Enhancement of Scaffold In Vivo Biodegradability for Bone Regeneration Using P28 Peptide Formulations
Abstract
:1. Introduction
2. Results and Discussion
2.1. Animal Husbandry
2.2. Histopathological Evaluation
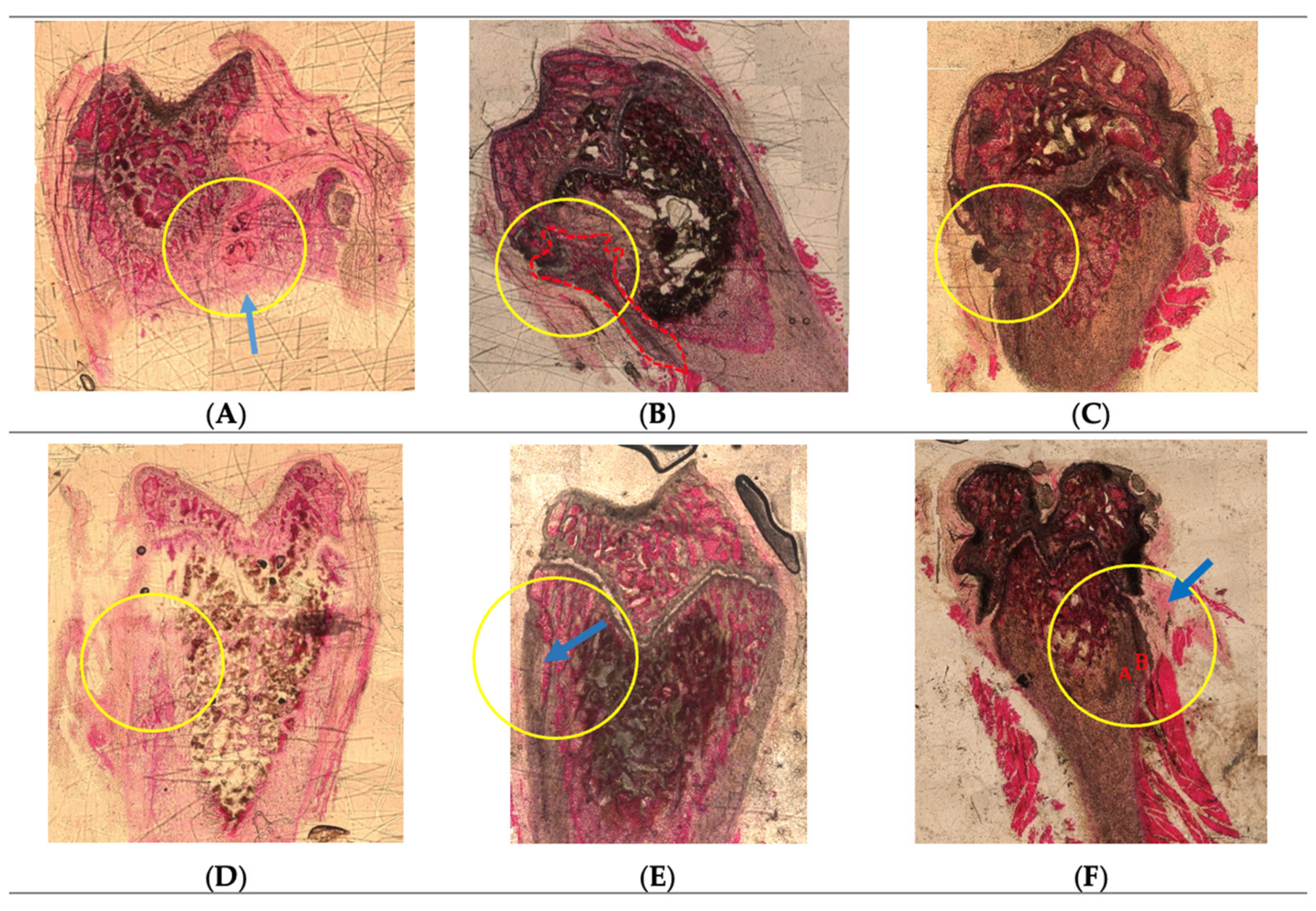
2.3. Histological Analysis
Hematoxylin-Eosin Staining
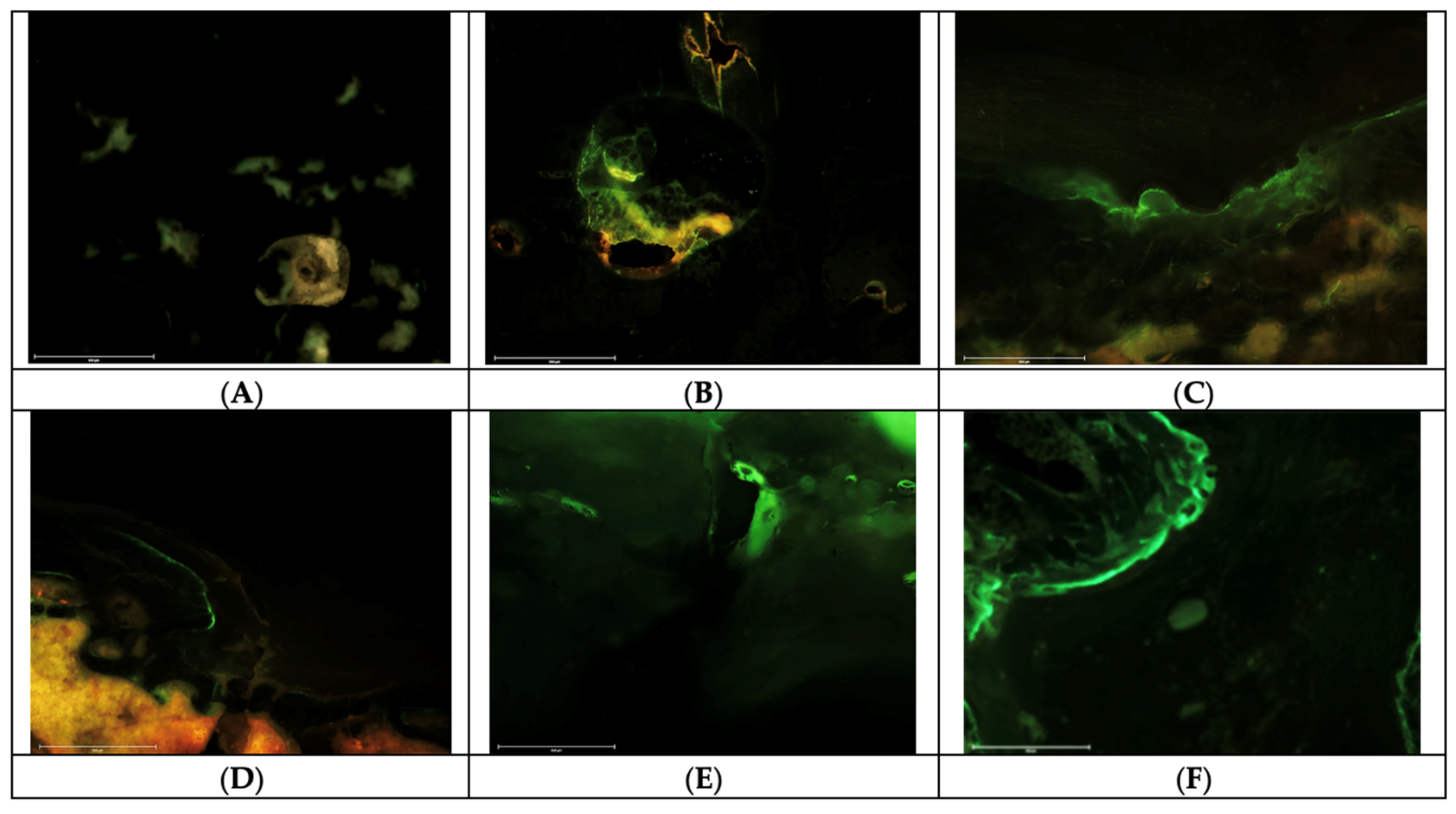
2.4. Fluorochrome Labelling Analyses
3. Materials and Methods
3.1. Scaffold Preparation
3.2. Animal Housing and Husbandry
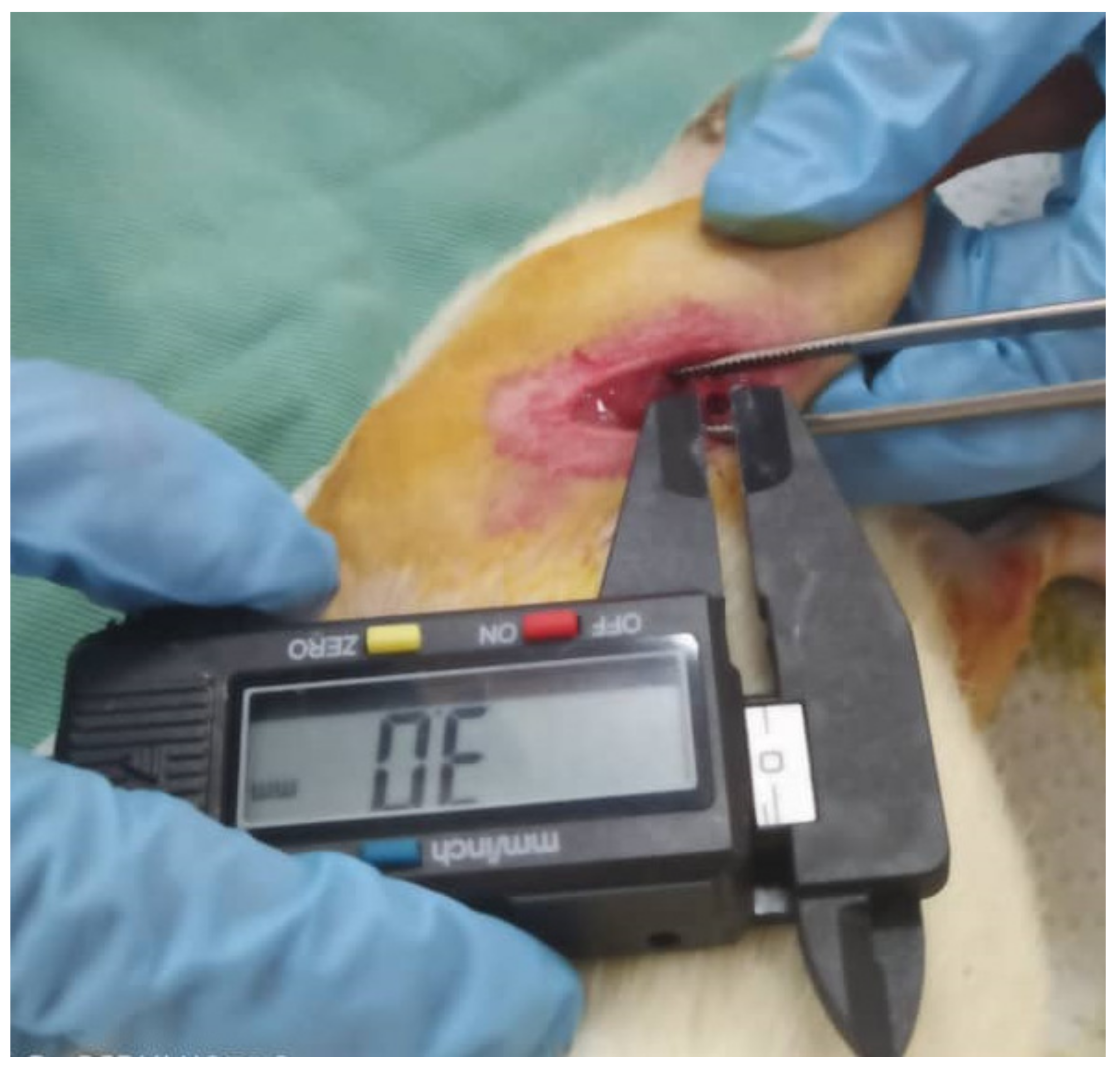
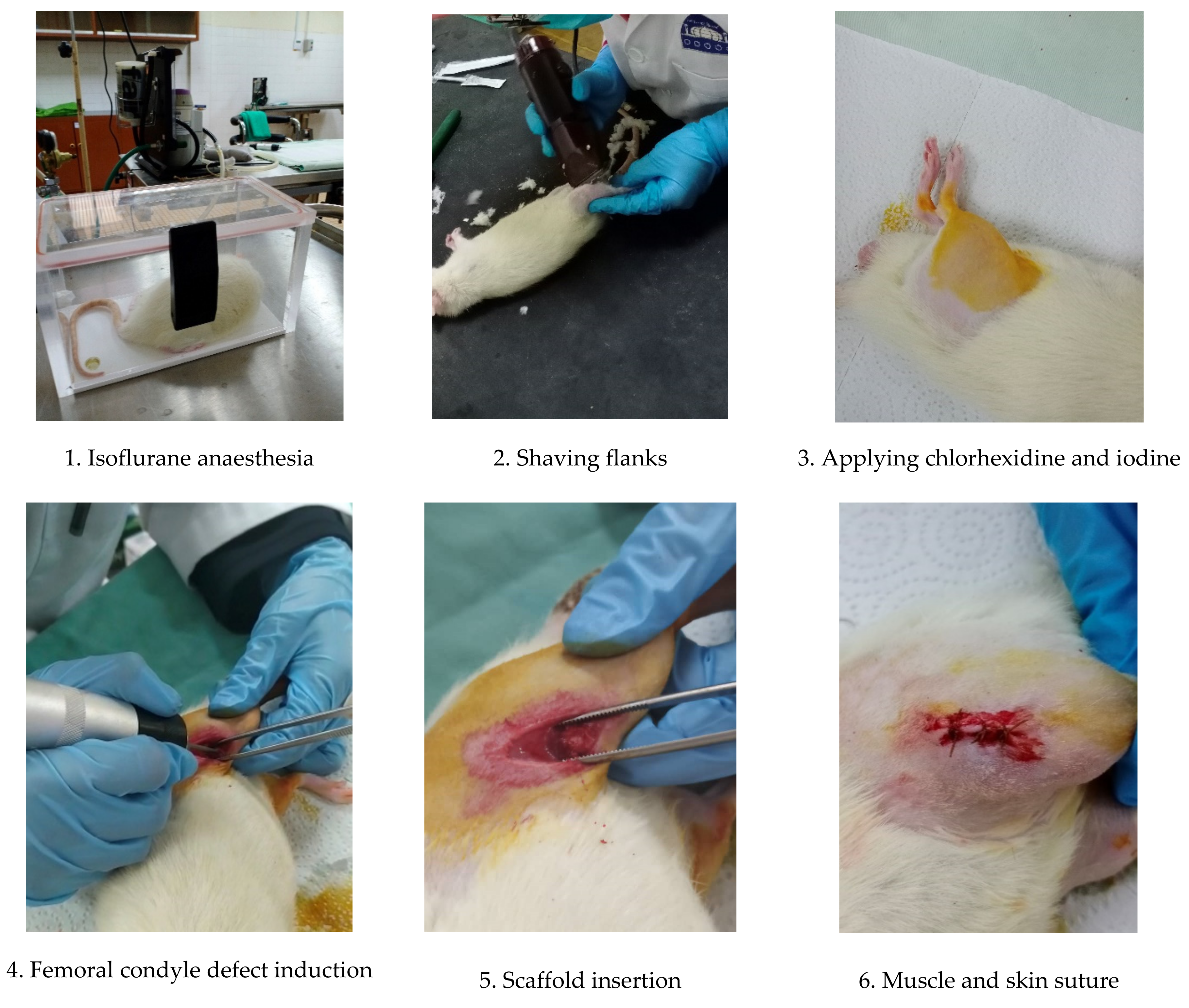
3.3. Femoral Condyle Defect Induction and Scaffold Implantation
3.4. Post-Operative Monitoring
- Weight loss >20% of the mean weight of rats;
- Severe lameness;
- Diarrhoea/blood in faecal material;
- Circling phenomenon;
- Severe necrosis at the implantation site;
- Persistent self-induced trauma five days after analgesic treatment as well as local and general treatment;
- Abnormal behaviour even in the presence of appropriate treatment (e.g., sign of pain following administration of analgesic).
3.5. Fluorescent Bone Labelling for Dynamic Bone Formation
3.6. Animal Euthanasia
3.7. Macroscopic Histopathological Evaluation
3.8. Histological Processing, Embedding and Cutting
3.9. Histological Staining (Hematoxylin/Eosin)
3.10. Fluorescent Imaging
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaliva, M.; Georgopoulou, A.; Dragatogiannis, D.A.; Charitidis, C.A.; Chatzinikolaidou, M.; Vamvakaki, M. Biodegradable Chitosan-Graft-Poly(L-Lactide) Copolymers for Bone Tissue Engineering. Polymers 2020, 12, 316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez-Salvador, J.L.; Balea, A.; Monte, M.C.; Negro, C.; Blanco, A. Chitosan Grafted/Cross-Linked with Biodegradable Polymers: A Review. Int. J. Biol. Macromol. 2021, 178, 325–343. [Google Scholar] [CrossRef] [PubMed]
- Dorati, R.; DeTrizio, A.; Modena, T.; Conti, B.; Benazzo, F.; Gastaldi, G.; Genta, I. Biodegradable Scaffolds for Bone Regeneration Combined with Drug-Delivery Systems in Osteomyelitis Therapy. Pharmaceuticals 2017, 10, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- e Silva, E.P.; Huang, B.; Helaehil, J.V.; Nalesso, P.R.L.; Bagne, L.; de Oliveira, M.A.; Albiazetti, G.C.C.; Aldalbahi, A.; El-Newehy, M.; Santamaria, M., Jr.; et al. In Vivo Study of Conductive 3D Printed PCL/MWCNTs Scaffolds with Electrical Stimulation for Bone Tissue Engineering. Bio-Des. Manuf. 2021, 4, 190–202. [Google Scholar] [CrossRef]
- Fournet, M.E.B.; Alwani, F.; Gunbay, S.; Chen, Y.Y.; Devine, D.M. Orthopedic 3D Printing in Orthopedic Medicine. In Polymer-Based Additive Manufaturing: Biomedical Application; Devine, D.M., Ed.; Springer: Cham, Switzerland, 2019; pp. 121–142. ISBN 978-3-030-24532-0. [Google Scholar]
- Choy, C.S.; Lee, W.F.; Lin, P.Y.; Wu, Y.F.; Huang, H.M.; Teng, N.C.; Pan, Y.H.; Salamanca, E.; Chang, W.J. Surface Modified β-Tricalcium Phosphate Enhanced Stem Cell Osteogenic Differentiation in vitro and Bone Regeneration in Vivo. Sci. Rep. 2021, 11, 9234. [Google Scholar] [CrossRef]
- Azaman, F.A.; Zhou, K.; del Blanes-Martínez, M.M.; Fournet, M.B.; Devine, D.M. Bioresorbable Chitosan-Based Bone Regeneration Scaffold Using Various Bioceramics and the Alteration of Photoinitiator Concentration in an Extended UV Photocrosslinking Reaction. Gels 2022, 8, 696. [Google Scholar] [CrossRef]
- Andrzejowski, P.; Giannoudis, P.V. The ‘Diamond Concept’ for Long Bone Non-Union Management. J. Orthop. Traumatol. 2019, 20, 21. [Google Scholar] [CrossRef] [Green Version]
- Baruffaldi, D.; Palmara, G.; Pirri, C.; Frascella, F. 3D Cell Culture: Recent Development in Materials with Tunable Stiffness. ACS Appl. Bio Mater. 2021, 4, 2233–2250. [Google Scholar] [CrossRef]
- Yamada, Y.; Yoshida, C.; Hamada, K.; Kikkawa, Y.; Nomizu, M. Development of Three-Dimensional Cell Culture Scaffolds Using Laminin Peptide-Conjugated Agarose Microgels. Biomacromolecules 2020, 21, 3765–3771. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, M.; He, J. A Review of Biomimetic Scaffolds for Bone Regeneration: Toward a Cell-free Strategy. Bioeng. Transl. Med. 2021, 6, e10206. [Google Scholar] [CrossRef]
- Wang, S.J.; Jiang, D.; Zhang, Z.Z.; Chen, Y.R.; Yang, Z.D.; Zhang, J.Y.; Shi, J.; Wang, X.; Yu, J.K. Biomimetic Nanosilica–Collagen Scaffolds for In Situ Bone Regeneration: Toward a Cell-Free, One-Step Surgery. Adv. Mater. 2019, 31, 1904341. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lu, H.; Zhao, Y.; Luo, J.; Yang, L.; Liu, W.; He, Q. Functionalized Cell-Free Scaffolds for Bone Defect Repair Inspired by Self-Healing of Bone Fractures: A Review and New Perspectives. Mater. Sci. Eng. C 2019, 98, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.; Wancket, L.; Metz, A. Current Considerations in Medical Device Pathology, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2020; ISBN 9780081026434. [Google Scholar]
- James, A.W.; LaChaud, G.; Shen, J.; Asatrian, G.; Nguyen, V.; Zhang, X.; Ting, K.; Soo, C. A Review of the Clinical Side Effects of Bone Morphogenetic Protein-2. Tissue Eng. Part B Rev. 2016, 22, 284–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biswas, D.; Ray, L.; Halquist, M. Development and Validation of a Stability-Indicating Method for Bone Morphogenetic Protein-2. Rev. Sep. Sci. 2019, 1, 3–16. [Google Scholar] [CrossRef] [Green Version]
- Arnold, P.M.; Vaccaro, A.R.; Sasso, R.C.; Fehlings, M.G.; Kopjar, B. Six-Year Follow-up of i-FACTOR® Peptide Enhanced Bone Graft vs. Autograft in Single Level ACDF in a Randomized Single Blinded FDA Investigational Device Exemption Study. Spine J. 2021, 21, S203. [Google Scholar] [CrossRef]
- Govoni, M.; Vivarelli, L.; Mazzotta, A.; Stagni, C.; Maso, A.; Dallari, D. Commercial Bone Grafts Claimed as an Alternative to Autografts: Current Trends for Clinical Applications in Orthopaedics. Materials 2021, 14, 3290. [Google Scholar] [CrossRef]
- Durham, E.L.; Howie, R.N.; Hall, S.; Larson, N.; Oakes, B.; Houck, R.; Grey, Z.; Steed, M.; Larue, A.C.; Muise-Helmericks, R.; et al. Optimizing Bone Wound Healing Using BMP2 with Absorbable Collagen Sponge and Talymed Nanofiber Scaffold. J. Transl. Med. 2018, 16, 321. [Google Scholar] [CrossRef] [PubMed]
- Azaman, F.A.; Daubiné, F.; Lebatard, A.; Fournet, M.E.B.; Devine, D.M. Chitosan/Hydroxyapatite Scaffolds with P28 as a Promising Osteoinductive Scaffold for Bone Healing Applications. Micro 2023, 3, 118–142. [Google Scholar] [CrossRef]
- Briquez, P.S.; Tsai, H.M.; Watkins, E.A.; Hubbell, J.A. Engineered Bridge Protein with Dual Affinity for Bone Morphogenetic Protein-2 and Collagen Enhances Bone Regeneration for Spinal Fusion. Sci. Adv. 2021, 7, 1–12. [Google Scholar] [CrossRef]
- Paxton, N.C.; Wong, C.S.; Desselle, M.R.; Allenby, M.C.; Woodruff, M.A. Bone Morphogenetic Protein–Assisted Bone Regeneration and Applications in Biofabrication; Woodhead Publishing Series in Biomaterials; Vrana, N.E., Knopf-Marques, H., Barthes, J., Eds.; Woodhead Publishing: Cambridge, UK, 2020; ISBN 9780081029060. [Google Scholar]
- Meng, C.; Su, W.; Liu, M.; Yao, S.; Ding, Q.; Yu, K.; Xiong, Z.; Chen, K.; Guo, X.; Bo, L.; et al. Controlled Delivery of Bone Morphogenic Protein-2-Related Peptide from Mineralised Extracellular Matrix-Based Scaffold Induces Bone Regeneration. Mater. Sci. Eng. C 2021, 126, 112182. [Google Scholar] [CrossRef]
- Bain, J.L.; Bonvallet, P.P.; Abou-Arraj, R.V.; Schupbach, P.; Reddy, M.S.; Bellis, S.L. Enhancement of the Regenerative Potential of Anorganic Bovine Bone Graft Utilizing a Polyglutamate-Modified BMP2 Peptide with Improved Binding to Calcium-Containing Materials. Tissue Eng. Part A 2015, 21, 2426–2436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, A.; Suzuki, Y.; Ogata, S.I.; Ohtsuki, C.; Tanihara, M. Activation of Osteo-Progenitor Cells by a Novel Synthetic Peptide Derived from the Bone Morphogenetic Protein-2 Knuckle Epitope. Biochim. Biophys. Acta Proteins Proteom. 2003, 1651, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Ahn, C.B.; Je, J.Y. Bone Health-Promoting Bioactive Peptides. J. Food Biochem. 2019, 43, e12529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madl, C.M.; Mehta, M.; Duda, G.N.; Heilshorn, S.C.; Mooney, D.J. Presentation of BMP-2 Mimicking Peptides in 3D Hydrogels Directs Cell Fate Commitment in Osteoblasts and Mesenchymal Stem Cells. Biomacromolecules 2014, 15, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Feng, Q.; Wang, M.; Guo, X.; Zheng, Q. Porous Nano-HA/Collagen/PLLA Scaffold Containing Chitosan Microspheres for Controlled Delivery of Synthetic Peptide Derived from BMP-2. J. Control. Release 2009, 134, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, X.; Liu, R.; Gong, Y.; Wang, M.; Huang, Q.; Feng, Q.; Yu, B. Zero-Order Controlled Release of BMP2-Derived Peptide P24 from the Chitosan Scaffold by Chemical Grafting Modification Technique for Promotion of Osteogenesis in Vitro and Enhancement of Bone Repair in vivo. Theranostics 2017, 7, 1072–1087. [Google Scholar] [CrossRef]
- Cui, W.; Sun, G.; Qu, Y.; Xiong, Y.; Sun, T.; Ji, Y.; Yang, L.; Shao, Z.; Ma, J.; Zhang, S.; et al. Repair of Rat Calvarial Defects Using Si-Doped Hydroxyapatite Scaffolds Loaded with a Bone Morphogenetic Protein-2-Related Peptide. J. Orthop. Res. 2016, 34, 1874–1882. [Google Scholar] [CrossRef] [Green Version]
- Chao, Y.L.; Lin, L.D.; Chang, H.H.; Wang, T.M. Preliminary Evaluation of BMP-2-Derived Peptide in Repairing a Peri-Implant Critical Size Defect: A Canine Model. J. Formos. Med. Assoc. 2021, 120, 1212–1220. [Google Scholar] [CrossRef]
- Bullock, G.; Atkinson, J.; Gentile, P.; Hatton, P.; Miller, C. Osteogenic Peptides and Attachment Methods Determine Tissue Regeneration in Modified Bone Graft Substitutes. J. Funct. Biomater. 2021, 12, 22. [Google Scholar] [CrossRef]
- Talbot, S.R.; Biernot, S.; Bleich, A.; van Dijk, R.M.; Ernst, L.; Häger, C.; Helgers, S.O.A.; Koegel, B.; Koska, I.; Kuhla, A.; et al. Defining Body-Weight Reduction as a Humane Endpoint: A Critical Appraisal. Lab. Anim. 2020, 54, 99–110. [Google Scholar] [CrossRef]
- Helgers, S.O.A.; Talbot, S.R.; Riedesel, A.K.; Wassermann, L.; Wu, Z.; Krauss, J.K.; Häger, C.; Bleich, A.; Schwabe, K. Body Weight Algorithm Predicts Humane Endpoint in an Intracranial Rat Glioma Model. Sci. Rep. 2020, 10, 9020. [Google Scholar] [CrossRef] [PubMed]
- Rudert, M.; Wilms, U.; Hoberg, M.; Wirth, C.J. Cell-Based Treatment of Osteochondral Defects in the Rabbit Knee with Natural and Synthetic Matrices: Cellular Seeding Determines the Outcome. Arch. Orthop. Trauma Surg. 2005, 125, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Malysheva, K.; Kwaśniak, K.; Gnilitskyi, I.; Barylyak, A.; Zinchenko, V.; Fahmi, A.; Korchynskyi, O.; Bobitski, Y. Functionalization of Polycaprolactone Electrospun Osteoplastic Scaffolds with Fluorapatite and Hydroxyapatite Nanoparticles: Biocompatibility Comparison of Human versus Mouse Mesenchymal Stem Cells. Materials 2021, 14, 1333. [Google Scholar] [CrossRef] [PubMed]
- Vidal, C.; Alves, P.; Alves, M.M.; Carmezim, M.J.; Fernandes, M.H.; Grenho, L.; Inácio, P.L.; Ferreira, F.B.; Santos, T.G.; Santos, C. Fabrication of a Biodegradable and Cytocompatible Magnesium/Nanohydroxyapatite/Fluorapatite Composite by Upward Friction Stir Processing for Biomedical Applications. J. Mech. Behav. Biomed. Mater. 2022, 129, 105137. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Qu, Y.; Cui, W.; Yang, L.; Ji, Y.; Yu, W.; Navinduth, R.; Shao, Z.; Yang, H.; Guo, X. Evaluation of Osteogenic Inductivity of a Novel BMP2-Mimicking Peptide P28 and P28-Containing Bone Composite. J. Biomed. Mater. Res. Part A 2017, 106, 210–220. [Google Scholar] [CrossRef]
- Zhou, J.; Xiong, Z.; Liu, M.; Yang, L.; Yao, S.; Chen, K.; Yu, K.; Qu, Y.; Sun, T.; Guo, X. Creation of Bony Microenvironment with Extracellular Matrix Doped-Bioactive Ceramics to Enhance Osteoblast Behavior and Delivery of Aspartic Acid-Modified Bmp-2 Peptides. Int. J. Nanomedicine 2020, 15, 8465–8478. [Google Scholar] [CrossRef]
- van Gaalen, S.M.; Kruyt, M.C.; Geuze, R.E.; de Bruijn, J.D.; Alblas, J.; Dhert, W.J.A. Use of Fluorochrome Labels in in vivo Bone Tissue Engineering Research. Tissue Eng. Part B. Rev. 2010, 16, 209–217. [Google Scholar] [CrossRef]
- Shanker, M.D. Utilisation of Injectable Fluorochromes for Quantification of Bone Growth in a Sheep Tibial. Master’s Thesis, Queensland University of Technology, Brisbane, Australia, 2019. [Google Scholar]
- Spicer, P.P.; Kretlow, J.D.; Young, S.; Jansen, J.A.; Kasper, F.K.; Mikos, A.G. Evaluation of Bone Regeneration Using the Rat Critical Size Calvarial Defect. Nat. Protoc. 2012, 7, 1918–1929. [Google Scholar] [CrossRef] [Green Version]
- Shih, Y.-R.; Padke, A.; Yamaguchi, T.; Kang, H.; Inoue, N.; Masuda, K.; Varghese, S. Synthetic Bone Mimetic Matrix-Mediated in situ Bone Tissue Formation through Host Cell Recruitment. Acta Biomater. 2017, 19, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Pautke, C.; Tischer, T.; Vogt, S.; Haczek, C.; Deppe, H.; Neff, A.; Horch, H.H.; Schieker, M.; Kolk, A. New Advances in Fluorochrome Sequential Labelling of Teeth Using Seven Different Fluorochromes and Spectral Image Analysis. J. Anat. 2007, 210, 117–121. [Google Scholar] [CrossRef]
- Pautke, C.; Vogt, S.; Tischer, T.; Wexel, G.; Deppe, H.; Milz, S.; Schieker, M.; Kolk, A. Polychrome Labeling of Bone with Seven Different Fluorochromes: Enhancing Fluorochrome Discrimination by Spectral Image Analysis. Bone 2005, 37, 441–445. [Google Scholar] [CrossRef]
- Pautke, C.; Vogt, S.; Kreutzer, K.; Haczek, C.; Wexel, G.; Kolk, A.; Imhoff, A.B.; Zitzelsberger, H.; Milz, S.; Tischer, T. Characterization of Eight Different Tetracyclines: Advances in Fluorescence Bone Labeling. J. Anat. 2010, 217, 76–82. [Google Scholar] [CrossRef]
- Zuncheddu, D.; Della Bella, E.; Schwab, A.; Petta, D.; Rocchitta, G.; Generelli, S.; Kurth, F.; Parrilli, A.; Verrier, S.; Rau, J.V.; et al. Quality Control Methods in Musculoskeletal Tissue Engineering: From Imaging to Biosensors. Bone Res. 2021, 9, 46. [Google Scholar] [CrossRef]
- Maglio, M.; Salamanna, F.; Brogini, S.; Borsari, V.; Pagani, S.; Nicoli Aldini, N.; Giavaresi, G.; Fini, M. Histological, Histomorphometrical, and Biomechanical Studies of Bone-Implanted Medical Devices: Hard Resin Embedding. Biomed Res. Int. 2020, 2020, 1804630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dempster, D.W.; Compston, J.E.; Drezner, M.K.; Kanis, F.H.G.; Malluche, J.A.; Ott, S.; Malluche, H.; Parfitt, A.M.; Recker, R.R.; Parfitt, M. Histomorphometry Nomenclature: A 2012 Update of the Report of the ASBMR Histomorphometry Nomenclature David. J. Bone Miner. Res. 2014, 28, 2–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohiuddin, O.A.; Campbell, B.; Poche, J.N.; Ma, M.; Rogers, E.; Gaupp, D.; Harrison, M.A.A.; Bunnell, B.A.; Hayes, D.J.; Gimble, J.M. Decellularized Adipose Tissue Hydrogel Promotes Bone Regeneration in Critical-Sized Mouse Femoral Defect Model. Front. Bioeng. Biotechnol. 2019, 7, 211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, A.; Baranowski, A.; Ritz, U.; Mack, C.; Götz, H.; Langendorf, E.; Al-Nawas, B.; Drees, P.; Rommens, P.M.; Hofmann, A. Effect of Bone Sialoprotein Coating on Progression of Bone Formation in a Femoral Defect Model in Rats. Eur. J. Trauma Emerg. Surg. 2019, 46, 277–286. [Google Scholar] [CrossRef]
- Porter, A.; Irwin, R.; Miller, J.; Horan, D.J.; Robling, A.G.; McCabe, L.R. Quick and Inexpensive Paraffin-Embedding Method for Dynamic Bone Formation Analyses. Sci. Rep. 2017, 7, 42505. [Google Scholar] [CrossRef] [Green Version]
- Ruvinov, E.; Tavor Re’em, T.; Witte, F.; Cohen, S. Articular Cartilage Regeneration Using Acellular Bioactive Affinity-Binding Alginate Hydrogel: A 6-Month Study in a Mini-Pig Model of Osteochondral Defects. J. Orthop. Transl. 2019, 16, 40–52. [Google Scholar] [CrossRef]
- McGregor, N.; Poulton, I.; Walker, E.; Sims, N. Testing Bone Formation Induction by Calvarial Injection Assay in vivo. Bio-Protocol 2020, 10, e3560. [Google Scholar] [CrossRef]
- Tosun, H.B.; Gürger, M.; Gümüştaş, S.A.; Uludag, A.; Üçer, Ö.; Serbest, S.; Çelik, S. The Effect of Sodium Hyaluronate–Chondroitin Sulfate Combined Solution on Cartilage Formation in Osteochondral Defects of the Rabbit Knee: An Experimental Study. Ther. Clin. Risk Manag. 2017, 13, 523–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdallah, A.N.; Shamaa, A.A.; El-Tookhy, O.S.; Abd El-Mottaleb, E.M. Evaluation of Low Level Laser-Activated Stromal Vascular Fraction as a Single Procedure for Treatment of Experimental Chondral Defects. Asian J. Anim. Sci. 2016, 10, 15–28. [Google Scholar] [CrossRef] [Green Version]
- Betsch, M.; Schneppendahl, J.; Thuns, S.; Herten, M.; Sager, M.; Jungbluth, P.; Hakimi, M.; Wild, M. Bone Marrow Aspiration Concentrate and Platelet Rich Plasma for Osteochondral Repair in a Porcine Osteochondral Defect Model. PLoS ONE 2013, 8, e71602. [Google Scholar] [CrossRef]
- Radzak, N.A.; Murali, M.R.; Kamarul, T. Assessing the Potential of Bone Marrow Concentrate for Cartilage Repair and Regeneration in Animal Models: A Systemic Review. Sains Malays. 2021, 50, 1727–1744. [Google Scholar] [CrossRef]
- Chan, J.K.C. The Wonderful Colors of the Hematoxylin-Eosin Stain in Diagnostic Surgical Pathology. Int. J. Surg. Pathol. 2014, 22, 12–32. [Google Scholar] [CrossRef]

| Samples | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R1L | R1R | R2L | R2R | R3L | R3R | R4L | R4R | R5L | R5R | R6L | R6R | R7L | R7R | R8L | R8R | R9L | R9R | |
| Total score for each sample | 5 | 3 | 5 | 5 | 3 | 3 | 6 | 3 | 5 | 3 | 7 | 3 | 5 | 3 | 7 | 6 | 3 | 6 |
| Standard deviation | 0.58 | 0 | 0.58 | 0.58 | 0 | 0 | 0 | 0 | 0.58 | 0 | 0.58 | 0 | 0.58 | 0 | 0.58 | 0 | 0 | 1 |
| Sample ID | Ratio HAp:FAp | Weight (g) | Volume (µL) | Volume (mL) | ||||
|---|---|---|---|---|---|---|---|---|
| (MW) CS | HAp | FAp | BP | PEG600 DMA | 5 mg/mL P28 | Acetic Acid | ||
| 12% LW CS/HAp/FAp 1:1 | 1:1 | (LW) 1.5 | 0.75 | 0.75 | 5 | 100 | 0 | 12.5 |
| 12% HW CS/HAp/FAp 1:1 | 1:1 | (HW) 1.5 | 0.75 | 0.75 | 5 | 100 | 0 | 12.5 |
| 12% HW CS/HAp/FAp 1:0.75 | 1:0.75 | (HW) 1.5 | 1 | 0.75 | 5 | 100 | 0 | 12.5 |
| 12% HW CS/HAp/FAp 1:1/P28 25 µg | 1:1 | (HW) 1.5 | 0.75 | 0.75 | 5 | 100 | 5 | 12.5 |
| 12% HW CS/HAp/FAp 1:1/P28 75 µg | 1:1 | (HW) 1.5 | 0.75 | 0.75 | 5 | 100 | 15 | 12.5 |
| 12% HW CS/HAp/FAp 1:1/P28 150 µg | 1:1 | (HW) 1.5 | 0.75 | 0.75 | 5 | 100 | 30 | 12.5 |
| Rat Tag | Left Condyle Treatments | Right Condyle Treatments |
|---|---|---|
| R1 | 12% LW CS/HAp/FAp 1:1 | 12% HW CS/HAp/FAp 1:1 |
| R2 | 12% LW CS/HAp/FAp 1:1 | 12% HW CS/HAp/FAp 1:0.75 |
| R3 | 12% LW CS/HAp/FAp 1:1 | 12% HW CS/HAp/FAp 1:1/P28 25 µg |
| R4 | 12% HW CS/HAp/FAp 1:1 | 12% HW CS/HAp/FAp 1:1/P28 25 µg |
| R5 | 12% HW CS/HAp/FAp 1:0.75 | 12% HW CS/HAp/FAp 1:1/P28 75 µg |
| R6 | 12% HW CS/HAp/FAp 1:1/P28 25 µg | 12% HW CS/HAp/FAp 1:1/P28 75 µg |
| R7 | 12% HW CS/HAp/FAp 1:1/P28 150 µg | 12% HW CS/HAp/FAp 1:0.75 |
| R8 | 12% HW CS/HAp/FAp 1:1/P28 150 µg | 12% HW CS/HAp/FAp 1:1 |
| R9 | 12% HW CS/HAp/FAp 1:1/P28 150 µg | 12% HW CS/HAp/FAp 1:1/P28 75 µg |
| Criterion | Score | Macroscopic Characteristics |
|---|---|---|
| Defect visibility | 1 | Appeared as small irregular bumps |
| 2 | Regular hole closed by a transparent tissue | |
| 3 | Completely closed | |
| Colour | 1 | Yellowish paste observed |
| 2 | White bony appearance | |
| Surface | 1 | Rough and bumpy |
| 2 | Smooth |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azaman, F.A.; Brennan Fournet, M.E.; Sheikh Ab Hamid, S.; Zawawi, M.S.F.; da Silva Junior, V.A.; Devine, D.M. Enhancement of Scaffold In Vivo Biodegradability for Bone Regeneration Using P28 Peptide Formulations. Pharmaceuticals 2023, 16, 876. https://doi.org/10.3390/ph16060876
Azaman FA, Brennan Fournet ME, Sheikh Ab Hamid S, Zawawi MSF, da Silva Junior VA, Devine DM. Enhancement of Scaffold In Vivo Biodegradability for Bone Regeneration Using P28 Peptide Formulations. Pharmaceuticals. 2023; 16(6):876. https://doi.org/10.3390/ph16060876
Chicago/Turabian StyleAzaman, Farah Alwani, Margaret E. Brennan Fournet, Suzina Sheikh Ab Hamid, Muhamad Syahrul Fitri Zawawi, Valdemiro Amaro da Silva Junior, and Declan M. Devine. 2023. "Enhancement of Scaffold In Vivo Biodegradability for Bone Regeneration Using P28 Peptide Formulations" Pharmaceuticals 16, no. 6: 876. https://doi.org/10.3390/ph16060876
APA StyleAzaman, F. A., Brennan Fournet, M. E., Sheikh Ab Hamid, S., Zawawi, M. S. F., da Silva Junior, V. A., & Devine, D. M. (2023). Enhancement of Scaffold In Vivo Biodegradability for Bone Regeneration Using P28 Peptide Formulations. Pharmaceuticals, 16(6), 876. https://doi.org/10.3390/ph16060876








