Monitoring of Levosimendan Administration in Patients with Pulmonary Hypertension Undergoing Cardiac Surgery and Effect of Two Different Dosing Schemes on Hemodynamic and Echocardiographic Parameters
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Randomization of Patients
4.3. Intraoperative Anesthetic Management
4.4. Intraoperative Hemodynamic Parameters
4.5. Transesophageal Echocardiographic Parameters
4.6. ICU Management and ICU Discharge Criteria
4.7. Levosimendan Administration and Blood Plasma Concentration Measurement
4.8. LC-MS/MS Methodology
4.8.1. Chemicals
4.8.2. Instrumentation and LC-MS/MS Conditions
4.8.3. Preparation of Stock Solutions, Calibration Standard Solutions, and Quality Control (QC) Solutions
4.8.4. Plasma Samples
4.9. Power Analysis
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Within Run Accuracy | Low QC | Middle QC | High QC | |||
|---|---|---|---|---|---|---|
| Mean Calculated Concentration (ng/mL) | Accuracy % | Mean Calculated Concentration (ng/mL) | Accuracy % | Mean Calculated Concentration (ng/mL) | Accuracy % | |
| 1st (n = 4) | ||||||
| LS | 0.218 | 110 | 20.0 | 99.8 | 37.5 | 93.7 |
| OR-1896 | 2.078 | 104 | 21.0 | 105 | 39.9 | 100 |
| 2nd (n = 2) | ||||||
| LS | 0.210 | 105 | 18.8 | 94.1 | 36.2 | 90.4 |
| OR-1896 | 1.915 | 95.7 | 17.9 | 89.6 | 35.2 | 87.9 |
| 3rd (n = 2) | ||||||
| LS | 0.198 | 98.8 | 19.1 | 95.2 | 40.1 | 100 |
| OR-1896 | 1.945 | 97.2 | 18.6 | 92.6 | 40.4 | 101 |
| Between Runs | Low QC | Middle QC | High QC | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean Calculated Concentration (ng/mL) | Accuracy % | Precision % | Mean Calculated Concentration (ng/mL) | Accuracy % | Precision % | Mean Calculated Concentration (ng/mL) | Accuracy % | Precision % | |
| LS | 0.209 | 105 | 4.8 | 19.3 | 96.4 | 3.2 | 37.9 | 94.7 | 5.2 |
| OR-1896 | 1.979 | 99.0 | 4.4 | 19.2 | 95.7 | 8.5 | 38.5 | 96.3 | 7.5 |
References
- Denault, A.; Deschamps, A.; Tardif, J.C.; Lambert, J.; Perrault, L. Pulmonary hypertension in cardiac surgery. Curr. Cardiol. Rev. 2010, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Nashef, S.A.; Roques, F.; Sharples, L.D.; Nilsson, J.; Smith, C.; Goldstone, A.R.; Lockowandt, U. EuroSCORE II. Eur. J. Cardiothorac. Surg. 2012, 41, 734–744, discussed in Eur. J. Cardiothorac. Surg. 2012, 41, 744–745. [Google Scholar] [CrossRef] [PubMed]
- Denault, A.Y.; Haddad, F.; Jacobsohn, E.; Deschamps, A. Perioperative right ventricular dysfunction. Curr. Opin. Anaesthesiol. 2013, 26, 71–81. [Google Scholar] [CrossRef]
- Jabagi, H.; Nantsios, A.; Ruel, M.; Mielniczuk, L.M.; Denault, A.Y.; Sun, L.Y. A standardized definition for right ventricular failure in cardiac surgery patients. ESC Heart Fail. 2022, 9, 1542–1552. [Google Scholar] [CrossRef] [PubMed]
- Foschi, M.; Di Mauro, M.; Tancredi, F.; Capparuccia, C.; Petroni, R.; Leonzio, L.; Romano, S.; Gallina, S.; Penco, M.; Cibelli, M.; et al. The Dark Side of the Moon: The Right Ventricle. J. Cardiovasc. Dev. Dis. 2017, 4, 18. [Google Scholar] [CrossRef]
- Haddad, F.; Couture, P.; Tousignant, C.; Denault, A.Y. The right ventricle in cardiac surgery, a perioperative perspective: II. Pathophysiology, clinical importance, and management. Anesth. Analg. 2009, 108, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Estrada, V.H.; Franco, D.L.; Moreno, A.A.; Gambasica, J.A.; Nunez, C.C. Postoperative Right Ventricular Failure in Cardiac Surgery. Cardiol. Res. 2016, 7, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Thunberg, C.A.; Gaitan, B.D.; Grewal, A.; Ramakrishna, H.; Stansbury, L.G.; Grigore, A.M. Pulmonary hypertension in patients undergoing cardiac surgery: Pathophysiology, perioperative management, and outcomes. J. Cardiothorac. Vasc. Anesth. 2013, 27, 551–572. [Google Scholar] [CrossRef]
- Al-Omary, M.S.; Sugito, S.; Boyle, A.J.; Sverdlov, A.L.; Collins, N.J. Pulmonary Hypertension Due to Left Heart Disease: Diagnosis, Pathophysiology, and Therapy. Hypertension 2020, 75, 1397–1408. [Google Scholar] [CrossRef]
- Hart, S.A.; Krasuski, R.A.; Wang, A.; Kisslo, K.; Harrison, J.K.; Bashore, T.M. Pulmonary hypertension and elevated transpulmonary gradient in patients with mitral stenosis. J. Heart Valve Dis. 2010, 19, 708–715. [Google Scholar]
- Weitsman, T.; Weisz, G.; Farkash, R.; Klutstein, M.; Butnaru, A.; Rosenmann, D.; Hasin, T. Pulmonary Hypertension with Left Heart Disease: Prevalence, Temporal Shifts in Etiologies and Outcome. Am. J. Med. 2017, 130, 1272–1279. [Google Scholar] [CrossRef] [PubMed]
- Graham, A.; Brown, C.H., 4th. Frailty, Aging, and Cardiovascular Surgery. Anesth. Analg. 2017, 124, 1053–1060. [Google Scholar] [CrossRef]
- Wan, S.; LeClerc, J.L.; Vincent, J.L. Inflammatory response to cardiopulmonary bypass: Mechanisms involved and possible therapeutic strategies. Chest 1997, 112, 676–692. [Google Scholar] [CrossRef]
- Hill, G.E. Cardiopulmonary bypass-induced inflammation: Is it important? J. Cardiothorac. Vasc. Anesth. 1998, 12 (Suppl. 1), 21–25. [Google Scholar] [PubMed]
- Souza, M.F.S.; Penha, J.G.; Maeda, N.Y.; Galas, F.R.B.G.; Abud, K.C.O.; Carvalho, E.S.; Thomaz, A.M.; Castro, C.R.P.; Pereira, J.; Lopes, A.A. Postoperative Pulmonary Hemodynamics and Systemic Inflammatory Response in Pediatric Patients Undergoing Surgery for Congenital Heart Defects. Mediat. Inflamm. 2022, 2022, 3977585. [Google Scholar] [CrossRef]
- Ocal, A.; Kiriş, I.; Erdinç, M.; Peker, O.; Yavuz, T.; Ibrişim, E. Efficiency of prostacyclin in the treatment of protamine-mediated right ventricular failure and acute pulmonary hypertension. Tohoku J. Exp. Med. 2005, 207, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Viaro, F.; Dalio, M.B.; Evora, P.R. Catastrophic cardiovascular adverse reactions to protamine are nitric oxide/cyclic guanosine monophosphate dependent and endothelium mediated: Should methylene blue be the treatment of choice? Chest 2002, 122, 1061–1066. [Google Scholar] [CrossRef]
- Baron-Stefaniak, J.; Leitner, G.C.; Küntzel, N.K.I.; Meyer, E.L.; Hiesmayr, M.J.; Ullrich, R.; Baron, D.M. Transfusion of standard-issue packed red blood cells induces pulmonary vasoconstriction in critically ill patients after cardiac surgery-A randomized, double-blinded, clinical trial. PLoS ONE 2019, 14, e0213000. [Google Scholar] [CrossRef]
- Baron, D.M.; Yu, B.; Lei, C.; Bagchi, A.; Beloiartsev, A.; Stowell, C.P.; Steinbicker, A.U.; Malhotra, R.; Bloch, K.D.; Zapol, W.M. Pulmonary hypertension in lambs transfused with stored blood is prevented by breathing nitric oxide. Anesthesiology 2012, 116, 637–647. [Google Scholar] [CrossRef]
- Magne, J.; Mathieu, P.; Dumesnil, J.G.; Tanné, D.; Dagenais, F.; Doyle, D.; Pibarot, P. Impact of prosthesis-patient mismatch on survival after mitral valve replacement. Circulation 2007, 115, 1417–1425. [Google Scholar] [CrossRef]
- Ammannaya, G.K.K.; Mishra, P.; Khandekar, J.V.; Mohapatra, C.K.R.; Seth, H.S.; Raut, C.; Shah, V.; Saini, J.S. Effect of prosthesis patient mismatch in mitral position on pulmonary hypertension. Eur. J. Cardiothorac. Surg. 2017, 52, 1168–1174. [Google Scholar] [CrossRef] [PubMed]
- Navaratnam, M.; DiNardo, J.A. Peri-operative right ventricular dysfunction-the anesthesiologist’s view. Cardiovasc. Diagn. Ther. 2020, 10, 1725–1734. [Google Scholar] [CrossRef]
- Sarkar, M.S.; Desai, P.M. Pulmonary hypertension and cardiac anesthesia: Anesthesiologist’s perspective. Ann. Card. Anaesth. 2018, 21, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Wanner, P.M.; Filipovic, M. The Right Ventricle-You May Forget it, but It Will Not Forget You. J. Clin. Med. 2020, 9, 432. [Google Scholar] [CrossRef] [PubMed]
- Theodoraki, K.; Thanopoulos, A.; Rellia, P.; Leontiadis, E.; Zarkalis, D.; Perreas, K.; Antoniou, T. A retrospective comparison of inhaled milrinone and iloprost in post-bypass pulmonary hypertension. Heart Vessels. 2017, 32, 1488–1497. [Google Scholar] [CrossRef]
- Antoniou, T.; Koletsis, E.N.; Prokakis, C.; Rellia, P.; Thanopoulos, A.; Theodoraki, K.; Zarkalis, D.; Sfyrakis, P. Hemodynamic effects of combination therapy with inhaled nitric oxide and iloprost in patients with pulmonary hypertension and right ventricular dysfunction after high-risk cardiac surgery. J. Cardiothorac. Vasc. Anesth. 2013, 27, 459–466. [Google Scholar] [CrossRef]
- Ventetuolo, C.E.; Klinger, J.R. Management of acute right ventricular failure in the intensive care unit. Ann. Am. Thorac. Soc. 2014, 1, 811–822. [Google Scholar] [CrossRef]
- De Luca, L.; Colucci, W.S.; Nieminen, M.S.; Massie, B.M.; Gheorghiade, M. Evidence-based use of levosimendan in different clinical settings. Eur. Heart J. 2006, 27, 1908–1920. [Google Scholar] [CrossRef]
- Ukkonen, H.; Saraste, M.; Akkila, J.; Knuuti, J.; Karanko, M.; Iida, H.; Lehikoinen, P.; Någren, K.; Lehtonen, L.; Voipio-Pulkki, L.M. Myocardial efficiency during levosimendan infusion in congestive heart failure. Clin. Pharmacol. Ther. 2000, 68, 522–531. [Google Scholar] [CrossRef]
- Parissis, J.T.; Rafouli-Stergiou, P.; Paraskevaidis, I.; Mebazaa, A. Levosimendan: From basic science to clinical practice. Heart Fail. Rev. 2009, 14, 265–275. [Google Scholar] [CrossRef]
- Figgitt, D.P.; Gillies, P.S.; Goa, K.L. Levosimendan. Drugs 2001, 61, 613–627, discussed in Drugs 2001, 61, 628–629. [Google Scholar] [CrossRef]
- Hansen, M.S.; Andersen, A.; Nielsen-Kudsk, J.E. Levosimendan in pulmonary hypertension and right heart failure. Pulm. Circ. 2018, 8, 2045894018790905. [Google Scholar] [CrossRef] [PubMed]
- Kundra, T.S.; Nagaraja, P.S.; Bharathi, K.S.; Kaur, P.; Manjunatha, N. Inhaled levosimendan versus intravenous levosimendan in patients with pulmonary hypertension undergoing mitral valve replacement. Ann. Card. Anaesth. 2018, 21, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.H.; Leimberger, J.D.; van Diepen, S.; Meza, J.; Wang, A.; Jankowich, R.; Harrison, R.W.; Hay, D.; Fremes, S.; Duncan, A.; et al. Levosimendan in Patients with Left Ventricular Dysfunction Undergoing Cardiac Surgery. N. Engl. J. Med. 2017, 376, 2032–2042. [Google Scholar] [CrossRef] [PubMed]
- Guarracino, F.; Heringlake, M.; Cholley, B.; Bettex, D.; Bouchez, S.; Lomivorotov, V.V.; Rajek, A.; Kivikko, M.; Pollesello, P. Use of Levosimendan in Cardiac Surgery: An Update After the LEVO-CTS, CHEETAH, and LICORN Trials in the Light of Clinical Practice. J. Cardiovasc. Pharmacol. 2018, 71, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kivikko, M.; Antila, S.; Eha, J.; Lehtonen, L.; Pentikäinen, P.J. Pharmacokinetics of levosimendan and its metabolites during and after a 24-hour continuous infusion in patients with severe heart failure. Int. J. Clin. Pharmacol. Ther. 2002, 40, 465–471. [Google Scholar] [CrossRef]
- Jiménez-Rivera, J.J.; Álvarez-Castillo, A.; Ferrer-Rodríguez, J.; Iribarren-Sarrías, J.L.; García-González, M.J.; Jorge-Pérez, P.; Lacalzada-Almeida, J.; Pérez-Hernández, R.; Montoto-López, J.; Martínez-Sanz, R. Preconditioning with levosimendan reduces postoperative low cardiac output in moderate-severe systolic dysfunction patients who will undergo elective coronary artery bypass graft surgery: A cost-effective strategy. J. Cardiothorac. Surg. 2020, 15, 108. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, X.; Liao, X.; Liu, B.; Yu, H. The efficacy and safety of prophylactic use of levosimendan on patients undergoing coronary artery bypass graft: A systematic review and meta-analysis. J. Anesth. 2019, 33, 543–550.e6. [Google Scholar] [CrossRef]
- Van Diepen, S.; Mehta, R.H.; Leimberger, J.D.; Goodman, S.G.; Fremes, S.; Jankowich, R.; Heringlake, M.; Anstrom, K.J.; Levy, J.H.; Luber, J.; et al. Levosimendan in patients with reduced left ventricular function undergoing isolated coronary or valve surgery. J. Thorac. Cardiovasc. Surg. 2020, 159, 2302–2309. [Google Scholar] [CrossRef]
- Immohr, M.B.; Akhyari, P.; Boettger, C.; Erbel, S.; Westenfeld, R.; Scheiber, D.; Tudorache, I.; Aubin, H.; Lichtenberg, A.; Boeken, U. Levosimendan for Treatment of Primary Graft Dysfunction After Heart Transplantation: Optimal Timing of Application. Exp. Clin. Transplant. 2021, 19, 473–480. [Google Scholar] [CrossRef]
- Ellouze, O.; Soudry Faure, A.; Radhouani, M.; Abou-Arab, O.; Besnier, E.; Moussa, M.; Cransac, A.; Ksiazek, E.; Fischer, M.O.; Mertes, P.M.; et al. Levosimendan in venoarterial ECMO weaning. Rational and design of a randomized double blind multicentre trial. ESC Heart Fail. 2021, 8, 3339–3347. [Google Scholar] [CrossRef] [PubMed]
- Ebade, A.A.; Khalil, M.A.; Mohamed, A.K. Levosimendan is superior to dobutamine as an inodilator in the treatment of pulmonary hypertension for children undergoing cardiac surgery. J. Anesth. 2013, 27, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Abdelbaser, I.; Mageed, N.A.; Elfayoumy, S.I.; Elgamal, M.F.; Elmorsy, M.M.; Taman, H.I. The direct comparison of inhaled versus intravenous levosimendan in children with pulmonary hypertension undergoing on-cardiopulmonary bypass cardiac surgery: A randomized, controlled, non-inferiority study. J. Clin. Anesth. 2021, 71, 110231. [Google Scholar] [CrossRef]
- Lang, R.M.; Bierig, M.; Devereux, R.B.; Flachskampf, F.A.; Foster, E.; Pellikka, P.A.; Picard, M.H.; Roman, M.J.; Seward, J.; Shanewise, J.S.; et al. Recommendations for chamber quantification: A report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J. Am. Soc. Echocardiogr. 2005, 18, 1440–1463. [Google Scholar] [CrossRef] [PubMed]
| Dose | P | ||
|---|---|---|---|
| 6 μg/kg (N = 15; 50%) | 12 μg/kg (N = 15; 50%) | ||
| N (%) | N (%) | ||
| Gender | |||
| Females | 9 (60.0) | 8 (53.3) | 0.713 + |
| Males | 6 (40.0) | 7 (46.7) | |
| Age (years), mean (SD) | 69.9 (14.3) | 67.9 (6.5) | 0.615 ‡ |
| BSA (m2), mean (SD) | 1.8 (0.2) | 1.9 (0.2) | 0.338 ‡ |
| Type of surgery | |||
| ASD | 0 (0.0) | 1 (6.7) | - |
| AVR | 2 (13.3) | 0 (0.0) | |
| AVR-MV repair-TV repair | 2 (13.3) | 2 (13.3) | |
| AVR-MVR | 2 (13.3) | 2 (13.3) | |
| AVR-MVR-GABG | 0 (0.0) | 1 (6.7) | |
| CABG + MV repair | 2 (13.3) | 2 (13.3) | |
| MV repair | 1 (6.7) | 1 (6.7) | |
| MV repair + TV repair | 1 (6.7) | 1 (6.7) | |
| MVR | 4 (26.7) | 5 (33.3) | |
| MVR REDO | 1 (6.7) | 0 (0.0) | |
| CPB duration (minutes), mean (SD) | 137.5 (46.6) | 150.5 (63.1) | 0.526 ‡ |
| Patients in need of norepinephrine administration | 9 (60.0) | 9 (60.0) | >0.999 + |
| Norepinephrine dose (μg/kg/min), mean (SD) | 0.1 (0.1) | 0.1 (0.1) | 0.522 ‡ |
| Patients in need of dobutamine administration | 9 (60) | 4 (26.7) | 0.065 + |
| Dobutamine dose (μg/kg/min), mean (SD) | 3.2 (1.7) | 3.8 (1.3) | 0.548 ‡ |
| Patients in need of epinephrine administration | 1 (7.7) | 0 (0.0) | >0.999 ++ |
| Epinephrine dose (μg/kg/min), mean (SD) | 0.1 (-) | 0 (-) | |
| ICU length of stay (hours), mean (SD) | 103.6 (53.1) | 137.3 (274.1) | 0.644 ‡ |
| Duration of hospitalization (days), median (IQR) | 9 (7–14) | 8 (6–10) | 0.118 ‡‡ |
| LS | ||||||
|---|---|---|---|---|---|---|
| 20 min after CPB (ng/mL) | 6 h (ng/mL) | 12 h (ng/mL) | Change to 12 h | |||
| Dose | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | P2 | P3 |
| 6 μg/kg | 5.29 (3.72) | 1.98 (1.38) | 0.18 (0.18) | −5.11 (3.71) | 0.001 | 0.108 |
| 12 μg/kg | 8.21 (5.56) | 3.33 (2.79) | 0.2 (0.23) | −8.01 (5.45) | <0.001 | |
| P1 | 0.102 | 0.104 | 0.786 | |||
| Baseline | 20 min after CPB | End of Surgery | ICU | Change to ICU | ||||
|---|---|---|---|---|---|---|---|---|
| Dose | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | P2 | P3 | |
| HR (beats/min) | 6 μg/kg | 76.9 (20.7) | 86.7 (7.3) | 85.1 (8.6) | 83.1 (11.9) | 6.3 (21.1) | 0.251 | 0.645 |
| 12 μg/kg | 69.2 (11.7) | 82.1 (10.9) | 82.4 (10.8) | 80.7 (9.9) | 11.5 (15.2) | 0.071 | ||
| P1 | 0.222 | 0.193 | 0.452 | 0.543 | ||||
| SAP (mm/Hg) | 6 μg/kg | 110.8 (18.9) | 108.1 (13.7) | 105.5 (12.9) | 116.2 (17.1) | 5.4 (24.1) | 0.115 | 0.141 |
| 12 μg/kg | 119.9 (20.1) | 106.7 (12.1) | 103.8 (14.3) | 107 (9.1) | −12.9 (20.7) | 0.087 | ||
| P1 | 0.214 | 0.758 | 0.73 | 0.077 | ||||
| DAP (mmHg) | 6 μg/kg | 64.9 (11.2) | 55.7 (7.9) | 59.7 (9) | 60.9 (11.1) | −3.9 (9.7) | 0.056 | 0.372 |
| 12 μg/kg | 64.8 (15.4) | 62.5 (10.2) | 59.2 (9.9) | 61.3 (8.3) | −3.5 (14.1) | 0.566 | ||
| P1 | 0.989 | 0.053 | 0.878 | 0.912 | ||||
| MAP (mmHg) | 6 μg/kg | 82.9 (12.6) | 71.9 (8.2) | 72.9 (9.3) | 78.6 (12.3) | −4.3 (14.6) | 0.038 | 0.509 |
| 12 μg/kg | 85.3 (15.8) | 69.2 (18.7) | 72.9 (9.7) | 72.4 (10) | −12.9 (18.6) | 0.026 | ||
| P1 | 0.658 | 0.608 | >0.999 | 0.142 | ||||
| CVP (mmHg) | 6 μg/kg | 15.9 (3.7) | 15.7 (2.8) | 16.1 (3) | 13.7 (2.7) | −2.2 (3.5) | 0.042 | 0.903 |
| 12 μg/kg | 15.1 (3.9) | 14.7 (3.6) | 14.8 (3.4) | 13.1 (2.5) | −1.9 (4) | 0.138 | ||
| P1 | 0.538 | 0.403 | 0.29 | 0.532 | ||||
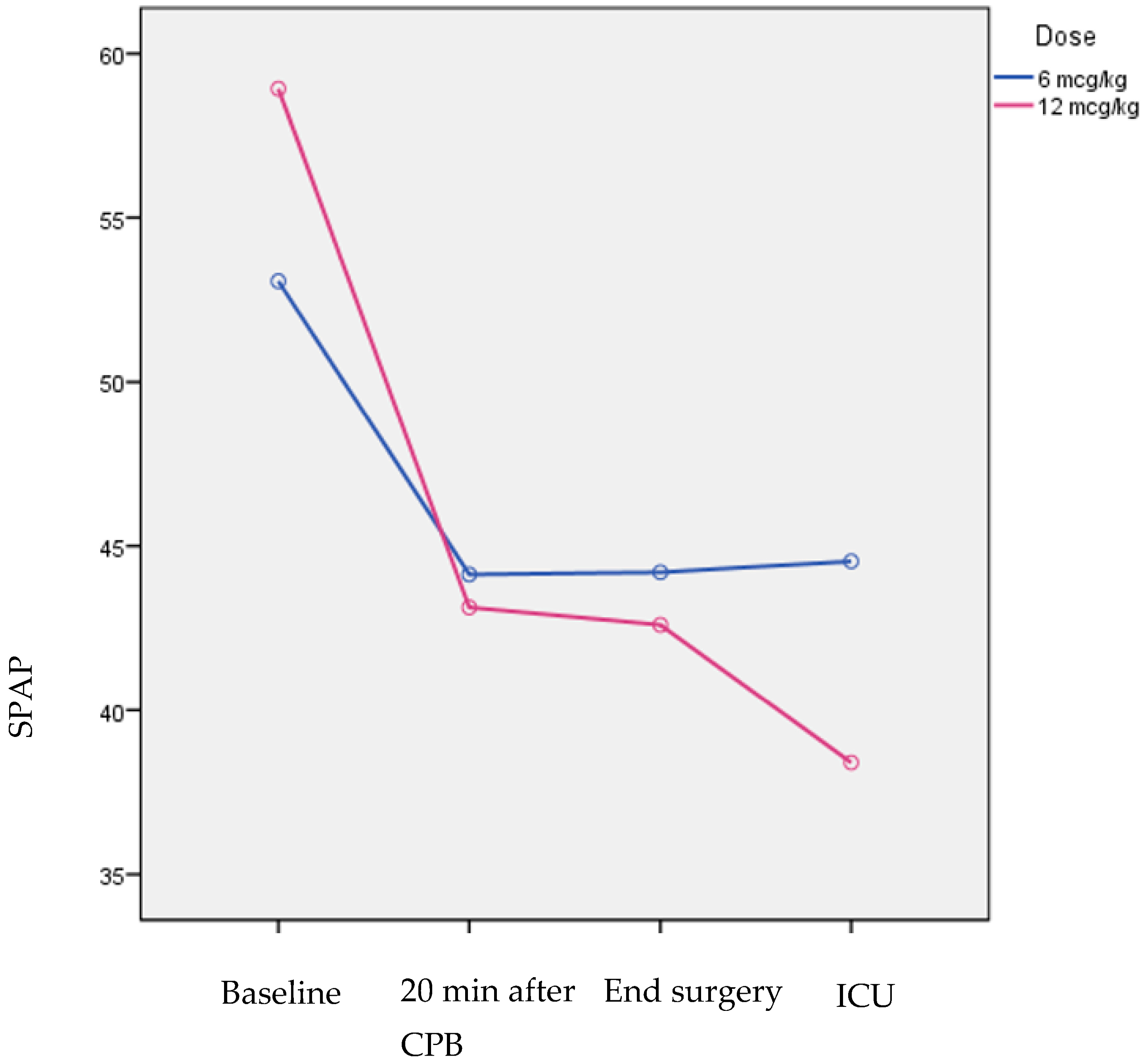
| SPAP (mmHg) | 6 μg/kg | 53.1 (7.2) | 44.1 (7.3) | 44.2 (7) | 44.5 (6.9) | −8.5 (10.4) | 0.021 | 0.015 |
| 12 μg/kg | 58.9 (12.1) | 43.1 (10.3) | 42.6 (12.4) | 38.4 (9.2) | −20.5 (10.7) | <0.001 | ||
| P1 | 0.116 | 0.762 | 0.668 | 0.049 | ||||
| DPAP (mmHg) | 6 μg/kg | 29.5 (5.4) | 25.1 (6.7) | 25.8 (4.9) | 23.1 (4.5) | −6.5 (6.4) | 0.004 | 0.814 |
| 12 μg/kg | 30.1 (7.4) | 24.3 (8.1) | 24.7 (7.3) | 21.8 (6.4) | −8.3 (5.5) | <0.001 | ||
| P1 | 0.824 | 0.771 | 0.643 | 0.535 | ||||
| MPAP (mmHg) | 6 μg/kg | 40 (5) | 31.9 (6.5) | 33.7 (5.3) | 31 (5.5) | −9 (7.8) | <0.001 | 0.046 |
| 12 μg/kg | 42.7 (8.5) | 31.7 (8.3) | 31.6 (8.2) | 27.8 (7.2) | −14.9 (6.6) | <0.001 | ||
| P1 | 0.291 | 0.961 | 0.405 | 0.182 | ||||
| MPAP/MAP | 6 μg/kg | 0.47 (0.09) | 0.44 (0.1) | 0.46 (0.09) | 0.39 (0.08) | −0.08 (0.13) | 0.008 | 0.048 |
| 12 μg/kg | 0.52 (0.15) | 0.43 (0.13) | 0.43 (0.1) | 0.34 (0.09) | −0.17 (0.10) | <0.001 | ||
| P1 | 0.361 | 0.681 | 0.350 | 0.157 | ||||
| PCWP (mmHg) | 6 μg/kg | 24.6 (3.5) | 23.7 (4.5) | 22.9 (4.6) | 20.1 (3.4) | −4.5 (3.4) | 0.001 | 0.523 |
| 12 μg/kg | 25.2 (4.9) | 22.4 (4.7) | 21.4 (4.2) | 18.4 (2.9) | −6.8 (4.7) | <0.001 | ||
| P1 | 0.701 | 0.434 | 0.347 | 0.165 | ||||
| CO (L/min) | 6 μg/kg | 3.54 (0.68) | 4.26 (0.96) | 4.27 (0.71) | 4.07 (0.72) | 0.53 (0.77) | 0.022 | 0.833 |
| 12 μg/kg | 4.14 (0.94) | 5.03 (0.78) | 4.95 (0.72) | 4.61 (0.78) | 0.47 (0.91) | 0.003 | ||
| P1 | 0.055 | 0.023 | 0.015 | 0.061 | ||||
| CI (L/min/m2) | 6 μg/kg | 1.96 (0.37) | 2.33 (0.49) | 2.36 (0.43) | 2.24 (0.46) | 0.28 (0.41) | 0.020 | 0.742 |
| 12 μg/kg | 2.18 (0.51) | 2.70 (0.37) | 2.61 (0.35) | 2.38 (0.39) | 0.2 (0.51) | 0.001 | ||
| P1 | 0.201 | 0.045 | 0.093 | 0.386 | ||||
| SV (mL) | 6 μg/kg | 49.5 (17.6) | 49.2 (11.4) | 49.8 (13) | 49.6 (10.9) | 0.1 (15.6) | 0.998 | 0.709 |
| 12 μg/kg | 59.9 (15.6) | 62.7 (11.7) | 60.7 (10.9) | 58 (11.3) | −1.9 (15.4) | 0.524 | ||
| P1 | 0.099 | 0.003 | 0.019 | 0.048 | ||||
| SVI (mL/m2) | 6 μg/kg | 26.5 (8.8) | 27.8 (6.8) | 34.9 (21.8) | 27.5 (7) | 0.9 (8.9) | 0.262 | 0.255 |
| 12 μg/kg | 31.7 (8.6) | 33.3 (7.5) | 32.2 (6.8) | 30.7 (6.3) | −1 (8) | 0.507 | ||
| P1 | 0.113 | 0.042 | 0.652 | 0.192 | ||||
| SVR (dyn*sec/cm5) | 6 μg/kg | 1520.3 (359.8) | 1104.1 (301.3) | 1074 (249.4) | 1347 (423.3) | −173.3 (378.2) | <0.001 | 0.768 |
| 12 μg/kg | 1389.9 (299.7) | 982.5 (260.2) | 956.4 (198.3) | 1122.7 (314.6) | −267.2 (323.3) | <0.001 | ||
| P1 | 0.29 | 0.247 | 0.164 | 0.111 | ||||
| SVRI (dyn*sec/cm5*m2) | 6 μg/kg | 2804 (664.4) | 2112.5 (750.3) | 1994.1 (578.1) | 2460.1 (813.9) | −343.9 (703.2) | <0.001 | 0.778 |
| 12 μg/kg | 2635.6 (625.5) | 1830.2 (372.9) | 1796.1 (318.3) | 2074.5 (456.8) | −561.1 (643.3) | <0.001 | ||
| P1 | 0.481 | 0.203 | 0.255 | 0.121 | ||||
| PVR (dyn*sec/cm5) | 6 μg/kg | 355.4 (100.8) | 159.6 (117) | 172.5 (73.8) | 225.8 (134.3) | −129.6 (179.3) | <0.001 | 0.654 |
| 12 μg/kg | 331.5 (146.7) | 147.9 (86.1) | 163.6 (88.4) | 168.8 (91.1) | −162.7 (129) | <0.001 | ||
| P1 | 0.608 | 0.757 | 0.766 | 0.185 | ||||
| PVRI (dyn*sec/cm5*m2) | 6 μg/kg | 650 (166.7) | 239.2 (195) | 315.5 (138.8) | 409 (239.2) | −241 (321.9) | <0.001 | 0.560 |
| 12 μg/kg | 619.5 (249.6) | 261.8 (194.5) | 316.1 (187.3) | 321.9 (183.5) | −297.7 (222.4) | <0.001 | ||
| P1 | 0.697 | 0.753 | 0.992 | 0.273 |
| Baseline | At the End of Surgery | ICU | Change Until ICU | ||||
|---|---|---|---|---|---|---|---|
| Dose | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | P2 | P3 | |
| LVEF (%) | 6 mcg/kg | 46 (7.6) | 47.3 (6.8) | 47.3 (7.5) | 1.3 (5.2) | 0.584 | 0.881 |
| 12 mcg/kg | 49.3 (7.3) | 50.3 (4.4) | 49.7 (7.7) | 0.3 (7.4) | 0.703 | ||
| P1 | 0.231 | 0.162 | 0.407 | ||||
| TAPSE (mm) | 6 mcg/kg | 15.7 (2.8) | 15.7 (2.8) | 16.1 (2.7) | 0.4 (2.6) | 0.264 | 0.042 |
| 12 mcg/kg | 14.5 (1.8) | 16.1 (1.4) | 16.2 (1.4) | 1.7 (0.9) | 0.008 | ||
| P1 | 0.198 | 0.626 | 0.866 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ftikos, P.; Falara, A.; Rellia, P.; Leontiadis, E.; Samanidis, G.; Kamperi, N.; Piperakis, A.; Tamvakopoulos, C.; Antoniou, T.; Theodoraki, K. Monitoring of Levosimendan Administration in Patients with Pulmonary Hypertension Undergoing Cardiac Surgery and Effect of Two Different Dosing Schemes on Hemodynamic and Echocardiographic Parameters. Pharmaceuticals 2023, 16, 815. https://doi.org/10.3390/ph16060815
Ftikos P, Falara A, Rellia P, Leontiadis E, Samanidis G, Kamperi N, Piperakis A, Tamvakopoulos C, Antoniou T, Theodoraki K. Monitoring of Levosimendan Administration in Patients with Pulmonary Hypertension Undergoing Cardiac Surgery and Effect of Two Different Dosing Schemes on Hemodynamic and Echocardiographic Parameters. Pharmaceuticals. 2023; 16(6):815. https://doi.org/10.3390/ph16060815
Chicago/Turabian StyleFtikos, Panagiotis, Areti Falara, Panagiota Rellia, Evangelos Leontiadis, George Samanidis, Natalia Kamperi, Artemios Piperakis, Constantin Tamvakopoulos, Theofani Antoniou, and Kassiani Theodoraki. 2023. "Monitoring of Levosimendan Administration in Patients with Pulmonary Hypertension Undergoing Cardiac Surgery and Effect of Two Different Dosing Schemes on Hemodynamic and Echocardiographic Parameters" Pharmaceuticals 16, no. 6: 815. https://doi.org/10.3390/ph16060815
APA StyleFtikos, P., Falara, A., Rellia, P., Leontiadis, E., Samanidis, G., Kamperi, N., Piperakis, A., Tamvakopoulos, C., Antoniou, T., & Theodoraki, K. (2023). Monitoring of Levosimendan Administration in Patients with Pulmonary Hypertension Undergoing Cardiac Surgery and Effect of Two Different Dosing Schemes on Hemodynamic and Echocardiographic Parameters. Pharmaceuticals, 16(6), 815. https://doi.org/10.3390/ph16060815






