Diversity of Solid Forms Promoted by Ball Milling: Characterization and Intrinsic Dissolution Studies of Pioglitazone Hydrochloride and Fluvastatin Sodium Drug–Drug Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. NG or LAG Solvent Screening (Stoichiometry Ratio 1:1)
2.2.2. Evaluation of the Formation of the Multicomponent Salt PGZ-FLV (EtOH, Stoichiometric Ratio 1:1) at Different Grinding Times
2.2.3. Evaluation of the Amorphization Ability of the PGZ·HCl
2.2.4. Evaluation of the Formation of the PGZ·HCl-FLV Solid Forms (1:2; 1:4; 1:6; 1:8 and 1:10)
2.2.5. Evaluation of the Formation of the PGZ·HCl-FLV Solid Forms (2:1; 4:1; 6:1; 8:1 and 10:1)
2.2.6. Thermal Analysis
2.2.7. X-ray Powder Diffraction (XRPD)
2.2.8. Nuclear Magnetic Resonance
2.2.9. FT-IR
2.2.10. Intrinsic Dissolution Studies
2.2.11. Eutectic Binary Mixture Screening by DSC Data
2.2.12. Scanning Electron Microscopy Studies (SEM)
2.2.13. X-ray Photoelectron Spectroscopy (XPS)
3. Results
3.1. NG and LAG Solvent Screening (Stoichiometry 1:1)
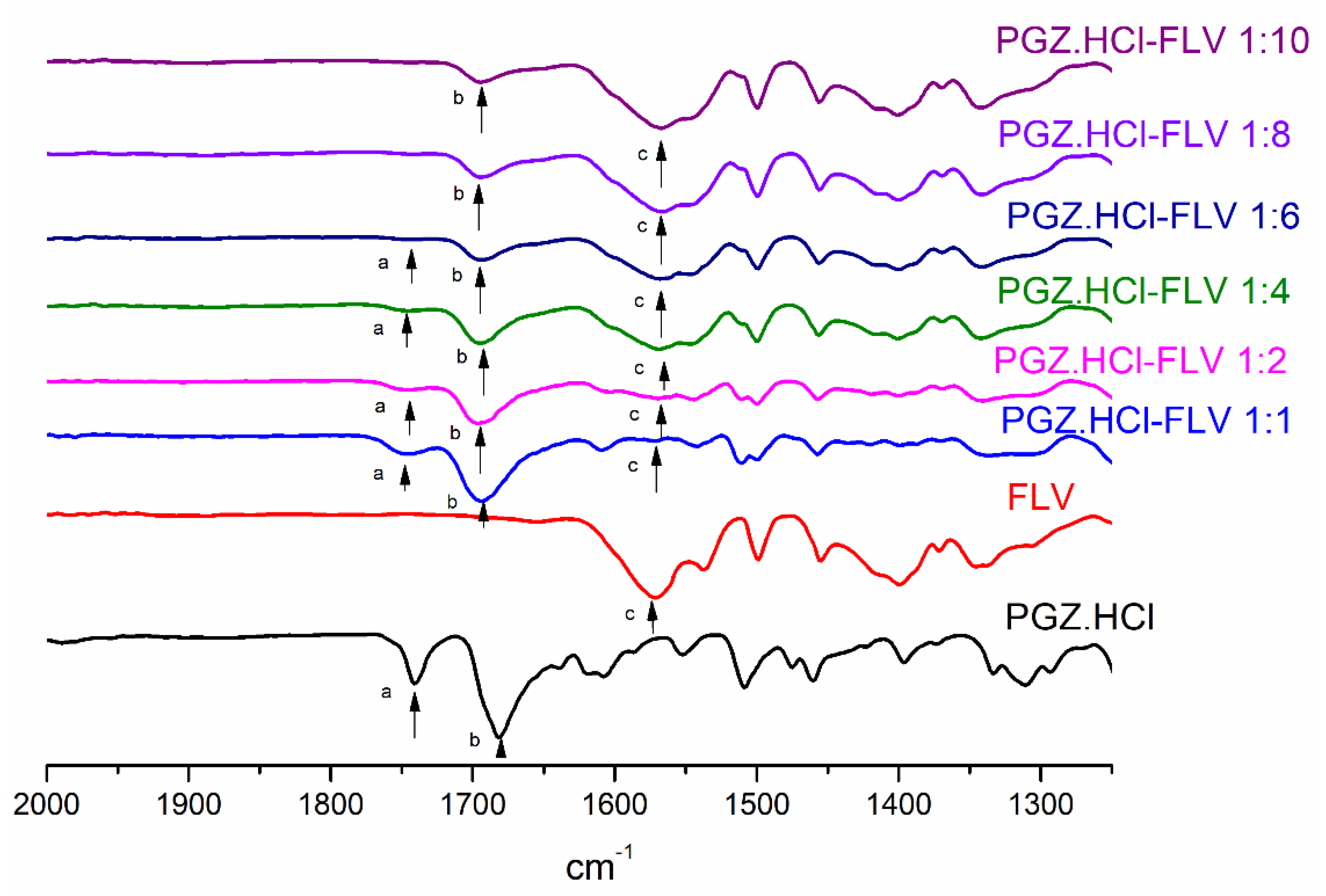
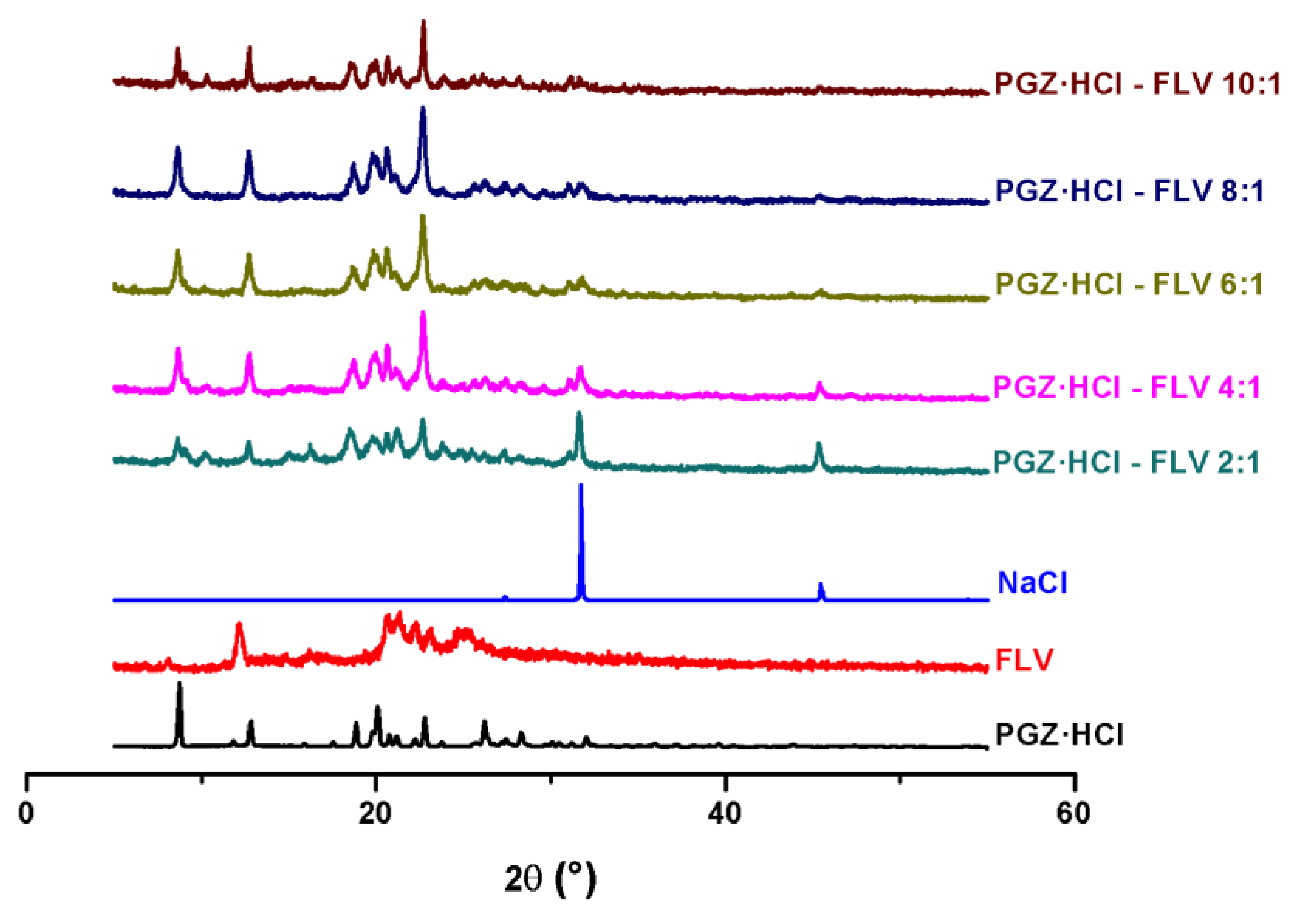
3.2. Evaluation of the Formation of the PGZ·HCl-FLV Solid Forms (1:2; 1:4; 1:6; 1:8 and 1:10)
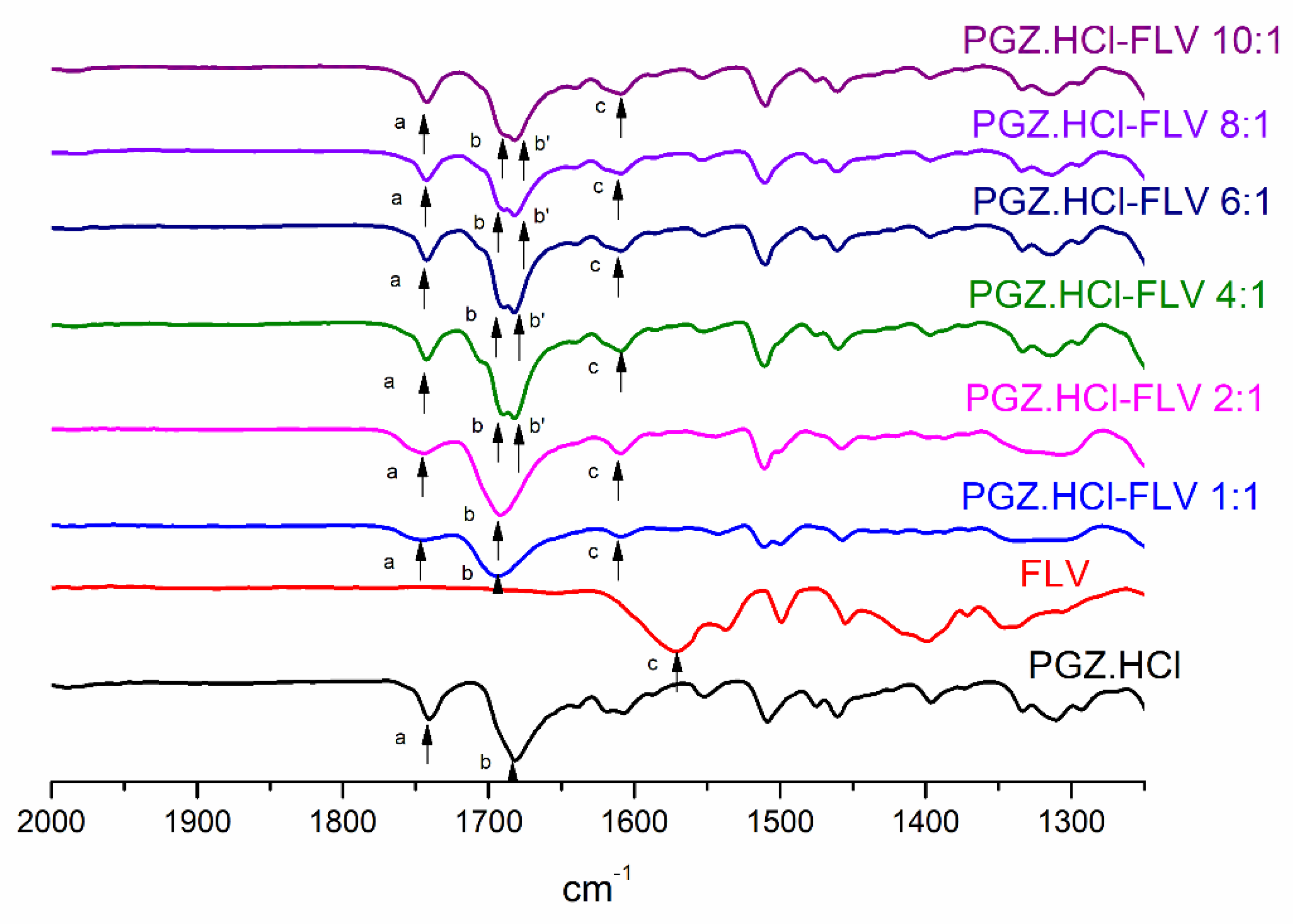
3.3. Evaluation of the Formation of the PGZ·HCl-FLV Solid Forms (2:1; 4:1; 6:1; 8:1 and 10:1)
3.4. Eutectic Screening to Predict the Most Stable Coamorphous Molar Ratio
3.5. SEM
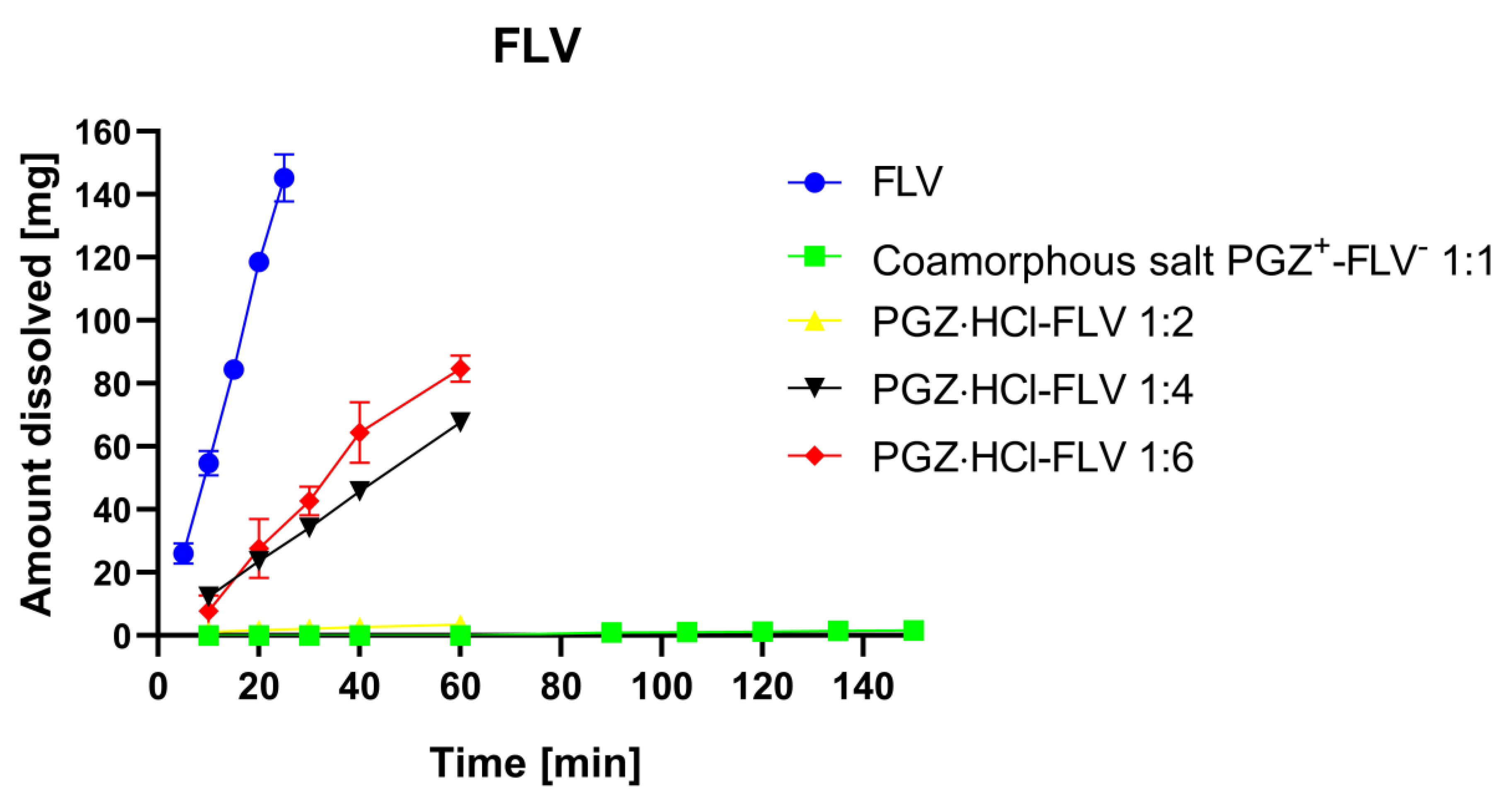
3.6. Determination of Dissolution Profiles
- (A)
- The drug dissolves and is released rapidly into the solution, precipitously raising the concentration of molecules, and subsequently, the drug is precipitated by the amorphous → crystal transformation.
- (B)
- Simultaneously, the drug and polymer are progressively released, while the drug remains amorphous on the surface of the undissolved particles.
- (C)
- The drug and polymer are progressively released; however, the drug is in the form of crystals on the surface of the undissolved particles.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koch, C. Statin Therapy. Curr. Pharm. Des. 2012, 18, 6284–6290. [Google Scholar] [CrossRef]
- Borgmann, S.H.M.; Bernardi, L.S.; Rauber, G.S.; Oliveira, P.R.; Campos, C.E.M.; Monti, G.; Cuffini, S.L.; Cardoso, S.G. Solid-state characterization and dissolution properties of Fluvastatin sodium salt hydrates. Pharm. Dev. Technol. 2013, 18, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Plosker, G.L.; Wagstaff, A.J. Fluvastatin. Drugs 1996, 51, 433–459. [Google Scholar] [CrossRef] [PubMed]
- Balakumar, P.; Mahadevan, N. Interplay between statins and PPARs in improving cardiovascular outcomes: A double-edged sword? Br. J. Pharmacol. 2012, 165, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Tonstad, S.; Retterstøl, K.; Ose, L.; Öhman, K.P.; Lindberg, M.B.; Svensson, M. The dual peroxisome proliferator-activated receptor α/γ agonist tesaglitazar further improves the lipid profile in dyslipidemic subjects treated with atorvastatin. Metabolism 2007, 56, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Barilla, D.; Prasad, P.; Hubert, M.; Gumbhir-Shah, K. Steady-state pharmacokinetics of fluvastatin in healthy subjects following a new extended release fluvastatin tablet, Lescol® XL. Biopharm. Drug Dispos. 2004, 25, 51–59. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, N.; Kaur, G. Central composite designed solid dispersion for dissolution enhancement of fluvastatin sodium by kneading technique. Ther. Deliv. 2020, 11, 313–328. [Google Scholar] [CrossRef]
- Sharma, N.; Singh, S.; Kaur, G.; Arora, S. Preformulation studies of fluvastatin sodium with polyvinyl pyrollidone K-30 and polyethylene glycol 6000. Plant Arch. 2019, 19, 1373–1377. [Google Scholar]
- El-Helw, A.R.M.; Fahmy, U.A. Improvement of fluvastatin bioavailability by loading on nanostructured lipid carriers. Int. J. Nanomed. 2015, 10, 5797–5804. [Google Scholar] [CrossRef]
- Papageorgiou, G.; Papadimitriou, S.; Karavas, E.; Georgarakis, E.; Docoslis, A.; Bikiaris, D. Improvement in Chemical and Physical Stability of Fluvastatin Drug Through Hydrogen Bonding Interactions with Different Polymer Matrices. Curr. Drug Deliv. 2009, 6, 101–112. [Google Scholar] [CrossRef]
- Panda, H.S.; Srivastava, R.; Bahadur, D. In-vitro release kinetics and stability of anticardiovascular drugs-intercalated layered double hydroxide nanohybrids. J. Phys. Chem. B 2009, 113, 15090–15100. [Google Scholar] [CrossRef]
- Solares-Briones, M.; Coyote-Dotor, G.; Páez-Franco, J.C.; Zermeño-Ortega, M.R.; de la O Contreras, C.M.; Canseco-González, D.; Avila-Sorrosa, A.; Morales-Morales, D.; Germán-Acacio, J.M. Mechanochemistry: A Green Approach in the Preparation of Pharmaceutical Cocrystals. Pharmaceutics 2021, 13, 790. [Google Scholar] [CrossRef]
- Muñoz Tecocoatzi, M.F.; Páez-Franco, J.C.; Coyote-Dotor, G.; Dorazco-González, A.; Miranda-Ruvalcaba, R.; Morales-Morales, D.; Germán-Acacio, J.M. Mecanoquímica: Una herramienta importante en la reactividad en el Estado Sólido Mechanochemistry: An important tool in solid-state reactivity. Tecnociencia Chihuahua 2022, 16, e973. [Google Scholar] [CrossRef]
- Karagianni, A.; Kachrimanis, K.; Nikolakakis, I. Co-Amorphous Solid Dispersions for Solubility and Absorption Improvement of Drugs: Composition, Preparation, Characterization and Formulations for Oral Delivery. Pharmaceutics 2018, 10, 98. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Moinuddin, S.M.; Cai, T. Advances in coamorphous drug delivery systems. Acta Pharm. Sin. B 2019, 9, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Chavan, R.B.; Thipparaboina, R.; Kumar, D.; Shastri, N.R. Co amorphous systems: A product development perspective. Int. J. Pharm. 2016, 515, 403–415. [Google Scholar] [CrossRef]
- Zakeri-Milani, P.; Barzegar-Jalali, M.; Azimi, M.; Valizadeh, H. Biopharmaceutical classification of drugs using intrinsic dissolution rate (IDR) and rat intestinal permeability. Eur. J. Pharm. Biopharm. 2009, 73, 102–106. [Google Scholar] [CrossRef]
- Newman, A.; Zografi, G. Commentary: Considerations in the Measurement of Glass Transition Temperatures of Pharmaceutical Amorphous Solids. AAPS PharmSciTech 2020, 21, 26. [Google Scholar] [CrossRef]
- Kapourani, A.; Vardaka, E.; Katopodis, K.; Kachrimanis, K.; Barmpalexis, P. Crystallization tendency of APIs possessing different thermal and glass related properties in amorphous solid dispersions. Int. J. Pharm. 2020, 579, 119149. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.chemsrc.com/en/cas/93957-54-1_237852.html (accessed on 13 December 2022).
- International Agency for Research on Cancer; WHO. Pioglitazone and Rosiglitazone; WHO: Geneva, Switzerland, 2016; Volume 108, pp. 319–373. [Google Scholar]
- Rawlinson, C.F.; Williams, A.C.; Timmins, P.; Grimsey, I. Polymer-mediated disruption of drug crystallinity. Int. J. Pharm. 2007, 336, 42–48. [Google Scholar] [CrossRef]
- Secretaría de Salud, Comisión Permanente de la Farmacopea de los Estados Unidos Mexicanos. Farmacopea de los Estados Unidos Mexicanos (FEUM), 13th ed.; Secretaría de Salud, Comisión permanente de la Farmacopea de los Estados Unidos Mexicanos: Colonia Cuauhtémoc, Mexico, 2022. [Google Scholar]
- Rycerz, L. Practical remarks concerning phase diagrams determination on the basis of differential scanning calorimetry measurements. J. Therm. Anal. Calorim. 2013, 113, 231–238. [Google Scholar] [CrossRef]
- Coyote-Dotor, G.; Páez-Franco, J.C.; Canseco-González, D.; Núñez-Pineda, A.; Dorazco-González, A.; Fuentes-Noriega, I.; Vilchis-Néstor, A.R.; Rodríguez-Hernández, J.; Morales-Morales, D.; Germán-Acacio, J.M. Synthesis, Characterization, and Intrinsic Dissolution Studies of Drug—Drug Eutectic Solid Forms of Metformin Hydrochloride and Thiazide Diuretics. Pharmaceutics 2021, 13, 1926. [Google Scholar] [CrossRef] [PubMed]
- Chadha, K.; Karan, M.; Chadha, R.; Bhalla, Y.; Vasisht, K. Is Failure of Cocrystallization Actually a Failure? Eutectic Formation in Cocrystal Screening of Hesperetin. J. Pharm. Sci. 2017, 106, 2026–2036. [Google Scholar] [CrossRef] [PubMed]
- Multipack, version 9.6.0.15, 2015-02-19, Ulvac-phi. Physical Electronics: Chanhassen, MN, USA, 1994–2014.
- Crist, B.V. 17 January 2004 SDP v 4.1 (32 bit) Copyright ©2004, XPS International, LLC., Compiled 17 January 2004. Available online: http://www.xpsdata.com (accessed on 8 December 2022).
- Poornima, B.; Prasad, K.V.S.R.G.; Bharathi, K. Solid-State Screening and Evaluation of Pioglitazone Hydrochloride. Curr. Pharm. Anal. 2017, 14, 8–16. [Google Scholar] [CrossRef]
- Newman, A.; Engers, D.; Bates, S.; Ivanisevic, I.; Kelly, R.C.; Zografi, G. Characterization of amorphous API:Polymer mixtures using X-ray powder diffraction. J. Pharm. Sci. 2008, 97, 4840–4856. [Google Scholar] [CrossRef] [PubMed]
- Shamblin, S.L.; Zografi, G. Enthalpy relaxation in binary amorphous mixtures containing sucrose. Pharm. Res. 1998, 15, 1828–1834. [Google Scholar] [CrossRef]
- Su, M.; Xia, Y.; Shen, Y.; Heng, W.; Wei, Y.; Zhang, L.; Gao, Y.; Zhang, J.; Qian, S. A novel drug-drug coamorphous system without molecular interactions: Improve the physicochemical properties of tadalafil and repaglinide. RSC Adv. 2019, 10, 565–583. [Google Scholar] [CrossRef]
- Jensen, K.T.; Blaabjerg, L.I.; Lenz, E.; Bohr, A.; Grohganz, H.; Kleinebudde, P.; Rades, T.; Löbmann, K. Preparation and characterization of spray-dried co-amorphous drug–amino acid salts. J. Pharm. Pharmacol. 2016, 68, 615–624. [Google Scholar] [CrossRef]
- Shayanfar, A.; Jouyban, A. Drug-drug coamorphous systems: Characterization and physicochemical properties of coamorphous atorvastatin with carvedilol and glibenclamide. J. Pharm. Innov. 2013, 8, 218–228. [Google Scholar] [CrossRef]
- Shamblin, S.L.; Huang, E.Y.; Zografi, G. The effects of co-lyophilized polymeric additives on the glass transition temperature and crystallization of amorphous sucrose. J. Therm. Anal. 1996, 47, 1567–1579. [Google Scholar] [CrossRef]
- Wu, W.; Wang, Y.; Löbmann, K.; Grohganz, H.; Rades, T. Transformations between Co-Amorphous and Co-Crystal Systems and Their Influence on the Formation and Physical Stability of Co-Amorphous Systems. Mol. Pharm. 2019, 16, 1294–1304. [Google Scholar] [CrossRef]
- Friščić, T. New opportunities for materials synthesis using mechanochemistry. J. Mater. Chem. 2010, 20, 7599–7605. [Google Scholar] [CrossRef]
- Jayasankar, A.; Somwangthanaroj, A.; Shao, Z.J.; Rodríguez-Hornedo, N. Cocrystal formation during cogrinding and storage is mediated by amorphous phase. Pharm. Res. 2006, 23, 2381. [Google Scholar] [CrossRef]
- Seefeldt, K.; Miller, J.; Alvarez-Nunez, F.; Rodriguez-Hornedo, N. Crystallization Pathways and Kinetics of Carbamazepine-Nicotinamide Cocrystals from the Amorphous State by In Situ Thermomicroscopy, Spectroscopy, and Calorimetry Studies. J. Pharm. Sci. 2007, 96, 1147–1158. [Google Scholar] [CrossRef] [PubMed]
- Tothadi, S.; Shaikh, T.R.; Gupta, S.; Dandela, R.; Vinod, C.P.; Nangia, A.K. Can We Identify the Salt–Cocrystal Continuum State Using XPS? Cryst. Growth Des. 2021, 21, 735–747. [Google Scholar] [CrossRef]
- Al-Majed, A.; Bakheit, A.H.H.; Abdel Aziz, H.A.; Alharbi, H.; Al-Jenoobi, F.I. Chapter Five—Pioglitazone. In Profiles of Drug Substances, Excipients and Related Methodology; Academic Press: Cambridge, MA, USA, 2016; Volume 41, pp. 379–438. ISBN 1871-5125. [Google Scholar]
- Levy, G.C.; Lichter, R.L. Nitrogen-15 Nuclear Magnetic Resonance Spectroscopy; Wiley: Hoboken, NJ, USA, 1979; ISBN 9780471029540. [Google Scholar]
- Stevens, J.S.; Byard, S.J.; Seaton, C.C.; Sadiq, G.; Davey, R.J.; Schroeder, S.L.M. Proton transfer and hydrogen bonding in the organic solid state: A combined XRD/XPS/ssNMR study of 17 organic acid-base complexes. Phys. Chem. Chem. Phys. 2014, 16, 1150–1160. [Google Scholar] [CrossRef] [PubMed]
- Skorupska, E.; Kaźmierski, S.; Potrzebowski, M.J. Solid State NMR Characterization of Ibuprofen: Nicotinamide Cocrystals and New Idea for Controlling Release of Drugs Embedded into Mesoporous Silica Particles. Mol. Pharm. 2017, 14, 1800–1810. [Google Scholar] [CrossRef]
- Cerreia Vioglio, P.; Catalano, L.; Vasylyeva, V.; Nervi, C.; Chierotti, M.R.; Resnati, G.; Gobetto, R.; Metrangolo, P. Natural Abundance15N and13C Solid-State NMR Chemical Shifts: High Sensitivity Probes of the Halogen Bond Geometry. Chem. A. Eur. J. 2016, 22, 16819–16828. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Chu, Y.; Wang, L.; Yu, K.; Zhang, H.; Deng, Z. Structure determination of the theophylline—Nicotinamide cocrystal: A combined powder calculation study. CrystEngComm 2014, 16, 3141–3147. [Google Scholar] [CrossRef]
- Solum, M.S.; Altmann, K.L.; Strohmeier, M.; Berges, D.a.; Zhang, Y.; Facelli, J.C.; Pugmire, R.J.; Grant, D.M. 15N Chemical Shift Principal Values in Nitrogen Heterocycles Chemical Shift Principal Values in Nitrogen Heterocycles. J. Am. Chem. Soc. 1997, 119, 9804–9809. [Google Scholar] [CrossRef]
- Marek, R.; Lycka, A.; Kolehmainen, E.; Sievänen, E.; Tousek, J. 15N NMR Spectroscopy in Structural Analysis: An Update (2001–2005). Curr. Org. Chem. 2007, 11, 1154–1205. [Google Scholar] [CrossRef]
- Li, Z.J.; Abramov, Y.; Bordner, J.; Leonard, J.; Medek, A.; Trask, A. V Solid-State Acid−Base Interactions in Complexes of Heterocyclic Bases with Dicarboxylic Acids: Crystallography, Hydrogen Bond Analysis, and 15N NMR Spectroscopy. J. Am. Chem. Soc. 2006, 128, 8199–8210. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Hanrahan, M.P.; Chakravarty, P.; DiPasquale, A.G.; Sirois, L.E.; Nagapudi, K.; Lubach, J.W.; Rossini, A.J. Characterization of Pharmaceutical Cocrystals and Salts by Dynamic Nuclear Polarization-Enhanced Solid-State NMR Spectroscopy. Cryst. Growth Des. 2018, 18, 2588–2601. [Google Scholar] [CrossRef]
- Bolla, G.; Nangia, A. Novel pharmaceutical salts of albendazole. CrystEngComm 2018, 20, 6394–6405. [Google Scholar] [CrossRef]
- Stevens, J.S.; Byard, S.J.; Schroeder, S.L.M. Salt or Co-Crystal? Determination of Protonation State by X-Ray Photoelectron Spectroscopy (XPS). J. Pharm. Sci. 2010, 99, 4453–4457. [Google Scholar] [CrossRef] [PubMed]
- Childs, S.L.; Stahly, G.P.; Park, A. The Salt–Cocrystal Continuum: The Influence of Crystal Structure on Ionization Stat. Mol. Pharm. 2007, 4, 323–338. [Google Scholar] [CrossRef]
- Available online: https://go.drugbank.com/salts/DBSALT000555 (accessed on 13 December 2022).
- Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Fluvastatin-sodium#section=Stability-Shelf-Life (accessed on 13 December 2022).
- Stevens, J.S.; Newton, L.K.; Jaye, C.; Muryn, C.A.; Fischer, D.A.; Schroeder, S.L.M. Proton Transfer, Hydrogen Bonding, and Disorder: Nitrogen Near-Edge X-ray Absorption Fine Structure and X-ray Photoelectron Spectroscopy of Bipyridine–Acid Salts and Co-crystals. Cryst. Growth Des. 2015, 15, 1776–1783. [Google Scholar] [CrossRef]
- Pandit, V.; Gorantla, R.; Devi, K.; Pai, R.S.; Sarasija, S. Preparation and Characterization of Pioglitazone Cyclodextrin Inclusion Complexes. J. Young Pharm. 2011, 3, 267–274. [Google Scholar] [CrossRef]
- Kissi, E.O.; Khorami, K.; Rades, T. Determination of stable co-amorphous drug–drug ratios from the eutectic behavior of crystalline physical mixtures. Pharmaceutics 2019, 11, 628. [Google Scholar] [CrossRef]
- Babu, N.J.; Nangia, A. Solubility advantage of amorphous drugs and pharmaceutical cocrystals. Cryst. Growth Des. 2011, 11, 2662–2679. [Google Scholar] [CrossRef]
- Alonzo, D.E.; Zhang, G.G.Z.; Zhou, D.; Gao, Y.; Taylor, L.S. Understanding the behavior of amorphous pharmaceutical systems during dissolution. Pharm. Res. 2010, 27, 608–618. [Google Scholar] [CrossRef] [PubMed]
- Brough, C.; Williams, R.O. Amorphous solid dispersions and nano-crystal technologies for poorly water-soluble drug delivery. Int. J. Pharm. 2013, 453, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, H.R.; Tawa, M.; Zhang, Z.; Ratanabanangkoon, P.; Shaw, P.; Gardner, C.R.; Chen, H.; Moreau, J.; Almarsson, Ö.; Remenar, J.F. Combined Use of Crystalline Salt Forms and Precipitation Inhibitors to Improve Oral Absorption of Celecoxib from Solid Oral Formulations. J. Pharm. Sci. 2007, 96, 2686–2702. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Dai, W.-G. Fundamental aspects of solid dispersion technology for poorly soluble drugs. Acta Pharm. Sin. B 2014, 4, 18–25. [Google Scholar] [CrossRef]
- Vullendula, S.K.A.; Nair, A.R.; Yarlagadda, D.L.; Navya Sree, K.S.; Bhat, K.; Dengale, S.J. Polymeric solid dispersion Vs co-amorphous technology: A critical comparison. J. Drug Deliv. Sci. Technol. 2022, 78, 103980. [Google Scholar] [CrossRef]
- Dengale, S.J.; Grohganz, H.; Rades, T.; Löbmann, K. Recent advances in co-amorphous drug formulations. Adv. Drug Deliv. Rev. 2016, 100, 116–125. [Google Scholar] [CrossRef]
- Jouyban, A.; Soltanpour, S. Solubility of pioglitazone hydrochloride in binary and ternary mixtures of water, propylene glycol, and polyethylene glycols 200, 400, and 600 at 298.2 K. AAPS PharmSciTech 2010, 11, 1713–1717. [Google Scholar] [CrossRef] [PubMed]
- Satheeshkumar, N.; Shantikumar, S.; Srinivas, R. Pioglitazone: A review of analytical methods. J. Pharm. Anal. 2014, 4, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Jouyban, A.; Soltanpour, S. Solubility of pioglitazone hydrochloride in binary mixtures of polyethylene glycol 400 with ethanol, propylene glycol, N-methyl-2-pyrrolidone, and water at 25 °C. Chem. Pharm. Bull. 2010, 58, 1132–1135. [Google Scholar] [CrossRef] [PubMed]
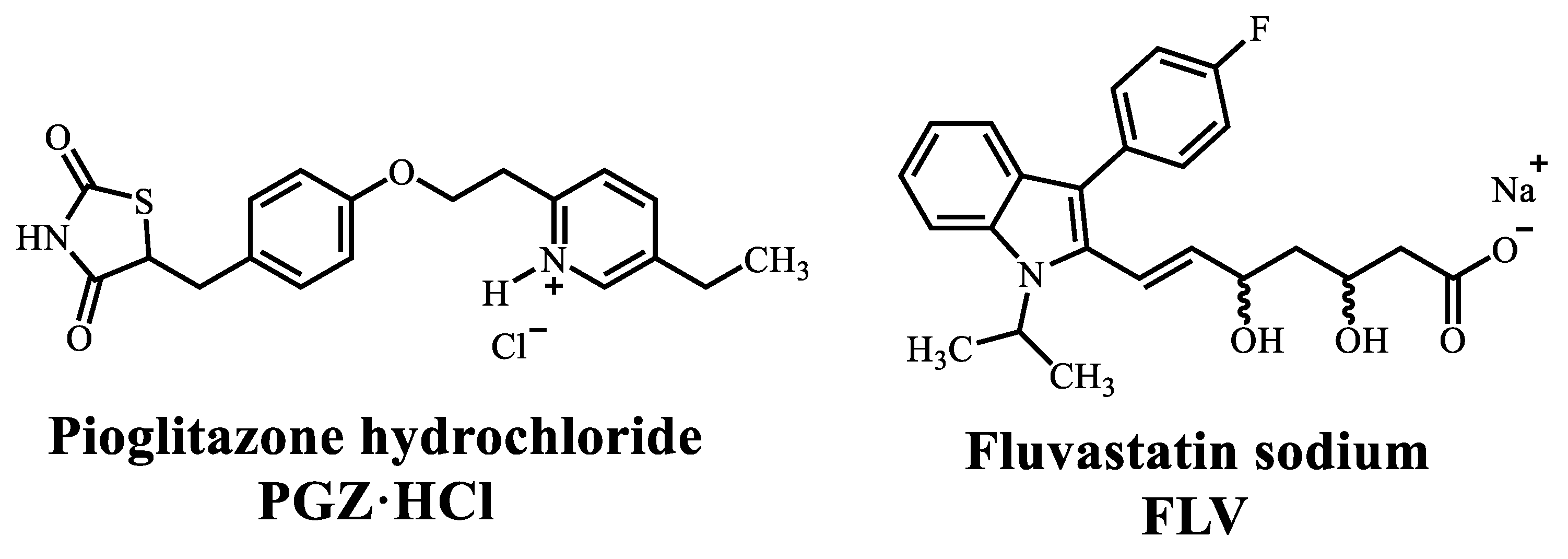
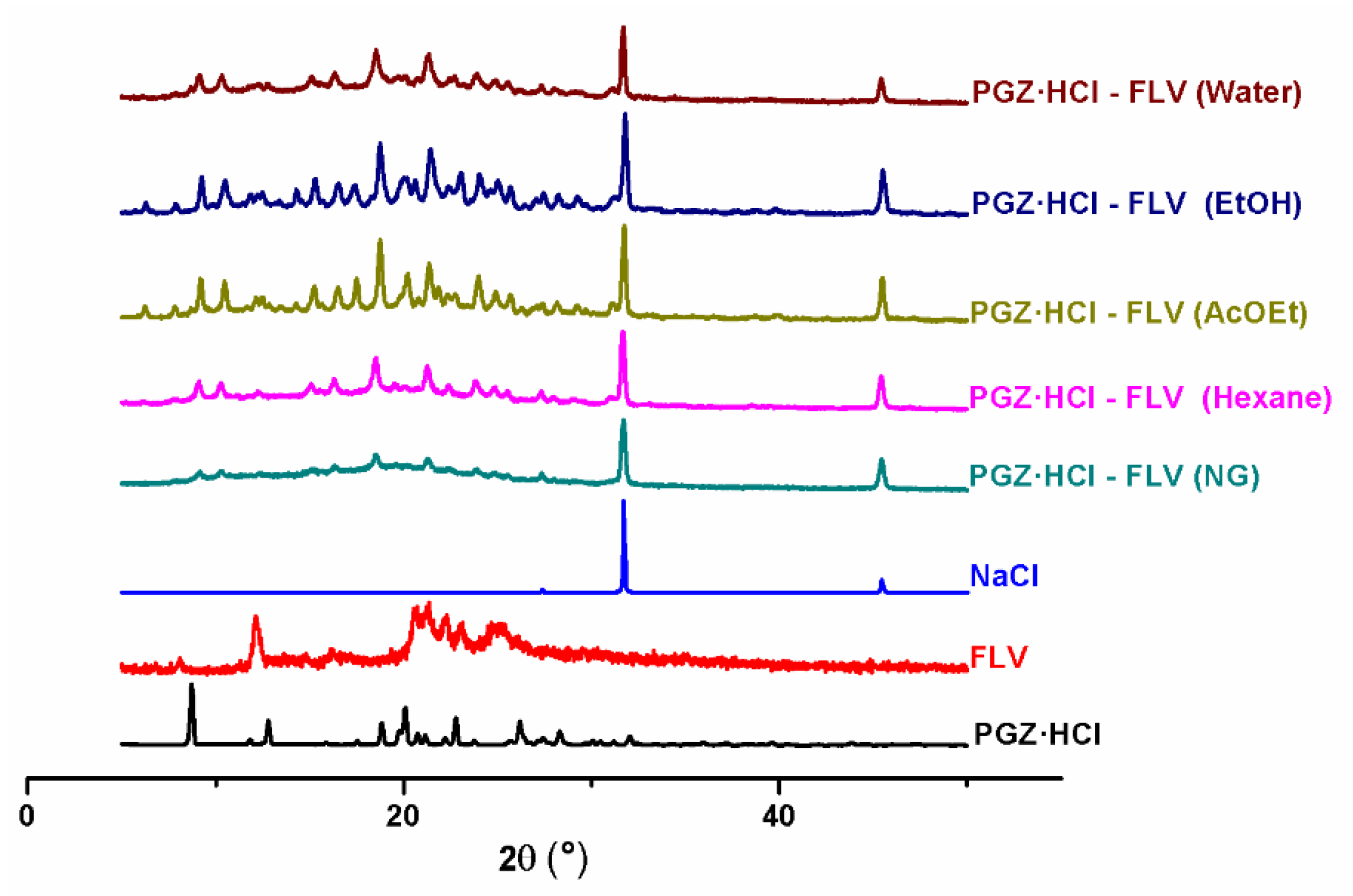

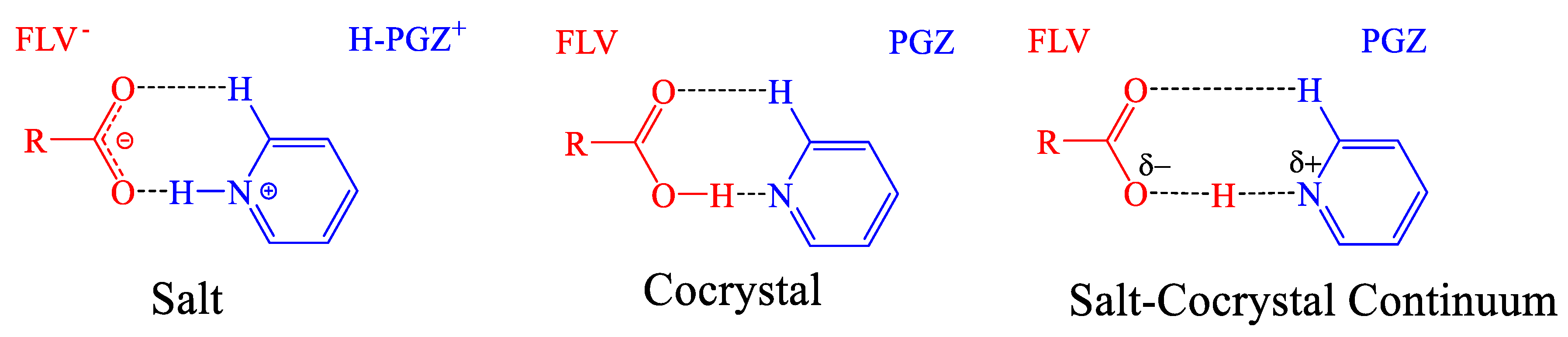
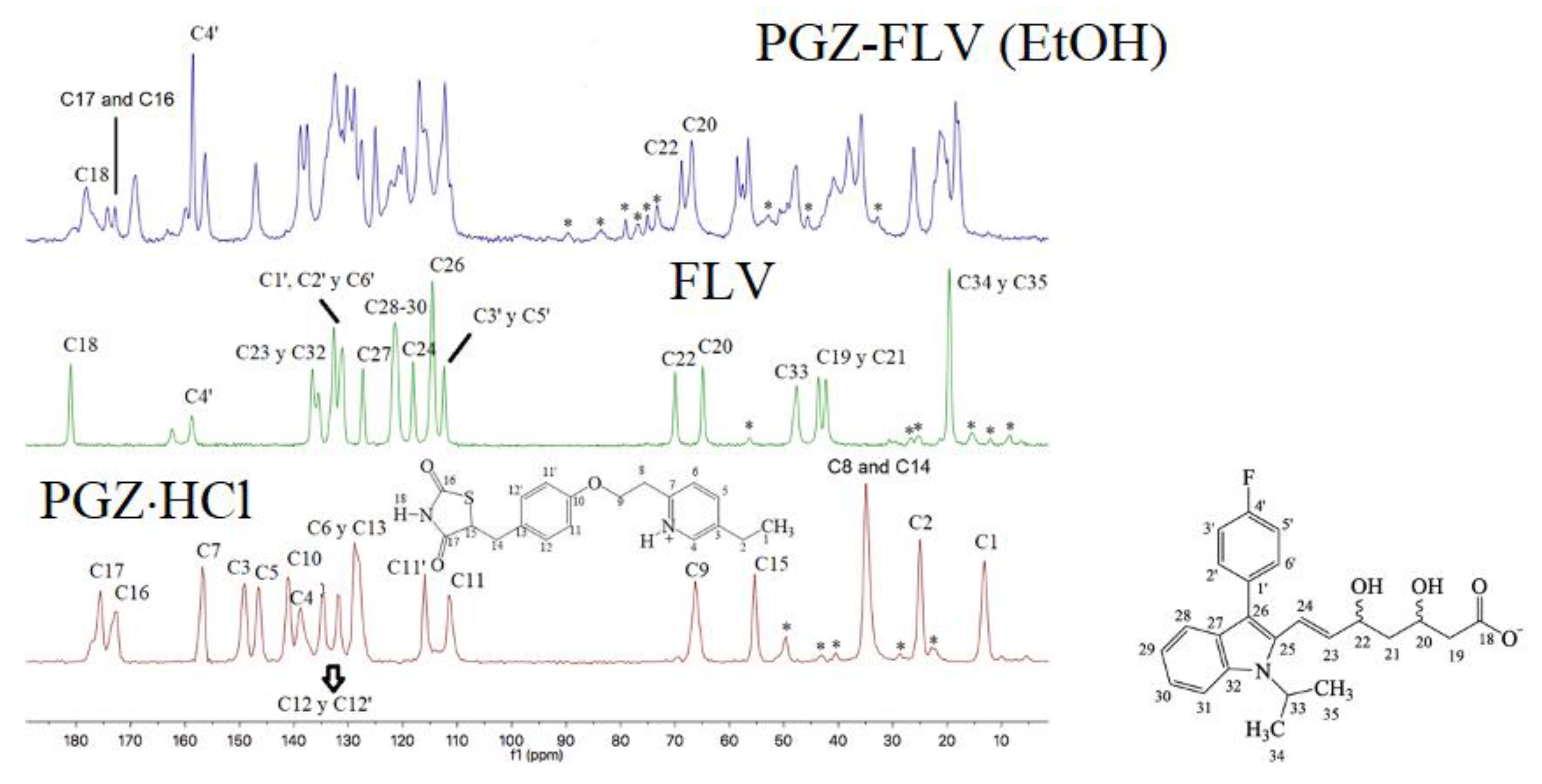




| Outcome NG or LAG Solvent Screening | PGZ⋅HCl (mg) | FLV (mg) | PGZ⋅HCl (%w) | FLV (%w) | Tfirst peak (°C) | Tonset second peak (°C) | Tm second peak (°C) | ΔHm second peak J/g | Tg exp/Tg clcd °C | %Cristallinity PGZ⋅HCl | %Cristallinity FLV |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PGZ⋅HCl | - | - | - | - | - | 190.0 | 197.8 | 125.5 | 64.4 [19] | - | - |
| FLV | - | - | - | 78.6 | 186.2 | 215.6 | 97.0 | 69.5 [10] | - | - | |
| NG | 97.46 | 107.49 | 46.86 | 53.14 | Tc: 95.8 exo | Tm: 140.6 | Tm: 154.5 | 38.73 | 56.2/67.07 | 65.85 | 75.13 |
| Hexane | 97.46 | 107.49 | 46.86 | 53.14 | - | Tm: 135.5 | Tm: 155.3 | 46.16 | 61.7/67.07 | 78.49 | 89.55 |
| AcOEt | 97.46 | 107.49 | 46.86 | 53.14 | Tm: 115.2 | - | Tm: 156.9 | 98.8 | - | - | - |
| EtOH | 97.46 | 107.49 | 46.86 | 53.14 | - | 188.4 | 196.5 | 223.6 | 108.8/44.28 | ||
| Water | 97.46 | 107.49 | 46.86 | 53.14 | - | 136.8 | 156.8 | 47.95 | 71.1 and 93.5/44.28 | - | - |
| Stoichiometric Ratios | PGZ⋅HCl (mg) | FLV (mg) | PGZ⋅HCl (%w) | FLV (%w) | Tfus first peak(°C) | Tc (°C) | Tonset melting (°C) | Tm (°C) | ΔHm J/g | Tg exp/ Tg clcd °C | %Cristallinity PGZ⋅HCl | %Cristallinity FLV |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1:2 | 94.5 | 205.7 | 31.48 | 68.52 | 59.3 | 116.0 | 132.2 | 154.2 | 39.12 | 42.4/67.86 | 99.01 | 58.85 |
| 1:4 | 58.3 | 252.7 | 18.75 | 81.25 | 68.5 | - | 119.4 | 164.6 | 64.71 | 46.0/68.52 | - | - |
| 1:6 | 47.4 | 304.5 | 13.47 | 86.53 | 66.6 | - | 135.3 | 167.0 | 54.68 | 48.7/68.79 | - | - |
| 1:8 | 33.9 | 267.3 | 11.25 | 88.75 | 69.1 | - | 122.5 | 169.1 | 52.59 | 49.9/68.91 | - | - |
| 1:10 | 31.5 | 332.0 | 8.67 | 91.33 | 69.4 | - | 119.8 | 170.1 | 48.2 | 50.9/69.04 | - | - |
| Vibrational Band Assignment | PGZ⋅HCl | FLV | PGZ⋅HCl-FLV (1:1) | PGZ⋅HCl-FLV (1:2) | PGZ⋅HCl-FLV (1:4) | PGZ⋅HCl-FLV (1:6) | PGZ⋅HCl-FLV (1:8) | PGZ⋅HCl-FLV (1:10) |
|---|---|---|---|---|---|---|---|---|
| −C=OPGZ (a, b) (Δν cm−1) | a: 1741 b: 1682 | a: 1743 (2) b: 1693 (9) | a: 1744 (3) b: 1697 (15) | a: 1743 (2) b: 1695 (13) | a: 1740 (1) b: 1693 (11) | a: 1740 (1) b: 1693 (11) | a: 1740 (1) b: 1693 (11) | |
| −C=OFLV (c) (Δν cm−1) | 1572 | 1548 (24) | 1546 (26) | 1568 (8) | 1565 (7) | 1567 (5) | 1565 (7) |
| Stoichiometric Ratios | PGZ⋅HCl (mg) | FLV (mg) | PGZ⋅HCl (%w) | FLV (%w) | Tfus first peak (°C) | Tonset second peak (°C) | Tm second peak (°C) | ΔHm second peak J/g | Tg exp/Tg clcd °C |
|---|---|---|---|---|---|---|---|---|---|
| 2:1 | 202.7 | 113.9 | 64.02 | 35.98 | - | N.D. | 155.9 | 18.5 | 47.2/66.20 |
| 4:1 | 251.6 | 69.6 | 78.33 | 21.67 | 155.7 | N.D. | 155.7 | 14.9 | 54.5/65.48 |
| 6:1 | 302.7 | 57.7 | 83.99 | 16.01 | 155.1 | N.D. | 155.1 | 9.34 | 59.6/65.19 |
| 8:1 | 303.5 | 43.5 | 87.46 | 12.54 | 156.3 | N.D. | 156.3 | 6.53 | 52.6/65.02 |
| 10:1 | 302.6 | 34.9 | 89.66 | 10.34 | 155.6 | N.D. | 155.6 | 5.28 | 52.0/64.91 |
| Vibrational Band Assignment | PGZ⋅HCl | FLV | PGZ⋅HCl-FLV (2:1) | PGZ⋅HCl-FLV (4:1) | PGZ⋅HCl-FLV (6:1) | PGZ⋅HCl-FLV (8:1) | PGZ⋅HCl-FLV (10:1) |
|---|---|---|---|---|---|---|---|
| −C=OPGZ (a,b,b’) (Δν cm−1) | a: 1741 b: 1682 | a: 1742 (1) b: 1692 (10) | a: 1742 (1) b: 1682 (0) b’: 1680 (2) | a: 1743 (2) b: 1682 (10) b’: 1680 (2) | a: 1743 (2) b: 1682 (0) b’: 1678 (4) | a: 1743 (2) b: 1684 (2) b’: 1680 (2) | |
| −C=OFLV (c) (Δν cm−1) | 1572 | 1609 (37) | 1609 (37) | 1608 (36) | 1609 (37) | 1608 (36) |
| . | Pure FLV | Pure PGZ⋅HCl | Coamorphous Salt (1:1) | PGZ⋅HCl-FLV (1:6) | PGZ⋅HCl-FLV (1:10) | PGZ⋅HCl-FLV (6:1) | PGZ⋅HCl-FLV (10:1) |
|---|---|---|---|---|---|---|---|
| Morphology | Flakes | Prism shaped | Compacted poor defined prismatic forms | Prismatic forms with different sizes | Rough shapes mixed with flakes | A mix of well-defined prismatic shapes with irregular prismatic | Prism-shaped poorly defined |
| Pure FLV | Coamorphous Salt PGZ-FLV (1:1) | PGZ⋅HCl-FLV (1:2) | PGZ⋅HCl-FLV (1:4) | PGZ⋅HCl-FLV (1:6) | |
|---|---|---|---|---|---|
| Kint mg/cm2⋅min−1 | 13.6270 ± 0.8127 | 0.0220 ± 0.0014 | 0.1057 ± 0.0113 | 2.4953 ± 0.0309 | 3.5049 ± 0.3547 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villeda-Villegas, M.; Páez-Franco, J.C.; Coyote-Dotor, G.; Núñez-Pineda, A.; Dorazco-González, A.; Fuentes-Noriega, I.; Rubio-Carrasco, K.; Toledo Jaldín, H.P.; Morales-Morales, D.; Germán-Acacio, J.M. Diversity of Solid Forms Promoted by Ball Milling: Characterization and Intrinsic Dissolution Studies of Pioglitazone Hydrochloride and Fluvastatin Sodium Drug–Drug Systems. Pharmaceuticals 2023, 16, 781. https://doi.org/10.3390/ph16060781
Villeda-Villegas M, Páez-Franco JC, Coyote-Dotor G, Núñez-Pineda A, Dorazco-González A, Fuentes-Noriega I, Rubio-Carrasco K, Toledo Jaldín HP, Morales-Morales D, Germán-Acacio JM. Diversity of Solid Forms Promoted by Ball Milling: Characterization and Intrinsic Dissolution Studies of Pioglitazone Hydrochloride and Fluvastatin Sodium Drug–Drug Systems. Pharmaceuticals. 2023; 16(6):781. https://doi.org/10.3390/ph16060781
Chicago/Turabian StyleVilleda-Villegas, Marco, José C. Páez-Franco, Guadalupe Coyote-Dotor, Alejandra Núñez-Pineda, Alejandro Dorazco-González, Inés Fuentes-Noriega, Kenneth Rubio-Carrasco, Helen P. Toledo Jaldín, David Morales-Morales, and Juan Manuel Germán-Acacio. 2023. "Diversity of Solid Forms Promoted by Ball Milling: Characterization and Intrinsic Dissolution Studies of Pioglitazone Hydrochloride and Fluvastatin Sodium Drug–Drug Systems" Pharmaceuticals 16, no. 6: 781. https://doi.org/10.3390/ph16060781
APA StyleVilleda-Villegas, M., Páez-Franco, J. C., Coyote-Dotor, G., Núñez-Pineda, A., Dorazco-González, A., Fuentes-Noriega, I., Rubio-Carrasco, K., Toledo Jaldín, H. P., Morales-Morales, D., & Germán-Acacio, J. M. (2023). Diversity of Solid Forms Promoted by Ball Milling: Characterization and Intrinsic Dissolution Studies of Pioglitazone Hydrochloride and Fluvastatin Sodium Drug–Drug Systems. Pharmaceuticals, 16(6), 781. https://doi.org/10.3390/ph16060781







