Phase II, Double-Blinded, Randomized, Placebo-Controlled Clinical Trial Investigating the Efficacy of Mebendazole in the Management of Symptomatic COVID-19 Patients
Abstract
:1. Introduction
2. Results
2.1. Study Participants
2.2. Drug Safety
2.3. Mebendazole Efficacy
2.4. Outcomes Comparison between the Groups of the Study in the Two Timeframes
2.5. Relationship of Different Parameters in the Drug and Placebo Groups
3. Discussion
4. Materials and Methods
4.1. Study Participants
4.2. Inclusion and Exclusion Criteria
4.3. Blinding, Randomization, and Sample Size Calculation
4.4. Recruitment of Study Participants
4.5. Trial Procedure
4.6. Efficacy and Safety Assessment
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chakraborty, I.; Maity, P. COVID-19 outbreak: Migration, effects on society, global environment and prevention. Sci. Total Environ. 2020, 728, 138882. [Google Scholar] [CrossRef] [PubMed]
- Domenico, C.; Vanelli, M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020, 91, 157–160. [Google Scholar]
- Worldometer. COVID-19 Coronavirus Pandemic. Available online: https://www.worldometers.info/coronavirus/ (accessed on 10 May 2022).
- Worldometer. CWG.Coronavirus Worldwide Graphs. Available online: https://www.worldometers.info/coronavirus/worldwide-graphs/#total-deaths (accessed on 25 May 2022).
- World Health Organization. Jordan: WHO Coronavirus Disease (COVID-19) Dashboard with Vaccination Data. Available online: https://covid19.who.int/region/emro/country/jo (accessed on 11 May 2022).
- Khader, Y.; Al Nsour, M. Excess mortality during the COVID-19 pandemic in Jordan: Secondary data analysis. JMIR Public Health Surveill. 2021, 7, e32559. [Google Scholar] [CrossRef] [PubMed]
- Al-Balas, M.; Al-Balas, H.I.; Alqassieh, R.; Al-Balas, H.; Al-Balas, R.; Al-Balas, S. Clinical features of COVID-19 patients in Jordan: A study of 508 patients. Open Respir. Med. J. 2021, 15, 28–34. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Cohen, M.S. Early treatment to prevent progression of SARS-CoV-2 infection. Lancet Respir. Med. 2022, 10, P930–P931. [Google Scholar] [CrossRef]
- Alamer, A.; Alrashed, A.A.; Alfaifi, M.; Alosaimi, B.; AlHassar, F.; Almutairi, M.; Howaidi, J.; Almutairi, W.; Mohzari, Y.; Sulaiman, T.; et al. Effectiveness and safety of favipiravir compared to supportive care in moderately to critically ill COVID-19 patients: A retrospective study with propensity score matching sensitivity analysis. Curr. Med. Res. Opin. 2021, 37, 1085–1097. [Google Scholar] [CrossRef]
- Udwadia, Z.F.; Singh, P.; Barkate, H.; Patil, S.; Rangwala, S.; Pendse, A.; Kadam, J.; Wu, W.; Caracta, C.F.; Tandon, M. Efficacy and safety of favipiravir, an oral RNA-dependent RNA polymerase inhibitor, in mild-to-moderate COVID-19: A randomized, comparative, open-label, multicenter, phase 3 clinical trial. Int. J. Infect. Dis. 2021, 103, 62–71. [Google Scholar] [CrossRef]
- Ministry of Health Saudi Arabia. Available online: https://www.moh.gov.sa/en/Pages/Default.aspx (accessed on 7 November 2021).
- Yamakawa, K.; Yamamoto, R.; Terayama, T.; Hashimoto, H.; Ishihara, T.; Ishimaru, G.; Imura, H.; Okano, H.; Narita, C.; Mayumi, T.; et al. Japanese Rapid/Living recommendations on drug management for COVID-19. Acute Med. Surg. 2021, 9, e789. [Google Scholar] [CrossRef]
- Bosaeed, M.; Alharbi, A.; Mahmoud, E.; Alrehily, S.; Bahlaq, M.; Gaifer, Z.; Alturkistani, H.; Alhagan, K.; Alshahrani, S.; Tolbah, A.; et al. Efficacy of favipiravir in adults with mild COVID-19: A randomized, double-blind, multicentre, placebo-controlled clinical trial. Clin. Microbiol. Infect. 2022, 28, 602–608. [Google Scholar] [CrossRef]
- Gupta, D.; Sahoo, A.K.; Singh, A. Ivermectin: Potential candidate for the treatment of COVID-19. Braz. J. Infect. Dis. 2020, 24, 369–371. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, T.; Cai, D.; Hu, Z.; Liao, H.; Zhi, L.; Wei, H.; Zhang, Z.; Qiu, Y.; Wang, J.; et al. Cytokine storm intervention in the early stages of COVID-19 pneumonia. Cytokine Growth Factor. Rev. 2020, 53, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Hu, C.; Hood, M.; Zhang, X.; Zhang, L.; Kan, J.; Du, J. A novel combination of vitamin C, curcumin and glycyrrhizic acid potentially regulates immune and inflammatory response associated with coronavirus infections: A perspective from system biology analysis. Nutrients 2020, 12, 1193. [Google Scholar] [CrossRef]
- Galal, M.W.; Ahmed, M.; Shao, Y.; Xing, C.; Ali, W.; Baly, A.E.; Elfiky, A.; Amer, K.; Schoggins, J.; Sadek, H.A.; et al. The Use of Mebendazole in COVID-19 Patients: An Observational Retrospective Single Center Study. Adv. Virol. 2022, 2022, 3014686. [Google Scholar] [CrossRef] [PubMed]
- Keystone, J.S.; Murdoch, J.K. Drugs Five Years Later: Mebendazole. Ann. Intern. Med. 1979, 91, 582–586. [Google Scholar] [CrossRef]
- Guerini, A.E.; Triggiani, L.; Maddalo, M.; Bonù, M.L.; Frassine, F.; Baiguini, A.; Alghisi, A.; Tomasini, D.; Borghetti, P.; Pasinetti, N.; et al. Mebendazole as a candidate for drug repurposing in oncology: An extensive review of current literature. Cancers 2019, 11, 1284. [Google Scholar] [CrossRef]
- Elayapillai, S.; Ramraj, S.; Benbrook, D.M.; Bieniasz, M.; Wang, L.; Pathuri, G.; Isingizwe, Z.R.; Kennedy, A.L.; Zhao, Y.D.; Lightfoot, S.; et al. Potential and mechanism of mebendazole for treatment and maintenance of ovarian cancer. Gynecol. Oncol. 2021, 160, 302–311. [Google Scholar] [CrossRef]
- Rushworth, L.K.; Hewit, K.; Munnings-Tomes, S.; Somani, S.; James, D.; Shanks, E.; Dufes, C.; Straube, A.; Patel, R.; Leung, H.Y. Repurposing screen identifies mebendazole as a clinical candidate to synergise with docetaxel for prostate cancer treatment. Br. J. Cancer 2020, 122, 517–527. [Google Scholar] [CrossRef]
- De Witt, M.; Gamble, A.; Hanson, D.; Markowitz, D.; Powell, C.; Symons, M.; Atlas, M.; Boockvar, J.; Ruggieri, R.; Symons, M. Repurposing mebendazole as a replacement for vincristine for the treatment of brain tumors. Mol. Med. 2017, 23, 50–56. [Google Scholar] [CrossRef]
- Li, Y.; Thomas, D.; Deutzmann, A.; Majeti, R.; Felsher, D.W.; Dill, D.L. Mebendazole for differentiation therapy of acute myeloid leukemia identified by a lineage maturation index. Sci. Rep. 2019, 9, 16775. [Google Scholar] [CrossRef]
- Zhang, L.; Bochkur, D.M.; Yazal, T.; Dong, K.; Nguyen, A.; Yu, G.; Dao, A.; Bochkur, D.M.; Duhachek-Muggy, S.; Bhat, K.; et al. Mebendazole potentiates radiation therapy in triple-negative breast cancer. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, S.K.; El-Azab, G.A.; Zakaria, F.; Mostafa, M.F.; El-Ghoneimy, R.A. Mebendazole; from an anti-parasitic drug to a promising candidate for drug repurposing in colorectal cancer. Life Sci. 2022, 299, 120536. [Google Scholar] [CrossRef]
- Kudo, N.; Kubota, H.; Gotoh, H.; Ishida, H.; Ikadai, H.; Oyamada, T. Efficacy of thiabendazole, mebendazole, levamisole and ivermectin against gullet worm, Gongylonema pulchrum: In vitro and in vivo studies. Vet. Parasitol. 2008, 151, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Hemphill, A.; Müller, J. Alveolar and cystic echinococcosis: Towards novel chemotherapeutical treatment options. J. Helminthol. 2009, 83, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Blom, K.; Rubin, J.; Berglund, M.; Jarvius, M.; Lenhammar, L.; Parrow, V.; Andersson, C.; Loskog, A.; Fryknäs, M.; Nygren, P.; et al. Mebendazole-induced M1 polarisation of THP-1 macrophages may involve DYRK1B inhibition. BMC Res. Notes 2019, 12, 234. [Google Scholar] [CrossRef] [PubMed]
- Andersson, C.; Selvin, T.; Blom, K.; Rubin, J.; Berglund, M.; Jarvius, M.; Lenhammar, L.; Parrow, V.; Loskog, A.; Fryknäs, M.; et al. Mebendazole is unique among tubulin-active drugs in activating the MEK–ERK pathway. Sci. Rep. 2020, 10, 13124. [Google Scholar] [CrossRef]
- Karuppagounder, S.S.; Zhai, Y.; Chen, Y.; He, R.; Ratan, R.R. The interferon response as a common final pathway for many preconditioning stimuli: Unexpected crosstalk between hypoxic adaptation and antiviral defense. Cond. Med. 2018, 1, 143–150. [Google Scholar]
- Law, J.N.; Akers, K.; Tasnina, N.; Santina, C.M.D.; Deutsch, S.; Kshirsagar, M.; Klein-Seetharaman, J.; Crovella, M.; Rajagopalan, P.; Kasif, S.; et al. Interpretable network propagation with application to expanding the repertoire of human proteins that interact with SARS-CoV-2. GigaScience 2021, 10, giab082. [Google Scholar] [CrossRef]
- Hajjo, R.; Tropsha, A. A systems biology workflow for drug and vaccine repurposing: Identifying small-molecule BCG mimics to reduce or prevent COVID-19 mortality. Pharm. Res. 2020, 37, 212. [Google Scholar] [CrossRef]
- National Institute of Diabetes and Digestive and Kidney Diseases. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012.
- Wilson, J.F.; Rausch, R.L. Mebendazole and alveolar hydatid disease. Ann. Trop. Med. Parasitol. 1982, 76, 165–173. [Google Scholar] [CrossRef]
- Tolomeo, M.; Colomba, C.; Meli, M.; Cascio, A. Hepatotoxicity caused by mebendazole in a patient with Gilbert’s syndrome. J. Clin. Pharm. Ther. 2019, 44, 985–987. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.Y.; Jung, B.K.; Hong, S.J. Albendazole and mebendazole as anti-parasitic and anti-cancer agents: An update. Korean J. Parasitol. 2021, 59, 189–225. [Google Scholar] [CrossRef] [PubMed]
- Seitz, R.; Schwerk, W.; Arnold, R. Hepatocellular drug reaction caused by mebendazole therapy in cystic echinococcosis. Z. Gastroenterol. 1983, 21, 324–329. [Google Scholar] [PubMed]
- Braithwaite, P.A.; Thomas, R.J.S.; Thompson, R.C.A. Hydatid disease: The alveolar variety in Australia. A case report with comment on the toxicity of mebendazole. Aust. N. Z. J. Surg. 1985, 55, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Puente, S.; Lago, M.; Subirats, M.; Sanz-Esteban, I.; Arsuaga, M.; Vicente, B.; Alonso-Sardon, M.; Belhassen-Garcia, M.; Muro, A. Imported Mansonella perstans infection in Spain. Infect. Dis. Poverty 2020, 9, 105. [Google Scholar] [CrossRef]
- Reis, G.; Silva, E.A.M.; Silva, D.C.M.; Thabane, L.; Singh, G.; Park, J.J.; Forrest, J.I.; Harari, O.; Dos Santos, C.V.Q.; De Almeida, A.P.F.G.; et al. Effect of early treatment with hydroxychloroquine or lopinavir and ritonavir on risk of hospitalization among patients with COVID-19: The TOGETHER randomized clinical trial. JAMA Netw. Open 2021, 4, e216468. [Google Scholar] [CrossRef]
- COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines; National Institutes of Health: Bethesda, MD, USA, 2022.
- Xu, J.; Cao, B. Lessons learnt from hydroxychloroquine/azithromycin in treatment of COVID-19. Eur. Respir. J. 2022, 59, 2102002. [Google Scholar] [CrossRef]
- Reis, G.; Silva, E.A.M.; Silva, D.C.M.; Thabane, L.; Milagres, A.C.; Ferreira, T.S.; Dos Santos, C.V.Q.; Campos, V.H.S.; Nogueira, A.M.R.; de Almeida, E.A.P.F.; et al. Effect of early treatment with ivermectin among patients with COVID-19. N. Engl. J. Med. 2022, 386, 1721–1731. [Google Scholar] [CrossRef]
- Dorward, J.; Yu, L.M.; Hayward, G.; Saville, B.R.; Gbinigie, O.; Van Hecke, O.; Ogburn, E.; Evans, P.H.; Thomas, N.P.; Patel, M.G.; et al. Colchicine for COVID-19 in the community (PRINCIPLE): A randomised, controlled, adaptive platform trial. Br. J. Gen. Pract. 2022, 72, e446–e455. [Google Scholar] [CrossRef]
- Surapat, B.; Kobpetchyok, W.; Kiertiburanakul, S.; Arnuntasupakul, V. Use of Favipiravir for the Treatment of Coronavirus Disease 2019 in the Setting of Hospitel. Int. J. Clin. Pract. 2022, 2022, 3098527. [Google Scholar] [CrossRef]
- Bosaeed, M.; Alharbi, A.; Hussein, M.; Abalkhail, M.; Sultana, K.; Musattat, A.; Alqahtani, H.; Alshamrani, M.; Mahmoud, E.; Alothman, A.; et al. Multicentre randomised double-blinded placebo-controlled trial of favipiravir in adults with mild COVID-19. BMJ Open 2021, 11, e047495. [Google Scholar] [CrossRef] [PubMed]
- Farag, A.; Wang, P.; Boys, I.N.; Eitson, J.L.; Ohlson, M.B.; Fan, W.; McDougal, M.B.; Ahmed, M.; Schoggins, J.W.; Sadek, H. Identification of Atovaquone, Ouabain and Mebendazole as FDA Approved Drugs Targeting SARS-CoV-2 (Version 4). ChemRxiv 2020. [Google Scholar] [CrossRef]
- Murer, L.; Volle, R.; Andriasyan, V.; Petkidis, A.; Gomez-Gonzalez, A.; Yang, L.; Meili, N.; Suomalainen, M.; Bauer, M.; Sequeira, D.P.; et al. Identification of broad anti-coronavirus chemical agents for repurposing against SARS-CoV-2 and variants of concern. Curr. Res. Virol. Sci. 2022, 3, 100019. [Google Scholar] [CrossRef] [PubMed]
- Yele, V.; Sanapalli, B.K.R.; Mohammed, A.A. Imidazoles and benzimidazoles as putative inhibitors of SARS-CoV-2 B. 1.1. 7 (Alpha) and P. 1 (Gamma) variant spike glycoproteins: A computational approach. Chem. Zvesti. 2022, 76, 1107–1117. [Google Scholar] [PubMed]
- Wang, Z.; Guo, K.; Gao, P.; Pu, Q.; Li, C.; Hur, J.; Wu, M. Repurposable drugs for SARS-CoV-2 and influenza sepsis with scRNA-seq data targeting post-transcription modifications. Precis. Clin. Med. 2021, 4, 215–230. [Google Scholar] [CrossRef]
- Tonelli, M.; Simone, M.; Tasso, B.; Novelli, F.; Boido, V.; Sparatore, F.; Paglietti, G.; Pricl, S.; Giliberti, G.; Blois, S.; et al. Antiviral activity of benzimidazole derivatives. II. Antiviral activity of 2-phenylbenzimidazole derivatives. Bioorg. Med. Chem. 2010, 18, 2937–2953. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Farag, A.B.; Boys, I.N.; Wang, P.; Menendez-Montes, I.; Nguyen, N.U.N.; Eitson, J.L.; Ohlson, M.B.; Fan, W.; McDougal, M.B.; et al. FDA approved drugs with antiviral activity against SARS-CoV-2: From structure-based repurposing to host-specific mechanisms. Biomed. Pharmacother. 2023, 162, 114614. [Google Scholar] [CrossRef]
- Panahi, Y.; Dadkhah, M.; Talei, S.; Gharari, Z.; Asghariazar, V.; Abdolmaleki, A.; Matin, S.; Molaei, S. Can anti-parasitic drugs help control COVID-19? Future Virol. 2022, 17, 315–339. [Google Scholar] [CrossRef]
- Patel, A.A.; Zhang, Y.; Fullerton, J.N.; Boelen, L.; Rongvaux, A.; Maini, A.A.; Bigley, V.; Flavell, R.A.; Gilroy, D.W.; Asquith, B.; et al. The fate and lifespan of human monocyte subsets in steady state and systemic inflammation. J. Exp. Med. 2017, 214, 1913–1923. [Google Scholar] [CrossRef]
- Ferrari, D.; Seveso, A.; Sabetta, E.; Ceriotti, D.; Carobene, A.; Banfi, G.; Locatelli, M.; Cabitza, F. Role of time-normalized laboratory findings in predicting COVID-19 outcome. Diagnosis 2020, 7, 387–394. [Google Scholar] [CrossRef]
- Villoteau, A.; Asfar, M.; Otekpo, M.; Loison, J.; Gautier, J.; Annweiler, C. GERIA-COVID study group, Elevated C-reactive protein in early COVID-19 predicts worse survival among hospitalized geriatric patients. PLoS ONE 2021, 16, e0256931. [Google Scholar] [CrossRef] [PubMed]
- Turner, L.; Shamseer, L.; Altman, D.G.; Weeks, L.; Peters, J.; Kober, T.; Dias, S.; Schulz, K.F.; Plint, A.C.; Moher, D. Consolidated standards of reporting trials (CONSORT) and the completeness of reporting of randomised controlled trials (RCTs) published in medical journals. Cochrane Database Syst. Rev. 2012, 11, mr000030. [Google Scholar] [CrossRef] [PubMed]



| Study Groups | Chi-Square | p-Value | |||
|---|---|---|---|---|---|
| Drug | Placebo | ||||
| Gender | Female | 21 | 19 | ||
| Male | 13 | 16 | 0.396 | 0.529 | |
| Total | 34 | 35 | |||
| Mebendazole Group (N = 34) | Placebo Group (N = 35) | p-Value * | |
|---|---|---|---|
| Age (years) | 42.7 ± 13.3 | 38.9 ± 15.6 | 0.278 |
| CRP (mg/L) | 6.20 ± 5.22 | 6.76 ± 5.16 | 0.655 |
| CT | 24.07 ± 3.82 | 23.93 ± 4.34 | 0.894 |
| Neutrophiles (%) | 53.9 ± 13.2 | 48.5 ± 12.3 | 0.078 |
| Lymphocytes (%) | 34.59 ± 12.99 | 39.54 ± 12.91 | 0.117 |
| Monocytes (%) | 8.68 ± 3.39 | 9.46 ± 3.76 | 0.368 |
| Urea (mg/dl) | 23.27 ± 6.79 | 23.88 ± 11.97 | 0.793 |
| Creatinine (mg/dl) | 0.78 ± 0.13 | 0.79 ± 0.18 | 0.949 |
| Sodium (mmol/dl) | 139.8 ± 2.17 | 140.1 ± 1.9 | 0.564 |
| Potassium (mmol/dl) | 4.39 ± 0.46 | 4.46 ± 0.49 | 0.560 |
| AST (U/L) | 22.68 ± 11.26 | 25.34 ± 16.47 | 0.434 |
| ALT (U/L) | 25.56 ± 16.73 | 23.17 ± 11.33 | 0.492 |
| Baseline Day (N = 34) | Day 3 (N = 31) | p-Value * | |
|---|---|---|---|
| Renal Function | |||
| Urea (mg/dL) | 23.27 ± 6.79 | 25.13 ± 6.62 | 0.269 |
| Creatinine (mg/dL) | 0.78 ± 0.13 | 0.80 ± 0.15 | 0.575 |
| Sodium (mmol/dL) | 139.8 ± 2.2 | 135.5 ± 25.1 | 0.351 |
| Potassium (mmol/dL) | 4.39 ± 0.46 | 10.05 ± 25.10 | 0.219 |
| Liver Function | |||
| AST | 22.68 ± 11.26 | 21.26 ± 11.10 | 0.611 |
| ALT | 25.56 ± 16.73 | 23.35 ± 11.78 | 0.539 |
| Parameter | Study Groups | N | Mean ± SD | p-Value * |
|---|---|---|---|---|
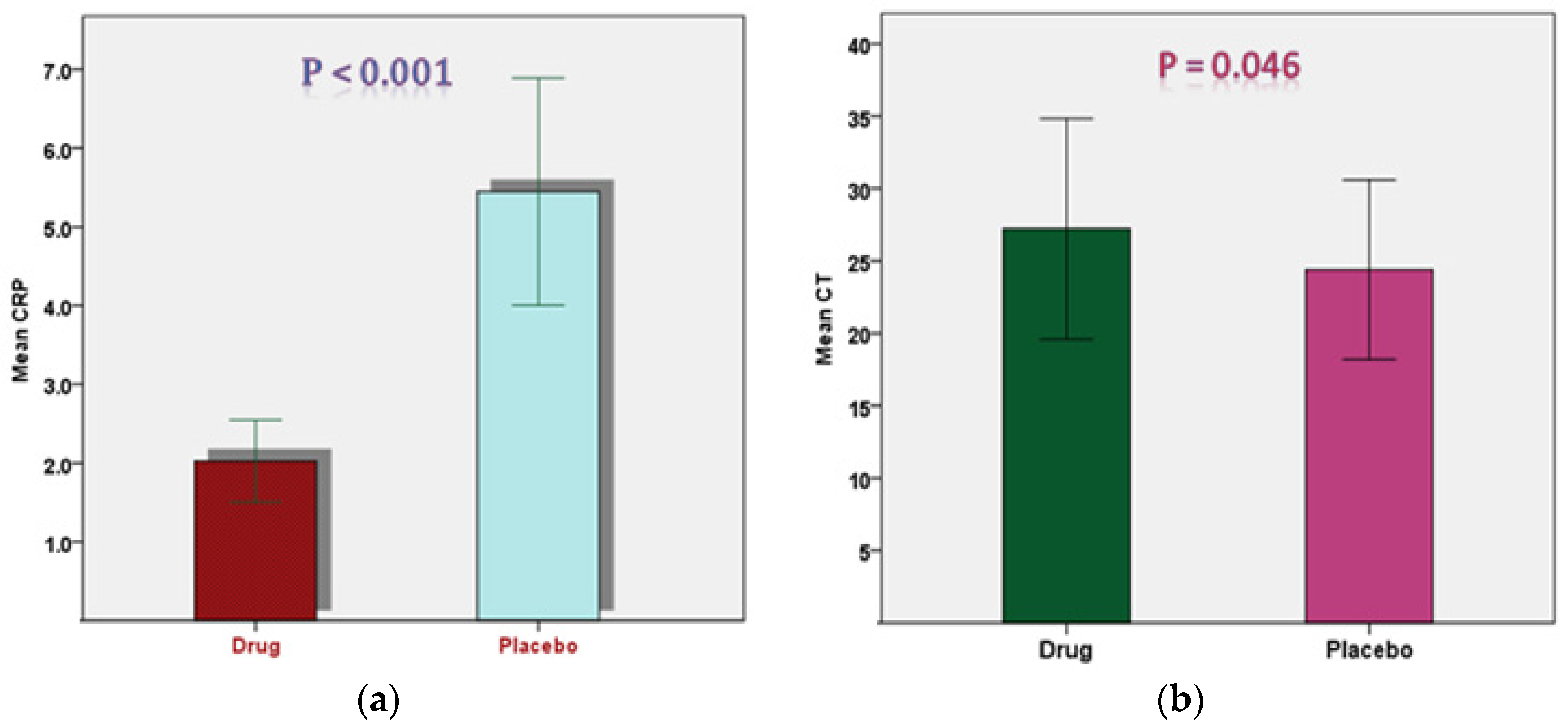
| CRP (mg/L) | Drug | 34 | 6.20 ± 5.22 | 0.655 |
| Placebo | 35 | 6.76 ± 5.16 | ||
| CT | Drug | 34 | 24.06 ± 3.82 | 0.984 |
| Placebo | 35 | 23.93 ± 4.34 |
| Study Groups | Lymphocytes vs. CT | |
|---|---|---|
| Pearson’s Correlation Coefficient (r) | p-Value | |
| Treatment group | −0.491 | 0.039 * |
| Placebo group | 0.051 | 0.888 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Tanani, M.; Ahmed, K.A.-A.; Shakya, A.K.; Ammari, W.G.; Al-Shudifat, A.-E. Phase II, Double-Blinded, Randomized, Placebo-Controlled Clinical Trial Investigating the Efficacy of Mebendazole in the Management of Symptomatic COVID-19 Patients. Pharmaceuticals 2023, 16, 799. https://doi.org/10.3390/ph16060799
El-Tanani M, Ahmed KA-A, Shakya AK, Ammari WG, Al-Shudifat A-E. Phase II, Double-Blinded, Randomized, Placebo-Controlled Clinical Trial Investigating the Efficacy of Mebendazole in the Management of Symptomatic COVID-19 Patients. Pharmaceuticals. 2023; 16(6):799. https://doi.org/10.3390/ph16060799
Chicago/Turabian StyleEl-Tanani, Mohamed, Khaled Abdul-Aziz Ahmed, Ashok K. Shakya, Wesam G. Ammari, and Abdel-Elah Al-Shudifat. 2023. "Phase II, Double-Blinded, Randomized, Placebo-Controlled Clinical Trial Investigating the Efficacy of Mebendazole in the Management of Symptomatic COVID-19 Patients" Pharmaceuticals 16, no. 6: 799. https://doi.org/10.3390/ph16060799
APA StyleEl-Tanani, M., Ahmed, K. A.-A., Shakya, A. K., Ammari, W. G., & Al-Shudifat, A.-E. (2023). Phase II, Double-Blinded, Randomized, Placebo-Controlled Clinical Trial Investigating the Efficacy of Mebendazole in the Management of Symptomatic COVID-19 Patients. Pharmaceuticals, 16(6), 799. https://doi.org/10.3390/ph16060799








