%) | 75 | Bacteriophage holin (IPR006485) | Holin |
| 62 | 40,781–41,083 | − | WP_017647841 | Hypothetical protein [S. agalactiae] | 100/100 (100%) | 100 | − | − |
| 63 | 41,092–41,292 | − | AMQ13761 | Hypothetical protein CUGBS08_00165 [S. agalactiae] | 66/66 (100%) | 66 | − | − |
| 64 | 41,243–41,635 | − | WP_000889196 | DUF1366 domain-containing protein [Streptococcus] | 130/130 (100%) | 130 | Protein of unknown function DUF1366 (IPR009796) | − |
| 65 c | 41,647–42,999 | − | WP_053515051 | DUF859 family phage minor structural protein [S. agalactiae] | 450/450 (100%) | 667 | Putative viral structural protein DUF859 (IPR008577) | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Megat Mazhar Khair, M.H.; Tee, A.N.; Wahab, N.F.; Othman, S.S.; Goh, Y.M.; Masarudin, M.J.; Chong, C.M.; In, L.L.A.; Gan, H.M.; Song, A.A.-L. Comprehensive Characterization of a Streptococcus agalactiae Phage Isolated from a Tilapia Farm in Selangor, Malaysia, and Its Potential for Phage Therapy. Pharmaceuticals 2023, 16, 698. https://doi.org/10.3390/ph16050698
Megat Mazhar Khair MH, Tee AN, Wahab NF, Othman SS, Goh YM, Masarudin MJ, Chong CM, In LLA, Gan HM, Song AA-L. Comprehensive Characterization of a Streptococcus agalactiae Phage Isolated from a Tilapia Farm in Selangor, Malaysia, and Its Potential for Phage Therapy. Pharmaceuticals. 2023; 16(5):698. https://doi.org/10.3390/ph16050698
Chicago/Turabian StyleMegat Mazhar Khair, Megat Hamzah, An Nie Tee, Nurul Fazlin Wahab, Siti Sarah Othman, Yong Meng Goh, Mas Jaffri Masarudin, Chou Min Chong, Lionel Lian Aun In, Han Ming Gan, and Adelene Ai-Lian Song. 2023. "Comprehensive Characterization of a Streptococcus agalactiae Phage Isolated from a Tilapia Farm in Selangor, Malaysia, and Its Potential for Phage Therapy" Pharmaceuticals 16, no. 5: 698. https://doi.org/10.3390/ph16050698
APA StyleMegat Mazhar Khair, M. H., Tee, A. N., Wahab, N. F., Othman, S. S., Goh, Y. M., Masarudin, M. J., Chong, C. M., In, L. L. A., Gan, H. M., & Song, A. A.-L. (2023). Comprehensive Characterization of a Streptococcus agalactiae Phage Isolated from a Tilapia Farm in Selangor, Malaysia, and Its Potential for Phage Therapy. Pharmaceuticals, 16(5), 698. https://doi.org/10.3390/ph16050698





