A Three-Step Process to Isolate Large Quantities of Bioactive Sesquiterpene Lactones from Cichorium intybus L. Roots and Semisynthesis of Chicory STLs Standards
Abstract
1. Introduction
2. Results
2.1. Identification of the Main STLs
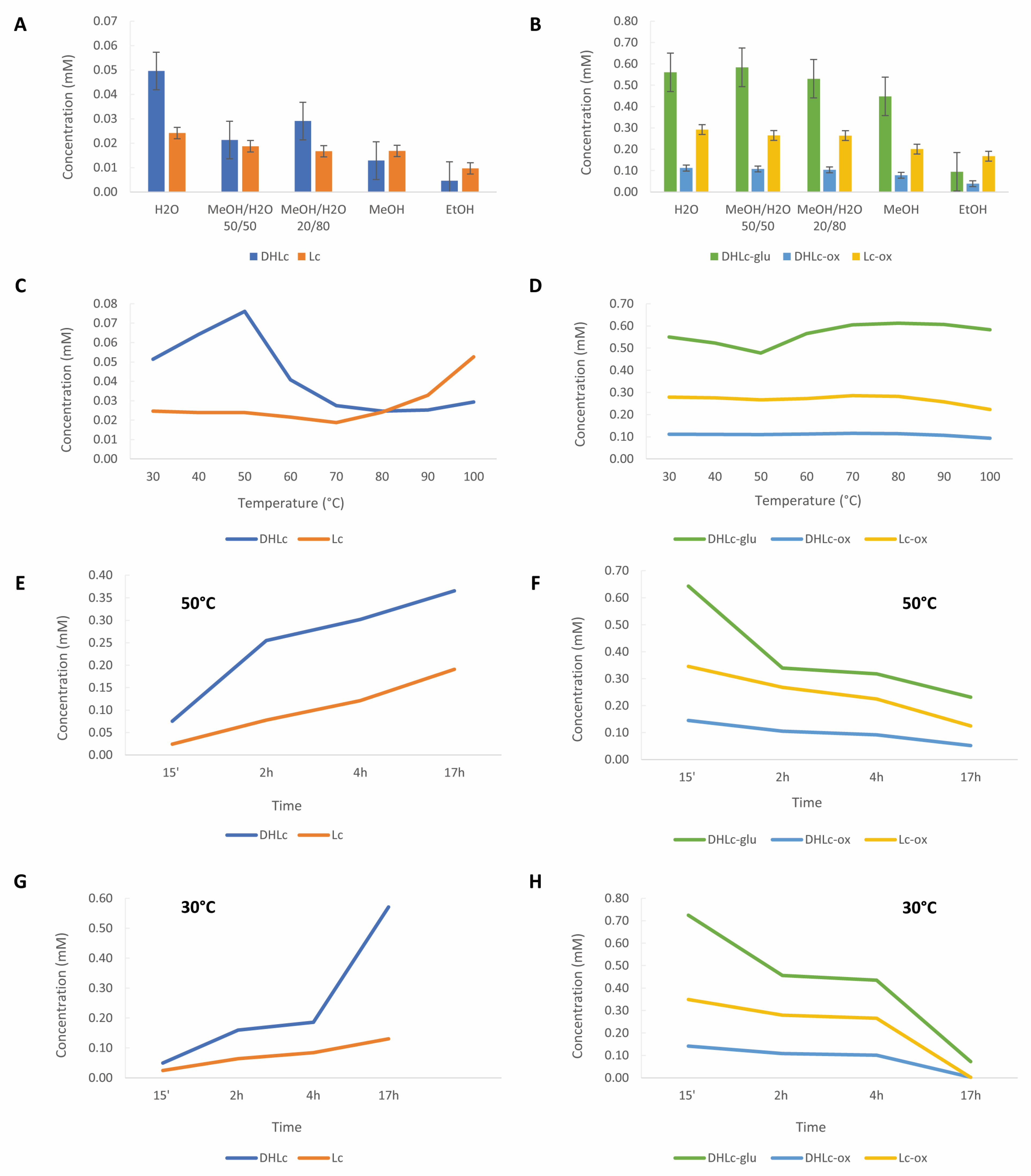
2.2. Method Development
Extraction Conditions Determination
2.3. Scaling-Up
2.4. Analytical Validation of STLs
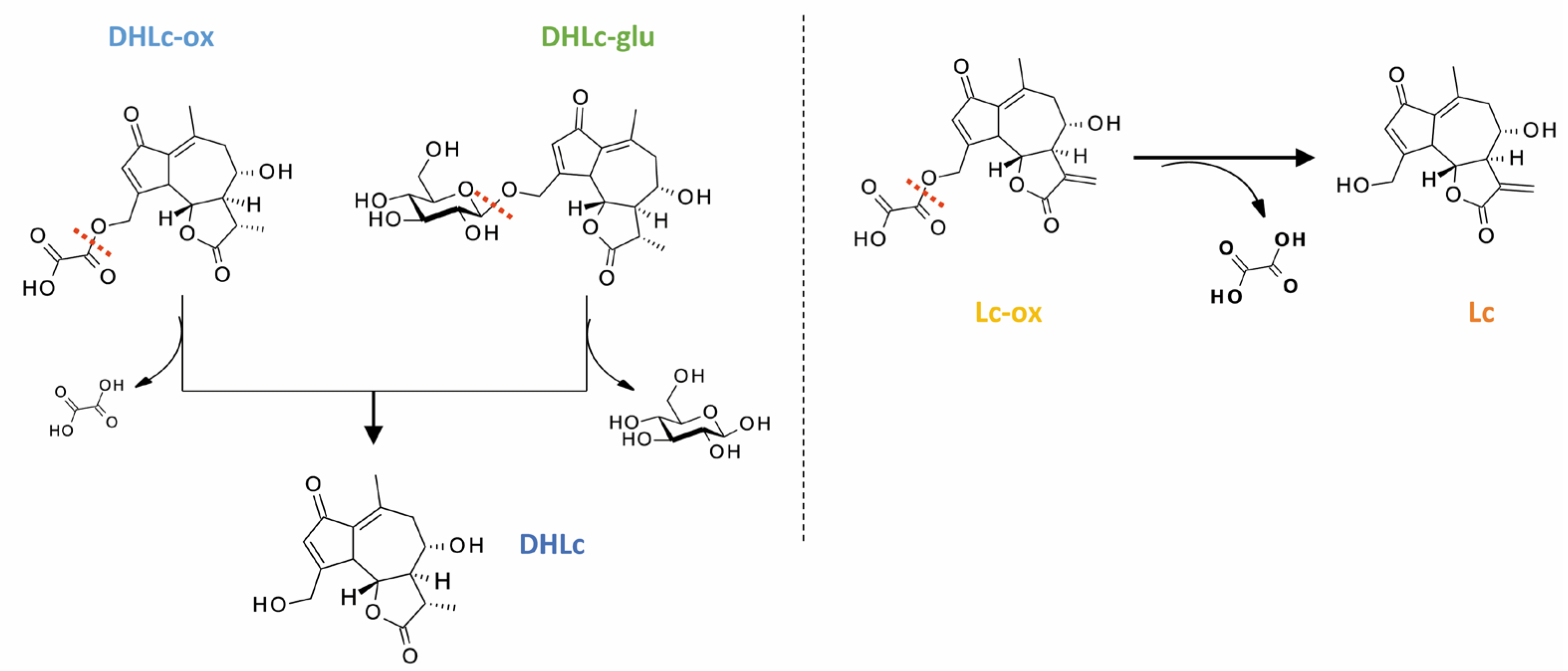
2.5. Isolation of 11β,13-Dihydrolactucin-glucoside
2.6. Synthesis of STLs Standards
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Reagents and Chemicals
4.3. Method Development
4.3.1. Extraction of STLs from Freeze-Dried Chicory Roots
4.3.2. Scaling-Up
Selective Fractionation of Free STLs and Removal of Sugars by Liquid–Liquid Extraction
4.3.3. The Standardized Big-Scale Process
4.4. 11β,13-Dihydrolactucin-glucoside Isolation
4.5. STLs Analysis
4.5.1. Quantification of STLs by UPLC-DAD
4.5.2. LC-QTOF-HRMS Analysis of STLs
4.5.3. NMR Analysis
4.5.4. X-ray Structural Determination
4.6. Chemistry
4.6.1. Chemical Synthesis
General Procedure for the Synthesis of DHLc-Me-oxalate and Lc-Me-oxalate
General Procedure for the Synthesis of DHLc-oxalate and Lc-oxalate
4.7. Statistical Assays
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Padilla-Gonzalez, G.F.; dos Santos, F.A.; Da Costa, F.B. Sesquiterpene Lactones: More Than Protective Plant Compounds with High Toxicity. Crit. Rev. Plant Sci. 2016, 35, 18–37. [Google Scholar] [CrossRef]
- Van Beek, T.A.; Maas, P.; King, B.M.; Leclercq, E.; Voragen, A.G.J.; De Groot, A. Bitter Sesquiterpene Lactones from Chicory Roots. J. Agric. Food Chem. 1990, 38, 1035–1038. [Google Scholar] [CrossRef]
- Graziani, G.; Ferracane, R.; Sambo, P.; Santagata, S.; Nicoletto, C.; Fogliano, V. Profiling Chicory Sesquiterpene Lactones by High Resolution Mass Spectrometry. Food Res. Int. 2015, 67, 193–198. [Google Scholar] [CrossRef]
- Ivanescu, B.; Miron, A.; Corciova, A. Sesquiterpene Lactones from Artemisia Genus: Biological Activities and Methods of Analysis. J. Anal. Methods Chem. 2015, 2015, 247685. [Google Scholar] [CrossRef]
- Schmidt, T.J. Structure-Activity Relationships of Sesquiterpene Lactones. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2006; Volume 33, pp. 309–392. ISBN 978-0-444-52717-2. [Google Scholar]
- Häkkinen, S.T.; Nohynek, L.; Ivanov, M.; Tsitko, I.; Matos, M.; Baixinho, J.P.; Ivasiv, V.; Fernández, N. Chicory Extracts and Sesquiterpene Lactones Show Potent Activity against Bacterial and Fungal Pathogens. Pharmaceuticals 2021, 14, 941. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, M.; Cai, G.H.; Chen, Y.; Shi, X.C.; Zhang, C.C.; Xia, B.; Xie, B.C.; Liu, H.; Zhang, R.X.; et al. A Potential Nutraceutical Candidate Lactucin Inhibits Adipogenesis through Downregulation of JAK2/STAT3 Signaling Pathway-Mediated Mitotic Clonal Expansion. Cells 2020, 9, 331. [Google Scholar] [CrossRef] [PubMed]
- Imam, K.M.S.U.; Tian, Y.; Xin, F.; Xie, Y.; Wen, B. Lactucin, a Bitter Sesquiterpene from Cichorium Intybus, Inhibits Cancer Cell Proliferation by Downregulating the MAPK and Central Carbon Metabolism Pathway. Molecules 2022, 27, 7358. [Google Scholar] [CrossRef]
- Bischoff, T.A.; Kelley, C.J.; Karchesy, Y.; Laurantos, M.; Nguyen-Dinh, P.; Arefi, A.G. Antimalarial Activity of Lactucin and Lactucopicrin: Sesquiterpene Lactones Isolated from Cichorium Intybus L. J. Ethnopharmacol. 2004, 95, 455–457. [Google Scholar] [CrossRef]
- Wesołowska, A.; Nikiforuk, A.; Michalska, K.; Kisiel, W.; Chojnacka-Wójcik, E. Analgesic and Sedative Activities of Lactucin and Some Lactucin-like Guaianolides in Mice. J. Ethnopharmacol. 2006, 107, 254–258. [Google Scholar] [CrossRef]
- Rojas-Silva, P. Leishmanicidal, Anti-Inflammatory and Anti-Obesity Properties of Natural Products from Common Medicinal and Edible Plants. Ph.D. Thesis, Rutgers University, New Brunswick, NJ, USA, 2014. [Google Scholar] [CrossRef]
- Jaśkiewicz, A.; Budryn, G.; Carmena-Bargueño, M.; Pérez-Sánchez, H. Evaluation of Activity of Sesquiterpene Lactones and Chicory Extracts as Acetylcholinesterase Inhibitors Assayed in Calorimetric and Docking Simulation Studies. Nutrients 2022, 14, 3633. [Google Scholar] [CrossRef]
- Sessa, R.A.; Bennett, M.H.; Lewis, M.J.; Mansfield, J.W.; Beale, M.H. Metabolite Profiling of Sesquiterpene Lactones from Lactuca Species. J. Biol. Chem. 2000, 275, 26877–26884. [Google Scholar] [CrossRef] [PubMed]
- Willeman, H.; Hance, P.; Fertin, A.; Voedts, N.; Duhal, N.; Goossens, J.-F.; Hilbert, J.-L. A Method for the Simultaneous Determination of Chlorogenic Acid and Sesquiterpene lactone Content in Industrial Chicory Root Foodstuffs. Sci. World J. 2014, 2014, 583180. [Google Scholar] [CrossRef] [PubMed]
- Chadni, M.; Isidore, E.; Diemer, E.; Ouguir, O.; Brunois, F.; Catteau, R.; Cassan, L.; Ioannou, I. Optimization of Extraction Conditions to Improve Chlorogenic Acid Content and Antioxidant Activity of Extracts from Forced Witloof Chicory Roots. Foods 2022, 11, 1217. [Google Scholar] [CrossRef] [PubMed]
- Milala, J.; Grzelak, K.; Król, B.; Juśkiewicz, J.; Zduńczyk, Z. Composition and Properties of Chicory Extracts Rich in Fructans and Polyphenols. Pol. J. Food Nutr. Sci. 2009, 59, 35–43. [Google Scholar]
- Sinkovič, L.; Hribar, J.; Žnidarčič, D.; Treutter, D.; Vidrih, R. Comparison of the Phenolics Profiles of Forced and Unforced Chicory (Cichorium Intybus L.) Cultivars. Agr. Conspec. Sci. 2014, 79, 133–137. [Google Scholar]
- Deng, Y.; Scott, L.; Swanson, D.; Snyder, J.K.; Sari, N.; Dogan, H. Guaianolide Sesquiterpene Lactones from Cichorium Intybus (Asteraceae) [1]. Z. Für Nat. B 2001, 56, 787–796. [Google Scholar] [CrossRef]
- Michalska, K.; Szneler, E.; Kisiel, W. Complete NMR Spectral Assignments of Two Lactucin-Type Sesquiterpene Lactone Glycosides from Picris Conyzoides: Sesquiterpene lactone Glycosides from Picris conyzoides. Magn. Reson. Chem. 2011, 49, 753–756. [Google Scholar] [CrossRef]
- Dang, T.; Zheng, G.; Zhang, Q.; Jin, P.; Zhang, H.; Su, L.; Qin, D.; Yao, G. Sesquiterpenoids with Diverse Carbon Skeletons from the Roots of Cichorium glandulosum and Their Anti-Inflammatory Activities. Fitoterapia 2019, 136, 104170. [Google Scholar] [CrossRef]
- Ruban, G.; Zabel, V.; Gensch, K.H.; Smalla, H. The Crystal Structure and Absolute Configuration of Lactucin. Acta Crystallogr. B Struct. Sci. 1978, 34, 1163–1167. [Google Scholar] [CrossRef]
- Escobar-Ledesma, F.R.; Sánchez-Moreno, V.E.; Vera, E.; Ciobotă, V.; Jentzsch, P.V.; Jaramillo, L.I. Extraction of Inulin from Andean Plants: An Approach to Non-Traditional Crops of Ecuador. Molecules 2020, 25, 5067. [Google Scholar] [CrossRef]
- 11beta,13-Dihydrolactucin 3810, CAS 83117-63-9-SESQUITERPENE. Available online: https://www.extrasynthese.com/sesquiterpene/290-11beta13-dihydrolactucin.html (accessed on 5 March 2023).
- Leclercq, E.; Netjes, J.J. Release of Sesquiterpene Lactones by Enzymatic Liquefaction of Chicory Roots. Z Leb. Unters. 1985, 181, 475–477. [Google Scholar] [CrossRef]
- Baixinho, J.P.; Anastácio, J.D.; Ivasiv, V.; Cankar, K.; Bosch, D.; Menezes, R.; de Roode, M.; dos Santos, C.N.; Matias, A.A.; Fernández, N. Supercritical CO2 Extraction as a Tool to Isolate Anti-Inflammatory Sesquiterpene Lactones from Cichorium intybus L. Roots. Molecules 2021, 26, 2583. [Google Scholar] [CrossRef] [PubMed]
- Ajiaikebaier, A.; Wei, Z.; Sun, G. Preparation Method of Lactucin. Chinese Patent CN111689935A, 22 September 2020. [Google Scholar]
- Katiyar, K.C.; Duggar, R.; Yadav, S.S.; Sharma, N.; Ray, A.; Shirumalla, R.; Bajpai, M.; Shukla, G.; Subhadra, B.; Kontham, L.; et al. Preparation Method and Application of Lactucin Extracted from Cichorium Intybus. Chinese Patent CN112939912A, 11 June 2021. [Google Scholar]
- Xin, X.; Ajiaikebaier, A.; Li, Y. Lactucin and Its Preparation Method and Application. Chinese Patent CN101099566A, 9 January 2008. [Google Scholar]
- Qin, D.; Zou, N.; Cai, G.; Wang, S.; Su, L.; Dang, T. Lactucin and Application Thereof as Anti-Inflammatory Component. Chinese Patent CN112079804A, 15 December 2020. [Google Scholar]
- De Oliveira, D.M.P.; Forde, B.M.; Kidd, T.J.; Harris, P.N.A.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walker, M.J. Antimicrobial Resistance in ESKAPE Pathogens. Clin. Microbiol. Rev. 2020, 33, e00181-19. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]




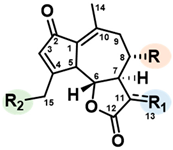 | |||
|---|---|---|---|
| R | R1 | R2 | |
| Lactucin | OH | CH2 | OH |
| Lactucin-15-oxalate | OH | CH2 | OCOCOOH |
| Lactucin-15-glucoside | OH | CH2 | OGlucose |
| 11β,13-dihydrolactucin | OH | CH3 | OH |
| 11β,13-dihydrolactucin-15-oxalate | OH | CH3 | OCOCOOH |
| 11β,13-dihydrolactucin-15-glucoside | OH | CH3 | OGlucose |
| 8-deoxylactucin | H | CH2 | OH |
| 8-deoxylactucin-15-oxalate | H | CH2 | OCOCOOH |
| 8-deoxylactucin-15-glucoside | H | CH2 | OGlucose |
| 11β,13-dihydro-8-deoxylactucin | H | CH3 | OH |
| 11β,13-dihydro-8-deoxylactucin-oxalate | H | CH3 | OCOCOOH |
| 11β,13-dihydro-8-deoxylactucin-glucoside | H | CH3 | OGlucose |
| Lactucopicrin | 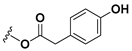 | CH2 | OH |
| Lactucopicrin-15-oxalate | CH2 | OCOCOOH | |
| Lactucopicrin-15-glucoside | CH2 | OGlucose | |
| 11β,13-dihydrolactucopicrin | 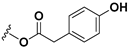 | CH3 | OH |
| 11β,13-dihydrolactucopicrin-15-oxalate | CH3 | OCOCOOH | |
| 11β,13-dihydrolactucopicrin-15-glucoside | CH3 | OGlucose | |
| Compound | Molecular Weight | Retention Time (min) | ESI Major Fragment Ions (m/z) |
|---|---|---|---|
| DHLc-glu | 440 | 16.9 | 463 (100) [M+Na]+; 441 (13) [M+H]+; 279 (18) [M-glucosyl+H]+; 261 (9) [M-glucosyl-H2O+H]+; 243 (10) [M-glucosyl-2H2O+H]+; 215 (8) [M-glucosyl-H2O-HCO2H+H]+ |
| DHLc-ox | 350 | 17.7 | 373 (57) [M+Na]+; 351 (100) [M+H]+; 261 (94) [M-oxaloyl-H2O+H]+; 243 (56) [M-oxaloyl-2H2O+H]+; 215 (39) [M-oxaloyl-H2O-HCO2H+H]+ |
| DHLc | 278 | 18.8 | 301 (100) [M+Na]+; 279 (59) [M+H]+; 261 (7) [M-H2O+H]+; 243 (8) [M-2H2O+H]+; 215 (17) [M-HCO2H-H2O+H]+ |
| Lc-ox | 348 | 19.7 | 371 (73) [M+Na]+; 349 (86) [M+H]+; 259 (100) [M-oxaloyl-H2O+H]+; 241 (96) [M-oxaloyl-2H2O+H]+; 213 (60) [M-oxaloyl-H2O-HCO2H+H]+ |
| Lc | 276 | 20.7 | 299 (100) [M+Na]+; 277 (44) [M+H]+; 259 (8) [M-H2O+H]+; 241 (17) [M-2H2O+H]+; 213 (18) [M-HCO2H-H2O+H]+ |
| STLs Concentration (mM) ± SD | ||||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | DHLc-glu | DHLc-ox | DHLc | Lc-ox | Lc | TOTAL Conjugated STLs | TOTAL Free STLs | |
| Solvent (15’, 30 °C) | H2O | 0.561 ± 0.012 | 0.112 ± 0.004 | 0.050 ± 0.003 | 0.292 ± 0.008 | 0.024 ± 0.002 | 0.965 ± 0.023 c | 0.0737 ± 0.005 d |
| MeOH/H2O 20/80 | 0.530 ± 0.099 | 0.104 ± 0.019 | 0.029 ± 0.004 | 0.264 ± 0.048 | 0.017 ± 0.004 | 0.898 ± 0.167 bc | 0.0457 ± 0.008 c | |
| MeOH/H2O 50/50 | 0.584 ± 0.010 | 0.108 ± 0.003 | 0.021 ± 0.001 | 0.265 ± 0.005 | 0.019 ± 0.002 | 0.957 ± 0.018 c | 0.0400 ± 0.003 bc | |
| MeOH | 0.448 ± 0.029 | 0.078 ± 0.005 | 0.013 ± 0.000 | 0.201 ± 0.016 | 0.017 ± 0.003 | 0.727 ± 0.049 b | 0.0297 ± 0.003 b | |
| EtOH | 0.095 ± 0.002 | 0.039 ± 0.006 | 0.005 ± 0.000 | 0.167 ± 0.005 | 0.009 ± 0.000 | 0.301 ± 0.008 a | 0.0143 ± 0.000 a | |
| Temperature (15’, H2O) | 30 °C | 0.550 ± 0.006 | 0.112 ± 0.002 | 0.051 ± 0.001 | 0.279 ± 0.006 | 0.025 ± 0.001 | 0.940 ± 0.011 ab | 0.076 ± 0.001 a |
| 40 °C | 0.523 ± 0.011 | 0.111 ± 0.004 | 0.064 ± 0.001 | 0.276 ± 0.008 | 0.024 ± 0.000 | 0.909 ± 0.023 bc | 0.088 ± 0.001 b | |
| 50 °C | 0.478 ± 0.010 | 0.110 ± 0.001 | 0.076 ± 0.001 | 0.267 ± 0.003 | 0.024 ± 0.000 | 0.855 ± 0.015 d | 0.100 ± 0.001 c | |
| 60 °C | 0.565 ± 0.013 | 0.112 ± 0.002 | 0.041 ± 0.000 | 0.272 ± 0.005 | 0.022 ± 0.001 | 0.950 ± 0.014 ac | 0.062 ± 0.002 d | |
| 70 °C | 0.605 ± 0.011 | 0.116 ± 0.004 | 0.028 ± 0.000 | 0.286 ± 0.004 | 0.019 ± 0.000 | 1.007 ± 0.022 e | 0.046 ± 0.001 e | |
| 80 °C | 0.612 ± 0.005 | 0.114 ± 0.002 | 0.025 ± 0.000 | 0.282 ± 0.004 | 0.024 ± 0.000 | 1.009 ± 0.006 e | 0.049 ± 0.001 e | |
| 90 °C | 0.607 ± 0.013 | 0.107 ± 0.003 | 0.025 ± 0.000 | 0.258 ± 0.002 | 0.033 ± 0.001 | 0.971 ± 0.017 ae | 0.058 ± 0.001 f | |
| 100 °C | 0.583 ± 0.008 | 0.093 ± 0.002 | 0.029 ± 0.001 | 0.223 ± 0.002 | 0.053 ± 0.053 | 0.900 ± 0.008 bd | 0.082 ± 0.001 g | |
| Time (H2O, 30 °C) | 15’ | 0.552 ± 0.005 | 0.113 ± 0.026 | 0.049 ± 0.006 | 0.291 ± 0.066 | 0.024 ± 0.003 | 0.955 ± 0.015 * | 0.074 ± 0.026 ns |
| 2 h | 0.456 ± 0.039 | 0.108 ± 0.008 | 0.160 ± 0.016 | 0.280 ± 0.022 | 0.064 ± 0.007 | 0.844 ± 0.069 ** | 0.224 ± 0.023 *** | |
| 4 h | 0.435 ± 0.048 | 0.101 ± 0.010 | 0.185 ± 0.011 | 0.265 ± 0.022 | 0.084 ± 0.008 | 0.801 ± 0.080 *** | 0.270 ± 0.019 **** | |
| 17 h | 0.072 ± 0.013 | 0.003 ± 0.000 | 0.571 ± 0.066 | 0.002 ± 0.000 | 0.131 ± 0.006 | 0.077 ± 0.014 **** | 0.701 ± 0.070 **** | |
| Time (H2O, 50 °C) | 15’ | 0.485 ± 0.014 | 0.110 ± 0.002 | 0.075 ± 0.001 | 0.269 ± 0.006 | 0.024 ± 0.001 | 0.864 ± 0.019 | 0.100 ± 0.001 |
| 2 h | 0.340 ± 0.017 | 0.105 ± 0.004 | 0.255 ± 0.008 | 0.268 ± 0.014 | 0.078 ± 0.004 | 0.712 ± 0.035 | 0.333 ± 0.012 | |
| 4 h | 0.318 ± 0.016 | 0.091 ± 0.001 | 0.302 ± 0.003 | 0.225 ± 0.003 | 0.121 ± 0.003 | 0.634 ± 0.020 | 0.423 ± 0.003 | |
| 17 h | 0.231 ± 0.011 | 0.052 ± 0.003 | 0.366 ± 0.015 | 0.125 ± 0.008 | 0.191 ± 0.011 | 0.408 ± 0.021 | 0.556 ± 0.025 | |
| STLs Concentration (mM) ± SD | ||
|---|---|---|
| DHLc | Lc | |
| Initial H2O | 0.571 ± 0.066 | 0.134 ± 0.005 |
| EtOAc 1 | 0.345 ± 0.010 | 0.081 ± 0.002 |
| EtOAc 2 | 0.153 ± 0.004 | 0.033 ± 0.003 |
| EtOAc 3 | 0.066 ± 0.000 | 0.013 ± 0.011 |
| FinalH2O | 0.004 ± 0.016 | 0.005 ± 0.003 |
| DHLc: (CD3)2SO | Lc: (CD3)2SO | |||
|---|---|---|---|---|
| Position | 1H (mult, J Hz) | 13C | 1H (mult, J Hz) | 13C |
| 1 | - | 132.0 | - | 132.3 |
| 2 | - | 194.9 | - | 194.8 |
| 3 | 6.27 (s) | 132.5 | 6.28 (d, 1.0) | 132.7 |
| 4 | - | 175.8 | - | 169.2 |
| 5 | 3.76–3.64 (m) | 48.4 | 3.83–3.67 (m) | 48.3 |
| 6 | 3.76–3.64 (m) | 80.9 | 3.83–3.67 (m) | 81.1 |
| 7 | 2.17–2.05 (m) | 60.5 | 3.12–3.03 (m) | 56.8 |
| 8 | 3.61–3.49 (m) | 68.4 | 3.83–3.67 (m) | 66.9 |
| 9 | 9: 2.74–2.55 (m) | 48.7 | 9: 2.74–2.55 (m) | 48.8 |
| 9’: 2.24 (dd, 13.6, 1.8) | 9’: 2.24 (dd, 13.6, 1.8) | |||
| 10 | - | 147.5 | - | 147.0 |
| 11 | 2.74–2.55 (m) | 40.9 | - | 138.4 |
| 12 | - | 178.3 | - | 175.4 |
| 13 | 1.25 (d, 7.0) | 15.7 | 13: 6.13 (dd, 3.0, 1.5) | 121.9 |
| 13’: 6.01 (dd, 3.2, 1.5) | ||||
| 14 | 2.33 (s) | 21.5 | 2.33 (s) | 21.5 |
| 15 | 15: 4.64 (ddd, 18.8, 5.8, 1.9) | 61.8 | 15: 4.66 (d, 18.7) | 61.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruggieri, F.; Hance, P.; Gioia, B.; Biela, A.; Roussel, P.; Hilbert, J.-L.; Willand, N. A Three-Step Process to Isolate Large Quantities of Bioactive Sesquiterpene Lactones from Cichorium intybus L. Roots and Semisynthesis of Chicory STLs Standards. Pharmaceuticals 2023, 16, 771. https://doi.org/10.3390/ph16050771
Ruggieri F, Hance P, Gioia B, Biela A, Roussel P, Hilbert J-L, Willand N. A Three-Step Process to Isolate Large Quantities of Bioactive Sesquiterpene Lactones from Cichorium intybus L. Roots and Semisynthesis of Chicory STLs Standards. Pharmaceuticals. 2023; 16(5):771. https://doi.org/10.3390/ph16050771
Chicago/Turabian StyleRuggieri, Francesca, Philippe Hance, Bruna Gioia, Alexandre Biela, Pascal Roussel, Jean-Louis Hilbert, and Nicolas Willand. 2023. "A Three-Step Process to Isolate Large Quantities of Bioactive Sesquiterpene Lactones from Cichorium intybus L. Roots and Semisynthesis of Chicory STLs Standards" Pharmaceuticals 16, no. 5: 771. https://doi.org/10.3390/ph16050771
APA StyleRuggieri, F., Hance, P., Gioia, B., Biela, A., Roussel, P., Hilbert, J.-L., & Willand, N. (2023). A Three-Step Process to Isolate Large Quantities of Bioactive Sesquiterpene Lactones from Cichorium intybus L. Roots and Semisynthesis of Chicory STLs Standards. Pharmaceuticals, 16(5), 771. https://doi.org/10.3390/ph16050771






