The Effect of Oleanolic Acid and Its Four New Semisynthetic Derivatives on Human MeWo and A375 Melanoma Cell Lines
Abstract
1. Introduction
2. Results
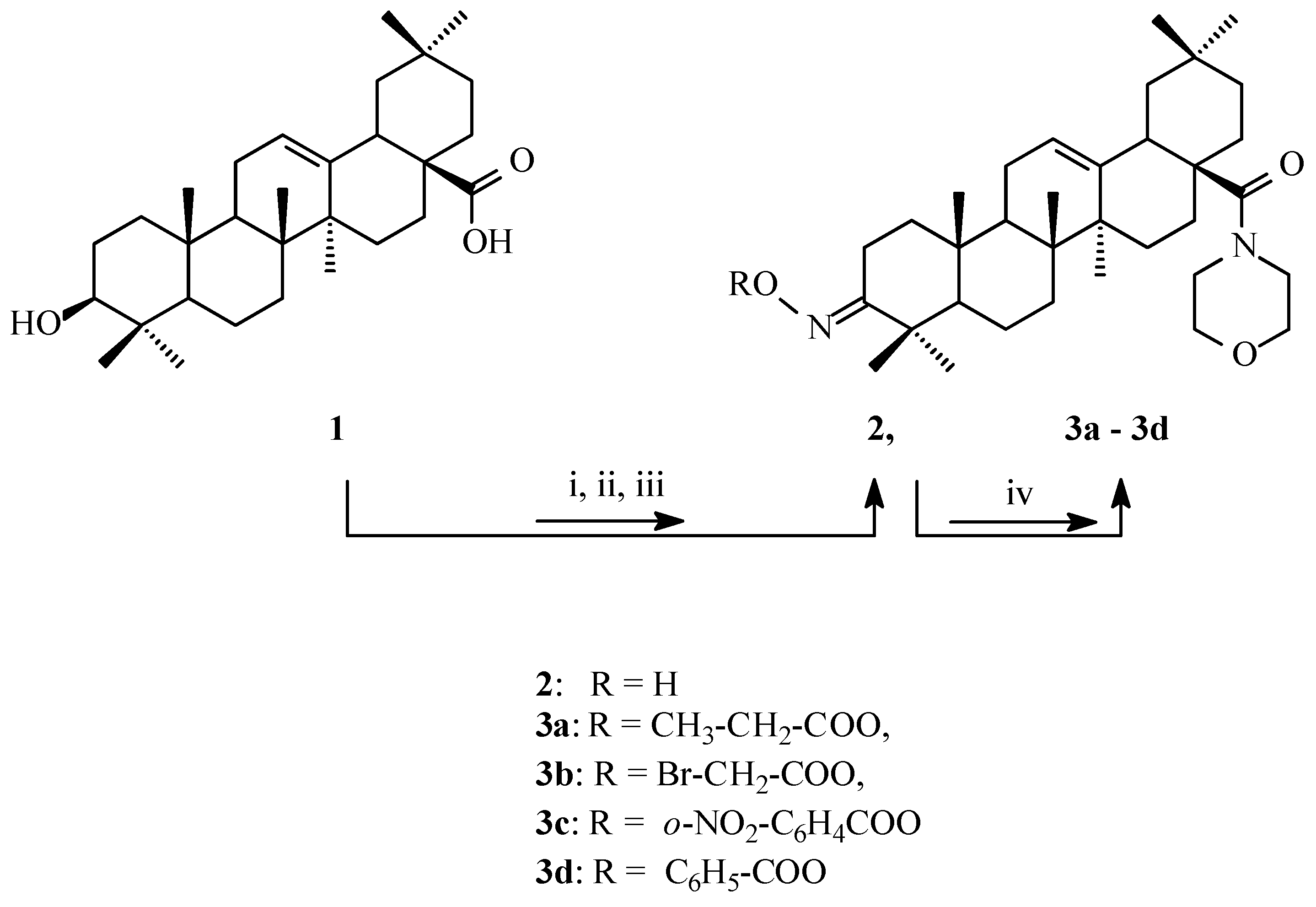
2.1. Synthesis of Cytotoxic Agents
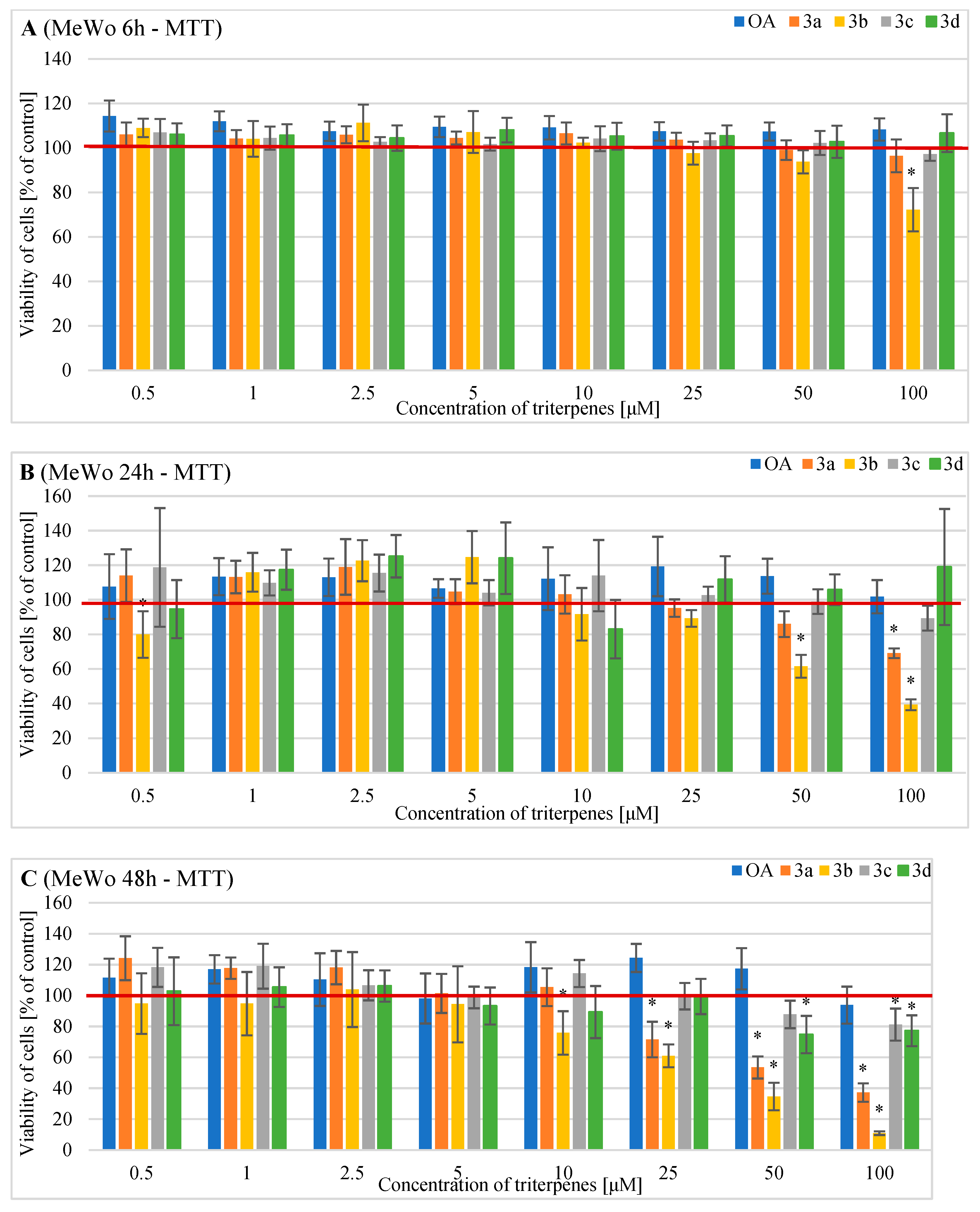
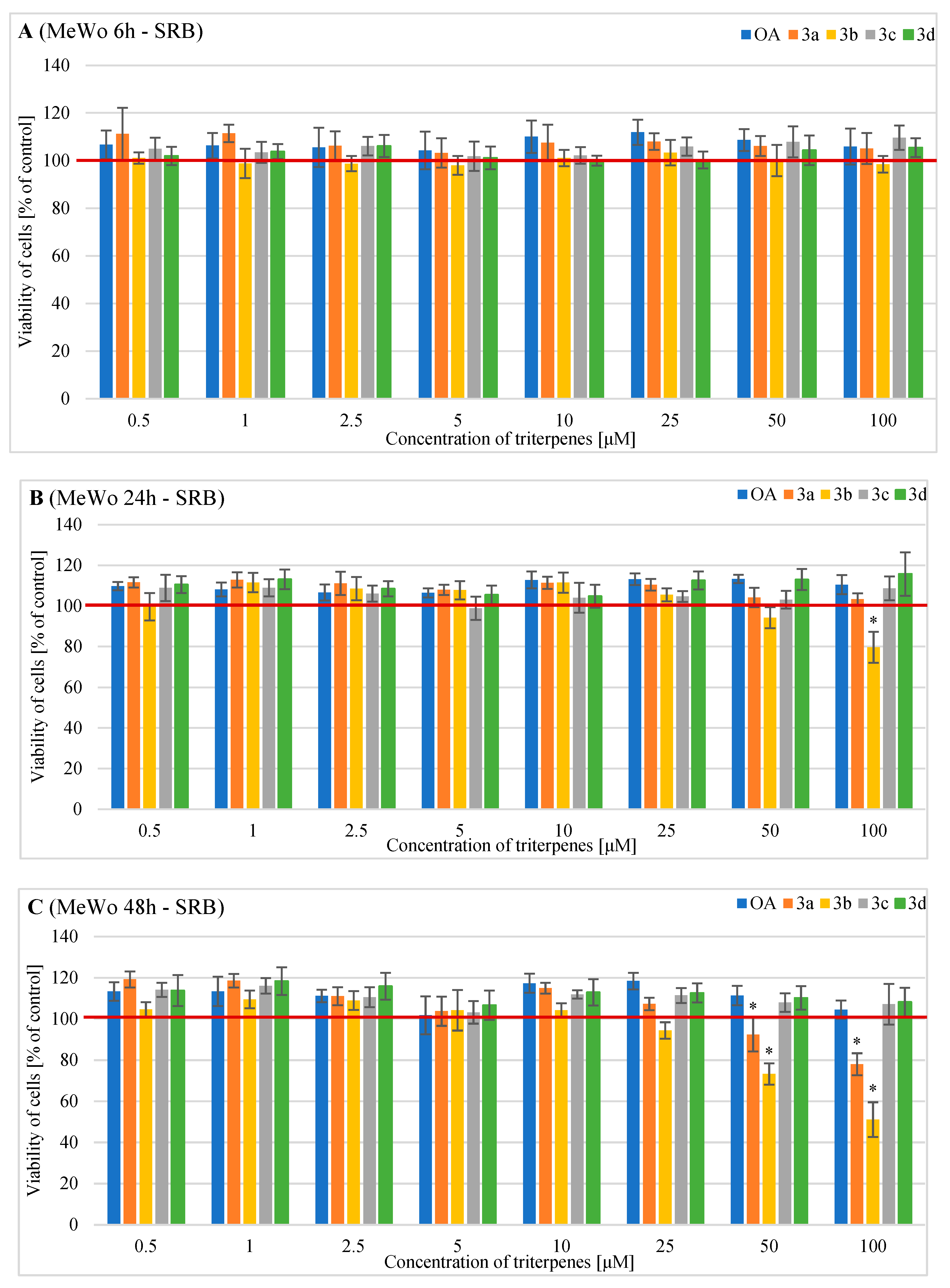
2.2. Effects of OA and Its Derivatives on the Viability of Human MeWo Cells—MTT and SRB Assays
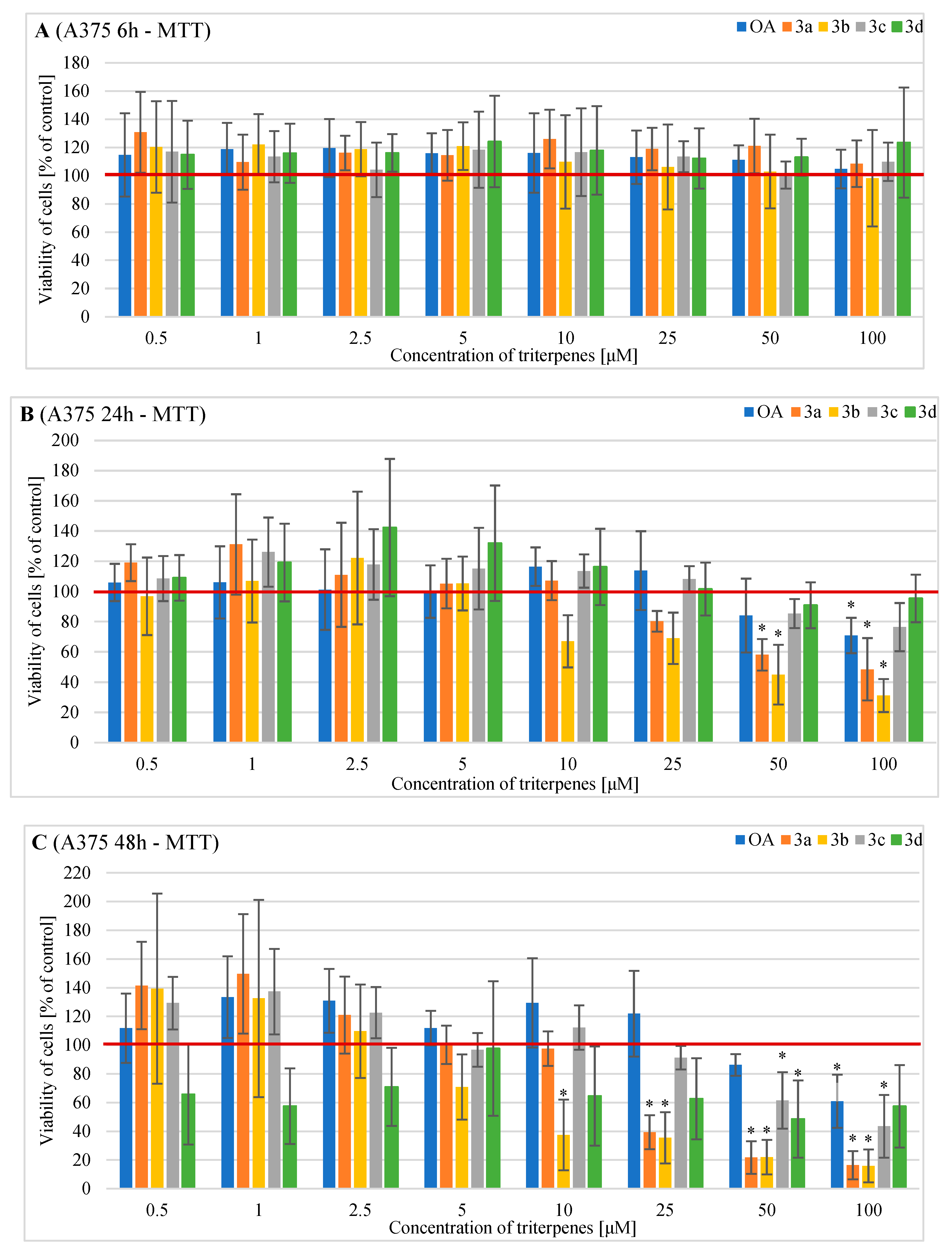
2.3. Effects of OA and Its Derivatives on Viability of Human A375 Cells—MTT and SRB Assays
3. Discussion
4. Materials and Methods
4.1. General Synthesis Procedure for Derivatives 3a, 3b, 3c and 3d
- General information
- Synthesis of oximes 2
- Acylation of oxime 2 with carboxylic acids
4.2. Cell Culture
4.3. Evaluation of Cell Metabolic Activity of Cells Treated with OA and Its Derivatives with the MTT Assay
4.4. Evaluation of the Cells Number after Exposure to OA and Its Derivatives with the Sulforhodamine B (SRB) Assay
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krutmann, J.; Schikowski, T.; Morita, A.; Berneburg, M. Environmentally-Induced (Extrinsic) Skin Aging: Exposomal Factors and Underlying Mechanisms. J. Investig. Dermatol. 2021, 141, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Intrinsic and Extrinsic Factors in Skin Ageing: A Review. Int. J. Cosmet. Sci. 2008, 30, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Van Der Leest, R.J.T.; Flohil, S.C.; Arends, L.R.; De Vries, E.; Nijsten, T. Risk of Subsequent Cutaneous Malignancy in Patients with Prior Melanoma: A Systematic Review and Meta-Analysis. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1053–1062. [Google Scholar] [CrossRef]
- Garbe, C.; Amaral, T.; Peris, K.; Hauschild, A.; Arenberger, P.; Basset-Seguin, N.; Bastholt, L.; Bataille, V.; del Marmol, V.; Dréno, B.; et al. European Consensus-Based Interdisciplinary Guideline for Melanoma. Part 1: Diagnostics: Update 2022. Eur. J. Cancer 2022, 170, 236–255. [Google Scholar] [CrossRef] [PubMed]
- Rashid, S.; Shaughnessy, M.; Tsao, H. Melanoma Classification and Management in the Era of Molecular Medicine. Dermatol. Clin. 2023, 41, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Barsouk, A. Epidemiology of Melanoma. Med. Sci. 2021, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Whiteman, D.C.; Green, A.C.; Olsen, C.M. The Growing Burden of Invasive Melanoma: Projections of Incidence Rates and Numbers of New Cases in Six Susceptible Populations through 2031. J. Investig. Dermatol. 2016, 136, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Balch, C.M.; Gershenwald, J.E.; Soong, S.J.; Thompson, J.F.; Atkins, M.B.; Byrd, D.R.; Buzaid, A.C.; Cochran, A.J.; Coit, D.G.; Ding, S.; et al. Final Version of 2009 AJCC Melanoma Staging and Classification. J. Clin. Oncol. 2009, 27, 6199. [Google Scholar] [CrossRef]
- Sundararajan, S.; Thida, A.M.; Yadlapati, S.; Koya, S. Metastatic Melanoma. StatPearls 2022, 2, 193–202. [Google Scholar]
- Kohoutova, D.; Worku, D.; Aziz, H.; Teare, J.; Weir, J.; Larkin, J. Malignant Melanoma of the Gastrointestinal Tract: Symptoms, Diagnosis, and Current Treatment Options. Cells 2021, 10, 327. [Google Scholar] [CrossRef]
- Dunn, J.; Watson, M.; Aitken, J.F.; Hyde, M.K. Systematic Review of Psychosocial Outcomes for Patients with Advanced Melanoma. Psychooncology 2017, 26, 1722–1731. [Google Scholar] [CrossRef] [PubMed]
- Garbe, C.; Amaral, T.; Peris, K.; Hauschild, A.; Arenberger, P.; Basset-Seguin, N.; Bastholt, L.; Bataille, V.; del Marmol, V.; Dréno, B.; et al. European Consensus-Based Interdisciplinary Guideline for Melanoma. Part 2: Treatment—Update 2022. Eur. J. Cancer 2022, 170, 256–284. [Google Scholar] [CrossRef]
- Winder, M.; Virós, A. Mechanisms of Drug Resistance in Melanoma. Handb. Exp. Pharmacol. 2018, 249, 91–108. [Google Scholar] [CrossRef] [PubMed]
- Schadendorf, D.; van Akkooi, A.C.J.; Berking, C.; Griewank, K.G.; Gutzmer, R.; Hauschild, A.; Stang, A.; Roesch, A.; Ugurel, S. Melanoma. Lancet 2018, 392, 971–984. [Google Scholar] [CrossRef] [PubMed]
- Sandru, A.; Voinea, S.; Panaitescu, E.; Blidaru, A. Survival Rates of Patients with Metastatic Malignant Melanoma. J. Med. Life 2014, 7, 572. [Google Scholar] [PubMed]
- Mioc, M.; Milan, A.; Malița, D.; Mioc, A.; Prodea, A.; Racoviceanu, R.; Ghiulai, R.; Cristea, A.; Căruntu, F.; Șoica, C. Recent Advances Regarding the Molecular Mechanisms of Triterpenic Acids: A Review (Part I). Int. J. Mol. Sci. 2022, 23, 7740. [Google Scholar] [CrossRef]
- Mioc, M.; Prodea, A.; Racoviceanu, R.; Mioc, A.; Ghiulai, R.; Milan, A.; Voicu, M.; Mardale, G.; Șoica, C. Recent Advances Regarding the Molecular Mechanisms of Triterpenic Acids: A Review (Part II). Int. J. Mol. Sci. 2022, 23, 8896. [Google Scholar] [CrossRef]
- Caunii, A.; Oprean, C.; Cristea, M.; Ivan, A.; Danciu, C.; Tatu, C.; Paunescu, V.; Marti, D.; Tzanakakis, G.; Spandidos, D.A.; et al. Effects of Ursolic and Oleanolic on SK-MEL-2 Melanoma Cells: In Vitro and in Vivo Assays. Int. J. Oncol. 2017, 51, 1651–1660. [Google Scholar] [CrossRef] [PubMed]
- Ayeleso, T.B.; Matumba, M.G.; Mukwevho, E. Oleanolic Acid and Its Derivatives: Biological Activities and Therapeutic Potential in Chronic Diseases. Molecules 2017, 22, 1915. [Google Scholar] [CrossRef]
- Ghante, M.H.; Jamkhande, P.G. Role of Pentacyclic Triterpenoids in Chemoprevention and Anticancer Treatment: An Overview on Targets and Underling Mechanisms. J. Pharmacopunct. 2019, 22, 55–67. [Google Scholar] [CrossRef]
- Ren, Y.; Douglas Kinghorn, A. Natural Product Triterpenoids and Their Semi-Synthetic Derivatives with Potential Anticancer Activity. Planta Med. 2019, 176, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Oprean, C.; Ivan, A.; Bojin, F.; Cristea, M.; Soica, C.; Drăghia, L.; Caunii, A.; Paunescu, V.; Tatu, C. Selective in Vitro Anti-Melanoma Activity of Ursolic and Oleanolic Acids. Toxicol. Mech. Methods 2018, 28, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Mioc, M.; Mioc, A.; Prodea, A.; Milan, A.; Balan-Porcarasu, M.; Racoviceanu, R.; Ghiulai, R.; Iovanescu, G.; Macasoi, I.; Draghici, G.; et al. Novel Triterpenic Acid—Benzotriazole Esters Act as Pro-Apoptotic Antimelanoma Agents. Int. J. Mol. Sci. 2022, 23, 9992. [Google Scholar] [CrossRef] [PubMed]
- Isaković-Vidović, S.; Dariš, B.; Knez, Ž.; Vidović, K.; Oprić, D.; Ferk, P. Antiproliferative Activity of Selected Triterpene Acids from Rosemary on Metastatic Melanoma Cell Line Wm-266-4. Open Access Maced. J. Med. Sci. 2021, 9, 515–521. [Google Scholar] [CrossRef]
- Lisiak, N.M.; Lewicka, I.; Kaczmarek, M.; Kujawski, J.; Bednarczyk-Cwynar, B.; Zaprutko, L.; Rubis, B. Oleanolic Acid’s Semisynthetic Derivatives Himoxol and Br-himolid Show Proautophagic Potential and Inhibit Migration of Her2-positive Breast Cancer Cells in Vitro. Int. J. Mol. Sci. 2021, 22, 11273. [Google Scholar] [CrossRef] [PubMed]
- Jannus, F.; Medina-O’donnell, M.; Rivas, F.; Díaz-Ruiz, L.; Rufino-Palomares, E.E.; Lupiáñez, J.A.; Parra, A.; Reyes-Zurita, F.J. A Diamine-PEGylated Oleanolic Acid Derivative Induced Efficient Apoptosis through a Death Receptor and Mitochondrial Apoptotic Pathway in HepG2 Human Hepatoma Cells. Biomolecules 2020, 10, 1375. [Google Scholar] [CrossRef]
- Reyes-Zurita, F.J.; Medina-O’Donnell, M.; Ferrer-Martin, R.M.; Rufino-Palomares, E.E.; Martin-Fonseca, S.; Rivas, F.; Martínez, A.; García-Granados, A.; Pérez-Jiménez, A.; García-Salguero, L.; et al. The Oleanolic Acid Derivative, 3-: O -Succinyl-28- O -Benzyl Oleanolate, Induces Apoptosis in B16-F10 Melanoma Cells via the Mitochondrial Apoptotic Pathway. RSC Adv. 2016, 6, 93590–93601. [Google Scholar] [CrossRef]
- Lisiak, N.; Paszel-Jaworska, A.; Bednarczyk-Cwynar, B.; Zaprutko, L.; Kaczmarek, M.; Rybczyńska, M. Methyl 3-Hydroxyimino-11-Oxoolean-12-En-28-Oate (HIMOXOL), a Synthetic Oleanolic Acid Derivative, Induces Both Apoptosis and Autophagy in MDA-MB-231 Breast Cancer Cells. Chem. Biol. Interact. 2014, 208, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Kalani, K.; Saxena, M.; Srivastava, S.K.; Agrawal, S.K.; Suri, N.; Saxena, A.K. Cytotoxic Evaluation of Semisynthetic Ester and Amide Derivatives of Oleanolic Acid. Nat. Prod. Commun. 2010, 5, 1567–1570. [Google Scholar] [CrossRef]
- Baer-Dubowska, W.; Narożna, M.; Krajka-Kuźniak, V. Anti-Cancer Potential of Synthetic Oleanolic Acid Derivatives and Their Conjugates with NSAIDs. Molecules 2021, 26, 4957. [Google Scholar] [CrossRef]
- Bednarczyk-Cwynar, B.; Ruszkowski, P.; Atamanyuk, D.; Lesyk, R.; Zaprutko, L. Hybrids of Oleanolic Acid with Norbornene-2,3-Dicarboximide-n- Carboxylic Acids as Potential Anticancer Agents. Acta. Pol. Pharm. Drug. Res. 2017, 74, 827–835. [Google Scholar]
- Duan, L.; Yang, Z.; Jiang, X.; Zhang, J.; Guo, X. Oleanolic Acid Inhibits Cell Proliferation Migration and Invasion and Induces SW579 Thyroid Cancer Cell Line Apoptosis by Targeting Forkhead Transcription Factor A. Anti-Cancer Drugs 2019, 30, 812–820. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Liu, X.; Huang, M.; Wei, Z.; Zhang, M.; He, M.; Zheng, Z.; Dong, H.; Liu, D. Oleanolic Acid Inhibits the Migration and Invasion of Hepatocellular Carcinoma Cells by Promoting MicroRNA-122 Expression. Pharmazie 2021, 76, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Han, B.; Luo, K.; Ren, Z.; Cai, L.; Sun, L. NOX2-ROS-HIF-1α Signaling Is Critical for the Inhibitory Effect of Oleanolic Acid on Rectal Cancer Cell Proliferation. Biomed. Pharmacother. 2017, 85, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Cao, Y.; Li, P.; Tang, X.; Yang, M.; Gu, S.; Xiong, K.; Li, T.; Xiao, T. Oleanolic Acid Induces Autophagy and Apoptosis via the AMPK-MTOR Signaling Pathway in Colon Cancer. J. Oncol. 2021, 2021, 8281718. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Louwman, M.; Wakkee, M.; van der Veldt, A.; Mooyaart, A.; Nijsten, T.; Hollestein, L.; Grünhagen, D.; Verhoef, C. Primary Melanoma Characteristics of Metastatic Disease: A Nationwide Cancer Registry Study. Cancers 2021, 13, 4431. [Google Scholar] [CrossRef] [PubMed]
- Oprean, C.; Mioc, M.; Csányi, E.; Ambrus, R.; Bojin, F.; Tatu, C.; Cristea, M.; Ivan, A.; Danciu, C.; Dehelean, C.; et al. Improvement of Ursolic and Oleanolic Acids’ Antitumor Activity by Complexation with Hydrophilic Cyclodextrins. Biomed. Pharmacother. 2016, 83, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Bednarczyk-Cwynar, B.; Ruszkowski, P.; Bobkiewicz-Kozlowska, T.; Zaprutko, L. Oleanolic Acid A-Lactams Inhibit the Growth of HeLa, KB, MCF-7 and Hep-G2 Cancer Cell Lines at Micromolar Concentrations. Anti-Cancer Agents Med. Chem. 2016, 16, 579–592. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bednarczyk-Cwynar, B.; Leśków, A.; Szczuka, I.; Zaprutko, L.; Diakowska, D. The Effect of Oleanolic Acid and Its Four New Semisynthetic Derivatives on Human MeWo and A375 Melanoma Cell Lines. Pharmaceuticals 2023, 16, 746. https://doi.org/10.3390/ph16050746
Bednarczyk-Cwynar B, Leśków A, Szczuka I, Zaprutko L, Diakowska D. The Effect of Oleanolic Acid and Its Four New Semisynthetic Derivatives on Human MeWo and A375 Melanoma Cell Lines. Pharmaceuticals. 2023; 16(5):746. https://doi.org/10.3390/ph16050746
Chicago/Turabian StyleBednarczyk-Cwynar, Barbara, Anna Leśków, Izabela Szczuka, Lucjusz Zaprutko, and Dorota Diakowska. 2023. "The Effect of Oleanolic Acid and Its Four New Semisynthetic Derivatives on Human MeWo and A375 Melanoma Cell Lines" Pharmaceuticals 16, no. 5: 746. https://doi.org/10.3390/ph16050746
APA StyleBednarczyk-Cwynar, B., Leśków, A., Szczuka, I., Zaprutko, L., & Diakowska, D. (2023). The Effect of Oleanolic Acid and Its Four New Semisynthetic Derivatives on Human MeWo and A375 Melanoma Cell Lines. Pharmaceuticals, 16(5), 746. https://doi.org/10.3390/ph16050746







