The Therapeutic Potential of Novel Carnosine Formulations: Perspectives for Drug Development
Abstract
1. Introduction
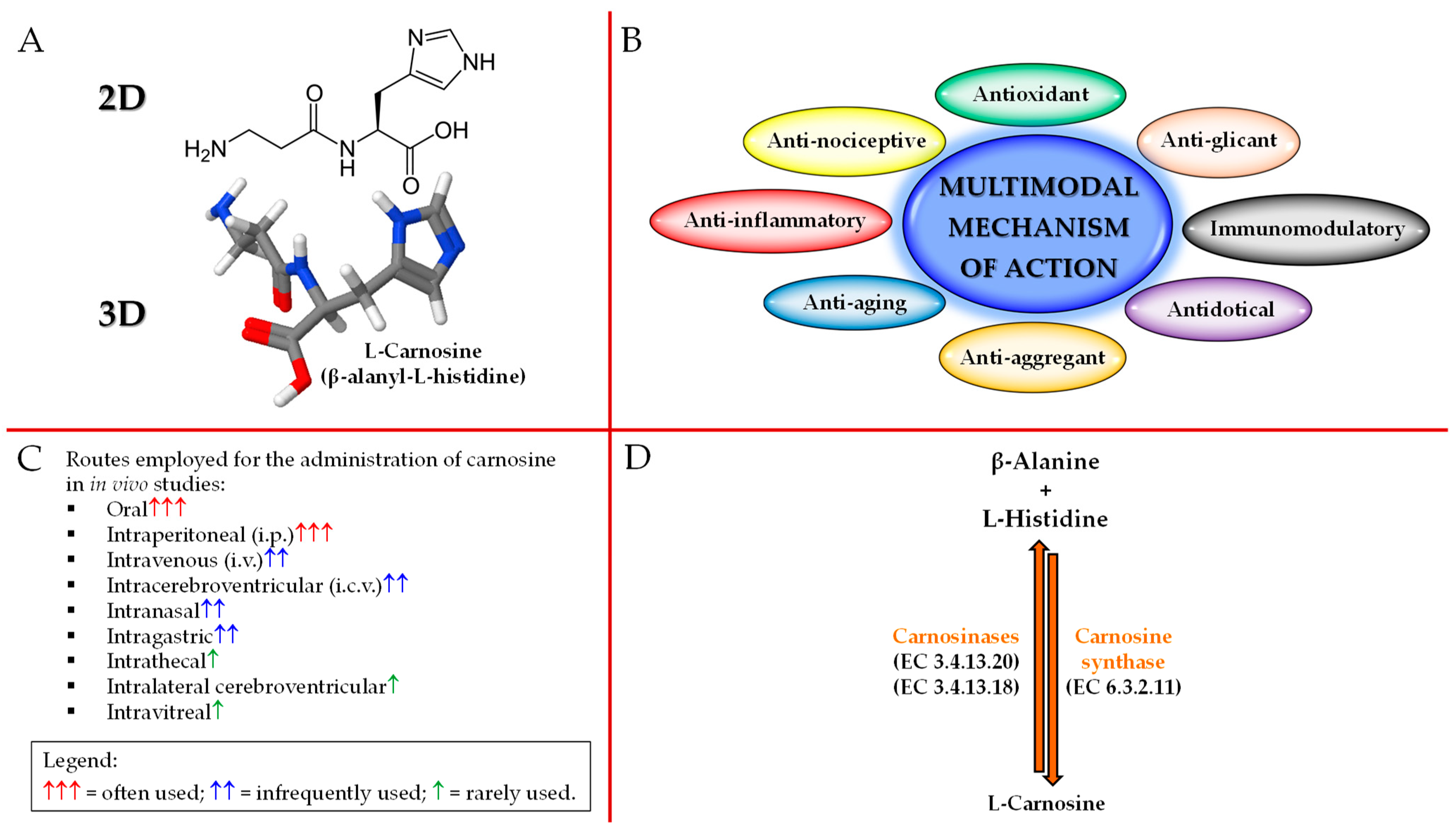
2. Carnosine Structure, Biological Activities, Administration Routes, and Metabolism
3. Drug Delivery Systems
3.1. Vesicular Systems: From Liposomes to Polymerosomes
3.2. Metallic Nanoparticles
3.3. Derivative Conjugates
4. Increasing Carnosine Bioavailability through DDS and/or Chemical Modifications
4.1. Vesicular Systems
4.2. Nanoparticles
4.3. Derivatives/Conjugates
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gulewitsch, W.; Amiradžibi, S. Ueber das carnosin, eine neue organische base des fleischextractes. Ber. Dtsch. Chem. Ges. 1900, 33, 1902–1903. [Google Scholar] [CrossRef]
- Boldyrev, A.A.; Aldini, G.; Derave, W. Physiology and pathophysiology of carnosine. Physiol. Rev. 2013, 93, 1803–1845. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Caraci, F.; Jolivet, R.B. Pivotal role of carnosine in the modulation of brain cells activity: Multimodal mechanism of action and therapeutic potential in neurodegenerative disorders. Prog. Neurobiol. 2019, 175, 35–53. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Fresta, C.G.; Musso, N.; Giambirtone, M.; Grasso, M.; Spampinato, S.F.; Merlo, S.; Drago, F.; Lazzarino, G.; Sortino, M.A.; et al. Carnosine prevents aβ-induced oxidative stress and inflammation in microglial cells: A key role of tgf-β1. Cells 2019, 8, 64. [Google Scholar] [CrossRef] [PubMed]
- Fleisher-Berkovich, S.; Abramovitch-Dahan, C.; Ben-Shabat, S.; Apte, R.; Beit-Yannai, E. Inhibitory effect of carnosine and n-acetyl carnosine on lps-induced microglial oxidative stress and inflammation. Peptides 2009, 30, 1306–1312. [Google Scholar] [CrossRef]
- Caruso, G.; Privitera, A.; Antunes, B.M.; Lazzarino, G.; Lunte, S.M.; Aldini, G.; Caraci, F. The therapeutic potential of carnosine as an antidote against drug-induced cardiotoxicity and neurotoxicity: Focus on nrf2 pathway. Molecules 2022, 27, 4452. [Google Scholar] [CrossRef] [PubMed]
- Fresta, C.G.; Fidilio, A.; Lazzarino, G.; Musso, N.; Grasso, M.; Merlo, S.; Amorini, A.M.; Bucolo, C.; Tavazzi, B.; Lazzarino, G.; et al. Modulation of pro-oxidant and pro-inflammatory activities of m1 macrophages by the natural dipeptide carnosine. Int. J. Mol. Sci. 2020, 21, 776. [Google Scholar] [CrossRef]
- Caruso, G.; Fresta, C.G.; Fidilio, A.; O’Donnell, F.; Musso, N.; Lazzarino, G.; Grasso, M.; Amorini, A.M.; Tascedda, F.; Bucolo, C.; et al. Carnosine decreases pma-induced oxidative stress and inflammation in murine macrophages. Antioxidants 2019, 8, 281. [Google Scholar] [CrossRef]
- Caruso, G.; Privitera, A.; Saab, M.W.; Musso, N.; Maugeri, S.; Fidilio, A.; Privitera, A.P.; Pittalà, A.; Jolivet, R.B.; Lanzanò, L.; et al. Characterization of carnosine effect on human microglial cells under basal conditions. Biomedicines 2023, 11, 474. [Google Scholar] [CrossRef]
- Agostinho, P.; Cunha, R.A.; Oliveira, C. Neuroinflammation, oxidative stress and the pathogenesis of alzheimer’s disease. Curr. Pharm. Des. 2010, 16, 2766–2778. [Google Scholar] [CrossRef]
- Caruso, G.; Grasso, M.; Fidilio, A.; Torrisi, S.A.; Musso, N.; Geraci, F.; Tropea, M.R.; Privitera, A.; Tascedda, F.; Puzzo, D.; et al. Antioxidant activity of fluoxetine and vortioxetine in a non-transgenic animal model of alzheimer’s disease. Front. Pharmacol. 2021, 12, 809541. [Google Scholar] [CrossRef]
- Caruso, G.; Spampinato, S.F.; Cardaci, V.; Caraci, F.; Sortino, M.A.; Merlo, S. Β-amyloid and oxidative stress: Perspectives in drug development. Curr. Pharm. Des. 2019, 25, 4771–4781. [Google Scholar] [CrossRef] [PubMed]
- Araminia, B.; Shalbafan, M.; Mortezaei, A.; Shirazi, E.; Ghaffari, S.; Sahebolzamani, E.; Mortazavi, S.H.; Shariati, B.; Ardebili, M.E.; Aqamolaei, A.; et al. L-carnosine combination therapy for major depressive disorder: A randomized, double-blind, placebo-controlled trial. J. Affect. Disord. 2020, 267, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Benatti, C.; Blom, J.M.C.; Caraci, F.; Tascedda, F. The many faces of mitochondrial dysfunction in depression: From pathology to treatment. Front. Pharmacol. 2019, 10, 995. [Google Scholar] [CrossRef]
- Hald, A.; Lotharius, J. Oxidative stress and inflammation in parkinson’s disease: Is there a causal link? Exp. Neurol. 2005, 193, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Niranjan, R. The role of inflammatory and oxidative stress mechanisms in the pathogenesis of parkinson’s disease: Focus on astrocytes. Mol. Neurobiol. 2014, 49, 28–38. [Google Scholar] [CrossRef]
- Bellia, F.; Vecchio, G.; Rizzarelli, E. Carnosinases, their substrates and diseases. Molecules 2014, 19, 2299–2329. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G. Unveiling the hidden therapeutic potential of carnosine, a molecule with a multimodal mechanism of action: A position paper. Molecules 2022, 27, 3303. [Google Scholar] [CrossRef]
- Grasso, M.; Caruso, G.; Godos, J.; Bonaccorso, A.; Carbone, C.; Castellano, S.; Currenti, W.; Grosso, G.; Musumeci, T.; Caraci, F. Improving cognition with nutraceuticals targeting tgf-β1 signaling. Antioxidants 2021, 10, 1075. [Google Scholar] [CrossRef]
- Aldawsari, H.M.; Badr-Eldin, S.M.; Assiri, N.Y.; Alhakamy, N.A.; Privitera, A.; Caraci, F.; Caruso, G. Surface-tailoring of emulsomes for boosting brain delivery of vinpocetine via intranasal route: In vitro optimization and in vivo pharmacokinetic assessment. Drug Deliv. 2022, 29, 2671–2684. [Google Scholar] [CrossRef]
- Bermúdez, M.L.; Skelton, M.R.; Genter, M.B. Intranasal carnosine attenuates transcriptomic alterations and improves mitochondrial function in the thy1-asyn mouse model of parkinson’s disease. Mol. Genet. Metab. 2018, 125, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, T.; Bonaccorso, A.; Puglisi, G. Epilepsy disease and nose-to-brain delivery of polymeric nanoparticles: An overview. Pharmaceutics 2019, 11, 118. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Deng, X.; Liang, J. Review of the application of nanovesicles and the human interstitial fluid in gastrointestinal premalignant lesion detection, diagnosis, prognosis and therapy. Int. J. Nanomed. 2019, 14, 9469–9482. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ren, L.; Li, S.; Li, W.; Zheng, X.; Yang, Y.; Fu, W.; Yi, J.; Wang, J.; Du, G. The biology, function, and applications of exosomes in cancer. Acta Pharm. Sin. B 2021, 11, 2783–2797. [Google Scholar] [CrossRef] [PubMed]
- Maestrelli, F.; Landucci, E.; De Luca, E.; Nerli, G.; Bergonzi, M.C.; Piazzini, V.; Pellegrini-Giampietro, D.E.; Gullo, F.; Becchetti, A.; Tadini-Buoninsegni, F.; et al. Niosomal formulation of a lipoyl-carnosine derivative targeting trpa1 channels in brain. Pharmaceutics 2019, 11, 669. [Google Scholar] [CrossRef]
- Mohd Zaffarin, A.S.; Ng, S.F.; Ng, M.H.; Hassan, H.; Alias, E. Pharmacology and pharmacokinetics of vitamin e: Nanoformulations to enhance bioavailability. Int. J. Nanomed. 2020, 15, 9961–9974. [Google Scholar] [CrossRef] [PubMed]
- Erdoğar, N.; Akkın, S.; Bilensoy, E. Nanocapsules for drug delivery: An updated review of the last decade. Recent Pat. Drug Deliv. Formul. 2018, 12, 252–266. [Google Scholar] [CrossRef]
- Laffleur, F.; Keckeis, V. Advances in drug delivery systems: Work in progress still needed? Int. J. Pharm. 2020, 590, 119912. [Google Scholar] [CrossRef]
- Miao, J.; Li, F.; Zhang, M.; Zhou, C.; Ren, W.; Hu, X.; Li, N.; Lei, L. Carnosine synthase 1 contributes to interferon gamma-induced arginine depletion via mitogen-activated protein kinase 11 signaling in bovine mammary epithelial cells. J. Interferon Cytokine Res. 2022, 42, 501–512. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Kiersztan, A.; Drozak, J. Biosynthesis of carnosine and related dipeptides in vertebrates. Curr. Protein Pept. Sci. 2018, 19, 771–789. [Google Scholar] [CrossRef]
- Parthasarathy, A.; Savka, M.A.; Hudson, A.O. The synthesis and role of β-alanine in plants. Front. Plant Sci. 2019, 10, 921. [Google Scholar] [CrossRef] [PubMed]
- Holeček, M. Histidine in health and disease: Metabolism, physiological importance, and use as a supplement. Nutrients 2020, 12, 848. [Google Scholar] [CrossRef]
- Junge, W.; McLaughlin, S. The role of fixed and mobile buffers in the kinetics of proton movement. Biochim. Biophys. Acta (BBA)-Bioenerg. 1987, 890, 1–5. [Google Scholar] [CrossRef]
- Swietach, P.; Youm, J.B.; Saegusa, N.; Leem, C.H.; Spitzer, K.W.; Vaughan-Jones, R.D. Coupled ca2+/h+ transport by cytoplasmic buffers regulates local ca2+ and h+ ion signaling. Proc. Natl. Acad. Sci. USA 2013, 110, E2064–E2073. [Google Scholar] [CrossRef] [PubMed]
- Dutka, T.L.; Lamboley, C.R.; McKenna, M.J.; Murphy, R.M.; Lamb, G.D. Effects of carnosine on contractile apparatus ca2+ sensitivity and sarcoplasmic reticulum ca2+ release in human skeletal muscle fibers. J. Appl. Physiol. 2012, 112, 728–736. [Google Scholar] [CrossRef]
- Severin, S.E.; Kirzon, M.V.; Kaftanova, T.M. Effect of carnosine and anserine on action of isolated frog muscles. Dokl. Akad. Nauk SSSR 1953, 91, 691–694. [Google Scholar]
- Sale, C.; Artioli, G.G.; Gualano, B.; Saunders, B.; Hobson, R.M.; Harris, R.C. Carnosine: From exercise performance to health. Amino Acids 2013, 44, 1477–1491. [Google Scholar] [CrossRef]
- Boldyrev, A.A.; Petukhov, V.B. Localization of carnosine effect on the fatigued muscle preparation. Gen. Pharmacol. 1978, 9, 17–20. [Google Scholar] [CrossRef]
- Brisola, G.M.P.; de Souza Malta, E.; Santiago, P.R.P.; Vieira, L.H.P.; Zagatto, A.M. Β-alanine supplementation’s improvement of high-intensity game activities in water polo. Int. J. Sport. Physiol. Perform. 2018, 13, 1208–1214. [Google Scholar] [CrossRef]
- de Andrade Kratz, C.; de Salles Painelli, V.; de Andrade Nemezio, K.M.; da Silva, R.P.; Franchini, E.; Zagatto, A.M.; Gualano, B.; Artioli, G.G. Beta-alanine supplementation enhances judo-related performance in highly-trained athletes. J. Sci. Med. Sport 2017, 20, 403–408. [Google Scholar] [CrossRef]
- Furst, T.; Massaro, A.; Miller, C.; Williams, B.T.; LaMacchia, Z.M.; Horvath, P.J. Β-alanine supplementation increased physical performance and improved executive function following endurance exercise in middle aged individuals. J. Int. Soc. Sport. Nutr. 2018, 15, 32. [Google Scholar] [CrossRef] [PubMed]
- Glenn, J.M.; Smith, K.; Moyen, N.E.; Binns, A.; Gray, M. Effects of acute beta-alanine supplementation on anaerobic performance in trained female cyclists. J. Nutr. Sci. Vitaminol. 2015, 61, 161–166. [Google Scholar] [CrossRef]
- Culbertson, J.Y.; Kreider, R.B.; Greenwood, M.; Cooke, M. Effects of beta-alanine on muscle carnosine and exercise performance: A review of the current literature. Nutrients 2010, 2, 75–98. [Google Scholar] [CrossRef] [PubMed]
- Privitera, A.; Cardaci, V.; Weerasekara, D.; Saab, M.W.; Diolosà, L.; Fidilio, A.; Jolivet, R.B.; Lazzarino, G.; Amorini, A.M.; Camarda, M. Microfluidic/hplc combination to study carnosine protective activity on challenged human microglia: Focus on oxidative stress and energy metabolism. Front. Pharmacol. 2023, 14, 667. [Google Scholar] [CrossRef] [PubMed]
- Tiedje, K.; Stevens, K.; Barnes, S.; Weaver, D. Β-alanine as a small molecule neurotransmitter. Neurochem. Int. 2010, 57, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Mal’tseva, V.V.; Sergienko, V.V.; Stvolinskii, S.L. The effect of carnosine on hematopoietic stem cell activity in irradiated animals. Biokhimiia 1992, 57, 1378–1382. [Google Scholar]
- de Courten, B.; Jakubova, M.; de Courten, M.P.; Kukurova, I.J.; Vallova, S.; Krumpolec, P.; Valkovic, L.; Kurdiova, T.; Garzon, D.; Barbaresi, S.; et al. Effects of carnosine supplementation on glucose metabolism: Pilot clinical trial. Obesity 2016, 24, 1027–1034. [Google Scholar] [CrossRef]
- Fresta, C.G.; Chakraborty, A.; Wijesinghe, M.B.; Amorini, A.M.; Lazzarino, G.; Lazzarino, G.; Tavazzi, B.; Lunte, S.M.; Caraci, F.; Dhar, P.; et al. Non-toxic engineered carbon nanodiamond concentrations induce oxidative/nitrosative stress, imbalance of energy metabolism, and mitochondrial dysfunction in microglial and alveolar basal epithelial cells. Cell Death Dis. 2018, 9, 245. [Google Scholar] [CrossRef]
- Caruso, G.; Fresta, C.G.; Martinez-Becerra, F.; Antonio, L.; Johnson, R.T.; de Campos, R.P.S.; Siegel, J.M.; Wijesinghe, M.B.; Lazzarino, G.; Lunte, S.M. Carnosine modulates nitric oxide in stimulated murine raw 264.7 macrophages. Mol. Cell. Biochem. 2017, 431, 197–210. [Google Scholar] [CrossRef]
- Caruso, G.; Benatti, C.; Musso, N.; Fresta, C.G.; Fidilio, A.; Spampinato, G.; Brunello, N.; Bucolo, C.; Drago, F.; Lunte, S.M.; et al. Carnosine protects macrophages against the toxicity of aβ1-42 oligomers by decreasing oxidative stress. Biomedicines 2021, 9, 477. [Google Scholar] [CrossRef]
- Nagai, K.; Suda, T.; Kawasaki, K.; Mathuura, S. Action of carnosine and beta-alanine on wound healing. Surgery 1986, 100, 815–821. [Google Scholar] [PubMed]
- Pepper, E.D.; Farrell, M.J.; Nord, G.; Finkel, S.E. Antiglycation effects of carnosine and other compounds on the long-term survival of escherichia coli. Appl. Environ. Microbiol. 2010, 76, 7925–7930. [Google Scholar] [CrossRef] [PubMed]
- Boldyrev, A.A.; Gallant, S.C.; Sukhich, G.T. Carnosine, the protective, anti-aging peptide. Biosci. Rep. 1999, 19, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Abe, H. Role of histidine-related compounds as intracellular proton buffering constituents in vertebrate muscle. Biochemistry 2000, 65, 757–765. [Google Scholar]
- Ouyang, L.; Tian, Y.; Bao, Y.; Xu, H.; Cheng, J.; Wang, B.; Shen, Y.; Chen, Z.; Lyu, J. Carnosine decreased neuronal cell death through targeting glutamate system and astrocyte mitochondrial bioenergetics in cultured neuron/astrocyte exposed to ogd/recovery. Brain Res. Bull. 2016, 124, 76–84. [Google Scholar] [CrossRef]
- Hasanein, P.; Felegari, Z. Chelating effects of carnosine in ameliorating nickel-induced nephrotoxicity in rats. Can. J. Physiol. Pharmacol. 2017, 95, 1426–1432. [Google Scholar] [CrossRef]
- Brown, C.E.; Antholine, W.E. Chelation chemistry of carnosine. Evidence that mixed complexes may occur in vivo. J. Phys. Chem. 1979, 83, 3314–3319. [Google Scholar] [CrossRef]
- Wang-Eckhardt, L.; Bastian, A.; Bruegmann, T.; Sasse, P.; Eckhardt, M. Carnosine synthase deficiency is compatible with normal skeletal muscle and olfactory function but causes reduced olfactory sensitivity in aging mice. J. Biol. Chem. 2020, 295, 17100–17113. [Google Scholar] [CrossRef]
- Wang-Eckhardt, L.; Becker, I.; Wang, Y.; Yuan, J.; Eckhardt, M. Absence of endogenous carnosine synthesis does not increase protein carbonylation and advanced lipoxidation end products in brain, kidney or muscle. Amino Acids 2022, 54, 1013–1023. [Google Scholar] [CrossRef]
- Park, H.; Otte, A.; Park, K. Evolution of drug delivery systems: From 1950 to 2020 and beyond. J. Control. Release 2022, 342, 53–65. [Google Scholar] [CrossRef]
- Park, K. Controlled drug delivery systems: Past forward and future back. J. Control. Release 2014, 190, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Benoit, D.S.; Overby, C.T.; Sims Jr, K.R.; Ackun-Farmmer, M.A. Drug delivery systems. In Biomaterials Science; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1237–1266. [Google Scholar]
- Witika, B.A.; Mweetwa, L.L.; Tshiamo, K.O.; Edler, K.; Matafwali, S.K.; Ntemi, P.V.; Chikukwa, M.T.; Makoni, P.A. Vesicular drug delivery for the treatment of topical disorders: Current and future perspectives. J. Pharm. Pharmacol. 2021, 73, 1427–1441. [Google Scholar] [CrossRef] [PubMed]
- Alhakamy, N.A.; Badr-Eldin, S.M.; Fahmy, U.A.; Alruwaili, N.K.; Awan, Z.A.; Caruso, G.; Alfaleh, M.A.; Alaofi, A.L.; Arif, F.O.; Ahmed, O.A.A.; et al. Thymoquinone-loaded soy-phospholipid-based phytosomes exhibit anticancer potential against human lung cancer cells. Pharmaceutics 2020, 12, 761. [Google Scholar] [CrossRef] [PubMed]
- Gangwar, M.; Singh, R.; Goel, R.; Nath, G. Recent advances in various emerging vescicular systems: An overview. Asian Pac. J. Trop. Biomed. 2012, 2, S1176–S1188. [Google Scholar] [CrossRef]
- El Maghraby, G.M.; Williams, A.C. Vesicular systems for delivering conventional small organic molecules and larger macromolecules to and through human skin. Expert Opin. Drug Deliv. 2009, 6, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, D.; Cavaco-Paulo, A.; Nogueira, E. Design of liposomes as drug delivery system for therapeutic applications. Int. J. Pharm. 2021, 601, 120571. [Google Scholar] [CrossRef]
- Bondu, C.; Yen, F.T. Nanoliposomes, from food industry to nutraceuticals: Interests and uses. Innov. Food Sci. Emerg. Technol. 2022, 81, 103140. [Google Scholar] [CrossRef]
- Hussain, A.; Singh, S.; Sharma, D.; Webster, T.J.; Shafaat, K.; Faruk, A. Elastic liposomes as novel carriers: Recent advances in drug delivery. Int. J. Nanomed. 2017, 12, 5087. [Google Scholar] [CrossRef]
- Trotta, M.; Peira, E.; Debernardi, F.; Gallarate, M. Elastic liposomes for skin delivery of dipotassium glycyrrhizinate. Int. J. Pharm. 2002, 241, 319–327. [Google Scholar] [CrossRef]
- El Maghraby, G.M.; Barry, B.W.; Williams, A. Liposomes and skin: From drug delivery to model membranes. Eur. J. Pharm. Sci. 2008, 34, 203–222. [Google Scholar] [CrossRef]
- Chen, J.; Lu, W.-L.; Gu, W.; Lu, S.-S.; Chen, Z.-P.; Cai, B.-C. Skin permeation behavior of elastic liposomes: Role of formulation ingredients. Expert Opin. Drug Deliv. 2013, 10, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Mishra, D.; Dubey, V.; Asthana, A.; Saraf, D.; Jain, N. Elastic liposomes mediated transcutaneous immunization against hepatitis b. Vaccine 2006, 24, 4847–4855. [Google Scholar] [CrossRef] [PubMed]
- Mishra, D.; Garg, M.; Dubey, V.; Jain, S.; Jain, N. Elastic liposomes mediated transdermal deliveryof an anti-hypertensive agent: Propranolol hydrochloride. J. Pharm. Sci. 2007, 96, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Altamimi, M.A.; Hussain, A.; AlRajhi, M.; Alshehri, S.; Imam, S.S.; Qamar, W. Luteolin-loaded elastic liposomes for transdermal delivery to control breast cancer: In vitro and ex vivo evaluations. Pharmaceuticals 2021, 14, 1143. [Google Scholar] [CrossRef] [PubMed]
- Montanari, J.; Maidana, C.; Esteva, M.I.; Salomon, C.; Morilla, M.J.; Romero, E.L. Sunlight triggered photodynamic ultradeformable liposomes against leishmania braziliensis are also leishmanicidal in the dark. J. Control. Release 2010, 147, 368–376. [Google Scholar] [CrossRef]
- Souto, E.B.; Macedo, A.S.; Dias-Ferreira, J.; Cano, A.; Zielińska, A.; Matos, C.M. Elastic and ultradeformable liposomes for transdermal delivery of active pharmaceutical ingredients (apis). Int. J. Mol. Sci. 2021, 22, 9743. [Google Scholar] [CrossRef]
- Du, X.; Huang, X.; Wang, L.; Mo, L.; Jing, H.; Bai, X.; Wang, H. Nanosized niosomes as effective delivery device to improve the stability and bioaccessibility of goat milk whey protein peptide. Food Res. Int. 2022, 161, 111729. [Google Scholar] [CrossRef]
- Barani, M.; Sangiovanni, E.; Angarano, M.; Rajizadeh, M.A.; Mehrabani, M.; Piazza, S.; Gangadharappa, H.V.; Pardakhty, A.; Mehrbani, M.; Dell’Agli, M. Phytosomes as innovative delivery systems for phytochemicals: A comprehensive review of literature. Int. J. Nanomed. 2021, 16, 6983. [Google Scholar] [CrossRef]
- Ge, X.; Wei, M.; He, S.; Yuan, W.-E. Advances of non-ionic surfactant vesicles (niosomes) and their application in drug delivery. Pharmaceutics 2019, 11, 55. [Google Scholar] [CrossRef]
- Chen, S.; Hanning, S.; Falconer, J.; Locke, M.; Wen, J. Recent advances in non-ionic surfactant vesicles (niosomes): Fabrication, characterization, pharmaceutical and cosmetic applications. Eur. J. Pharm. Biopharm. 2019, 144, 18–39. [Google Scholar] [CrossRef]
- Marianecci, C.; Di Marzio, L.; Rinaldi, F.; Celia, C.; Paolino, D.; Alhaique, F.; Esposito, S.; Carafa, M. Niosomes from 80s to present: The state of the art. Adv. Colloid Interface Sci. 2014, 205, 187–206. [Google Scholar] [CrossRef] [PubMed]
- Hoque, M.; Agarwal, S.; Gupta, S.; Garg, S.; Syed, I.; Rupesh, A.; Mohapatra, N.; Bose, S.; Sarkar, P. Lipid nanostructures in food applications. Innov. Food Sci. Emerg. Technol. 2021, 565–579. [Google Scholar]
- Patel, J.; Patel, R.; Khambholja, K.; Patel, N. An overview of phytosomes as an advanced herbal drug delivery system. Asian J. Pharm. Sci. 2009, 4, 363–371. [Google Scholar]
- Azeez, N.A.; Deepa, V.S.; Sivapriya, V. Phytosomes: Emergent promising nano vesicular drug delivery system for targeted tumor therapy. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 033001. [Google Scholar] [CrossRef]
- Alharbi, W.S.; Almughem, F.A.; Almehmady, A.M.; Jarallah, S.J.; Alsharif, W.K.; Alzahrani, N.M.; Alshehri, A.A. Phytosomes as an emerging nanotechnology platform for the topical delivery of bioactive phytochemicals. Pharmaceutics 2021, 13, 1475. [Google Scholar] [CrossRef]
- Panda, V.S.; Naik, S.R. Evaluation of cardioprotective activity of ginkgo biloba and ocimum sanctum in rodents. Altern. Med. Rev. 2009, 14, 161. [Google Scholar]
- Zhang, X.-Y.; Zhang, P.-Y. Polymersomes in nanomedicine—A review. Curr. Nanosci. 2017, 13, 124–129. [Google Scholar] [CrossRef]
- Lee, J.S.; Feijen, J. Polymersomes for drug delivery: Design, formation and characterization. J. Control. Release 2012, 161, 473–483. [Google Scholar] [CrossRef]
- Meerovich, I.; Dash, A.K. Polymersomes for drug delivery and other biomedical applications. In Materials for Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 269–309. [Google Scholar]
- Liu, Q.; Song, L.; Chen, S.; Gao, J.; Zhao, P.; Du, J. A superparamagnetic polymersome with extremely high t2 relaxivity for mri and cancer-targeted drug delivery. Biomaterials 2017, 114, 23–33. [Google Scholar] [CrossRef]
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The history of nanoscience and nanotechnology: From chemical–physical applications to nanomedicine. Molecules 2019, 25, 112. [Google Scholar] [CrossRef]
- Harris, N.; Blaber, M.; Schatz, G. Optical properties of metal nanoparticles. Encycl. Nanotechnol. 2012, 481, 9751–9754. [Google Scholar]
- Chandrakala, V.; Aruna, V.; Angajala, G. Review on metal nanoparticles as nanocarriers: Current challenges and perspectives in drug delivery systems. Emergent Mater. 2022, 5, 1593–1615. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Jary, J.; Machnicka, B. Pharmacokinetics of magnetic iron oxide nanoparticles for medical applications. J. Nanobiotechnology 2022, 20, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.-Y.; Zhang, J.-W.; Li, R.-F.; Wang, Z.-X.; Wang, W.-J.; Wang, W. Unique roles of gold nanoparticles in drug delivery, targeting and imaging applications. Molecules 2017, 22, 1445. [Google Scholar] [CrossRef] [PubMed]
- Martinkova, P.; Brtnicky, M.; Kynicky, J.; Pohanka, M. Iron oxide nanoparticles: Innovative tool in cancer diagnosis and therapy. Adv. Healthc. Mater. 2018, 7, 1700932. [Google Scholar] [CrossRef] [PubMed]
- Amina, S.J.; Guo, B. A review on the synthesis and functionalization of gold nanoparticles as a drug delivery vehicle. Int. J. Nanomed. 2020, 15, 9823–9857. [Google Scholar] [CrossRef] [PubMed]
- Daraee, H.; Eatemadi, A.; Abbasi, E.; Fekri Aval, S.; Kouhi, M.; Akbarzadeh, A. Application of gold nanoparticles in biomedical and drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 410–422. [Google Scholar] [CrossRef]
- Hammami, I.; Alabdallah, N.M. Gold nanoparticles: Synthesis properties and applications. J. King Saud Univ.-Sci. 2021, 33, 101560. [Google Scholar] [CrossRef]
- Arora, S.; Sharma, P.; Kumar, S.; Nayan, R.; Khanna, P.K.; Zaidi, M.G.H. Gold-nanoparticle induced enhancement in growth and seed yield of Brassica juncea. Plant growth regulation. Plant Growth Regul. 2012, 66, 303–310. [Google Scholar] [CrossRef]
- Garton, M.; Nim, S.; Stone, T.A.; Wang, K.E.; Deber, C.M.; Kim, P.M. Method to generate highly stable d-amino acid analogs of bioactive helical peptides using a mirror image of the entire pdb. Proc. Natl. Acad. Sci. USA 2018, 115, 1505–1510. [Google Scholar] [CrossRef]
- Liu, A.; Krushnamurthy, P.; Subramanya, K.; Mitchell, D.A.; Mahanta, N. Enzymatic thioamidation of peptide backbones. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2021; Volume 656, pp. 459–494. [Google Scholar]
- Bartling, C.R.O.; Alexopoulou, F.; Kuschert, S.; Chin, Y.K.; Jia, X.; Sereikaite, V.; Özcelik, D.; Jensen, T.M.; Jain, P.; Nygaard, M.M.; et al. Comprehensive peptide cyclization examination yields optimized app scaffolds with improved affinity toward mint2. J. Med. Chem. 2023, 66, 3045–3057. [Google Scholar] [CrossRef] [PubMed]
- Armas, F.; Di Stasi, A.; Mardirossian, M.; Romani, A.A.; Benincasa, M.; Scocchi, M. Effects of lipidation on a proline-rich antibacterial peptide. Int. J. Mol. Sci. 2021, 22, 7959. [Google Scholar] [CrossRef] [PubMed]
- Moreira Brito, J.C.; Carvalho, L.R.; Neves de Souza, A.; Carneiro, G.; Magalhães, P.P.; Farias, L.M.; Guimarães, N.R.; Verly, R.M.; Resende, J.M.; Elena de Lima, M. Pegylation of the antimicrobial peptide lyetx i-b maintains structure-related biological properties and improves selectivity. Front. Mol. Biosci. 2022, 9, 1001508. [Google Scholar] [CrossRef] [PubMed]
- Wijesinghe, A.; Kumari, S.; Booth, V. Conjugates for use in peptide therapeutics: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0255753. [Google Scholar] [CrossRef]
- Lanza, V.; Bellia, F.; D’Agata, R.; Grasso, G.; Rizzarelli, E.; Vecchio, G. New glycoside derivatives of carnosine and analogs resistant to carnosinase hydrolysis: Synthesis and characterization of their copper(ii) complexes. J. Inorg. Biochem. 2011, 105, 181–188. [Google Scholar] [CrossRef]
- Cooper, B.M.; Iegre, J.; DH, O.D.; Ölwegård Halvarsson, M.; Spring, D.R. Peptides as a platform for targeted therapeutics for cancer: Peptide-drug conjugates (pdcs). Chem. Soc. Rev. 2021, 50, 1480–1494. [Google Scholar] [CrossRef]
- Kulikova, O.I.; Stvolinsky, S.L.; Migulin, V.A.; Andreeva, L.A.; Nagaev, I.Y.; Lopacheva, O.M.; Kulichenkova, K.N.; Lopachev, A.V.; Trubitsina, I.E.; Fedorova, T.N. A new derivative of acetylsalicylic acid and carnosine: Synthesis, physical and chemical properties, biological activity. DARU J. Pharm. Sci. 2020, 28, 119–130. [Google Scholar] [CrossRef]
- Qiu, J.; Hauske, S.J.; Zhang, S.; Rodriguez-Niño, A.; Albrecht, T.; Pastene, D.O.; van den Born, J.; van Goor, H.; Ruf, S.; Kohlmann, M.; et al. Identification and characterisation of carnostatine (san9812), a potent and selective carnosinase (cn1) inhibitor with in vivo activity. Amino Acids 2019, 51, 7–16. [Google Scholar] [CrossRef]
- Peters, V.; Schmitt, C.P.; Weigand, T.; Klingbeil, K.; Thiel, C.; van den Berg, A.; Calabrese, V.; Nawroth, P.; Fleming, T.; Forsberg, E.; et al. Allosteric inhibition of carnosinase (cn1) by inducing a conformational shift. J. Enzym. Inhib. Med. Chem. 2017, 32, 1102–1110. [Google Scholar] [CrossRef]
- Maherani, B.; Arab-Tehrany, E.; Kheirolomoom, A.; Cleymand, F.; Linder, M. Influence of lipid composition on physicochemical properties of nanoliposomes encapsulating natural dipeptide antioxidant l-carnosine. Food Chem. 2012, 134, 632–640. [Google Scholar] [CrossRef]
- Slovák, L.; Poništ, S.; Fedorova, T.; Logvinenko, A.; Levacheva, I.; Samsonova, O.; Bakowsky, U.; Pašková, Ľ.; Čavojský, T.; Tsiklauri, L.; et al. Evaluation of liposomal carnosine in adjuvant arthritis. Gen. Physiol. Biophys. 2017, 36, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A.; Cha, J.-H.; Noh, A.R.; Qureshi, O.S.; Kim, K.-W.; Choe, Y.-H.; Shin, D.; Shah, F.A.; Majid, A.; Bae, O.-N. Neuroprotective effects of carnosine-loaded elastic liposomes in cerebral ischemia rat model. J. Pharm. Investig. 2020, 50, 373–381. [Google Scholar] [CrossRef]
- Moulahoum, H.; Sanli, S.; Timur, S.; Zihnioglu, F. Potential effect of carnosine encapsulated niosomes in bovine serum albumin modifications. Int. J. Biol. Macromol. 2019, 137, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Lundie, M.M. Formulation and Topical Delivery of Niosomes and Proniosomes Containing Carnosine. Ph.D. Thesis, Potchefstroom Campus, North-West University (South Africa), Potchefstroom, South Africa, 2016. [Google Scholar]
- Kim, E.-S.; Kim, D.; Nyberg, S.; Poma, A.; Cecchin, D.; Jain, S.A.; Kim, K.-A.; Shin, Y.-J.; Kim, E.-H.; Kim, M. Lrp-1 functionalized polymersomes enhance the efficacy of carnosine in experimental stroke. Sci. Rep. 2020, 10, 699. [Google Scholar] [CrossRef] [PubMed]
- Abdelkader, H.; Longman, M.R.; Alany, R.G.; Pierscionek, B. Phytosome-hyaluronic acid systems for ocular delivery of l-carnosine. Int. J. Nanomed. 2016, 11, 2815. [Google Scholar] [CrossRef]
- Darvishi, B.; Dinarvand, R.; Mohammadpour, H.; Kamarul, T.; Sharifi, A.M. Dual l-carnosine/aloe vera nanophytosomes with synergistically enhanced protective effects against methylglyoxal-induced angiogenesis impairment. Mol. Pharm. 2021, 18, 3302–3325. [Google Scholar] [CrossRef]
- Farid, R.M.; Gaafar, P.M.E.; Hazzah, H.A.; Helmy, M.W.; Abdallah, O.Y. Chemotherapeutic potential of l-carnosine from stimuli-responsive magnetic nanoparticles against breast cancer model. Nanomedicine 2020, 15, 891–911. [Google Scholar] [CrossRef]
- Durmus, Z.; Kavas, H.; Baykal, A.; Sozeri, H.; Alpsoy, L.; Çelik, S.; Toprak, M. Synthesis and characterization of l-carnosine coated iron oxide nanoparticles. J. Alloy. Compd. 2011, 509, 2555–2561. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, Y.; Wang, L.; Li, G.; Gao, J.; Wang, Y. Development of l-carnosine functionalized iron oxide nanoparticles loaded with dexamethasone for simultaneous therapeutic potential of blood brain barrier crossing and ischemic stroke treatment. Drug Deliv. 2021, 28, 380–389. [Google Scholar] [CrossRef]
- Khramtsov, P.; Barkina, I.; Kropaneva, M.; Bochkova, M.; Timganova, V.; Nechaev, A.; Byzov, I.; Zamorina, S.; Yermakov, A.; Rayev, M. Magnetic nanoclusters coated with albumin, casein, and gelatin: Size tuning, relaxivity, stability, protein corona, and application in nuclear magnetic resonance immunoassay. Nanomaterials 2019, 9, 1345. [Google Scholar] [CrossRef]
- Bellia, F.; Oliveri, V.; Rizzarelli, E.; Vecchio, G. New derivative of carnosine for nanoparticle assemblies. Eur. J. Med. Chem. 2013, 70, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xia, R.; Hu, H.; Peng, T. Biosynthesis, characterization and cytotoxicity of gold nanoparticles and their loading with n-acetylcarnosine for cataract treatment. J. Photochem. Photobiol. B Biol. 2018, 187, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Habra, K.; Morris, R.H.; McArdle, S.E.B.; Cave, G.W.V. Controlled release of carnosine from poly(lactic-co-glycolic acid) beads using nanomechanical magnetic trigger towards the treatment of glioblastoma. Nanoscale Adv. 2022, 4, 2242–2249. [Google Scholar] [CrossRef]
- Habra, K.; McArdle, S.E.B.; Morris, R.H.; Cave, G.W.V. Synthesis and functionalisation of superparamagnetic nano-rods towards the treatment of glioblastoma brain tumours. Nanomaterials 2021, 11, 2157. [Google Scholar] [CrossRef] [PubMed]
- Gaafar, P.M.E.; El-Salamouni, N.S.; Farid, R.M.; Hazzah, H.A.; Helmy, M.W.; Abdallah, O.Y. Pegylated liquisomes: A novel combined passive targeting nanoplatform of l-carnosine for breast cancer. Int. J. Pharm. 2021, 602, 120666. [Google Scholar] [CrossRef] [PubMed]
- Calcagni, A.; Ciattini, P.G.; Di Stefano, A.; Duprè, S.; Luisi, G.; Pinnen, F.; Rossi, D.; Spirito, A. Ψ (so2nh) transition state isosteres of peptides. Synthesis and bioactivity of sulfonamido pseudopeptides related to carnosine. Il Farm. 1999, 54, 673–677. [Google Scholar] [CrossRef]
- Vecchio, G.; La Mendola, D.; Rizzarelli, E. The synthesis and conformation of β-cyclodextrins functionalized with enantiomers of boc-carnosine. J. Supramol. Chem. 2001, 1, 87–95. [Google Scholar] [CrossRef]
- Amorini, A.M.; Bellia, F.; Di Pietro, V.; Giardina, B.; La Mendola, D.; Lazzarino, G.; Sortino, S.; Tavazzi, B.; Rizzarelli, E.; Vecchio, G. Synthesis and antioxidant activity of new homocarnosine beta-cyclodextrin conjugates. Eur. J. Med. Chem. 2007, 42, 910–920. [Google Scholar] [CrossRef]
- La Mendola, D.; Sortino, S.; Vecchio, G.; Rizzarelli, E. Synthesis of new carnosine derivatives of β-cyclodextrin and their hydroxyl radical scavenger ability. Helv. Chim. Acta 2002, 85, 1633–1643. [Google Scholar] [CrossRef]
- Mineo, P.; Vitalini, D.; La Mendola, D.; Rizzarelli, E.; Scamporrino, E.; Vecchio, G. Coordination features of difunctionalized β-cyclodextrins with carnosine: Esi-ms and spectroscopic investigations on 6a, 6d-di-(β-alanyl-l-histidine)-6a, 6d-dideoxy-β-cyclodextrin and 6a, 6c-di-(β-alanyl-l-histidine)-6a, 6c-dideoxy-β-cyclodextrin and their copper (ii) complexes. J. Inorg. Biochem. 2004, 98, 254–265. [Google Scholar]
- Babizhayev, M.A.; Deyev, A.I.; Yermakova, V.N.; Semiletov, Y.A.; Davydova, N.G.; Kurysheva, N.I.; Zhukotskii, A.V.; Goldman, I.M. N-acetylcarnosine, a natural histidine-containing dipeptide, as a potent ophthalmic drug in treatment of human cataracts. Peptides 2001, 22, 979–994. [Google Scholar] [CrossRef] [PubMed]
- Babizhayev, M.A. Biological activities of the natural imidazole-containing peptidomimetics n-acetylcarnosine, carcinine and l-carnosine in ophthalmic and skin care products. Life Sci. 2006, 78, 2343–2357. [Google Scholar] [CrossRef] [PubMed]
- Babizhayev, M.A.; Guiotto, A.; Kasus-Jacobi, A. N-acetylcarnosine and histidyl-hydrazide are potent agents for multitargeted ophthalmic therapy of senile cataracts and diabetic ocular complications. J. Drug Target. 2009, 17, 36–63. [Google Scholar] [CrossRef] [PubMed]
- Castelletto, V.; Cheng, G.; Stain, C.; Connon, C.J.; Hamley, I.W. Self-assembly of a peptide amphiphile containing l-carnosine and its mixtures with a multilamellar vesicle forming lipid. Langmuir 2012, 28, 11599–11608. [Google Scholar] [CrossRef]
- Pal, A.; Shrivastava, S.; Dey, J. Salt, ph and thermoresponsive supramolecular hydrogel of n-(4-n-tetradecyloxybenzoyl)-l-carnosine. Chem. Commun. 2009, 45, 6997–6999. [Google Scholar] [CrossRef]
- Gizzi, P.; Pasc, A.; Dupuy, N.; Parant, S.; Henry, B.; Gérardin, C. Molecular Tailored Histidine-Based Complexing Surfactants: From Micelles to Hydrogels; Wiley Online Library: Hoboken, NJ, USA, 2009. [Google Scholar]
- Stvolinsky, S.; Antonova, N.; Kulikova, O.; Lopachev, A.; Abaimov, D.; Al-Baidani, I.; Lopacheva, O.; Fedorova, T.; Kaplun, A.; Sorokoumova, G. Lipoilcarnosine: Synthesis, study of physico-chemical and antioxidant properties, biological activity. Biomeditsinskaia Khimiia 2018, 64, 268–275. [Google Scholar] [CrossRef]
- Stvolinsky, S.; Bulygina, E.; Fedorova, T.; Meguro, K.; Sato, T.; Tyulina, O.; Abe, H.; Boldyrev, A. Biological activity of novel synthetic derivatives of carnosine. Cell. Mol. Neurobiol. 2010, 30, 395–404. [Google Scholar] [CrossRef]
- Astete, C.E.; Songe Meador, D.; Spivak, D.; Sabliov, C. Synthesis of vitamin e-carnosine (vecar): New antioxidant molecule with potential application in atherosclerosis. Synth. Commun. 2013, 43, 1299–1313. [Google Scholar] [CrossRef]
- Grasso, G.I.; Bellia, F.; Arena, G.; Satriano, C.; Vecchio, G.; Rizzarelli, E. Multitarget trehalose-carnosine conjugates inhibit aβ aggregation, tune copper(ii) activity and decrease acrolein toxicity. Eur. J. Med. Chem. 2017, 135, 447–457. [Google Scholar] [CrossRef]
- Naletova, I.; Greco, V.; Sciuto, S.; Attanasio, F.; Rizzarelli, E. Ionophore ability of carnosine and its trehalose conjugate assists copper signal in triggering brain-derived neurotrophic factor and vascular endothelial growth factor activation in vitro. Int. J. Mol. Sci. 2021, 22, 13504. [Google Scholar] [CrossRef]
- Greco, V.; Naletova, I.; Ahmed, I.M.M.; Vaccaro, S.; Messina, L.; La Mendola, D.; Bellia, F.; Sciuto, S.; Satriano, C.; Rizzarelli, E. Hyaluronan-carnosine conjugates inhibit aβ aggregation and toxicity. Sci. Rep. 2020, 10, 15998. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.J.; Vistoli, G.; Katunga, L.A.; Funai, K.; Regazzoni, L.; Monroe, T.B.; Gilardoni, E.; Cannizzaro, L.; Colzani, M.; De Maddis, D. A carnosine analog mitigates metabolic disorders of obesity by reducing carbonyl stress. J. Clin. Investig. 2018, 128, 5280–5293. [Google Scholar] [CrossRef] [PubMed]
- Bertinaria, M.; Rolando, B.; Giorgis, M.; Montanaro, G.; Guglielmo, S.; Buonsanti, M.F.; Carabelli, V.; Gavello, D.; Daniele, P.G.; Fruttero, R. Synthesis, physicochemical characterization, and biological activities of new carnosine derivatives stable in human serum as potential neuroprotective agents. J. Med. Chem. 2011, 54, 611–621. [Google Scholar] [CrossRef]
- Bertinaria, M.; Rolando, B.; Giorgis, M.; Montanaro, G.; Marini, E.; Collino, M.; Benetti, E.; Daniele, P.G.; Fruttero, R.; Gasco, A. Carnosine analogues containing no-donor substructures: Synthesis, physico-chemical characterization and preliminary pharmacological profile. Eur. J. Med. Chem. 2012, 54, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Iacobini, C.; Menini, S.; Blasetti Fantauzzi, C.; Pesce, C.M.; Giaccari, A.; Salomone, E.; Lapolla, A.; Orioli, M.; Aldini, G.; Pugliese, G. Fl-926-16, a novel bioavailable carnosinase-resistant carnosine derivative, prevents onset and stops progression of diabetic nephropathy in db/db mice. Br. J. Pharmacol. 2018, 175, 53–66. [Google Scholar] [CrossRef]





| Advantages | Limitations | ||
|---|---|---|---|
| Vesicular Systems | Liposome | Made of natural ingredients; Biodegradable and biocompatible; Similarity to biomembrane. | Physical instability during storage; Susceptible to oxidation; Rigid liposomes remain confined to the stratum corneum. |
| Elastic Liposome | Highly deforming ability and flexibility ensure deeper skin penetration and biomembrane crossing ability. | On prolonged storage, due to increased elasticity and flexibility, it tends to be less stable and lose the entrapped drug, which complicates the scaling process. | |
| Niosome | Chemical stability. | Lower biocompatibility. | |
| Phytosome | Enhancement of pharmacokinetic and pharmacodynamic properties of herbal-originated polyphenolic compounds; Improves skin absorption of phytoconstituents; | Despite the easy scale-up production of phytosomes, the high pH sensitivity of some components could limit the large-scale synthesis of such formulations and should be considered during the manufacturing. | |
| Better stability of incorporated compounds owing to the chemical interaction. | |||
| Polymerosome | More stable than liposomes; Allows greater control of chemical and structural properties; Can be used to obtain controlled release kinetics by stimuli-response triggers. | In case of charged polymers, the self-assembled polymersome could induce stronger immune response and therefore be less tolerable for medical applications. | |
| Metallic NPs | Multiple shapes; Conductivity; Localized surface plasmon resonance; Ability to direct uptake through external magnetic stimulation. | Chemical contaminants from synthesis can cause toxicity issues. | |
| Drug-conjugate derivatives | Increased compound half-life; Increased target specificity; Increase drug stability. | Modification can reduce the potency, especially for small peptides and proteins; Any covalent modification of peptides or proteins presents a potential risk of increased immunogenicity. | |
| Formulation Name | Basic Description | Mode of Action | Ref. |
|---|---|---|---|
| Nanoliposomes | Carnosine incorporated into nanoliposomes could represent an innovative approach to overcoming the issues related to the direct application of this antioxidant peptide in food. |
| [113] |
| Liposomes | Liposomes are nanosized vesicles with a spherical shape that can be produced starting from natural or synthetic phospholipids. Encapsulation of antioxidants into liposomes has been shown to improve their therapeutic potential against oxidant-induced tissue injuries, facilitating intracellular delivery and extending the retention time of incorporated agents inside the cell. |
| [114] |
| Elastic liposomes | EL encapsulated with carnosine represent a promising strategy to enhance the transport into the brain, protecting the dipeptide against enzymatic hydrolysis. |
| [115] |
| Niosome derivatized with lipoyl-carnosine | Niosomes are nanovesicles coupled to specific ligands selectively recognized by transporters expressed on the BBB that could promote the delivery of drugs (e.g., carnosine) at brain level. |
| [25] |
| Niosome | Carnosine-encapsulated niosomes represent a powerful drug delivery tool allowing it to reach specific organs such as the brain. |
| [116,117] |
| Proniosome | Proniosomes are non-hydrated niosomes, which, upon hydration, form niosomes characterized by physical stability that overcome some problems presented by other vesicular systems, such as leaking, fusion, and aggregation. |
| [117] |
| Polymerosome | Polymersomes are synthetic vesicles formed through the self-assembly of amphiphilic co-polymers in aqueous conditions. Carnosine encapsulated in polymersomes could exert an enhanced neuroprotective potential. |
| [118] |
| Phytosome | Carnosine loaded into lipid-based phytosomes represent an alternative for the prodrug N-acetyl-carnosine as a novel delivery system to the lens. |
| [119] |
| Nanophytosome | Nanophytosomes represent one of the novel nanocarriers that could provide potent applications in both food and pharmaceutical fields. A novel nanophytosomal formulation obtained by physical mixture of two compounds, carnosine and Aloe vera, has shown a synergic effect in counteracting cell toxicity. |
| [120] |
| Formulation Name | Basic Description | Mode of Action | Ref. |
|---|---|---|---|
| Fe3O4 | Carnosine-coated Fe3O4 NPs have been prepared via co-precipitation of Fe3O4 in the presence of carnosine. They are commonly used because of their superparamagnetic properties allowing potential applications in many fields. |
| [122] |
| Fe3O4 NPs/poly(lactic-co-glycolic acid) (PLGA) polymer-loaded dexamethasone functionalized with carnosine | PLGA functionalized Fe3O4 NPs with carnosine peptide composite loaded with dexamethasone represent suitable drug delivery carriers for biomedical applications able to improve the therapeutic efficiency of carnosine. |
| [123] |
| Magnetic | Carnosine-coated MNPs were developed to enhance the chemotherapeutic activity of this dipeptide. |
| [121] |
| [124] | ||
| AuNPs/biotin | A new carnosine derivative with biotin was synthesized and structurally characterized. The binding affinity of the new molecular entity to avidin and streptavidin was exploited to functionalize avidin- and streptavidin-AuNPs with the carnosine–biotin conjugate. |
| [125] |
| AuNPs/N-acetyl-carnosine | NPs loaded with N-acetyl-carnosine were synthesized, characterized, and tested for cataract treatment. The AuNPs were biofabricated and characterized by using Coccinia grandis bark extract. |
| [126] |
| Poly (lactic-co-glycolic acid) microbeads | The Fe3O4 NPs have been encapsulated, along with carnosine, inside porous poly(lactic-co-glycolic acid) microbeads. These new drug-delivery vesicles have the potential to pave the way towards the safe and triggered release of onsite drug delivery as part of a theragnostic treatment for cancer. |
| [127] |
| Formulation Name | Basic Description | Mode of Action | Ref. |
|---|---|---|---|
| Derivatized with β-cyclodextrins | β-cyclodextrin is a heptasaccharide derived from glucose. Ciclodextrins are particularly used in pharmaceutical science for their ability to include and/or stabilize drugs. Glycoconjugate derivatives obtained by functionalization with carnosine in different positions of the sugar or the cyclodextrin are widely used because of their decreased susceptibility to degradation by carnosinases. |
| [131,132,133] |
| [134] | ||
| N-acetylcarnosine | N-acetyl-carnosine is obtained by the addition of an acetyl group to carnosine structure, which makes the dipeptide more resistant to the degradation exerted by carnosinases. |
| [135] |
| [136] | ||
| [137] | ||
| [110] | ||
| Derivatized by acylation with palmitoyl chain | Palmitic acid is a fatty acid with a 16-carbon chain. This compound is commonly used as a structure-directing agent to induce the fibrillization of carnosine. Its long lipid chains are able to drive self-assembly due to amphiphilicity, showing restricted dynamics and/or crystallization. |
| [138] |
| Derivatized by acylation with benzoic acid | Benzoic acid is a compound comprising a benzene ring core carrying a carboxylic acid substituent. N-(4-n-tetradecyloxybenzoyl)-L-carnosine represents a carnosine-based amphiphilic hydrogelator that efficiently gelates water and exhibits salt, pH, and thermoresponsive gelation properties. |
| [139] |
| Histidine-based derivatives | Novel histidine-based complexing surfactants containing trifunctional moduli (peptidic/hydrophilic/hydrophobic). It is possible to establish various links between the different parts, allowing the modulation of the lipophilic/hydrophilic balance and obtaining amphiphilic compounds with complexing properties and surfactive or gelator properties. |
| [140] |
| Lipoilcarnosine | Lipoic acid is an organosulfur compound derived from caprylic acid. Lipoilcarnosine is a conjugated molecule obtained by coupling α-lipoic acid to carnosine. |
| [141] |
| Derivatized with trolox | Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) is a water-soluble analog of vitamin E. (S)-trolox-L-carnosine (STC) and (R)-trolox-L-carnosine (RTC) represent novel derivatives of carnosine synthesized by N-acylation of carnosine with (S)- and (R)-trolox, respectively. |
| [142] |
| Derivatized with vitamin E-carnosine (VECAR) | VECAR is a novel heterodimer of α-tocopherol (vitamin E) and carnosine that was designed by using 13-carbon phytyl-chain to link carnosine to Trolox at the C2 carbon position, maintaining the antioxidant activities of the two components. |
| [143] |
| Derivatized with acetylsalicylic acid | Acetylsalicylic acid is a nonsteroidal anti-inflammatory drug (NSAID) used to reduce pain, fever, and/or inflammation and as an antithrombotic. Salicyl-carnosine was synthesized by condensation of acetylsalicylic acid and carnosine. Its properties are particularly promising for the potential development of new anti-inflammatory and antithrombotic drugs. |
| [110] |
| Derivatized with trehalose | Trehalose is a sugar consisting of two molecules of glucose. The glyco-conjugate trehalose-carnosine (TrCar), differently from carnosine, is not hydrolyzed by human carnosinases. Particular attention has been paid to the characterization of the Cu2+ binding features of TrCar. |
| [144] |
| [145] | ||
| Derivatized with hyaluronic acid | Hyaluronic acid is a linear glycosaminoglycan, an anionic, gel-like polymer, found in the extracellular matrix of epithelial and connective tissues. A derivative obtained from hyaluronic acid and carnosine was considered a pharmacological approach to cure and/or prevent the onset of neurodegenerative disorders. |
| [146] |
| Carnosinol | A derivative of carnosine with high oral bioavailability because of its resistance to carnosinases. Carnosinol displayed a suitable ADMET (absorption, distribution, metabolism, excretion, and toxicity) profile and the greatest potency and selectivity toward α,β-unsaturated aldehydes. |
| [147] |
| Amide derivatives | New family of amide derivatives that are not significantly hydrolyzed by carnosinases. In these derivatives, the sugar moiety can act as a recognition element. |
| [108] |
| [148] | ||
| Carnosine analogues containing NO-donor substructures | Carnosine analogs containing NO-donor substructures of which the physico-chemical characterization and preliminary pharmacological profile were carried out. These analogs are characterized by higher resistance to carnosinases’ degradation. |
| [149] |
| Derivatized with sulfamido pseudopeptides | These compounds, characterized by the presence of a sulfonamido junction, present several interesting aspects which relate to the biological relevance of taurine and the stability toward enzymatic hydrolysis. The high polar character and the sulfur tetrahedral structure make these compounds suitable for the design of tight-binding enzyme inhibitors. |
| [130] |
| FL-926-16 | A novel, rationally designed carnosine peptidomimetic with a favorable pharmacokinetic profile, which might be suitable for testing in human subjects. |
| [150] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonaccorso, A.; Privitera, A.; Grasso, M.; Salamone, S.; Carbone, C.; Pignatello, R.; Musumeci, T.; Caraci, F.; Caruso, G. The Therapeutic Potential of Novel Carnosine Formulations: Perspectives for Drug Development. Pharmaceuticals 2023, 16, 778. https://doi.org/10.3390/ph16060778
Bonaccorso A, Privitera A, Grasso M, Salamone S, Carbone C, Pignatello R, Musumeci T, Caraci F, Caruso G. The Therapeutic Potential of Novel Carnosine Formulations: Perspectives for Drug Development. Pharmaceuticals. 2023; 16(6):778. https://doi.org/10.3390/ph16060778
Chicago/Turabian StyleBonaccorso, Angela, Anna Privitera, Margherita Grasso, Sonya Salamone, Claudia Carbone, Rosario Pignatello, Teresa Musumeci, Filippo Caraci, and Giuseppe Caruso. 2023. "The Therapeutic Potential of Novel Carnosine Formulations: Perspectives for Drug Development" Pharmaceuticals 16, no. 6: 778. https://doi.org/10.3390/ph16060778
APA StyleBonaccorso, A., Privitera, A., Grasso, M., Salamone, S., Carbone, C., Pignatello, R., Musumeci, T., Caraci, F., & Caruso, G. (2023). The Therapeutic Potential of Novel Carnosine Formulations: Perspectives for Drug Development. Pharmaceuticals, 16(6), 778. https://doi.org/10.3390/ph16060778













