Phytochemicals as Antimicrobials: Prospecting Himalayan Medicinal Plants as Source of Alternate Medicine to Combat Antimicrobial Resistance
Abstract
:1. Introduction
Emergence of Antimicrobial Resistance
2. Mechanisms of Antimicrobial Resistance
2.1. Bacterial Efflux Pumps
2.2. Enzymatic Modification and Degradation of Antibiotics
2.2.1. Hydrolysis
β-Lactamases
Macrolide Esterases
Epoxidases
2.3. Group Transfer
2.4. Miscellaneous Mechanisms of Antibiotic Degradation
2.4.1. Redox Enzymes
2.4.2. Lyases
2.5. Target Site Modification
2.5.1. Enzymatic Alteration of Target Site
2.5.2. Replacement or Bypass of Antimicrobial Binding Site
2.5.3. Mutations in the Genes Encoding Target Sites
2.6. Porin Modification
3. Antimicrobial Resistance Modulation via Natural Products
3.1. Multiple-Compound Synergy vs. Single-Compound Therapy
3.2. Plant Secondary Metabolites as Antimicrobials
3.2.1. Alkaloids
3.2.2. Phenols
3.2.3. Organosulfur Compounds
3.2.4. Terpenes
4. Himalayan Medicinal Plants as a Reservoir of Phytochemicals for Novel Antimicrobial Drug Discovery
4.1. Plant Diversity of Indian Himalayas
4.2. Medicinal Plant Resources of Himalayas and Alternate Systems of Medicine
5. Antimicrobial Profile of Himalayan Medicinal Plants
6. Challenges of Using Phytochemicals as Medicine
6.1. Effects of Climate Change
6.2. Toxicity of Herbal Medicine
6.3. Other Challenges and Regulation
7. Methodology
8. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aminov, R.I. A brief history of the antibiotic era: Lessons learned and challenges for the future. Front. Microbiol. 2010, 1, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christensen, S.B. Drugs that changed society: History and current status of the early antibiotics: Salvarsan, sulfonamides, and β-lactams. Molecules 2021, 26, 6057. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro da Cunha, B.; Fonseca, L.P.; Calado, C.R.C. Antibiotic discovery: Where have we come from, where do we go? Antibiotics 2019, 8, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef]
- Cook, M.A.; Wright, G.D. The past, present, and future of antibiotics. Sci. Transl. Med. 2022, 14, eabo7793. [Google Scholar] [CrossRef]
- Uddin, T.M.; Chakraborty, A.J.; Khusro, A.; Zidan, B.R.M.; Mitra, S.; Bin Emran, T.; Dhama, K.; Ripon, K.H.; Gajdács, M.; Sahibzada, M.U.K.; et al. Antibiotic resistance in microbes: History, mechanisms, therapeutic strategies and future prospects. J. Infect. Public Health 2021, 14, 1750–1766. [Google Scholar] [CrossRef]
- Said, M.S.; Saleem, I.; Hashmi, A.M.; Ullah, I.; Khan, A.H. Rational use of antibiotics and requisition of pharmacist. Int. J. Nat. Med. Health Sci. 2022, 1, 21–24. [Google Scholar] [CrossRef]
- Aslam, B.; Khurshid, M.; Arshad, M.I.; Muzammil, S.; Rasool, M.; Yasmeen, N.; Shah, T.; Chaudhry, T.H.; Rasool, M.H.; Shahid, A.; et al. Antibiotic resistance: One health one world outlook. Front. Cell. Infect. Microbiol. 2021, 11, 1153. [Google Scholar] [CrossRef]
- Samreen; Ahmad, I.; Malak, H.A.; Abulreesh, H.H. Environmental antimicrobial resistance and its drivers: A potential threat to public health. J. Glob. Antimicrob. Resist. 2021, 27, 101–111. [Google Scholar] [CrossRef]
- Kongkham, B.; Prabakaran, D.; Puttaswamy, H. Opportunities and challenges in managing antibiotic resistance in bacteria using plant secondary metabolites. Fitoterapia 2020, 147, 104762. [Google Scholar] [CrossRef]
- Larsson, D.G.J.; Flach, C.-F. Antibiotic resistance in the environment. Nat. Rev. Microbiol. 2022, 20, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Manesh, A.; Varghese, G.M. CENDRIC Investigators and Collaborators. Rising antimicrobial resistance: An evolving epidemic in a pandemic. Lancet Microbe. 2021, 2, 419–420. [Google Scholar] [CrossRef] [PubMed]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsakris, Z.; Rozos, G.; Tsigalou, C.; Bezirtzoglou, E. Interactions between medical plant-derived bioactive compounds: Focus on antimicrobial combination effects. Antibiotics 2022, 11, 1014. [Google Scholar] [CrossRef] [PubMed]
- Arsalan, H.M.; Javed, H.; Farheen, N. Antibacterial activity of some medicinal plants against human pathogens. Int. J. Nat. Med. Health Sci. 2022, 1, 82–88. [Google Scholar] [CrossRef]
- Anand, U.; Jacobo-Herrera, N.; Altemimi, A.; Lakhssassi, N. A comprehensive review on medicinal plants as antimicrobial therapeutics: Potential avenues of biocompatible drug discovery. Metabolites 2019, 9, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chhetri, D.R. Medicinal Plants of the Himalaya: Production Technology and Utilization, 1st ed.; Agrobios: Jodhpur, India, 2015; ISBN 9788177545586. [Google Scholar]
- Shiva, M.P. Inventory of Forest Resources for Sustainable Management & Biodiversity Conservation with Lists of Multipurpose Tree Species Yielding Both Timber & Non-Timber Forest Products (Ntfps), and Shrub & Herb Species of Ntfp Importance, 1st ed.; Indus Publishing Company: New Delhi, India, 1998; ISBN 9788173870910. [Google Scholar]
- Singh, D.K.; Pusalkar, P.K. Floristic diversity of the Indian Himalaya. In Biodiversity of the Himalaya: Jammu and Kashmir State; Dar, G.H., Khuroo, A.A., Eds.; Springer: Singapore, 2020; Volume 18, pp. 93–126. [Google Scholar]
- Bhat, M.N.; Singh, B.; Surmal, O.; Singh, B.; Shivgotra, V.; Musarella, C.M. Ethnobotany of the Himalayas: Safeguarding medical practices and traditional uses of Kashmir regions. Biology 2021, 10, 851. [Google Scholar] [CrossRef]
- Abraham, E.P.; Chain, E. An enzyme from bacteria able to destroy penicillin. Nature 1940, 146, 837. [Google Scholar] [CrossRef]
- Woodruff, H.B.; Selman, A. Waksman, winner of the 1952 Nobel prize for physiology or medicine. Appl. Environ. Microbiol. 2014, 80, 2–8. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Myers, A.G. Development of a platform for the discovery and practical synthesis of new tetracycline antibiotics. Curr. Opin. Chem. Biol. 2016, 32, 48–57. [Google Scholar] [CrossRef] [Green Version]
- Wiest, D.B.; Cochran, J.B.; Tecklenburg, F.W. Chloramphenicol toxicity revisited: A 12-year-old patient with a brain abscess. J. Pediatr. Pharmacol. Ther. 2012, 17, 182–188. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.S.; de Lencastre, H.; Garau, J.; Kluytmans, J.; Malhotra-Kumar, S.; Peschel, A.; Harbarth, S. Methicillin-resistant Staphylococcus aureus. Nat. Rev. Dis. Primers 2018, 4, 18033. [Google Scholar] [CrossRef] [Green Version]
- Christaki, E.; Marcou, M.; Tofarides, A. Antimicrobial resistance in bacteria: Mechanisms, evolution, and persistence. J. Molecular Evolution 2020, 88, 26–40. [Google Scholar] [CrossRef]
- Munita, J.M.; Arias, C.A. Mechanisms of antibiotic resistance. Microbiol. Spectr. 2016, 4, 481–511. [Google Scholar] [CrossRef] [Green Version]
- Shriram, V.; Khare, T.; Bhagwat, R.; Shukla, R.; Kumar, V. Inhibiting bacterial drug efflux pumps via phyto-therapeutics to combat threatening antimicrobial resistance. Front. Microbiol. 2018, 9, 2990. [Google Scholar] [CrossRef] [PubMed]
- Du, D.; Wang-Kan, X.; Neuberger, A.; van Veen, H.W.; Pos, K.M.; Piddock LJ, V.; Luisi, B.F. Multidrug efflux pumps: Structure, function and regulation. Nat. Rev. Microbiol. 2018, 16, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Putman, M.; van Veen, H.W.; Konings, W.N. Molecular properties of bacterial multidrug transporters. Microbiol. Mol. Biol. Rev. 2000, 64, 672–693. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Deng, Z.; Yan, A. Bacterial multidrug efflux pumps: Mechanisms, physiology and pharmacological exploitations. Biochem. Biophys. Res. Commun. 2014, 453, 254–267. [Google Scholar] [CrossRef] [Green Version]
- Poole, K. Efflux-Mediated Multiresistance in Gram-Negative Bacteria. Clin. Microbiol. Infect. 2004, 10, 12–26. [Google Scholar] [CrossRef] [Green Version]
- Stavri, M.; Piddock, L.J.V.; Gibbons, S. Bacterial efflux pump inhibitors from natural sources. J. Antimicrob. Chemother. 2007, 59, 1247–1260. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.W.; Huda, M.N.; Kuroda, T.; Mizushima, T.; Tsuchiya, T. EfrAB, an ABC multidrug efflux pump in Enterococcus faecalis. Antimicrob. Agents Chemother. 2003, 47, 3733–3738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wendlandt, S.; Kadlec, K.; Feßler, A.T.; Schwarz, S. Identification of ABC transporter genes conferring combined pleuromutilin–lincosamide–streptogramin A resistance in bovine methicillin-resistant Staphylococcus aureus and coagulase-negative Staphylococci. Vet. Microbiol. 2015, 177, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Kaatz, G.W.; McAleese, F.; Seo, S.M. Multidrug resistance in Staphylococcus aureus due to overexpression of a novel multidrug and toxin extrusion (MATE) transport protein. Antimicrob. Agents Chemother. 2005, 49, 1857–1864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, H.; Bogaki, M.; Nakamura, S.; Ubukata, K.; Konno, A.M. Nucleotide sequence and characterization of the Staphylococcus aureus NorA gene, which confers resistance to quinolones. J. Bacteriol. 1990, 172, 6942–6949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Littlejohn, T.G.; Paulsen, I.T.; Gillespie, M.T.; Tennent, J.M.; Midgley, M.; Jones, I.G.; Purewal, A.S.; Skurray, R.A. Substrate specificity and energetics of antiseptic and disinfectant resistance in Staphylococcus aureus. FEMS Microbiol. Lett. 1992, 95, 259–265. [Google Scholar] [CrossRef]
- Ogawa, W.; Minato, Y.; Dodan, H.; Onishi, M.; Tsuchiya, T.; Kuroda, T. Characterization of MATE-type multidrug efflux pumps from Klebsiella pneumoniae MGH78578. PLoS ONE 2015, 10, e0121619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srinivasan, V.B.; Singh, B.B.; Priyadarshi, N.; Chauhan, N.K.; Rajamohan, G. Role of novel multidrug efflux pump involved in drug resistance in Klebsiella Pneumoniae. PLoS ONE 2014, 9, e96288. [Google Scholar] [CrossRef] [Green Version]
- Hansen, L.H.; Johannesen, E.; Burmølle, M.; Sørensen, A.H.; Sørensen, S.J. Plasmid-encoded multidrug efflux pump conferring resistance to olaquindox in Escherichia coli. Antimicrob. Agents Chemother. 2004, 48, 3332–3337. [Google Scholar] [CrossRef] [Green Version]
- Okada, U.; Yamashita, E.; Neuberger, A.; Morimoto, M.; van Veen, H.W.; Murakami, S. Crystal Structure of tripartite-type ABC transporter MacB from Acinetobacter baumannii. Nat. Commun. 2017, 8, 1336. [Google Scholar] [CrossRef] [Green Version]
- Su, X.Z.; Chen, J.; Mizushima, T.; Kuroda, T.; Tsuchiya, T. AbeM, an H+-coupled Acinetobacter baumannii multidrug efflux pump belonging to the MATE family of transporters. Antimicrob. Agents Chemother. 2005, 49, 4362–4364. [Google Scholar] [CrossRef] [Green Version]
- Roca, I.; Marti, S.; Espinal, P.; Martínez, P.; Gibert, I.; Vila, J. CraA, a major facilitator superfamily efflux pump associated with chloramphenicol resistance in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2009, 53, 4013–4014. [Google Scholar] [CrossRef] [Green Version]
- Coyne, S.; Rosenfeld, N.; Lambert, T.; Courvalin, P.; Périchon, B. Overexpression of resistance-nodulation-cell division pump AdeFGH confers multidrug resistance in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2010, 54, 4389–4393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajamohan, G.; Srinivasan, V.B.; Gebreyes, W.A. Novel role of Acinetobacter baumannii RND efflux transporters in mediating decreased susceptibility to biocides. J. Antimicrob. Chemother. 2009, 65, 228–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poole, K.; Gotoh, N.; Tsujimoto, H.; Zhao, Q.; Wada, A.; Yamasaki, T.; Neshat, S.; Yamagishi, J.-I.; Li, X.-Z.; Nishino, T. Overexpression of the MexC-MexD-OprJ efflux operon in NfxB-type multidrug-resistant strains of Pseudomonas aeruginosa. Mol. Microbiol. 1996, 21, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Köhler, T.; Pechère, J.-C.; Plésiat, P. Bacterial antibiotic efflux systems of medical importance. Cell. Mol. Life Sci. 1999, 56, 771–778. [Google Scholar] [CrossRef]
- Kobayashi, N.; Nishino, K.; Yamaguchi, A. Novel macrolide-specific ABC-type efflux transporter in Escherichia coli. J. Bacteriol. 2001, 183, 5639–5644. [Google Scholar] [CrossRef] [Green Version]
- Nishino, K.; Latifi, T.; Groisman, E.A. Virulence and drug resistance roles of multidrug efflux systems of Salmonella enterica serovar Typhimurium. Mol. Microbiol. 2006, 59, 126–141. [Google Scholar] [CrossRef]
- Cattoir, V.; Poirel, L.; Nordmann, P. Plasmid-mediated quinolone resistance pump QepA2 in an Escherichia coli isolate from France. Antimicrob. Agents Chemother. 2008, 52, 3801–3804. [Google Scholar] [CrossRef] [Green Version]
- Swick, M.C.; Morgan-Linnell, S.K.; Carlson, K.M.; Zechiedrich, L. Expression of multidrug efflux pump genes AcrAB-TolC, MdfA, and NorE in Escherichia coli clinical isolates as a function of fluoroquinolone and multidrug resistance. Antimicrob. Agents Chemother. 2011, 55, 921–924. [Google Scholar] [CrossRef] [Green Version]
- Lennen, R.M.; Politz, M.G.; Kruziki, M.A.; Pfleger, B.F. Identification of transport proteins involved in free fatty acid efflux in Escherichia coli. J. Bacteriol. 2013, 195, 135–144. [Google Scholar] [CrossRef] [Green Version]
- Schuldiner, S. EmrE, a model for studying evolution and mechanism of ion-coupled transporters. Biochim. Et Biophys. Acta Proteins Proteom. 2009, 1794, 748–762. [Google Scholar] [CrossRef]
- Beketskaia, M.S.; Bay, D.C.; Turner, R.J. Outer membrane protein OmpW participates with small multidrug resistance protein member EmrE in quaternary cationic compound efflux. J. Bacteriol. 2014, 196, 1908–1914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, G.D. Bacterial resistance to antibiotics: Enzymatic degradation and modification. Adv. Drug Deliv. Rev. 2005, 57, 1451–1470. [Google Scholar] [CrossRef] [PubMed]
- Tooke, C.L.; Hinchliffe, P.; Bragginton, E.C.; Colenso, C.K.; Hirvonen, V.H.A.; Takebayashi, Y.; Spencer, J. β-Lactamases and β-Lactamase inhibitors in the 21st Century. J. Mol. Biol. 2019, 431, 3472–3500. [Google Scholar] [CrossRef] [PubMed]
- Khare, T.; Anand, U.; Dey, A.; Assaraf, Y.G.; Chen, Z.-S.; Liu, Z.; Kumar, V. Exploring phytochemicals for combating antibiotic resistance in microbial pathogens. Front. Pharmacol. 2021, 12, 720726. [Google Scholar] [CrossRef]
- Bonomo, R.A. β-Lactamases: A focus on current challenges. Cold Spring Harb. Perspect. Med. 2017, 7, a025239. [Google Scholar] [CrossRef]
- Hall, B.G.; Barlow, M. Revised ambler classification of β-lactamases. J. Antimicrob. Chemother. 2005, 55, 1050–1051. [Google Scholar] [CrossRef] [Green Version]
- Bush, K. Past and present perspectives on β-lactamases. Antimicrob. Agents Chemother. 2018, 62, e01076-18. [Google Scholar] [CrossRef] [Green Version]
- Bush, K. Proliferation and significance of clinically relevant β-lactamases. Ann. N. Y. Acad. Sci. 2013, 1277, 84–90. [Google Scholar] [CrossRef]
- Orencia, M.C.; Yoon, J.S.; Ness, J.E.; Stemmer, W.P.C.; Stevens, R.C. Predicting the emergence of antibiotic resistance by directed evolution and structural analysis. Nat. Struct. Biol. 2001, 8, 238–242. [Google Scholar] [CrossRef]
- Egorov, A.M.; Ulyashova, M.M.; Rubtsova, M.Y. Bacterial enzymes and antibiotic resistance. Acta Nat. 2018, 10, 33–48. [Google Scholar] [CrossRef] [Green Version]
- Philippon, A.; Arlet, G.; Jacoby, G.A. Plasmid-determined AmpC-type β-Lactamases. Antimicrob. Agents Chemother. 2002, 46, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bebrone, C. Metallo-β-lactamases (classification, activity, genetic organization, structure, zinc coordination) and their superfamily. Biochem. Pharmacol. 2007, 74, 1686–1701. [Google Scholar] [CrossRef]
- Naas, T.; Oueslati, S.; Bonnin, R.A.; Dabos, M.L.; Zavala, A.; Dortet, L.; Retailleau, P.; Iorga, B.I. β-Lactamase Database (BLDB)–structure and function. J. Enzym. Inhib. Med. Chem. 2017, 32, 917–919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morar, M.; Pengelly, K.; Koteva, K.; Wright, G.D. Mechanism and diversity of the erythromycin esterase family of enzymes. Biochemistry 2012, 51, 1740–1751. [Google Scholar] [CrossRef] [PubMed]
- Fyfe, C.; Grossman, T.H.; Kerstein, K.; Sutcliffe, J. Resistance to macrolide antibiotics in public health pathogens. Cold Spring Harb. Perspect. Med. 2016, 6, a025395. [Google Scholar] [CrossRef] [Green Version]
- Gomes, C.; Martínez-Puchol, S.; Palma, N.; Horna, G.; Ruiz-Roldán, L.; Pons, M.J.; Ruiz, J. Macrolide resistance mechanisms in Enterobacteriaceae: Focus on azithromycin. Crit. Rev. Microbiol. 2017, 43, 1–30. [Google Scholar] [CrossRef]
- Morar, M.; Wright, G.D. The genomic enzymology of antibiotic resistance. Annu. Rev. Genet. 2010, 44, 25–51. [Google Scholar] [CrossRef]
- Schwarz, S.; Shen, J.; Kadlec, K.; Wang, Y.; Michael, G.B.; Feßler, A.T.; Vester, B. Lincosamides, streptogramins, phenicols, and pleuromutilins: Mode of action and mechanisms of resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a027037. [Google Scholar] [CrossRef] [Green Version]
- Park, B.H.; Levy, S.B. The cryptic tetracycline resistance determinant on Tn4400 mediates tetracycline degradation as well as tetracycline efflux. Antimicrob. Agents Chemother. 1988, 32, 1797–1800. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Moore, I.F.; Koteva, K.P.; Bareich, D.C.; Hughes, D.W.; Wright, G.D. TetX is a flavin-dependent monooxygenase conferring resistance to tetracycline antibiotics. J. Biol. Chem. 2004, 279, 52346–52352. [Google Scholar] [CrossRef] [Green Version]
- Speer, B.S.; Salyers, A.A. Characterization of a novel tetracycline resistance that functions only in aerobically grown Escherichia coli. J. Bacteriol. 1988, 170, 1423–1429. [Google Scholar] [CrossRef] [Green Version]
- Korczynska, M.; Mukhtar, T.A.; Wright, G.D.; Berghuis, A.M. Structural basis for streptogramin B resistance in Staphylococcus aureus by virginiamycin B lyase. Proc. Natl. Acad. Sci. USA 2007, 104, 10388–10393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allignet, J.; Loncle, V.; Mazodier, P.; el Solh, N. Nucleotide sequence of a Staphylococcal plasmid gene, Vgb, encoding a hydrolase inactivating the B components of virginiamycin-like antibiotics. Plasmid 1988, 20, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Luthra, S.; Rominski, A.; Sander, P. The role of antibiotic-target-modifying and antibiotic-modifying enzymes in Mycobacterium abscessus drug resistance. Front. Microbiol. 2018, 9, 2179. [Google Scholar] [CrossRef]
- Weisblum, B. Minireview erythromycin resistance by ribosome modification. Antimicrob. Agents Chemother. 1995, 39, 577–585. [Google Scholar] [CrossRef] [Green Version]
- Leclercq, R. Mechanisms of resistance to macrolides and lincosamides: Nature of the Resistance Elements and Their Clinical Implications. Clin. Infect. Dis. 2002, 34, 482–492. [Google Scholar] [CrossRef] [Green Version]
- Bush, K.; Bradford, P.A. β-Lactams and β-lactamase inhibitors: An overview. Cold Spring Harb. Perspect. Med. 2016, 6, a025247. [Google Scholar] [CrossRef]
- Miller, W.R.; Munita, J.M.; Arias, C.A. Mechanisms of antibiotic resistance in Enterococci. Expert Rev. Anti-Infect. Ther. 2014, 12, 1221–1236. [Google Scholar] [CrossRef]
- Campbell, E.A.; Korzheva, N.; Mustaev, A.; Murakami, K.; Nair, S.; Goldfarb, A.; Darst, S.A. Structural mechanism for rifampicin inhibition of bacterial RNA polymerase. Cell 2001, 104, 901–912. [Google Scholar] [CrossRef] [PubMed]
- Floss, H.G.; Yu, T.W. Rifamycin—Mode of action, resistance, and biosynthesis. Chem. Rev. 2005, 105, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Michaud, C.M. Global burden of infectious diseases. Encycl. Microbiol. 2009, 444–454. [Google Scholar] [CrossRef]
- Iskandar, K.; Murugaiyan, J.; Hammoudi Halat, D.; el Hage, S.; Chibabhai, V.; Adukkadukkam, S.; Roques, C.; Molinier, L.; Salameh, P.; van Dongen, M. Antibiotic discovery and resistance: The chase and the race. Antibiotics 2022, 11, 182. [Google Scholar] [CrossRef]
- Baessa, M.; Rodrigues, M.J.; Pereira, C.; Santos, T.; da Rosa Neng, N.; Nogueira, J.M.F.; Barreira, L.; Varela, J.; Ahmed, H.; Asif, S.; et al. A comparative study of the in vitro enzyme inhibitory and antioxidant activities of Butea monosperma (Lam.) Taub. and Sesbania grandiflora (L.) poiret from Pakistan: New sources of natural products for public health problems. South Afr. J. Bot. 2019, 120, 146–156. [Google Scholar] [CrossRef]
- Rasoanaivo, P.; Wright, C.W.; Willcox, M.L.; Gilbert, B. Whole plant extracts versus single compounds for the treatment of malaria: Synergy and positive interactions. Malar. J. 2011, 10, S4. [Google Scholar] [CrossRef] [Green Version]
- Choohan, M.A.; Jabeen, R.; Bibi, N. Extraction and quantification of antimicrobial peptides from medicinal plants through TrisNaCl and PBS buffer. Int. J. Nat. Med. Health Sci. 2022, 1, 1–5. [Google Scholar] [CrossRef]
- Archana, H.; Geetha Bose, V. Evaluation of phytoconstituents from selected medicinal plants and its synergistic antimicrobial activity. Chemosphere 2022, 287, 132276. [Google Scholar] [CrossRef] [PubMed]
- Gruessner, B.M.; Cornet-Vernet, L.; Desrosiers, M.R.; Lutgen, P.; Towler, M.J.; Weathers, P.J. It is not just Artemisinin: Artemisia sp. for treating diseases including malaria and schistosomiasis. Phytochem. Rev. 2019, 18, 1509–1527. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.D. β-Lactamases and β-Lactamase inhibitors. Int. J. Antimicrob. Agents 1999, 12, S3–S7. [Google Scholar] [CrossRef] [PubMed]
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Fazly Bazzaz, B.S. Review on plant antimicrobials: A mechanistic viewpoint. Antimicrob. Resist. Infect. Control 2019, 8, 118. [Google Scholar] [CrossRef] [Green Version]
- Hochma, E.; Yarmolinsky, L.; Khalfin, B.; Nisnevitch, M.; Ben-Shabat, S.; Nakonechny, F. Antimicrobial effect of phytochemicals from edible plants. Processes 2021, 9, 2089. [Google Scholar] [CrossRef]
- Adhikari, B.; Marasini, B.P.; Rayamajhee, B.; Bhattarai, B.R.; Lamichhane, G.; Khadayat, K.; Adhikari, A.; Khanal, S.; Parajuli, N. Potential roles of medicinal plants for the treatment of viral diseases focusing on COVID-19: A review. Phytother. Res. 2021, 35, 1298–1312. [Google Scholar] [CrossRef] [PubMed]
- Houghton, P.J. Old yet new—Pharmaceuticals from plants. J. Chem. Educ. 2001, 78, 175. [Google Scholar] [CrossRef]
- Mothana, R.A.A.; Lindequist, U. Antimicrobial activity of some medicinal plants of the island Soqotra. J. Ethnopharmacol. 2005, 96, 177–181. [Google Scholar] [CrossRef]
- Schmitz, R. Friedrich Wilhelm Sertürner and the discovery of Morphine. Pharm. Hist. 1985, 27, 61–74. [Google Scholar] [PubMed]
- Gibbons, S. Plants as a source of bacterial resistance modulators and anti-infective agents. Phytochem. Rev. 2005, 4, 63–78. [Google Scholar] [CrossRef]
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Et Biophys. Acta (BBA) Gen. Subj. 2013, 1830, 3670–3695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Z.; Tang, J.; Khare, T.; Kumar, V. The alarming antimicrobial resistance in ESKAPEE pathogens: Can essential oils come to the rescue? Fitoterapia 2020, 140, 104433. [Google Scholar] [CrossRef]
- Mohamed, T.A.; Abd El Aty, A.A.; Shahat, A.A.; Abdel-Azim, N.S.; Shams, K.A.; Elshamy, A.A.; Ahmed, M.M.; Youns, S.H.H.; El-Wassimy, T.M.; El-Toumy, S.A.; et al. New antimicrobial metabolites from the medicinal herb Artemisia herba-alba. Nat. Prod. Res. 2021, 35, 1959–1967. [Google Scholar] [CrossRef]
- Dahiya, P.; Purkayastha, S. Phytochemical screening and antimicrobial activity of some medicinal plants against multi-drug resistant bacteria from clinical isolates. Indian J. Pharm. Sci. 2012, 74, 443. [Google Scholar]
- Fujita, M.; Shiota, S.; Kuroda, T.; Hatano, T.; Yoshida, T.; Mizushima, T.; Tsuchiya, T. Remarkable synergies between baicalein and Tetracycline, and Baicalein and β-lactams against methicillin-resistant Staphylococcus aureus. Microbiol. Immunol. 2005, 49, 391–396. [Google Scholar] [CrossRef]
- Chan, B.C.L.; Ip, M.; Lau, C.B.S.; Lui, S.L.; Jolivalt, C.; Ganem-Elbaz, C.; Litaudon, M.; Reiner, N.E.; Gong, H.; See, R.H.; et al. Synergistic effects of Baicalein with Ciprofloxacin against NorA over-expressed methicillin-resistant Staphylococcus aureus (MRSA) and Inhibition of MRSA pyruvate kinase. J. Ethnopharmacol. 2011, 137, 767–773. [Google Scholar] [CrossRef]
- Iwasa, K.; Moriyasu, M.; Yamori, T.; Turuo, T.; Lee, D.-U.; Wiegrebe, W. In vitro cytotoxicity of the protoberberine-type alkaloids. J. Nat. Prod. 2001, 64, 896–898. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, Y.; Niu, X.; Wang, T.; Li, J.; Liu, Z.; Wang, J.; Tang, S.; Wang, Y.; Deng, X. Magnolol restores the activity of meropenem against NDM-1-producing Escherichia coli by inhibiting the activity of metallo-beta-lactamase. Cell Death Discov. 2018, 4, 28. [Google Scholar] [CrossRef] [Green Version]
- Shriram, V.; Jahagirdar, S.; Latha, C.; Kumar, V.; Puranik, V.; Rojatkar, S.; Dhakephalkar, P.K.; Shitole, M.G. A potential plasmid-curing agent, 8-Epidiosbulbin E Acetate, from Dioscorea bulbifera L. against multidrug-resistant bacteria. Int. J. Antimicrob. Agents 2008, 32, 405–410. [Google Scholar] [CrossRef]
- Verpoorte, R.; Heijden, R.; Schripsema, J.; Hoge, J.; Hoopen, H. Plant cell biotechnology for the production of alkaloids: Present status and prospects. J. Nat. Prod. 2004, 56, 186–207. [Google Scholar] [CrossRef]
- Heinrich, M.; Mah, J.; Amirkia, V. Alkaloids used as medicines: Structural phytochemistry meets biodiversity—An update and forward look. Molecules 2021, 26, 1836. [Google Scholar] [CrossRef] [PubMed]
- Malik, T.A.; Kamili, A.N.; Chishti, M.Z.; Ahad, S.; Tantry, M.A.; Hussain, P.R.; Johri, R.K. Breaking the resistance of Escherichia coli: Antimicrobial activity of Berberis lycium Royle. Microb. Pathog. 2017, 102, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Tse-Dinh, Y.C. Targeting bacterial topoisomerase I to meet the challenge of finding new antibiotics. Future Med. Chem. 2015, 7, 459–471. [Google Scholar] [CrossRef] [Green Version]
- Aqil, F.; Khan, M.S.A.; Owais, M.; Ahmad, I. Effect of certain bioactive plant extracts on clinical isolates of β-lactamase producing methicillin resistant Staphylococcus aureus. J. Basic Microbiol. 2005, 45, 106–114. [Google Scholar] [CrossRef]
- Kumar, A.; Khan, I.A.; Koul, S.; Koul, J.L.; Taneja, S.C.; Ali, I.; Ali, F.; Sharma, S.; Mirza, Z.M.; Kumar, M.; et al. Novel structural analogues of Piperine as inhibitors of the NorA efflux pump of Staphylococcus aureus. J. Antimicrob. Chemother. 2008, 61, 1270–1276. [Google Scholar] [CrossRef] [Green Version]
- Khameneh, B.; Diab, R.; Ghazvini, K.; Fazly Bazzaz, B.S. Breakthroughs in bacterial resistance mechanisms and the potential ways to combat them. Microb. Pathog. 2016, 95, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Chopra, B.; Dhingra, K.A.; Dhar, K.L. Synthesis and characterization of Piperine analogues as potent Staphylococcus aureus NorA efflux pump inhibitors. Chem. Methodol. 2019, 3, 104–114. [Google Scholar]
- Heeb, S.; Fletcher, M.P.; Chhabra, S.R.; Diggle, S.P.; Williams, P.; Cámara, M. Quinolones: From antibiotics to autoinducers. FEMS Microbiol. Rev. 2011, 35, 247–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmood, H.Y.; Jamshidi, S.; Mark Sutton, J.M.; Rahman, K. Current advances in developing inhibitors of bacterial multidrug efflux pumps. Curr. Med. Chem. 2016, 23, 1062–1081. [Google Scholar] [CrossRef]
- Guay, I.; Boulanger, S.; Isabelle, C.; Brouillette, E.; Chagnon, F.; Bouarab, K.; Marsault, E.; Malouin, F. Tomatidine and analog FC04-100 possess bactericidal activities against Listeria, Bacillus and Staphylococcus sp. BMC Pharmacol. Toxicol. 2018, 19, 7. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Li, H.; Wei, S.; Zhou, X.; Xiao, X. Antimicrobial effects of chemical compounds isolated from traditional chinese herbal medicine (TCHM) against drug-resistant bacteria: A review paper. Mini-Rev. Med. Chem. 2018, 19, 125–137. [Google Scholar] [CrossRef]
- Siriyong, T.; Chusri, S.; Srimanote, P.; Tipmanee, V.; Voravuthikunchai, S.P. Holarrhena Antidysenterica extract and its steroidal alkaloid, Conessine, as resistance-modifying agents against extensively drug-resistant Acinetobacter baumannii. Microb. Drug Resist. 2016, 22, 273–282. [Google Scholar] [CrossRef]
- Obiang-Obounou, B.-W.; Kang, O.-H.; Choi, J.-G.; Keum, J.-H.; Kim, S.-B.; Mun, S.-H.; Shin, D.-W.; Woo Kim, K.; Park, C.-B.; Kim, Y.-G.; et al. The mechanism of action of Sanguinarine against methicillin-resistant Staphylococcus aureus. J. Toxicol. Sci. 2011, 36, 277–283. [Google Scholar] [CrossRef] [Green Version]
- Al-Ani, I.; Zimmermann, S.; Reichling, J.; Wink, M. Pharmacological synergism of bee venom and melittin with antibiotics and plant secondary metabolites against multi-drug resistant microbial pathogens. Phytomedicine 2015, 22, 245–255. [Google Scholar] [CrossRef]
- Newton, S.M.; Lau, C.; Gurcha, S.S.; Besra, G.S.; Wright, C.W. The evaluation of forty-three plant species for in vitro antimycobacterial activities; isolation of active constituents from Psoralea corylifolia and Sanguinaria canadensis. J. Ethnopharmacol. 2002, 79, 57–67. [Google Scholar] [CrossRef]
- Alibi, S.; Crespo, D.; Navas, J. Plant-derivatives small molecules with antibacterial activity. Antibiotics 2021, 10, 231. [Google Scholar] [CrossRef]
- Maurya, A.; Dwivedi, G.R.; Darokar, M.P.; Srivastava, S.K. Antibacterial and synergy of clavine alkaloid lysergol and its derivatives against nalidixic acid-resistant Escherichia coli. Chem. Biol. Drug Des. 2013, 81, 484–490. [Google Scholar] [CrossRef]
- Ravichandiran, P.; Sheet, S.; Premnath, D.; Kim, A.R.; Yoo, D.J. 1,4-Naphthoquinone analogues: Potent antibacterial agents and mode of action evaluation. Molecules 2019, 24, 1437. [Google Scholar] [CrossRef] [Green Version]
- Yap, J.K.Y.; Tan, S.Y.Y.; Tang, S.Q.; Thien, V.K.; Chan, E.W.L. Synergistic antibacterial activity between 1,4-naphthoquinone and β-lactam antibiotics against methicillin-resistant Staphylococcus aureus. Microb. Drug Resist. 2021, 27, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Siriyong, T.; Srimanote, P.; Chusri, S.; Yingyongnarongkul, B.E.; Suaisom, C.; Tipmanee, V.; Voravuthikunchai, S.P. Conessine as a novel inhibitor of multidrug efflux pump systems in Pseudomonas aeruginosa. BMC Complement. Altern. Med. 2017, 17, 405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boberek, J.M.; Stach, J.; Good, L. Genetic evidence for inhibition of bacterial division protein FtsZ by Berberine. PLoS ONE 2010, 5, e13745. [Google Scholar] [CrossRef] [PubMed]
- Zorić, N.; Kosalec, I.; Tomić, S.; Bobnjarić, I.; Jug, M.; Vlainić, T.; Vlainić, J. Membrane of Candida albicans as a target of Berberine. BMC Complement. Altern. Med. 2017, 17, 268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sridevi, D.; Shankar, C.; Prakash, P.; Park, J.H.; Thamaraiselvi, K. Inhibitory effects of Reserpine against efflux pump activity of antibiotic resistance bacteria. Chem. Biol. Lett. 2017, 4, 69–72. [Google Scholar]
- Boulet, M.L.; Isabelle, C.; Guay, I.; Brouillette, E.; Langlois, J.P.; Jacques, P.É.; Rodrigue, S.; Brzezinski, R.; Beauregard, P.B.; Bouarab, K.; et al. Tomatidine is a lead antibiotic molecule that targets Staphylococcus aureus ATP synthase subunit C. Antimicrob. Agents Chemother. 2018, 62, e02197-17. [Google Scholar]
- Yan, Y.; Li, X.; Zhang, C.; Lv, L.; Gao, B.; Li, M. Research progress on antibacterial activities and mechanisms of natural alkaloids: A review. Antibiotics 2021, 10, 318. [Google Scholar] [CrossRef]
- Gorlenko, C.L.; Kiselev, H.Y.; Budanova, E.V.; Zamyatnin, A.A.; Ikryannikova, L.N. Plant secondary metabolites in the battle of drugs and drug-resistant bacteria: New heroes or worse clones of antibiotics? Antibiotics 2020, 9, 170. [Google Scholar] [CrossRef] [Green Version]
- Misiurek, J.; Plech, T.; Kaproń, B.; Makuch-Kocka, A.; Szultka-Młyńska, M.; Buszewski, B.; Petruczynik, A. Determination of Some Isoquinoline Alkaloids in Extracts Obtained from Selected Plants of the Ranunculaceae, Papaveraceae and Fumarioideae Families by Liquid Chromatography and In Vitro and In Vivo Investigations of Their Cytotoxic Activity. Molecules 2023, 28, 3503. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.F.; Qin, X.J.; Yu, H.F.; Liu, Y.P.; Wang, X.H.; Luo, X.D. Thalicfoetine, a novel isoquinoline alkaloid with antibacterial activity from Thalictrum foetidum. Tetrahedron. Lett. 2019, 60, 151135. [Google Scholar] [CrossRef]
- Zacchino, S.A.; Butassi, E.; di Liberto, M.; Raimondi, M.; Postigo, A.; Sortino, M. Plant phenolics and terpenoids as adjuvants of antibacterial and antifungal drugs. Phytomedicine 2017, 37, 27–48. [Google Scholar] [CrossRef]
- Lima, M.C.; Paiva de Sousa, C.; Fernandez-Prada, C.; Harel, J.; Dubreuil, J.D.; de Souza, E.L. A review of the current evidence of fruit phenolic compounds as potential antimicrobials against pathogenic bacteria. Microb. Pathog. 2019, 130, 259–270. [Google Scholar] [CrossRef]
- Ecevit, K.; Barros, A.A.; Silva, J.M.; Reis, R.L. Preventing microbial infections with natural phenolic compounds. Future Pharmacol. 2022, 2, 460–498. [Google Scholar] [CrossRef]
- Mehmood, A.; Javid, S.; Khan, M.F.; Ahmad, K.S.; Mustafa, A. In vitro total phenolics, total flavonoids, antioxidant and antibacterial activities of selected medicinal plants using different solvent systems. BMC Chem. 2022, 16, 64. [Google Scholar] [CrossRef] [PubMed]
- Kocaçalışkan, I.; Talan, I.; Terzi, I. Antimicrobial activity of catechol and pyrogallol as allelochemicals. J. Biosci. 2006, 61, 639–642. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.P.; Ma, T.W.; Fu, W.C.; Peng, X.C.; Zhang, A.H.; Zhu, H.L. The synthesis, structure and activity evaluation of pyrogallol and catechol derivatives as Helicobacter pylori urease inhibitors. Eur. J. Med. Chem. 2010, 45, 5064–5070. [Google Scholar] [CrossRef]
- Ma, C.; He, N.; Zhao, Y.; Xia, D.; Wei, J.; Kang, W. Antimicrobial mechanism of hydroquinone. Appl. Biochem. Biotechnol. 2019, 189, 1291–1303. [Google Scholar] [CrossRef]
- Lechner, D.; Gibbons, S.; Bucar, F. Modulation of isoniazid susceptibility by flavonoids in Mycobacterium. Phytochem. Lett. 2008, 1, 71–75. [Google Scholar] [CrossRef]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simões, M. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef]
- Wu, Y.; Bai, J.; Zhong, K.; Huang, Y.; Qi, H.; Jiang, Y.; Gao, H. Antibacterial activity and membrane-disruptive mechanism of 3-p-Trans-Coumaroyl-2-Hydroxyquinic acid, a novel phenolic compound from pine needles of Cedrus deodara, against Staphylococcus aureus. Molecules 2016, 21, 1084. [Google Scholar] [CrossRef] [Green Version]
- Patel, K.; Tyagi, C.; Goyal, S.; Jamal, S.; Wahi, D.; Jain, R.; Bharadvaja, N.; Grover, A. Identification of chebulinic acid as potent natural inhibitor of Mycobacterium tuberculosis dna gyrase and molecular insights into its binding mode of action. Comput. Biol. Chem. 2015, 59, 37–47. [Google Scholar] [CrossRef]
- Wu, D.; Kong, Y.; Han, C.; Chen, J.; Hu, L.; Jiang, H.; Shen, X. D-Alanine:D-Alanine ligase as a new target for the flavonoids Quercetin and Apigenin. Int. J. Antimicrob. Agents 2008, 32, 421–426. [Google Scholar] [CrossRef]
- Zou, D.; Xie, K.; Wang, H.; Chen, Y.; Xie, M. Inhibitory effects of Biochanin a on the efflux pump of methicillin-resistant Staphylococcus aureus (MRSA). Wei Sheng Wu Xue Bao 2014, 54, 1204–1211. [Google Scholar]
- Shao, J.; Zhang, M.; Wang, T.; Li, Y.; Wang, C. The roles of CDR1, CDR2, and MDR1 in Kaempferol-induced suppression with fluconazole-resistant Candida albicans. Pharm. Biol. 2016, 54, 984–992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Randhawa, H.K.; Hundal, K.K.; Ahirrao, P.N.; Jachak, S.M.; Nandanwar, H.S. Efflux pump inhibitory activity of flavonoids isolated from Alpinia calcarata against methicillin-resistant Staphylococcus aureus. Biologia 2016, 71, 484–493. [Google Scholar] [CrossRef]
- Gopal, J.; Muthu, M.; Paul, D.; Kim, D.H.; Chun, S. Bactericidal activity of green tea extracts: The importance of catechin containing nano particles. Sci. Rep. 2016, 6, 19710. [Google Scholar] [CrossRef] [Green Version]
- Gradišar, H.; Pristovšek, P.; Plaper, A.; Jerala, R. Green tea catechins inhibit bacterial DNA gyrase by interaction with its ATP binding site. J. Med. Chem. 2007, 50, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, B. Tannic acid with antiviral and antibacterial activity as a promising component of biomaterials—A minireview. Materials 2020, 13, 3224. [Google Scholar] [CrossRef]
- Tyagi, P.; Singh, M.; Kumari, H.; Kumari, A.; Mukhopadhyay, K. Bactericidal activity of Curcumin I is associated with damaging of bacterial membrane. PLoS ONE 2015, 10, e0121313. [Google Scholar] [CrossRef] [Green Version]
- Belofsky, G.; Percivill, D.; Lewis, K.; Tegos, G.P.; Ekart, J. Phenolic metabolites of Dalea Versicolor that enhance antibiotic activity against model pathogenic bacteria. J. Nat. Prod. 2004, 67, 481–484. [Google Scholar] [CrossRef]
- Duan, F.; Xin, G.; Niu, H.; Huang, W. Chlorinated Emodin as a natural antibacterial agent against drug-resistant bacteria through dual influence on bacterial cell membranes and DNA. Sci. Rep. 2017, 7, 12721. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.C.; Wang, J.; Kasman, L.; Jiang, X.; Haley-Zitlin, V. Activities of muscadine grape skin and Quercetin against Helicobacter pylori infection in mice. J. Appl. Microbiol. 2011, 110, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Klančnik, A.; Šikić Pogačar, M.; Trošt, K.; Tušek Žnidarič, M.; Mozetič Vodopivec, B.; Smole Možina, S. Anti-Campylobacter activity of Resveratrol and an extract from waste pinot noir grape skins and seeds, and resistance of Campylobacter jejuni planktonic and biofilm cells, mediated via the CmeABC efflux pump. J. Appl. Microbiol. 2017, 122, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, G.; Qu, H.; Wang, K.; Jing, S.; Guan, S.; Su, L.; Li, Q.; Wang, D. Taxifolin, an inhibitor of sortase a, interferes with the adhesion of methicillin-resistant Staphylococcal aureus. Front. Microbiol. 2021, 12, 686864. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.W.; Lee, J.Y.; Kang, D.I.; Lee, J.U.; Shin, S.Y.; Kim, Y. Screening of flavonoids as candidate antibiotics against Enterococcus faecalis. J. Nat. Prod. 2009, 72, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Tan, N.; Yazıcı-Tütüniş, S.; Bilgin, M.; Tan, E.; Miski, M. Antibacterial activities of pyrenylated coumarins from the roots of Prangos hulusii. Molecules 2017, 22, 1098. [Google Scholar] [CrossRef]
- Sun, M.; Sun, M.; Zhang, J. Osthole: An overview of its sources, biological activities, and modification development. Med. Chem. Res. 2021, 30, 1767–1794. [Google Scholar] [CrossRef]
- Fazly Bazzaz, B.S.; Memariani, Z.; Khashiarmanesh, Z.; Iranshahi, M.; Naderinasab, M. Effect of galbanic acid, a sesquiterpene coumarin from ferula szowitsiana, as an inhibitor of efflux mechanism in resistant clinical isolates of Staphylococcus aureus. Braz. J. Microbiol. 2010, 41, 574–580. [Google Scholar] [CrossRef] [Green Version]
- El-Seedi, H.R. Antimicrobial arylcoumarins from Asphodelus microcarpus. J. Nat. Prod. 2007, 70, 118–120. [Google Scholar] [CrossRef] [PubMed]
- Basile, A.; Sorbo, S.; Spadaro, V.; Bruno, M.; Maggio, A.; Faraone, N.; Rosselli, S. Antimicrobial and antioxidant activities of coumarins from the roots of Ferulago campestris (Apiaceae). Molecules 2009, 14, 939–952. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez, S.; Morán, A.; Martínez-Blanco, H.; Ferrero, M.A.; Rodríguez-Aparicio, L.B. The usefulness of non-toxic plant metabolites in the control of bacterial proliferation. Probiotics Antimicrob. Proteins 2017, 9, 323–333. [Google Scholar] [CrossRef]
- Nakamoto, M.; Kunimura, K.; Suzuki, J.; Kodera, Y. Antimicrobial properties of hydrophobic compounds in garlic: Allicin, Vinyldithiin, Ajoene and Diallyl Polysulfides (review). Exp. Ther. Med. 2019, 19, 1550–1553. [Google Scholar] [CrossRef] [Green Version]
- Dwivedi, V.P.; Bhattacharya, D.; Singh, M.; Bhaskar, A.; Kumar, S.; Fatima, S.; Sobia, P.; van Kaer, L.; Das, G. Allicin Enhances Antimicrobial Activity of Macrophages during Mycobacterium tuberculosis infection. J. Ethnopharmacol. 2019, 243, 111634. [Google Scholar] [CrossRef]
- Dufour, V.; Stahl, M.; Baysse, C. The Antibacterial Properties of Isothiocyanates. Microbiology 2015, 161, 229–243. [Google Scholar] [CrossRef] [Green Version]
- Sagdic, O.; Tornuk, F. Antimicrobial properties of organosulfur compounds. In Dietary Phytochemicals and Microbes; Springer: Dordrecht, The Netherlands, 2012; Volume 9789400739260, pp. 127–156. [Google Scholar]
- Boghrati, Z.; Iranshahi, M. Ferula species: A rich source of antimicrobial compounds. J. Herb. Med. 2019, 16, 100244. [Google Scholar] [CrossRef]
- Reiter, J.; Levina, N.; der Linden, M.; Gruhlke, M.; Martin, C.; Slusarenko, A.J. Diallylthiosulfinate (Allicin), a volatile antimicrobial from garlic (Allium sativum), kills human lung pathogenic bacteria, including MDR strains, as a vapor. Molecules 2017, 22, 1711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feldberg, R.S.; Chang, S.C.; Kotik, A.N.; Nadler, M.; Neuwirth, Z.; Sundstrom, D.C. In vitro mechanism of inhibition of bacterial cell growth by allicin Antimicrob. Agents Chemother. 1988, 32, 1763–1768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, A.; Eller, J.; Albrecht, F.; Prochnow, P.; Kuhlmann, K.; Bandow, J.E.; Slusarenko, A.J.; Leichert, L.I.O. Allicin induces thiol stress in bacteria through S-allylmercapto modification of protein cysteines. J. Biol. Chem. 2016, 291, 11477–11490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sofrata, A.; Santangelo, E.M.; Azeem, M.; Borg-Karlson, A.K.; Gustafsson, A.; Pütsep, K. Benzyl isothiocyanate, a major component from the roots of Salvadora persica is highly active against gram-negative bacteria. PLoS ONE 2011, 6, e23045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.-W.; Choi, K.-D.; Shin, I.-S. Antimicrobial activity of Isothiocyanates (ITCs) extracted from Horseradish (Armoracia rusticana) root against oral microorganisms. Biocontrol. Sci. 2013, 18, 163–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choo, S.; Chin, V.K.; Wong, E.H.; Madhavan, P.; Tay, S.T.; Yong, P.V.C.; Chong, P.P. Review: Antimicrobial properties of Allicin used alone or in combination with other medications. Folia Microbiol. 2020, 65, 451–465. [Google Scholar] [CrossRef]
- Kyung, K.H. Antimicrobial properties of Allium species. Curr. Opin. Biotechnol. 2012, 23, 142–147. [Google Scholar] [CrossRef]
- Rehman, F.; Mairaj, S. Antimicrobial Studies of Allicin and Ajoene. Int. J. Pharm. Bio Sci. 2013, 4, 1095–1105. [Google Scholar]
- Abukhabta, S.; Khalil Ghawi, S.; Karatzas, K.A.; Charalampopoulos, D.; McDougall, G.; Allwood, J.W.; Verrall, S.; Lavery, S.; Latimer, C.; Pourshahidi, L.K.; et al. Sulforaphane-enriched extracts from glucoraphanin-rich broccoli exert antimicrobial activity against gut pathogens in vitro and innovative cooking methods increase in vivo intestinal delivery of sulforaphane. Eur. J. Nutr. 2021, 60, 1263–1276. [Google Scholar] [CrossRef]
- Chen, H.; Yang, N.; Yu, L.; Li, J.; Zhang, H.; Zheng, Y.; Xu, M.; Liu, Y.; Yang, Y.; Li, J. Synergistic Microbicidal effect of AUR and PEITC against Staphylococcus aureus skin infection. Front. Cell. Infect. Microbiol. 2022, 12, 741. [Google Scholar] [CrossRef]
- Oldfield, E.; Lin, F.-Y. Terpene biosynthesis: Modularity rules. Angew. Chem. Int. Ed. 2012, 51, 1124–1137. [Google Scholar] [CrossRef] [Green Version]
- Paduch, R.; Kandefer-Szerszeń, M.; Trytek, M.; Fiedurek, J. Terpenes: Substances useful in human healthcare. Arch. Immunol. Ther. Exp. 2007, 55, 315. [Google Scholar] [CrossRef]
- Knowles, J.R.; Roller, S.; Murray, D.B.; Naidu, A.S. Antimicrobial action of Carvacrol at different stages of dual-species biofilm development by Staphylococcus aureus and Salmonella enterica serovar Typhimurium. Appl. Env. Microbiol. 2005, 71, 797–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Astani, A.; Reichling, J.; Schnitzler, P. Comparative study on the antiviral activity of selected monoterpenes derived from essential oils. Phytother. Res. 2010, 24, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Helander, I.M.; Alakomi, H.-L.; Latva-Kala, K.; Mattila-Sandholm, T.; Pol, I.; Smid, E.J.; Gorris, L.G.M.; von Wright, A. Characterization of the action of selected essential oil components on gram-negative bacteria. J. Agric. Food Chem. 1998, 46, 3590–3595. [Google Scholar] [CrossRef]
- Mahizan, N.A.; Yang, S.K.; Moo, C.L.; Song AA, L.; Chong, C.M.; Chong, C.W.; Abushelaibi, A.; Erin Lim, S.H.; Lai, K.S. Terpene derivatives as a potential agent against antimicrobial resistance (AMR) pathogens. Molecules 2019, 24, 2631. [Google Scholar] [CrossRef] [Green Version]
- Broniatowski, M.; Mastalerz, P.; Flasiński, M. Studies of the interactions of ursane-type bioactive terpenes with the model of Escherichia coli Inner Membrane—Langmuir Monolayer Approach. Biochim. Biophys. Acta Biomembr. 2015, 1848, 469–476. [Google Scholar] [CrossRef] [Green Version]
- Yadav, M.K.; Chae, S.W.; Im, G.J.; Chung, J.W.; Song, J.J. Eugenol: A phyto-compound effective against methicillin-resistant and methicillin-sensitive Staphylococcus aureus Clinical Strain Biofilms. PLoS ONE 2015, 10, e0119564. [Google Scholar] [CrossRef] [Green Version]
- Ali, S.M.; Khan, A.A.; Ahmed, I.; Musaddiq, M.; Ahmed, K.S.; Polasa, H.; Rao, L.V.; Habibullah, C.M.; Sechi, L.A.; Ahmed, N. Antimicrobial activities of Eugenol and Cinnamaldehyde against the human gastric pathogen Helicobacter pylori. Ann. Clin. Microbiol. Antimicrob. 2005, 4, 20. [Google Scholar] [CrossRef] [Green Version]
- Rathinam, P.; Vijay Kumar, H.S.; Viswanathan, P. Eugenol exhibits anti-virulence properties by competitively binding to quorum sensing receptors. Biofouling 2017, 33, 624–639. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Wang, Y.; Jiang, P.; Quek, S. Antibacterial activity and mechanism of cinnamon essential oil against Escherichia coli and Staphylococcus aureus. Food Control 2016, 59, 282–289. [Google Scholar] [CrossRef]
- Togashi, N.; Hamashima, H.; Shiraishi, A.; Inoue, Y.; Takano, A. Antibacterial activities against Staphylococcus aureus of terpene alcohols with aliphatic carbon chains. J. Essent. Oil Res. 2010, 22, 263–269. [Google Scholar] [CrossRef]
- Althunibat, O.Y.; Qaralleh, H.; Yassin, S.; Al-Dalin, A.; Abboud, M.; Khleifat, K.; Majali, I.S.; Aldal’in, H.K.H.; Rayyan, W.A.; Jaafraa, A. Effect of thymol and carvacrol, the major components of Thymus capitatus on the growth of Pseudomonas aeruginosa. Jr. Pure Appl. Microbiol. 2016, 10, 367–374. [Google Scholar]
- Qiu, J.; Wang, D.; Xiang, H.; Feng, H.; Jiang, Y.; Xia, L.; Dong, J.; Lu, J.; Yu, L.; Deng, X. Subinhibitory Concentrations of Thymol reduce enterotoxins A and B and α-Hemolysin production in Staphylococcus aureus isolates. PLoS ONE 2010, 5, e9736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mittermeier, R.A.; Gil, P.R.; Hoffmann, M.; Pilgrim, J.; Brooks, T.; Mittermeier, C.G.; Lamourex, J.; Fonseca, G.A.B. Earth’s Biologically Richest and Most Endangered Terrestrial Ecoregions, 1st ed.; CEMEX: Mexico City, Mexico, 2004; p. 390. [Google Scholar]
- Nayar, M.P. Hot spots of endemic plants of India, Nepal and Bhutan. In Thiruvananthapuram, 1st ed.; Tropical Botanic Garden and Research Institute: Trivandrum, India, 1996; pp. 19–252. ISBN 8190039717/9788190039710. [Google Scholar]
- Singh, K.P.; Gymnosperms, M.V. Floristic Diversity and Conservation Strategies in India; Mudgal, V., Hajra, P.K., Eds.; Botanical Survey of India, Ministry of Environment and Forests: Calcutta, India, 1997; pp. 443–472. [Google Scholar]
- Kholia, B.S.; Balkrishna, A. Pteridophytes used by peoples of indian Himalayan region and Northern India: An overview. In Ferns; Springer: Singapore, 2022; pp. 379–412. [Google Scholar] [CrossRef]
- Poddar Sarkar, M.; Biswas Raha, A.; Datta, J.; Mitra, S. Chemotaxonomic and evolutionary perspectives of Bryophyta based on multivariate analysis of fatty acid fingerprints of eastern Himalayan mosses. Protoplasma 2022, 259, 1125–1137. [Google Scholar] [CrossRef] [PubMed]
- Negi, V.S.; Pathak, R.; Thakur, S.; Joshi, R.K.; Bhatt, I.D.; Rawal, R.S. Scoping the Need of Mainstreaming Indigenous Knowledge for Sustainable Use of Bioresources in the Indian Himalayan Region. Environ. Manag. 2021, 72, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Hajar, R. The medicine of old India. Heart Views 2013, 14, 92. [Google Scholar] [CrossRef]
- Samant, S.S.; Pant, S. Diversity, distribution pattern and conservation status of the plants used in liver diseases/ailments in indian Himalayan region. J. Mt. Sci. 2006, 3, 28–47. [Google Scholar] [CrossRef]
- Wani, Z.A.; Pant, S. Ethnomedicinal study of plants used to cure skin diseases and healing of wounds in Gulmarg Wildlife Sanctuary (GWLS), Jammu & Kashmir. Indian Jr. Trad. Knowl. 2020, 19, 327–334. [Google Scholar]
- Mukherjee, P.K.; Harwansh, R.K.; Bahadur, S.; Banerjee, S.; Kar, A. Evidence-based validation of Indian traditional medicine: Way forward. In From Ayurveda to Chinese Medicine; World Scientific Publishing Co. Pte Ltd.: Singapore, 2017; pp. 137–167. [Google Scholar]
- Nema, N.K.; Dalai, M.K.; Mukherjee, P.K. Ayush herbs and status quo in herbal industries. Pharma. Rev. 2011, 141, 148. [Google Scholar]
- Ahmad, S.; Zahiruddin, S.; Parveen, B.; Basist, P.; Parveen, A.; Gaurav; Parveen, R.; Ahmad, M. Indian medicinal plants and formulations and their potential against COVID-19 preclinical and clinical research. Front. Pharmacol. 2021, 11, 2470. [Google Scholar] [CrossRef]
- Mukherjee, P.K. Quality Control of Herbal Drugs—An Approach to Evaluation of Botanicals, 1st ed.; Business Horizons: New Delhi, India, 2002; pp. 10–800. [Google Scholar]
- Kunwar, R.M.; Nepal, B.K.; Kshhetri, H.B.; Rai, S.K.; Bussmann, R.W. Ethnomedicine in Himalaya: A case study from Dolpa, Humla, Jumla and Mustang districts of Nepal. J. Ethnobiol. Ethnomed. 2006, 2, 27. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, K.S.; Hamid, A.; Nawaz, F.; Hameed, M.; Ahmad, F.; Deng, J.; Akhtar, N.; Wazarat, A.; Mahroof, S. Ethnopharmacological studies of indigenous plants in Kel village, Neelum valley, Azad Kashmir, Pakistan. J. Ethnobiol. Ethnomed. 2017, 13, 68. [Google Scholar] [CrossRef] [PubMed]
- Kausar, F.; Kim, K.-H.; Farooqi, H.M.U.; Farooqi, M.A.; Kaleem, M.; Waqar, R.; Khalil, A.A.K.; Khuda, F.; Abdul Rahim, C.S.; Hyun, K.; et al. Evaluation of antimicrobial and anticancer activities of selected medicinal plants of Himalayas, Pakistan. Plants 2022, 11, 48. [Google Scholar] [CrossRef] [PubMed]
- Das Prajapati, N.; Purohit, S.S.; Sharma, A.K.; Kumar, T. A Handbook of Medicinal Plants: A Complete Source Book, 1st ed.; Agrobios: Jodhpur, India, 2003; p. 554. ISBN 817754134X. [Google Scholar]
- Pandey, M.M.; Rastogi, S.; Rawat, A.K.S. Indian traditional Ayurvedic system of medicine and nutritional supplementation. Evid.-Based Complement. Altern. Med. 2013, 2013, 376327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhat, J.A.; Kumar, M.; Bussmann, R.W. Ecological status and traditional knowledge of medicinal plants in Kedarnath Wildlife Sanctuary of Garhwal Himalaya, India. J. Ethnobiol. Ethnomed. 2013, 9, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinrich, M.; Jiang, H.; Scotti, F.; Booker, A.; Walt, H.; Weckerle, C.; Maake, C. Medicinal plants from the Himalayan region for potential novel antimicrobial and anti-inflammatory skin treatments. J. Pharm. Pharmacol. 2021, 73, 956–967. [Google Scholar] [CrossRef] [PubMed]
- Adnan, M.; Patel, M.; Deshpande, S.; Alreshidi, M.; Siddiqui, A.J.; Reddy, M.N.; Emira, N.; de Feo, V. Effect of Adiantum philippense extract on biofilm formation, adhesion with its antibacterial activities against foodborne pathogens, and characterization of bioactive metabolites: An in vitro-in Silico Approach. Front. Microbiol. 2020, 11, 823. [Google Scholar] [CrossRef]
- Alam, F.; Khan, S.H.A.; Asad, M.H.H. bin. Phytochemical, antimicrobial, antioxidant and enzyme inhibitory potential of medicinal plant Dryopteris ramosa (Hope) C. Chr. BMC Complement. Med. Ther. 2021, 21, 197. [Google Scholar] [CrossRef]
- Radulović, N.; Stojanović, G.; Palić, R. Composition and antimicrobial activity of Equisetum arvense L. Essential Oil. Phytother. Res. 2006, 20, 85–88. [Google Scholar] [CrossRef]
- Frahm, J.-P. An evaluation of the Bryophyte flora of the Azores. Bryophyt. Divers Evol. 2005, 26, 57–79. [Google Scholar] [CrossRef] [Green Version]
- Alam, A. Some Indian Bryophytes known for their biologically active compounds. Int. J. Appl. Biol. Pharmceutical Technol. 2012, 3, 239–246. [Google Scholar]
- Mewari, N.; Kumar, P. Antimicrobial activity of extracts of Marchantia polymorpha. Pharm. Biol. 2008, 46, 819–822. [Google Scholar] [CrossRef]
- Li, Y.; Wu, J.-W.; Tan, H.-B.; Li, B.-L.; Qiu, S.-X. Three new pterocarpans from the aerial parts of Abrus precatorius. Nat. Prod. Res. 2020, 34, 1836–1844. [Google Scholar] [CrossRef]
- Tian, C.; Zhang, P.; Yang, C.; Gao, X.; Wang, H.; Guo, Y.; Liu, M. Extraction process, component analysis, and in vitro antioxidant, antibacterial, and anti-inflammatory activities of total flavonoid extracts from Abutilon Theophrasti Medic. Leaves. Mediat. Inflamm. 2018, 2018, 3508506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Kalamouni, C.; Venskutonis, P.; Zebib, B.; Merah, O.; Raynaud, C.; Talou, T. Antioxidant and antimicrobial activities of the essential oil of Achillea millefolium L. Grown in France. Medicines 2017, 4, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, D. Antibacterial activity of alkaloids present in plant Achyranthes aspera. Pharma Innov. J. 2018, 7, 147–153. [Google Scholar]
- Khan, F.A.; Khan, S.; Khan, N.M.; Khan, H.; Khan, S.; Ahmad, S.; Rehman, N.; Aziz, R.; Khan, F.A.; Khan, S.; et al. Antimicrobial and antioxidant role of the aerial parts of Aconitum violaceum. J. Mex. Chem. Soc. 2021, 65, 84–93. [Google Scholar] [CrossRef]
- Ahmad, M.; Ahmad, W.; Ahmad, M.; Zeeshan, M.; Obaidullah; Shaheen, F. Norditerpenoid Alkaloids from the roots of Aconitum heterophyllum Wall with antibacterial activity. J. Enzyme Inhib. Med. Chem. 2008, 23, 1018–1022. [Google Scholar] [CrossRef] [PubMed]
- Satyal, P.; Dosoky, N.S.; Poudel, A.; Setzer, W.N. Essential oil constituents and their biological activities from the leaves of Cassia fistula growing in Nepal. Open Access J. Med. Aromat. Plants 2017, 3, 1–4. [Google Scholar]
- Yuan, Q.; Wang, J.; Ruan, J. Screening for bioactive compounds from Adiantum capillus-veneris L. J. Chem. Soc. Pak. 2012, 34, 207. [Google Scholar]
- Karthik, M.R.M.G.; Chandrappa, C.P.; Shilpashree, C.B.; Sadananda, T.S. Antibacterial and antioxidant activities of Adiantum pedatum L. J. Phytol. 2011, 3, 26–32. [Google Scholar]
- Verma, R.S.; Padalia, R.C.; Chauhan, A. Essential oil composition of Aegle marmelos (L.) Correa: Chemotypic and seasonal variations. J. Sci. Food Agric. 2014, 94, 1904–1913. [Google Scholar] [CrossRef] [PubMed]
- Kurade, N.P.; Jaitak, V.; Kaul, V.K.; Sharma, O.P. Chemical composition and antibacterial activity of essential oils of Lantana camara, Ageratum houstonianum and Eupatorium adenophorum. Pharm. Biol. 2010, 48, 539–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, N.; Ahmad, M.; Riaz, M.; Jahan, N.; Ahmad, R. Phytochemical, antimicrobial, insecticidal and brine shrimp lethality bioassay of the crude methanolic extract of Ajuga parviflora Benth. Pak. J. Pharm. Sci. 2013, 26, 751–756. [Google Scholar] [PubMed]
- Karunakaran, G.; Jagathambal, M.; Gusev, A.; Torres, J.A.L.; Kolesnikov, E.; Kuznetsov, D. Rapid biosynthesis of AgNPs using soil bacterium Azotobacter vinelandii with promising antioxidant and antibacterial activities for biomedical applications. JOM 2017, 69, 1206–1212. [Google Scholar] [CrossRef]
- Ahiabor, C.; Gordon, A.; Ayittey, K.; Agyare, R. In vitro assessment of antibacterial activity of crude extracts of onion (Allium cepa L.) and shallot (Allium aescalonicum L.) on isolates of Escherichia coli (ATCC 25922), Staphylococcus aureus (ATCC 25923), and Salmonella typhi (ATCC 19430). Int. J. Appl. Res. 2016, 2, 1029–1032. [Google Scholar]
- Carminate, B.; Martin, G.B.; Barcelos, R.M.; Gontijo, I.; Almeida, M.S.; Belinelo, V.J. Evaluation of antifungal activity of Amaranthus viridis L. (Amaranthaceae) on Fusariosis by Piper nigrum L. and on Anthracnose by Musa sp. Agric. J. 2012, 7, 215–219. [Google Scholar]
- Satyal, P.; Dosoky, N.S.; Kincer, B.L.; Setzer, W.N. Chemical compositions and biological activities of Amomum subulatum essential oils from Nepal. Nat. Prod. Commun. 2012, 7, 1233–1236. [Google Scholar] [CrossRef] [Green Version]
- Joshi, R.K. Angelica (Angelica glauca and Angelica archangelica) oils. In Essential Oils in Food Preservation, Flavor and Safety; Academic Press: Cambridge, MA, USA, 2015; pp. 203–208. [Google Scholar]
- Petkova, N.; Hambarlyiska, I.; Tumbarski, Y.; Vrancheva, R.; Raeva, M.; Ivanov, I. Phytochemical composition and antimicrobial properties of Burdock (Arctium lappa L.) roots extracts. Biointerface Res. Appl. Chem. 2022, 12, 2826–2842. [Google Scholar]
- Shameem, N.; Kamili, A.N.; Parray, J.A.; Hamid, R.; Bandh, S.A. Antimicrobial and antioxidant activity of methanol extracts of Arnebia benthamii (Wall Ex. G. Don) Johnston—A critically endangered medicinal plant of North Western Himalaya. J. Anal. Sci. Technol. 2015, 6, 36. [Google Scholar] [CrossRef] [Green Version]
- Rashid, S.; Rather, M.A.; Shah, W.A.; Bhat, B.A. Chemical composition, antimicrobial, cytotoxic and antioxidant activities of the essential oil of Artemisia indica Willd. Food Chem. 2013, 138, 693–700. [Google Scholar] [CrossRef]
- Haider, S.Z.; Mohan, M.; Andola, H. Constituents of Artemisia indica Willd. from Uttarakhand Himalaya: A source of Davanone. Pharmacogn. Res. 2014, 6, 257. [Google Scholar]
- Shrestha, R.; Shakya, A.; Kumar Shrestha, K. Phytochemical screening and antimicrobial activity of Asparagus racemosus Willd. and Asparagus curillus Buch.-Ham. Ex Roxb. J. Nat. Hist. Mus. 2015, 29, 91–102. [Google Scholar] [CrossRef] [Green Version]
- Rahman, K.; Khan, S.U.; Fahad, S.; Shinwari, Z.K.; Khan, D.; Kamal, S.; Ullah, I.; Anjum, S.I.; Man, S.; Khan, A.J.; et al. In vitro biological screening of a critically endangered medicinal plant, Atropa acuminata Royle Ex Lindl of North Western Himalaya. Sci. Rep. 2018, 8, 11028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munir, N.; Iqbal, A.; Altaf, I.; Bashir, R.; Sharif, N.; Saleem, F.; Naz, S. Evaluation of antioxidant and antimicrobial potential of two endangered plant species Atropa belladonna & Matricaria chamomilla. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 111. [Google Scholar]
- Bin Emran, T.; Rahman, M.A.; Uddin, M.M.N.; Dash, R.; Hossen, M.F.; Mohiuddin, M.; Alam, M.R. Molecular docking and inhibition studies on the interactions of Bacopa monnieri’s potent phytochemicals against pathogenic Staphylococcus aureus. DARU J. Pharm. Sci. 2015, 23, 26. [Google Scholar] [CrossRef] [Green Version]
- Raveesha, H.R.; Sushma, B.K. Antimicrobial activity of Baliospermum montanum (Wild.) Muell. Arg. J. Mycopathol. Res. 2018, 56, 35–39. [Google Scholar]
- Zafar, R.; Ullah, H.; Zahoor, M.; Sadiq, A. Isolation of bioactive compounds from Bergenia ciliata (Haw.) Sternb rhizome and their antioxidant and anticholinesterase activities. BMC Complement. Altern. Med. 2019, 19, 296. [Google Scholar] [CrossRef] [Green Version]
- Pal, M.; Mishra, T.; Kumar, A.; Baleshwar; Upreti, D.K.; Rana, T.S. Chemical constituents and antimicrobial potential of essential oil from Betula utilis growing in high altitude of Himalaya (India). J. Essent. Oil-Bear. Plants 2015, 18, 1078–1082. [Google Scholar] [CrossRef]
- Zahara, K.; Bibi, Y.; Qayyum, A.; Nisa, S. Investigation of antimicrobial and antioxidant properties of Bidens Biternata. Iranian J. Sci. Technol. Trans. A Sci. 2019, 43, 725–734. [Google Scholar] [CrossRef]
- Satyal, P.; Chhetri, B.K.; Dosoky, N.S.; Shrestha, S.; Poudel, A.; Setzer, W.N. Chemical composition of Blumea lacera essential oil from Nepal. biological activities of the essential oil and (Z)-Lachnophyllum ester. Nat. Prod. Commun. 2015, 10, 1749–1750. [Google Scholar] [CrossRef] [Green Version]
- Owk, A.K.; Lagudu, M.N. Bridelia retusa (L.) Spreng. Fruits: Antimicrobial efficiency and their phytochemical constituents. Not. Sci. Biol. 2016, 8, 33–36. [Google Scholar] [CrossRef]
- Hernández-Díaz, J.A.; Garza-García, J.J.; León-Morales, J.M.; Zamudio-Ojeda, A.; Arratia-Quijada, J.; Velázquez-Juárez, G.; López-Velázquez, J.C.; García-Morales, S. Antibacterial activity of biosynthesized selenium nanoparticles using extracts of Calendula officinalis against potentially clinical bacterial strains. Molecules 2021, 26, 5929. [Google Scholar] [CrossRef] [PubMed]
- Saddiq, A.A.; Tag, H.M.; Doleib, N.M.; Salman, A.S.; Hagagy, N. Antimicrobial, antigenotoxicity, and characterization of Calotropis procera and its rhizosphere-inhabiting Actinobacteria: In vitro and in vivo studies. Molecules 2022, 27, 3123. [Google Scholar] [CrossRef] [PubMed]
- Mubashir, S.; Yousuf Dar, M.; Lone, B.A.; Zargar, M.I.; Shah, W.A. Anthelmintic, antimicrobial, antioxidant and cytotoxic activity of Caltha palustris Var. Alba Kashmir, India. Chin. J. Nat. Med. 2014, 12, 567–572. [Google Scholar] [CrossRef]
- Blaskovich, M.A.T.; Kavanagh, A.M.; Elliott, A.G.; Zhang, B.; Ramu, S.; Amado, M.; Lowe, G.J.; Hinton, A.O.; Pham, D.M.T.; Zuegg, J.; et al. The Antimicrobial potential of Cannabidiol. Commun Biol 2021, 4, 7. [Google Scholar] [CrossRef]
- Chaudhary, A.K.; Ahmad, S.; Mazumder, A. Cedrus deodara (Roxb.) Loud.: A review on its ethnobotany, phytochemical and pharmacological profile. Pharmacogn. J. 2011, 3, 12–17. [Google Scholar] [CrossRef] [Green Version]
- Joshi, S.; Subedi, P.C. Phytochemical and biological studies on essential oil and leaf extracts of Gaultheria fragrantissima Wall. Nepal J. Sci. Technol. 2013, 14, 59–64. [Google Scholar] [CrossRef]
- Shaikh, T.; Rub, R.A.; Sasikumar, S. Antimicrobial screening of Cichorium intybus seed extracts. Arab. J. Chem. 2016, 9, S1569–S1573. [Google Scholar] [CrossRef] [Green Version]
- Singh, C.; Singh, S.; Pande, C.; Tewari, G.; Pande, V.; Sharma, P. Exploration of antimicrobial potential of essential oils of Cinnamomum glanduliferum, Feronia elephantum, Bupleurum hamiltonii and Cyclospermum leptophyllum against foodborne pathogens. Pharm. Biol. 2013, 51, 1607–1610. [Google Scholar] [CrossRef]
- Ngoci, N.S.; Ramadhan, M.; Ngari, M.S.; Leonard, O.P. Screening for antimicrobial activity of Cissampelos pareira L. methanol root extract. Eur. J. Med. Plants 2012, 4, 45–51. [Google Scholar] [CrossRef]
- Ghori, N.-H.; Khan, M.; Hayat, M.Q. Phytochemical analyses for antibacterial activity and therapeutic compounds of Convolvulus arvensis L., collected from the salt range of Pakistan. Adv. Life Sci. 2015, 2, 83–90. [Google Scholar]
- Kačániová, M.; Galovičová, L.; Ivanišová, E.; Vukovic, N.L.; Štefániková, J.; Valková, V.; Borotová, P.; Žiarovská, J.; Terentjeva, M.; Felšöciová, S.; et al. Antioxidant, antimicrobial and antibiofilm activity of Coriander (Coriandrum sativum L.) essential oil for its application in foods. Foods 2020, 9, 282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mykhailenko, O.; Kovalyov, V.; Goryacha, O.; Ivanauskas, L.; Georgiyants, V. Biologically active compounds and pharmacological activities of species of the genus Crocus: A review. Phytochemistry 2019, 162, 56–89. [Google Scholar] [CrossRef] [PubMed]
- Essien, E.; Newby, J.; Walker, T.; Setzer, W.; Ekundayo, O. Chemotaxonomic characterization and in-vitro antimicrobial and cytotoxic activities of the leaf essential oil of Curcuma longa grown in Southern Nigeria. Medicines 2015, 2, 340–349. [Google Scholar] [CrossRef] [Green Version]
- Paudel, P.; Satyal, P.; Maharjan, S.; Shrestha, N.; Setzer, W.N. Volatile analysis and antimicrobial screening of the parasitic plant Cuscuta reflexa Roxb. from Nepal. Nat. Prod. Res. 2014, 28, 106–110. [Google Scholar] [CrossRef]
- Shah, G.; Shri, R.; Panchal, V.; Sharma, N.; Singh, B.; Mann, A. Scientific basis for the therapeutic use of Cymbopogon citratus, Stapf (lemon grass). J. Adv. Pharm. Technol. Res. 2011, 2, 3. [Google Scholar] [CrossRef]
- Eltayeib, A.A.; Um Ismaeel, H. Extraction of Cyperus rotundus rhizomes oil, identification of chemical constituents and evaluation of antimicrobial activity of the oil in North Kordofan state. Int. J. Adv. Res. Chem. Sci. 2014, 1, 18–29. [Google Scholar]
- Cheypratub, P.; Leeanansaksiri, W.; Eumkeb, G. The synergy and mode of action of Cyperus Rotundus L. Extract plus ampicillin against ampicillin-resistant Staphylococcus aureus. Evid.-Based Complement. Altern. Med. 2018, 2018, 3438453. [Google Scholar] [CrossRef] [Green Version]
- Bawazeer, S.; Rauf, A. In vitro antibacterial and antifungal potential of amyrin-type triterpenoid isolated from Datura metel Linnaeus. Biomed. Res. Int. 2021, 2021, 1543574. [Google Scholar] [CrossRef]
- Solomon, B.; Nega, B.; Wagaw, S.; Lianzhong, A. Antibacterial activity of Datura stramonium against standard and clinical isolate pathogenic microorganisms. J. Med. Plants Res. 2017, 11, 501–506. [Google Scholar] [CrossRef] [Green Version]
- Dedieu, L.; Brunel, J.M.; Lorenzi, V.; Muselli, A.; Berti, L.; Bolla, J.M. Antibacterial mode of action of the Daucus carota essential oil active compounds against Campylobacter jejuni and efflux-mediated drug resistance in gram-negative bacteria. Molecules 2020, 25, 5448. [Google Scholar] [CrossRef]
- Kuete, V.; BetrandTeponno, R.; Mbaveng, A.T.; Tapondjou, L.A.; Meyer, J.J.M.; Barboni, L.; Lall, N. Antibacterial activities of the extracts, fractions and compounds from Dioscorea bulbifera. BMC Complement. Altern. Med. 2012, 12, 228. [Google Scholar] [CrossRef] [Green Version]
- Joshi, S.C.; Verma, A.R.; Mathela, C.S. Antioxidant and antibacterial activities of the leaf essential oils of Himalayan Lauraceae species. Food Chem. Toxicol. 2010, 48, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Al-Snafi, P.D.A.E. A review on Dodonaea viscosa: A potential medicinal plant. IOSR J. Pharm. 2017, 7, 10–21. [Google Scholar] [CrossRef]
- Munir, N.; Mahmood, Z.; Riaz, M.; Tahir, I.M.; Ali Shah, S.M.; Bacha, U.; Mehmood, R.; Hussain, S. In vitro evaluation of antimicrobial and cytotoxic potential of Epimedium grandiflorum hydroethanolic extract as natural medicine. Int. J. Nat. Med. Health Sci. 2022, 2, 25–31. [Google Scholar] [CrossRef]
- Mir, M.; Ashraf, W.; Mir, B.; Biomater. Antimicrobial and antifungal and phytochemical analysis of various extracts of Equisetum diffusum. Biomater. Artif. Cells Artif. Organs. 2021, 35, 186–189. [Google Scholar]
- Salehi, B.; Iriti, M.; Vitalini, S.; Antolak, H.; Pawlikowska, E.; Kręgiel, D.; Sharifi-Rad, J.; Oyeleye, S.I.; Ademiluyi, A.O.; Czopek, K.; et al. Euphorbia-derived natural products with potential for use in health maintenance. Biomolecules 2019, 9, 337. [Google Scholar] [CrossRef] [Green Version]
- Kwon, Y.S.; Choi, W.G.; Kim, W.J.; Kim, W.K.; Kim, M.J.; Kang, W.H.; Kim, C.M. Antimicrobial constituents of Foeniculum vulgare. Arch. Pharm. Res. 2002, 25, 154–157. [Google Scholar] [CrossRef]
- Bhat, B.A.; Mir, W.R.; Sheikh, B.A.; Rather, M.A.; Dar Tul, H.; Mir, M.A. In Vitro and in silico evaluation of antimicrobial properties of Delphinium cashmerianum L., a medicinal herb growing in Kashmir, India. J. Ethnopharmacol. 2022, 291, 115046. [Google Scholar] [CrossRef]
- Jameel, M.; Islamuddin, M.; Ali, A.; Afrin, F.; Ali, M. Isolation, characterization and antimicrobial evaluation of a novel compound N-Octacosan 7β Ol, from Fumaria parviflora Lam. BMC Complement. Altern. Med. 2014, 14, 98. [Google Scholar] [CrossRef] [Green Version]
- Vlase, L.; Mocana, A.; Hanganu, D.; Benedec, D.; Gheldiu, A.-M.; Crişan, G. Comparative study of polyphenolic content, antioxidant and antimicrobial activity of four Galium species (Rubiaceae). Dig. J. Nanomater. Biostruct. 2014, 9, 1085–1094. [Google Scholar]
- Skinder, B.; Ganai, B.; Wani, A. Scientific study of Gentiana kurroo Royle. Medicines 2017, 4, 74. [Google Scholar] [CrossRef] [Green Version]
- Abbasi, B.A.; Iqbal, J.; Ahmad, R.; Zia, L.; Kanwal, S.; Mahmood, T.; Wang, C.; Chen, J.-T. Bioactivities of Geranium wallichianum leaf extracts conjugated with zinc oxide nanoparticles. Biomolecules 2019, 10, 38. [Google Scholar] [CrossRef] [Green Version]
- Njogu, P.M.; Thoithi, G.N.; Mwangi, J.W.; Kamau, F.N.; Kibwage, I.O.; Kariuki, S.T.; Yenesew, A.; Mugo, H.N.; Mwalukumbi, J.M. Phytochemical and antimicrobial investigation of Girardinia diversifolia (Link) Friis (Urticaceae). East Cent. Afr. J. Pharm. Sci. 2011, 14, 89–94. [Google Scholar]
- Rawat, S.; Jugran, A.K.; Bahukhandi, A.; Bahuguna, A.; Bhatt, I.D.; Rawal, R.S.; Dhar, U. Anti-oxidant and anti-microbial properties of some ethno-therapeutically important medicinal plants of Indian Himalayan Region. 3 Biotech 2016, 6, 154. [Google Scholar] [CrossRef] [Green Version]
- Arora, R.; Mazumder, A. Phytochemical screening and antimicrobial activity of rhizomes of Hedychium spicatum. Pharmacogn. J. 2017, 9, s64–s68. [Google Scholar] [CrossRef]
- Begum, S.; Saxena, B.; Goyal, M.; Ranjan, R.; Joshi, V.B.; Rao, C.V.; Krishnamurthy, S.; Sahai, M. Study of anti-inflammatory, analgesic and antipyretic activities of seeds of Hyoscyamus niger and Isolation of a New Coumarinolignan. Fitoterapia 2010, 81, 178–184. [Google Scholar] [CrossRef]
- Nazlı, O.; Baygar, T.; Demirci Dönmez, Ç.E.; Dere, Ö.; Uysal, A.İ.; Aksözek, A.; Işık, C.; Aktürk, S. Antimicrobial and antibiofilm activity of polyurethane/Hypericum perforatum extract (PHPE) composite. Bioorg. Chem. 2019, 82, 224–228. [Google Scholar] [CrossRef]
- Priydarshi, R.; Melkani, A.B.; Mohan, L.; Pant, C.C. Terpenoid composition and antibacterial activity of the essential oil from Inula cappa (Buch-Ham. Ex. D. Don) DC. J. Essent. Oil Res. 2016, 28, 172–176. [Google Scholar] [CrossRef]
- Liu, C.; Mishra, A.K.; He, B.; Tan, R. Antimicrobial activities of Isoalantolactone, a major sesquiterpene lactone OfInula racemosa. Chin. Sci. Bull. 2001, 46, 498–501. [Google Scholar] [CrossRef]
- Wagay, J.I.; Jain, K. Phytochemical analysis and antimicrobial activity of Iris Kashmiriana and Iris ensata extracts against selected microorganisms. J. Drug Deliv. Ther. 2018, 8, 28–34. [Google Scholar] [CrossRef]
- Zakir, M.; Kumar, N. Phytochemical screening and antimicrobial activity of extracts of Iris nepalensis species growing in Karnah Vallay, Kupwara, Jammu and Kashmir, India. SSR Inst. Int. J. Life Sci. 2021, 7, 2754–2762. [Google Scholar] [CrossRef]
- Gunasekara, T.; Radhika, N.; Ragunathan, K.; Gunathilaka, D.; Weerasekera, M.; Hewageegana, H.; Arawwawala, L.; Fernando, S. Determination of antimicrobial potential of five herbs used in ayurveda practices against Candida albicans, Candida parapsilosis and methicillin resistant Staphylococcus aureus. Anc. Sci. Life 2017, 36, 187. [Google Scholar] [CrossRef]
- Rather, M.A.; Dar, B.A.; Dar, M.Y.; Wani, B.A.; Shah, W.A.; Bhat, B.A.; Ganai, B.A.; Bhat, K.A.; Anand, R.; Qurishi, M.A. Chemical composition, antioxidant and antibacterial activities of the leaf essential oil of Juglans regia L. and its constituents. Phytomedicine 2012, 19, 1185–1190. [Google Scholar] [CrossRef]
- Stappen, I.; Tabanca, N.; Ali, A.; Wedge, D.E.; Wanner, J.; Kaul, V.K.; Lal, B.; Jaitak, V.; Gochev, V.K.; Schmidt, E. Chemical composition and biological activity of essential oils from wild growing aromatic plant species of Skimmia laureola and Juniperus macropoda from Western Himalaya. Nat. Prod. Commun. 2015, 10, 1071–1074. [Google Scholar] [CrossRef] [PubMed]
- Menpara, D.; Desai, D.; Rathod, T.; Chanda, S. Evaluation of Nutraceutical Bottle Gourd (Lagenaria siceraria) as a potential source of natural antimicrobial agent. Am. J. Phytomed. Clin. Ther. 2014, 2, 375–389. [Google Scholar]
- Insawang, S.; Pripdeevech, P.; Tanapichatsakul, C.; Khruengsai, S.; Monggoot, S.; Nakham, T.; Artrod, A.; D’Souza, P.E.; Panuwet, P. Essential oil compositions and antibacterial and antioxidant activities of five Lavandula stoechas Cultivars Grown in Thailand. Chem. Biodivers 2019, 16, e1900371. [Google Scholar] [CrossRef]
- Comai, S.; Dall’Acqua, S.; Grillo, A.; Castagliuolo, I.; Gurung, K.; Innocenti, G. essential Oil of Lindera neesiana Fruit: Chemical analysis and its potential use in topical applications. Fitoterapia 2010, 81, 11–16. [Google Scholar] [CrossRef]
- Gangwar, M.; Goel, R.K.; Nath, G. Mallotus philippinensis Muell. Arg (Euphorbiaceae): Ethnopharmacology and phytochemistry review. BioMed Res. Int. 2014, 2014, 213973. [Google Scholar] [CrossRef] [Green Version]
- Bilal, M.; Parveen, A.; Fiaz, A.; Mazhar, M. In vitro phytochemical analysis, antimicrobial and antioxidant activity of Mallotus philippinensis. Int. J. Nat. Med. Health Sci. 2022, 2, 11–16. [Google Scholar] [CrossRef]
- Saleem, U.; Khalid, S.; Zaib, S.; Anwar, F.; Ahmad, B.; Ullah, I.; Zeb, A.; Ayaz, M. Phytochemical analysis and wound healing studies on ethnomedicinally important plant Malva neglecta Wallr. J. Ethnopharmacol. 2020, 249, 112401. [Google Scholar] [CrossRef]
- Aćimović, M.; Jeremić, K.; Salaj, N.; Gavarić, N.; Kiprovski, B.; Sikora, V.; Zeremski, T. Marrubium vulgare L.: A phytochemical and pharmacological overview. Molecules 2020, 25, 2898. [Google Scholar] [CrossRef]
- Khan, A.V.; Ahmed, Q.U.; Mir, M.R.; Shukla, I.; Khan, A.A. Antibacterial efficacy of the seed extracts of Melia azedarach against some hospital isolated human pathogenic bacterial strains. Asian Pac. J. Trop. Biomed. 2011, 1, 452–455. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Varshney, V.K.; Prasad, R.; Rawat MS, M.; Stashenko, E.E. In vitro antioxidant, antifungal and antibacterial activities of essential oil of Morina longifolia Wall. leaves. J. Biol. Act. Prod. Nat. 2013, 3, 183–193. [Google Scholar]
- Joshi, R.K.; Mathela, C.S. Volatile oil composition of Morina longifolia Wall. Ex. Dc. from Himalayan region of Uttarakhand. Asian J. Res. Pharm. Sci. 2013, 3, 12–14. [Google Scholar]
- Ghosh, A.; Zhu, E.V.; Wang, H.; Zurek, L.; Zhu, J.J. Antibacterial activities of Nepetalactones against public health-related pathogens. Nat. Prod. Commun. 2021, 16, 1934578X211004875. [Google Scholar] [CrossRef]
- Adiguzel, A.; Ozer, H.; Sokmen, M.; Gulluce, M.; Sokmen, A.; Kilic, H.; Sahin, F.; Baris, O. Antimicrobial and antioxidant activity of the essential oil and methanol extract of Nepeta cataria. Pol. J. Microbiol. 2009, 58, 69–76. [Google Scholar] [PubMed]
- Satyal, P.; Chhetri, B.K.; Dosoky, N.S.; Poudel, A.; Setzer, W.N. Chemical composition of Nardostachys grandiflora rhizome oil from Nepal– A contribution to the chemotaxonomy and bioactivity of Nardostachys. Nat. Prod. Commun. 2015, 10, 1067–1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, S.; Koley, H.; Barman, S.; Mitra, S.; Datta, S.; Ghosh, S.; Paul, D.; Dhar, P. Oxalis corniculata (Oxalidaceae) leaf extract exerts in vitro antimicrobial and in vivo anticolonizing activities against Shigella dysenteriae 1 (NT4907) and Shigella flexneri 2a (2457T) in induced diarrhea in suckling mice. J. Med. Food. 2013, 16, 801–809. [Google Scholar] [CrossRef]
- Ahmad, M.; Malik, K.; Tariq, A.; Zhang, G.; Yaseen, G.; Rashid, N.; Sultana, S.; Zafar, M.; Ullah, K.; Khan, M.P.Z. Botany, ethnomedicines, phytochemistry and pharmacology of Himalayan Paeony (Paeonia emodi Royle.). J. Ethnopharmacol. 2018, 220, 197–219. [Google Scholar] [CrossRef]
- Huq, A.K.M.M.; Jamal, J.A.; Stanslas, J. Ethnobotanical, phytochemical, pharmacological, and toxicological aspects of Persicaria hydropiper (L.) Delarbre. Evid.-Based Complement. Altern. Med. 2014, 2014, 782830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adom, M.B.; Taher, M.; Mutalabisin, M.F.; Amri, M.S.; Abdul Kudos, M.B.; Wan Sulaiman, M.W.A.; Sengupta, P.; Susanti, D. Chemical constituents and medical benefits of Plantago major. Biomed. Pharmacother. 2017, 96, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Kalam, M.A.; Malik, A.H.; Ganie, A.H.; Butt, T.A. Medicinal importance of Papra (Podophyllum hexandrum Royle) in Unani system of medicine. J. Complement. Integr. Med. 2021, 18, 485–490. [Google Scholar] [CrossRef]
- Li, M.; Zhou, L.; Yang, D.; Li, T.; Li, W. Biochemical composition and antioxidant capacity of extracts from Podophyllum hexandrum rhizome. BMC Complement. Altern. Med. 2012, 12, 263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gosset-Erard, C.; Zhao, M.; Lordel-Madeleine, S.; Ennahar, S. Identification of Punicalagin as the bioactive compound behind the antimicrobial activity of Pomegranate (Punica granatum L.) peels. Food Chem. 2021, 352, 129396. [Google Scholar] [CrossRef]
- Silvan, J.M.; Michalska-Ciechanowska, A.; Martinez-Rodriguez, A.J. Modulation of antibacterial, antioxidant, and anti-inflammatory properties by drying of Prunus domestica L. plum juice extracts. Microorganisms 2020, 8, 119. [Google Scholar] [CrossRef] [Green Version]
- Rolta, R.; Kumar, V.; Sourirajan, A.; Upadhyay, N.K.; Dev, K. Bioassay guided fractionation of rhizome extract of Rheum emodi Wall as bio-availability enhancer of antibiotics against bacterial and fungal pathogens. J. Ethnopharmacol. 2020, 257, 112867. [Google Scholar] [CrossRef]
- Innocenti, G.; Dall’Acqua, S.; Scialino, G.; Banfi, E.; Sosa, S.; Gurung, K.; Barbera, M.; Carrara, M. Chemical composition and biological properties of Rhododendron anthopogon essential oil. Molecules 2010, 15, 2326–2338. [Google Scholar] [CrossRef]
- Mishra, A.P.; Sharifi-Rad, M.; Shariati, M.A.; Mabkhot, Y.N.; Al-Showiman, S.S.; Rauf, A.; Salehi, B.; Župunski, M.; Gusain, P.; Sharifi-Rad, J. Bioactive compounds and health benefits of edible Rumex species-A review. Cell. Mol. Biol. 2018, 64, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Tawfeek, N.; Mahmoud, M.F.; Hamdan, D.I.; Sobeh, M.; Farrag, N.; Wink, M.; El-Shazly, A.M. Phytochemistry, pharmacology and medicinal uses of plants of the genus Salix: An updated review. Front. Pharmacol. 2021, 12, 593856. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, X.; Zhou, H.; Zhao, C.; Lin, L. Antimicrobial activity and mechanisms of Salvia sclarea essential oil. Bot. Stud. 2015, 56, 16. [Google Scholar] [CrossRef] [Green Version]
- Khuda, F.; Ul Haq, Z.; Ilahi, I.; Ullah, R.; Khan, A.; Fouad, H.; Ali Khan Khalil, A.; Ullah, Z.; Umar Khayam Sahibzada, M.; Shah, Y.; et al. Synthesis of gold nanoparticles using Sambucus wightiana extract and investigation of its antimicrobial, anti-inflammatory, antioxidant and analgesic activities. Arab. J. Chem. 2021, 14, 103343. [Google Scholar] [CrossRef]
- Omer, R.E.E.; Koua, F.H.M.; Abdelhag, I.M.; Ismail, A.M. Gas chromatography/mass spectrometry profiling of the Costus plant Saussurea lappa (Decne.) CB Clarke root extracts and their anti-bacterial activity. J. Appl. Pharm. Sci. 2019, 9, 73–81. [Google Scholar]
- Shah, W.A.; Dar, M.Y.; Zagar, M.I.; Agnihotri, V.K.; Qurishi, M.A.; Singh, B. Chemical composition and antimicrobial activity of the leaf essential oil of Skimmia laureola growing wild in Jammu and Kashmir, India. Nat. Prod. Res. 2013, 27, 1023–1027. [Google Scholar] [CrossRef]
- Bártová, V.; Bárta, J.; Jarošová, M. Antifungal and antimicrobial proteins and peptides of potato (Solanum tuberosum L.) tubers and their applications. Appl. Microbiol. Biotechnol. 2019, 103, 5533–5547. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.Z.; Yu, X.F.; Zhu, Z.Y.; Zou, Z.D. Antioxidant and antibacterial activity of six edible wild plants (Sonchus sp.) in China. Nat. Prod. Res. 2011, 25, 1893–1901. [Google Scholar] [CrossRef]
- Semwal, D.; Rawat, U. Antimicrobial Hasubanalactam alkaloid from Stephania glabra. Planta. Med. 2009, 75, 378–380. [Google Scholar] [CrossRef] [PubMed]
- Kenny, O.; Brunton, N.P.; Walsh, D.; Hewage, C.M.; McLoughlin, P.; Smyth, T.J. Characterisation of antimicrobial extracts from Dandelion root (Taraxacum officinale) using LC-SPE-NMR. Phytother. Res. 2015, 29, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Q.; Gupta, N.; Kumar, A.; Nimesh, S. Antibacterial efficacy of silver nanoparticles synthesized employing Terminalia arjuna bark extract. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1192–1200. [Google Scholar] [CrossRef] [Green Version]
- Bag, A.; Bhattacharyya, S.K.; Chattopadhyay, R.R. The development of Terminalia chebula Retz. (Combretaceae) in clinical research. Asian Pac. J. Trop. Biomed. 2013, 3, 244–252. [Google Scholar] [CrossRef] [Green Version]
- Mandeville, A.; Cock, I.E. Terminalia chebula Retz. fruit extracts inhibit bacterial triggers of some autoimmune diseases and potentiate the activity of tetracycline. Indian J. Microbiol. 2018, 58, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Kher, M.N.; Sheth, N.R.; Bhatt, V.D. In vitro antibacterial evaluation of Terminalia chebula as an alternative of antibiotics against Bovine subclinical Mastitis. Anim. Biotechnol. 2019, 30, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Rather, A.M.; Nawchoo, I.A.; Ganie, A.H.; Singh, H.; Dutt, B.; Wani, A.A. Bioactive compounds & medicinal properties of Valeriana jatamansi Jones-a review. Life Sci. J. 2012, 9, 847–850. [Google Scholar]
- Mahdavi, S.; Amiradalat, M.; Babashpour, M.; Sheikhlooei, H.; Miransari, M. The antioxidant, anticarcinogenic and antimicrobial properties of Verbascum thapsus L. Med. Chem. 2020, 16, 991–995. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S.S.; Bithel, N.; Kumar, S.; Painuly, D.; Singh, J. A new derivative of ionone from aerial parts of Viola odorata Linn. and its antibacterial role against respiratory pathogens. Clin. Phytosci. 2017, 2, 1–5. [Google Scholar] [CrossRef] [Green Version]
- García-García, J.D.; Anguiano-Cabello, J.C.; Arredondo-Valdés, R.; Candido del Toro, C.A.; Martínez-Hernández, J.L.; Segura-Ceniceros, E.P.; Govea-Salas, M.; González-Chávez, M.L.; Ramos-González, R.; Esparza-González, S.C. Phytochemical characterization of Phoradendron bollanum and Viscum album Subs. Austriacum as mexican mistletoe plants with antimicrobial activity. Plants 2021, 10, 1299. [Google Scholar] [CrossRef]
- Kamruzzaman, M.; Bari, S.M.N.; Faruque, S.M. In vitro and in vivo bactericidal activity of Vitex negundo leaf extract against diverse multidrug resistant enteric bacterial pathogens. Asian Pac. J. Trop. Med. 2013, 6, 352–359. [Google Scholar] [CrossRef] [Green Version]
- Applequist, W.L.; Brinckmann, J.A.; Cunningham, A.B.; Hart, R.E.; Heinrich, M.; Katerere, D.R.; van Andel, T. Scientists’ Warning on Climate Change and Medicinal Plants. Planta Med. 2020, 86, 10–18. [Google Scholar]
- Sobuj, N.; Virjamo, V.; Zhang, Y.; Nybakken, L.; Julkunen-Tiitto, R. Impacts of elevated temperature and CO2 concentration on growth and phenolics in the sexually dimorphic Populus tremula (L. ). Environ. Exp. Bot. 2018, 146, 34–44. [Google Scholar] [CrossRef]
- Arab, L.; Kreuzwieser, J.; Kruse, J.; Zimmer, I.; Ache, P.; Alfarraj, S.; Al-Rasheid, K.A.S.; Schnitzler, J.-P.; Hedrich, R.; Rennenberg, H. Acclimation to heat and drought—Lessons to learn from the date palm (Phoenix dactylifera). Environ. Exp. Bot. 2016, 125, 20–30. [Google Scholar] [CrossRef] [Green Version]
- Rehman, S.; Iqbal, Z.; Qureshi, R.; Ur Rahman, I.; Khan, M.A.; Elshaer, M.M.A.; Al Farraj, D.A.; Elshikh, M.S.; Younas, M.; Sakhi, S.; et al. Ethnogynaecological knowledge of traditional medicinal plants used by the indigenous communities of north waziristan, Pakistan. Evid. Based Complement. Alternat. Med. 2022, 2022, 6528264. [Google Scholar] [CrossRef]
- Allard, T.; Wenner, T.; Greten, H.J.; Efferth, T. Mechanisms of herb-induced nephrotoxicity. Curr. Med. Chem. 2013, 20, 2812–2819. [Google Scholar] [CrossRef] [PubMed]
- Wattanathorn, J.; Uabundit, N.; Itarat, W.; Mucimapura, S.; Laopatarakasem, P.; Sripanidkulchai, B. Neurotoxicity of Coscinium fenestratum stem, a medicinal plant used in traditional medicine. Food Chem. Toxicol. 2006, l44, 1327–1333. [Google Scholar] [CrossRef]
- Parez, B.E.; Rodriquez, O.R.; Sanchez, V.M.C. Toxic plants: Brugmansia (floripondio) neurotoxicity. Arch. Med. Urg. Mex. 2012, 4, 119–124. [Google Scholar]
- Maffè, S.; Paffoni, P.; Laura Colombo, M.; Davanzo, F.; Dellavesa, P.; Cucchi, L.; Zenone, F.; Paino, A.M.; Franchetti Pardo, N.; Bergamasco, L.; et al. Herbs and cardiotoxic effects. G. Ital. Cardiol. 2013, 14, 445–455. [Google Scholar]
- Andrade, R.J.; Robles, M.; Fernández-Castañer, A.; López-Ortega, S.; López-Vega, M.C.; Lucena, M.I. Assessment of drug-induced hepatotoxicity in clinical practice: A challenge for gastroenterologists. World J. Gastroenterol. 2013, 13, 329–340. [Google Scholar] [CrossRef]
- Vinogradova, N.; Glukhov, A.; Chaplygin, V.; Kumar, P.; Mandzhieva, S.; Minkina, T.; Rajput, V.D. The Content of Heavy Metals in Medicinal Plants in Various Environmental Conditions: A Review. Horticulturae 2023, 9, 239. [Google Scholar] [CrossRef]
- Roufogalis, B.D. Challenges in integrating herbal medicine in healthcare systems. A Perspect. Focus Altern. Complement. Ther. 2015, 20, 34–35. [Google Scholar] [CrossRef]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, S. Current status of herbal product: Regulatory overview. Jr. Pharm. Bioallied Sci. 2015, 7, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Jogalekar, S.; Gibson, A. Regulation of natural health products in Canada. J. Ethanopharmacol. 2014, 158 Pt B, 507–510. [Google Scholar] [CrossRef]
- Harnett, J.; McIntyre, E.; Adams, J.; Addison, T.; Bannerman, H.; Egelton, L.; Ma, J.; Zabakly, L.; Steel, A. Prevalence and Characteristics of Australians Complementary Medicine Product Use, and Concurrent Use with Prescription and Over-the-Counter Medications-A Cross Sectional Study. Nutrients 2023, 15, 327. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, N.; Manchikanti, P. Herbal drug regulation and commercialization: An Indian industry perspective. J. Alt. Comp. Med. 2013, 19, 957–963. [Google Scholar] [CrossRef] [PubMed]
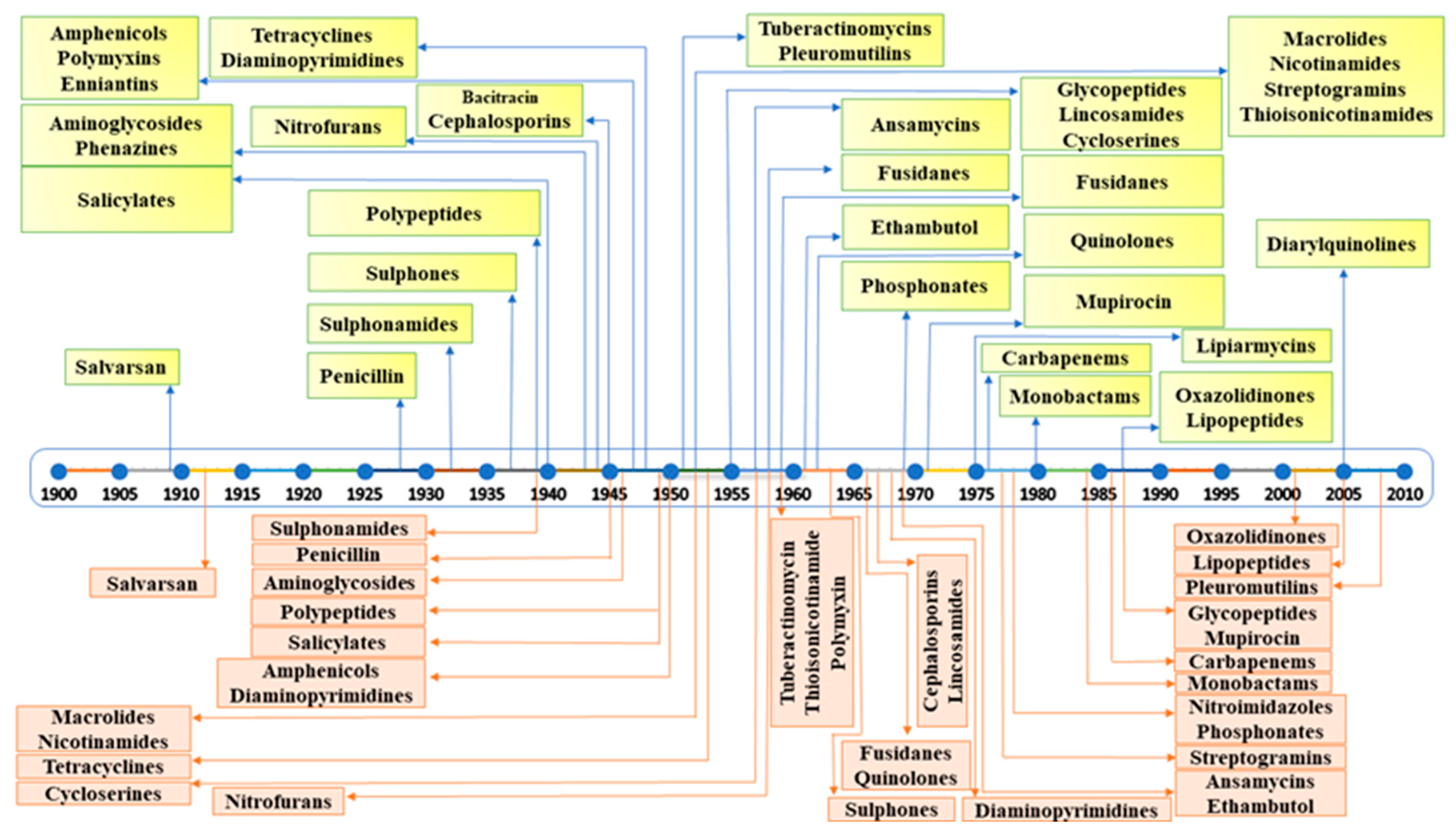
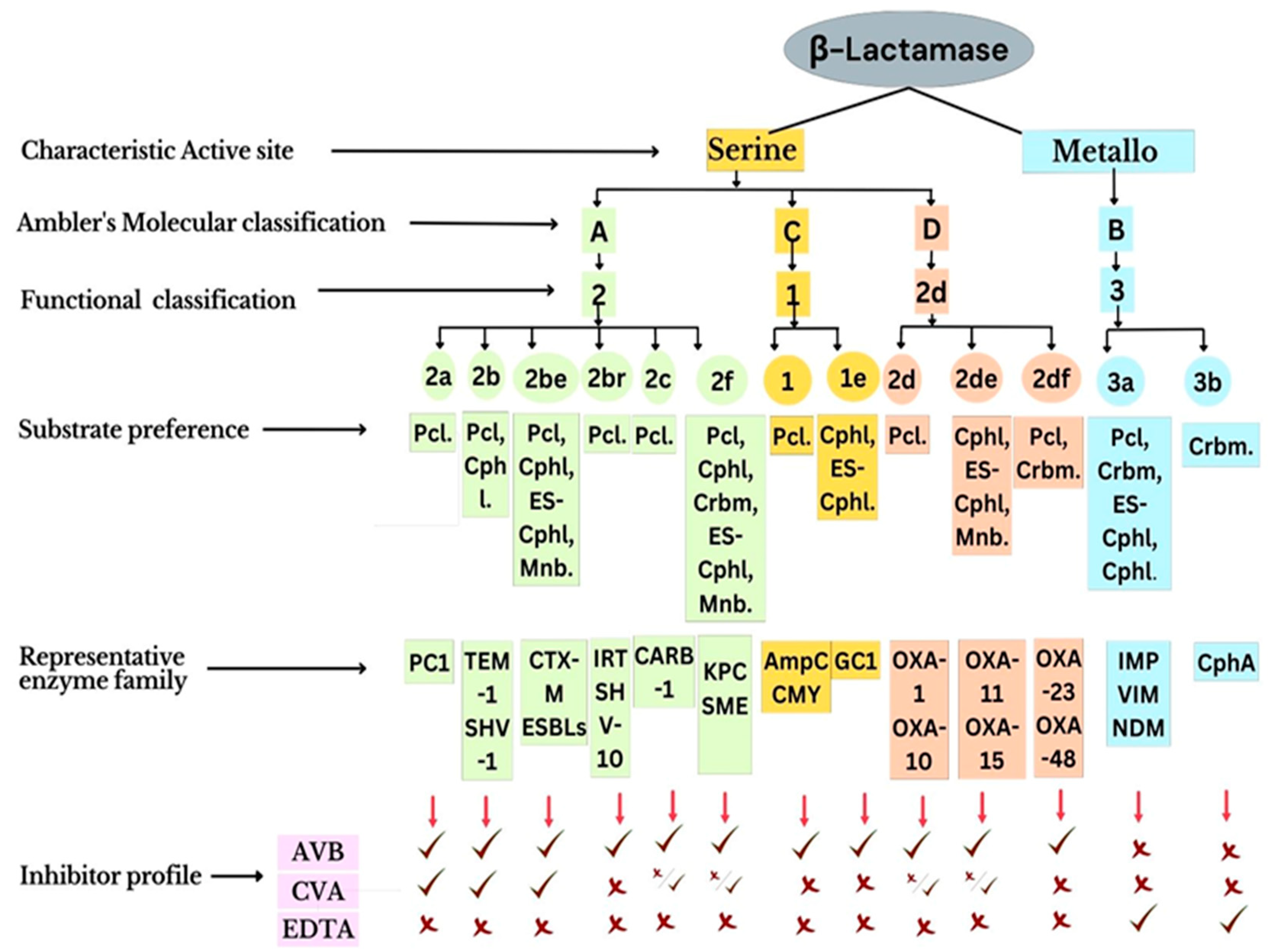
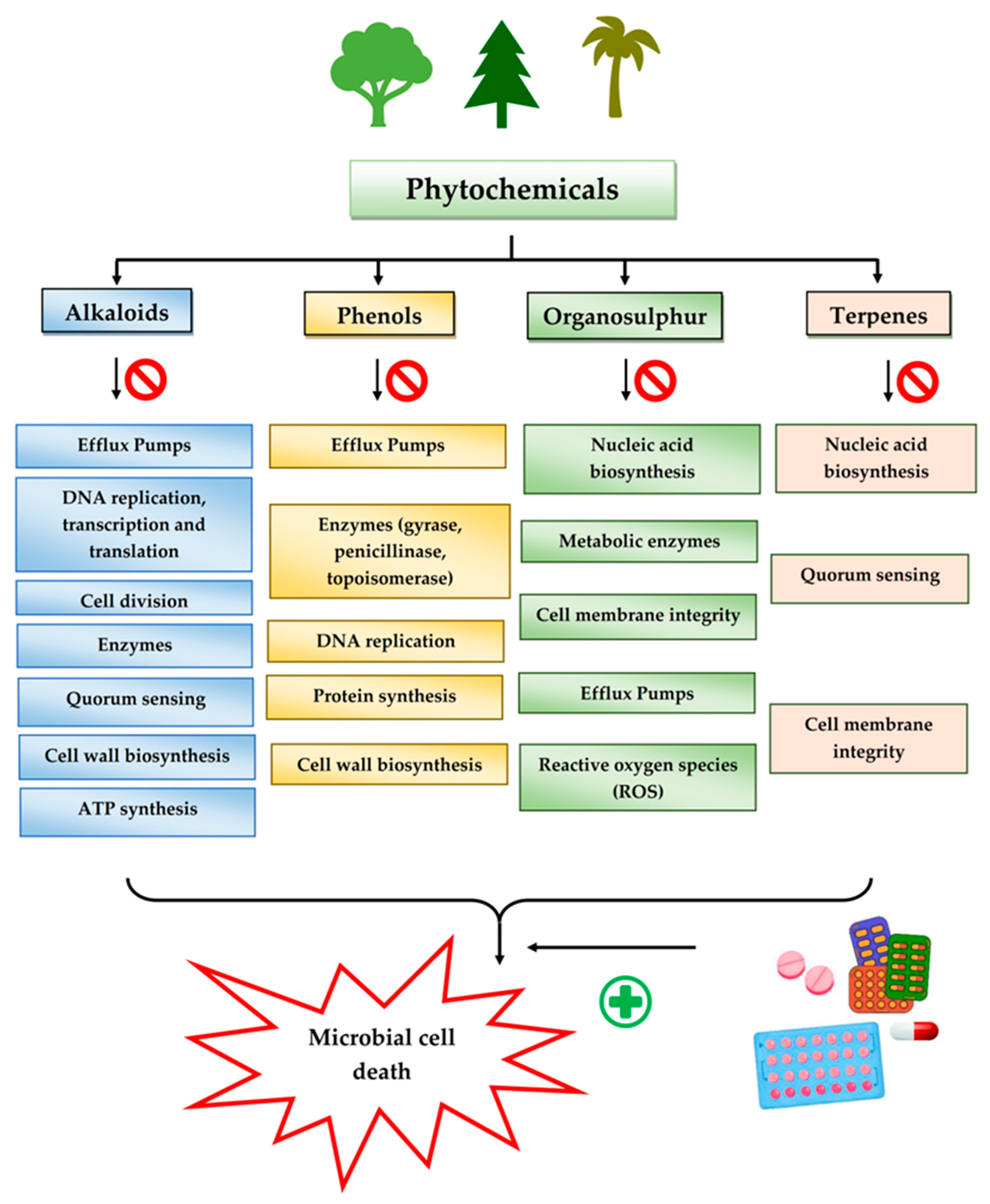
| Pathogenic Bacteria | Efflux Pump Family | Representative of Efflux Pump | Antibiotic Effluxed | References |
|---|---|---|---|---|
| Enterococcus faecium | ABC | EfrAB | Acriflavine, ciprofloxacin, daunomycin, doxorubicin, doxycycline, norfloxacin, tetraphenylphosphonium | [33] |
| Staphylococcus aureus | ABC | Isa(E) | Linosamide, pleuromutilin, streptogramin A | [34] |
| Msr(A) | Macrolides, telithromycin | [34] | ||
| MATE | MepA | Biocides, ethidium bromide, fluoroquinolones | [35] | |
| MFS | NorA | Fluoroquinolones | [36,37] | |
| QacA | Acriflavine, chlorhexidine, ethidium bromide, quaternary ammonium compounds | [37] | ||
| Klebsiella pneumoniae | MATE | KetM | 4, 6 Diamidino- 2- phenyl indole | [38] |
| MFS | KpnGH | Ceftazidime, cefepime, streptomycin, tetracycline | [39] | |
| RND | OqxAB | Chloramphenicol, fluoroquinolones | [40] | |
| SMR | KnpEF | Benzalkonium chloride, cefepime, chlorhexidine, erythromycin, streptomycin, tetracycline, triclosan | [39] | |
| Acinetobacter baumannii | ABC | MacAB- TolC | Macrolides | [41] |
| MATE | AbeM | Acriflavine, aminoglycosides, daunomycin, doxorubicin, fluoroquinolone | [42] | |
| MFS | CraA | Chloramphenicol | [43] | |
| RND | AdeABC, AdeFGH, AdeIJK | Aminoglycosides, beta-lactams, fluoroquinolones, macrolides, tetracycline, biocides | [44,45] | |
| Pseudomonas aeruginosa | RND | MexAB- OprM, | Aminoglycosides, beta-lactams, chloramphenicol, fluoroquinolones, macrolides, sulfonamides, tetracyclines, tigecycline | [46] |
| MexXY- OprM/A, MexCD- OprJ, MexEF- OprN | Trimethoprim, biocides, ethidium bromide | [47] | ||
| Escherichia coli | ABC | MacAB- TolC | Macrolides | [48] |
| MFS | MdfA | Chloramphenicol, doxorubicin, norfloxacin, tetracycline | [49] | |
| QepA/QepA2 | Fluoroquinolones | [50] | ||
| RND | AcrAB- TolC | β-lactams, chloramphenicol, fluoroquinolones, macrolides, novobiocin, tetracycline, tigecycline | [51,52] | |
| OqxAB | Chloramphenicol, fluoroquinolones | [40] | ||
| SMR | EmrE | Acriflavine, ethidium bromide, quaternary ammonium compounds | [53,54] |
| Enzyme Class | Type | Substrate Antibiotic Class | Representative |
|---|---|---|---|
| Hydrolases | β-lactamases | β-lactam | Penicillin |
| Cephalosporin | |||
| Carbapenem | |||
| Macrolide esterases | Macrolide | Erythromycin | |
| Roxithromycin | |||
| Azithromycin | |||
| Epoxidases | Epoxide | Fosfomycin | |
| Transferases | Acetyltransferases | Aminoglycoside | Gentamicin |
| Kanamycin | |||
| Amikacin | |||
| Chloramphenicol | Chloramphenicol | ||
| Streptogramin | Group A streptogramins | ||
| Phosphotransferases | Aminoglycoside | Gentamicin | |
| Kanamycin | |||
| Amikacin | |||
| Macrolide | Erythromycin | ||
| Roxithromycin | |||
| Azithromycin | |||
| Rifamycin | Rifampin | ||
| Rifabutin | |||
| Rifapentine | |||
| Peptide | Colistin | ||
| Polymixin B | |||
| Thiol S-transferases | Epoxide | Fosfomycin | |
| Nucleotidyltransferases | Aminoglycoside | Gentamicin | |
| Kanamycin | |||
| Amikacin | |||
| Lincosamide | Lincomycin | ||
| Clindamycin | |||
| Pirlimycin | |||
| ADP-ribosyltransferases | Rifamycin | Rifampin | |
| Rifabutin | |||
| Rifapentine | |||
| Glycosyltransferases | Macrolide | Erythromycin | |
| Roxithromycin | |||
| Azithromycin | |||
| Rifamycin | Rifampin | ||
| Rifabutin | |||
| Rifapentine | |||
| Redox enzymes | Monooxygenases | Tetracycline | Tetracycline |
| Oxytetracycline | |||
| Doxycycline | |||
| Rifamycin | Rifampin | ||
| Rifabutin | |||
| Rifapentine | |||
| Streptogramin | Group A streptogramins | ||
| Lyases | Lyases (Virginiamycin B lyase) | Streptogramin | Group B streptogramins |
| Bioactive Compound | Chemical Formula | PubChem CID | Chemical Structure * | Plant Source | Target Pathogen | Mode of Action | References |
|---|---|---|---|---|---|---|---|
| Conessine | C24H40N2 | 441082 |  | Holarrhena antidysenterica (G. Don) Wall. ex A. DC., Holarrhena floribunda (G.Don) T. Durand & Schinz, Holarrhena pubescens Wall. ex G. Don, Funtumia elastica (Preuss) Stapf. | Pseudomonas aeruginosa | Efflux pump inhibitor | [120,128] |
| Piperine | C17H19NO3 | 638024 | 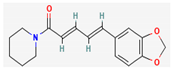 | Piper sylvaticum Roxb. | Methicillin- resistant Staphylococcus aureus (MRSA) | Efflux pump inhibitor | [92,114] |
| Berberine | C20H18NO4+ | 2353 | 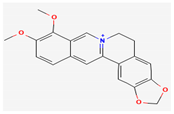 | Berberis lycium Royle | Escherichia coli | Cell division inhibitor, protein, and DNA synthesis inhibitor | [129,130] |
| Lysergol | C16H18N2O | 14987 |  | Convolvulaceae Juss. | Escherichia coli | Efflux pump inhibitor | [125] |
| 8-epidiosbulbin E-acetate | C21H24O7 | 131751666 |  | Dioscorea bulbifera L. | Escherichia coli, Enterococcus faecalis, Pseudomonas aeruginosa, Shigella. | Plasmid curing (R-plasmids in Escherichia coli and Enterococcus faecalis) | [57,107] |
| Reserpine | C33H40N2O9 | 5770 | 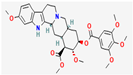 | Rauvolfia serpentina (L.) Benth. ex Kurz | Staphylococcus sp., Streptococcus sp. | Efflux pump inhibitor | [131] |
| Tomatidine | C27H45NO2 | 65576 | 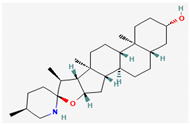 | Solanum L. sp. | Listeria, Bacillus and Staphylococcus sp. | ATP synthase inhibitor | [118,132] |
| Dictamnine | C12H9NO2 | 68085 | 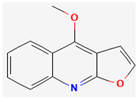 | Teclea afzelii (Engl.) | Escherichia coli, Microsporum audorium, Bacillus subtilis, Mycobacterium smegmatis | Inhibition of Type II topoisomerase enzyme and inhibition of DNA replication | [92] |
| Kokusagine | C13H9NO4 | 5318829 | 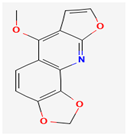 | Teclea afzelii (Engl.) | |||
| Maculine | C13H9NO4 | 68232 |  | Teclea afzelii (Engl.) | |||
| Sanguinarine | C20H14NO4+ | 5154 | 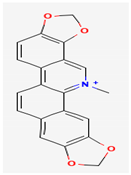 | Chelidonium majus L., Sanguinaria canadensis L., Macleaya cordata (Willd.) R. Br. | MRSA, Mycobacterium aurum and Mycobacterium smegmatis | Compromising cytoplasmic membrane, cell lysis, replication, and transcription inhibition | [92] |
| Chanoclavine | C16H20N2O | 5281381 | 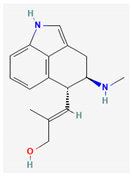 | Ipomoea muricata (L.) Jacq. | MDR Escherichia coli | Efflux pump inhibition | [92] |
| Caffeine | C8H10N4O2 | 2519 | 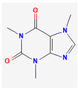 | Camellia sinensis (L.) Kuntze | Pseudomonas aeruginosa | Inhibition of quorum-sensing proteins LasR and LasI and inhibition of bacterial virulence factors | [124] |
| Caranine | C16H17NO3 | 441589 | 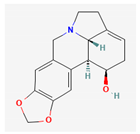 | Clivia miniata (Lindl.) Verschaff., Crinum bulbispermum (Burm.f.) Milne-Redh. & Schweick. | Candida dubliniensis | NA | [133] |
| Evodiamine | C19H17N3O | 442088 |  | Evodia aromatica (Sonn.) Pers. | Streptococcus pneumoniae | Inhibition of ATP-dependent MurE ligase of Mycobacterium tuberculosis, an enzyme required for the biosynthesis of peptidoglycan | [134] |
| Chanoclavine | C16H20N2O | 5281381 | 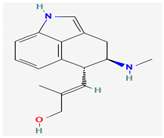 | Ipomoea muricata (L.) Jacq. | MDR Escherichia coli | Efflux pump inhibition | [92] |
| Evocarpine | C23H33NO | 5317303 | 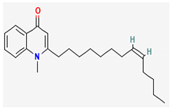 | Evodia aromatica (Sonn.) Pers. | Streptococcus pneumoniae | Inhibition of ATP-dependent MurE ligase of Mycobacterium tuberculosis, an enzyme required for the biosynthesis of peptidoglycan | [134] |
| Voacafricines A & B | Fruits of Voacanga Africana Stapf. | Staphylococcus aureus | NA | [133] | |||
| Thalicfoetine | Roots of Thalictrum foetidum L. | Bacillus subtilis | NA | [135,136] |
| Bioactive Compound | Chemical Formula | PubChem CID | Chemical Structure * | Plant Source | Target Pathogen | Mode of Action | References |
|---|---|---|---|---|---|---|---|
| Myricetin | C15H10O8 | 5281672 | 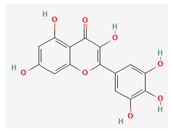 | Myricaceae Rich. ex Kunth., Anacardiaceae R.Br., Polygonaceae Juss., Pinaceae Spreng. ex F.Rudolphi., Primulaceae Batsch ex Borkh. | Mycobacterium smegmatis | Efflux pump inhibitor | [144] |
| Baicalein | C15H10O5 | 5281605 | 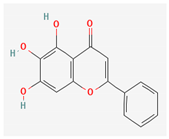 | Thymus vulgaris L., Scutellaria baicalensis Georgi, Scutellaria lateriflora L. | Methicillin-resistant Staphylococcus aureus | Inhibition of the NorA efflux Pump | [103,104] |
| 4′,6′-Dihydroxy-3′,5′-dimethyl-2′-methoxychalcone | C18H18O4 | 10424762 | 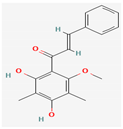 | Dalea versicolor Zucc. | Staphylococcus aureus, Bacillus cereus. | Inhibition of NorA efflux pump | [92,156] |
| Epigallocatechin gallate | C22H18O11 | 65064 | 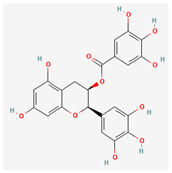 | Camellia sinensis (L.) Kuntze | Methicillin-resistant Staphylococcus aureus | Inhibition of NorA efflux pump, inhibition of chromosomal penicillinase and DNA gyrase | [98,153] |
| Chebulinic acid | C41H32O27 | 72284 | 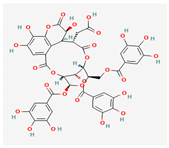 | Terminalia chebula Retz. | Quinolone-resistant mutants of Mycobacterium tuberculosis | Inhibition of DNA gyrase | [147] |
| Emodin | C15H10O5 | 3220 | 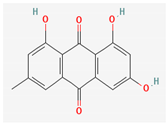 | Rheum palmatum L. | Methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus faecium | Inhibition of DNA gyrase | [157] |
| Curcumin | C21H20O6 | 969516 | 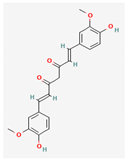 | Curcuma longa L. | Staphylococcus aureus, Escherichia coli. | Enhancing membrane permeability, inhibition of enzyme sortase A | [155] |
| Quercetin | C15H10O7 | 5280343 | 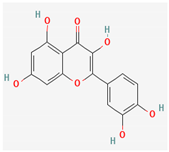 | Vitaceae Juss. Brassicaceae Burnett, Amaryllidaceae J.St.-Hil., Rutaceae Juss. | Staphylococcus aureus, Escherichia coli, Helicobacter pylori | Efflux pump inhibitor, inhibition of d-alanine:d-alanine ligase in Helicobacter pylori and Escherichia coli | [148,158] |
| Kaempferol | C15H10O6 | 5280863 | 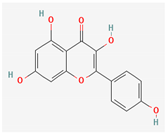 | Alpinia calcarata–Roscoe. | Methicillin-resistant Staphylococcus aureus, Candida albicans | Efflux pump inhibitor | [150,151] |
| Resveratrol | C14H12O3 | 445154 | 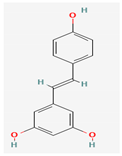 | Vitis vinifera L. | Campylobacter jejuni | Efflux pump inhibitor | [159] |
| Taxifolin/dihydroquercetin | C15H12O7 | 439533 | 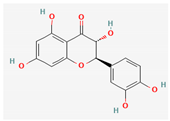 | Conifers-like Larix sibirica Ledeb., Pinus roxburghii Sarg., Cedrus deodara (Roxb. ex D.Don) G.Don, Taxus chinensis (Pilg.) Rehder. | Methicillin-resistant Staphylococcus aureus, Enterococcus faecalis | Cysteine transpeptidase sortase A (SrtA) inhibitor, β-ketoacyl acyl carrier protein synthase inhibitor | [160,161] |
| Osthole | C15H16O3 | 10228 | 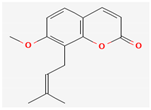 | Prangos hulusii (Şenol, Yıldırım & Seçmen), Cnidium monnieri (L.) Cusson ex Juss., Angelica pubescens Maxim. | Bacillus subtilis, Staphylococcus aureus, Klebsiella pneumoniae | DNA gyrase inhibitor, MCR-1 inhibitor | [162,163] |
| Galbanic acid | C24H30O5 | 7082474 | 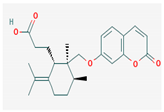 | Ferula szowitsiana DC. | Staphylococcus aureus | Efflux pump inhibitor | [164] |
| Asphodelin A | C15H10O6 | 54679752 | 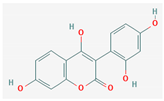 | Asphodelus microcarpus Rchb. | Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Candida albicans, Botrytis cinerea | DNA gyrase inhibitor | [165] |
| Aegelinol | C14H14O4 | 600671 | 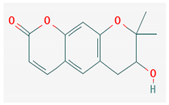 | Phlojodicarpus villosus (Turcz. ex Fisch. & C.A.Mey.) Turcz. ex Ledeb., Peucedanum praeruptorum Dunn, Ferulago galbanifera (Mill.) W.D.J.Koch | Salmonella enterica serovar typhi, Enterobacter aerogenes, Enterobacter cloacae, Staphylococcus aureus | DNA gyrase inhibitor | [92,166] |
| 3,4,5-trihydroxybenzoic acid (Gallic acid) | C7H6O5 | 370 | 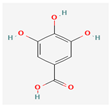 | Mimosa bimucronata (DC.) Kuntze, Punica granatum L. | Staphylococcus aureus, Escherichia coli, Listeria monocytogenes, Pseudomonas aeruginosa | Cell membrane disintegration | [145] |
| Ferulic acid | C10H10O4 | 445858 |  | Commelinaceae Mirb. | Staphylococcus aureus, Escherichia coli, Listeria monocytogenes, Pseudomonas aeruginosa | Cell membrane disintegration | [145] |
| Apigenin | C15H10O5 | 5280443 | 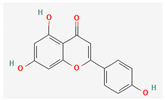 | Matricaria chamomilla L. | Pseudomonas aeruginosa | NA | [134,148] |
| Genistein | C15H10O5 | 5280961 | 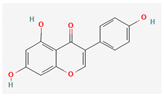 | Glycine max (L.) Merr. | Pseudomonas aeruginosa | NA | [167] |
| Eriodictyol | C15H12O6 | 440735 | 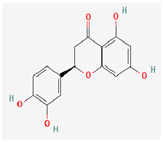 | Eriodictyon californicum (Hook. & Arn.) Decne. | Enterococcus faecalis | NA | [161] |
| Agasyllin | C19H20O5 | 15596603 | 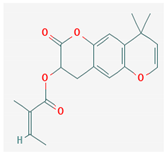 | Ferulago galbanifera (Mill.) W.D.J.Koch | Campylobacter species | DNA gyrase inhibitor | [166] |
| Bioactive Compound | Chemical Formula | PubChem CID | Chemical Structure * | Plant Source | Target Pathogen | Mode of Action | References |
|---|---|---|---|---|---|---|---|
| Allicin | C6H10OS2 | 65036 | 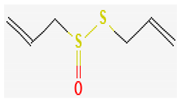 | Allium sativum L. | Salmonella typhimurium, Staphylococcus aureus, Bacillus subtilis, Bacillus typhosus, Bacillus paratyphosus, Morganella morganii, Bacillus enteritidis, Shigella dysenteriae, Vibrio cholera, Escherichia. coli, Listeria monocytogenes, Helicobacter pylori, drug-resistant strains of Mycobacterium tuberculosis | Sulfhydryl-dependent enzyme inhibitor, DNA/RNA synthesis inhibitor, inhibitor of acetyl-CoA synthases in yeasts | [169,174,178,179] |
| Ajoene | C9H14OS3 | 5386591 | 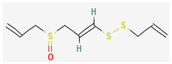 | Allium sativum L. | Campylobacter jejuni, Staphylococcus aureus, Escherichia coli, Helicobacter pylori | Sulfhydryl-dependent enzyme | [168,180] |
| Sulforaphane | C6H11NOS2 | 5350 | 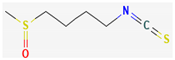 | Brassicaceae Burnett. | Bacillus cereus, Escherichia coli | Membrane destruction, ATP synthase inhibitor, DNA/protein synthesis inhibitor | [181] |
| Allyl isothiocyanates (AITCs) | C4H5NS | 5971 | 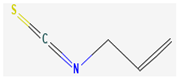 | Armoracia rusticana G.Gaertn., B.Mey., & Scherb. | Oral pathogens, Helicobacter pylori, Escherichia coli | Inhibition of urease, reducing the inflammatory component of Helicobacter infections, inhibition of ATP binding sites of P-ATPase in bacteria | [92,170] |
| Benzyl isothiocyanate (BITC) | C8H7NS | 2346 | 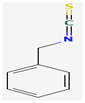 | Alliaria petiolata (M.Bieb.) Cavara & Grande | Methicillin-resistant Staphylococcus aureus | Disruption of membrane integrity | [176] |
| Phenethyl isothiocyanate(PEITC) | C9H9NS | 16741 | 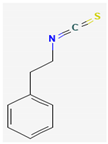 | Brassica campestris L., Brassica rapa L. | Gram-positive bacteria | Intracellular accumulation of reactive oxygen species (ROS), depolarization of mitochondrial membrane | [92,182] |
| Berteroin | C7H13NS2 | 206037 |  | Brassica oleracea L. | Bacillus subtilis, Escherichia coli, Helicobacter pylori | NA | [93] |
| Cheirolin | C5H9NO2S2 | 10454 |  | Cheiranthus cheiri L. | Helicobacter pylori | NA | [134] |
| Alyssin | C7H13NOS2 | 206035 |  | Alyssum L. sp. | Helicobacter pylori | NA | [134] |
| Bioactive Compound | Chemical Formula | PubChem CID | Chemical Structure * | Plant Source | Target Pathogen | Mode of Action | References |
|---|---|---|---|---|---|---|---|
| Eugenol | C10H12O2 | 3314 |  | Syzygium aromaticum (L.) Merr. & L.M.Perr, Cinnamomum zeylanicum Blume. | Helicobacter pylori, Methicillin-resistant Staphylococcus aureus, Methicillin-sensitive Staphylococcus aureus, Pseudomonas aeruginosa | Inhibits biofilm construction, interrupts cell-to-cell communication, eradicates the pre-established biofilms, and kills the bacteria in biofilms | [190,192] |
| Cinnamaldehyde | C9H8O | 637511 | 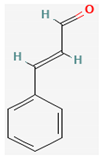 | Cinnamomum verum J. Presl. | Escherichia coli, Staphylococcus aureus | Membrane damage | [193] |
| Ursolic acid | C30H48O3 | 64945 | 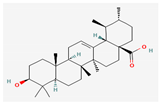 | Salvia rosmarinus Spenn., Salvia officinalis L. | Escherichia coli | Cell membrane disturbance | [189] |
| Farnesol | C15H26O | 445070 | 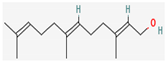 | Vachellia farnesiana (L.) Wight & Arn. | Staphylococcus aureus including MRSA | Membrane damage | [194] |
| Carvacrol | C10H14O | 10364 | 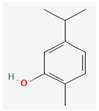 | Thymus capitatus (L.) Hoffmanns. & Link., Thymus vulgaris L. | Escherichia coli | Cell membrane damage | [167,195] |
| Nerolidol | C15H26O | 5284507 | 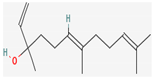 | Cannabis sativa L. | Staphylococcus aureus including MRSA | Cell membrane damage | [194] |
| Thymol | C10H14O | 6989 | 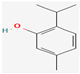 | Thymus capitatus (L.) Hoffmanns. & Link. | Staphylococcus aureus including MRSA | NA | [195,196] |
| Plant Name | Bioactive Compounds | Target Pathogen | References |
|---|---|---|---|
| Abrus precatorius L. | 6-propionyloxymethyl-4′,5,7-trihydroxyisoflavanone | Bacillus cereus, Escherichia coli | [223] |
| Abutilon theophrasti Medik. | Rutin, | Salmonella enterica, Escherichia coli, Streptococcus pneumoniae, Staphylococcus aureus | [224] |
| quercetin 7-o-β-glucoside, | |||
| kaempferol 3-o-α-rhamnopyranosyl (1→6)-β-glucopyranoside, | |||
| luteolin, | |||
| apigenin 7-o-β-diglucoside, | |||
| poncirin, | |||
| tiliroside | |||
| Achillea millefolium L. | Camphor, germacrene-d, (e)-nerolidol, sabinene, (e)-p-mentha-2,8-dien-1-ol, 1,8-cineole | Salmonella typhimurium, Salmonella agona | [225] |
| Achyranthes aspera L. | Achyranthine, betaine, betanin, isobetanin | Bacillus subtilis, Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus | [226] |
| Aconitum violaceum Jacq. ex Stapf. | Ethyl acetate fraction | Escherichia coli, Shigella flexneri, Bacillus subtilis, Staphylococcus aureus | [227] |
| Aconitum heterophyllum Wall. ex Royle | 6-dehydroacetylsepaconitine, | Staphylococcus aureus, Salmonella typhi, Pseudomonas aeruginosa | [228] |
| 13-hydroxylappaconitine, lycoctonine, | |||
| lappaconitine | |||
| Acorus calamus L. | Asarone | Aspergillus niger, Candida albicans | [229] |
| Adiantum capillus-veneris L. | 3-p-coumaroylquinic acid, kaempferol 3-o-glucoside | Staphylococcus aureus, Staphylococcus epidermidis, β-hemolytic Streptococcus, Enterococcus faecalis, Escherichia coli, Pseudomonas aeruginosa | [230] |
| Adiantum pedatum | Ethyl and acetone extracts | Staphylococcus aureus, Klebsiella pneumoniae, Pseudomonas aeruginosa, Escherichia coli | [231] |
| Aegle marmelos (L.) Corrêa | Limonene, β-ocimene, germacrene, α-phellandrene | Caenorhabditis elegans | [232] |
| Ageratum houstonianum Mill. | Ageratochromene, demothoxyageratochromene, β-caryophyllene | Micrococcus luteus, Rhodococcus rhodochrous | [233] |
| Ajuga parviflora Benth. | Ajugin A, ajugin B | Citrobacter sp., Pseudomonas aeruginosa | [234] |
| Allamanda cathartica L. | Silver nanoparticles of flower aqueous extracts | Salmonella typhimurium, Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae | [235] |
| Allium cepa L. | Allicin | Salmonella typhimurium, Staphylococcus aureus, Escherichia coli | [236] |
| Allium sativum L. | Allicin, | Aspergillus versicolor, Penicillium citrinum, Burkholderia cepacia, Staphylococcus aureus, Escherichia coli, Bacillus subtilis, Penicillium funiculosum, Candida albicans, Helicobacter pylori | [179] |
| diallyl sulfide, | |||
| diallyl disulfide, | |||
| diallyl trisulfide, | |||
| e/z-ajoene, | |||
| s-allyl-cysteine, | |||
| s-allyl-cysteine sulfoxide. | |||
| Amaranthus caudatus L. | Ferulic acid | Escherichia coli | [145] |
| Amaranthus viridis L. | Rutin, quercetin, spinosterol, amasterol | Staphylococcus aureus, Escherichia coli, c, Rhizopus oligosporus, Colletotrichum musae, Fusarium solani | [237] |
| Amomum subulatum Roxb. | 1,8-cineole, α-terpineol, α-pinene, β-pinene | Aspergillus niger | [238] |
| Angelica glauca Edgew. | β-phellandrene, (z)-ligustilide methyl octane, limonene, β-phellandrene, β-pinene, (z)-3-butylidene-phthalide, (z)-ligustilide, (e)-ligustilide, citronellyl acetate | Clostridium difficile, Clostridium perfringens, Enterococcus faecalis, Eubacterium limosum, Peptostreptococcus anaerobius, Candida albicans | [239] |
| Arctium lappa L. | Chlorogenic acid, caffeic acids | Pseudomonas aeruginosa, Bacillus cereus | [240] |
| Arnebia benthamii (Wall. ex G.Don) I.M.Johnst. | Shikonin, | Escherichia coli, Pseudomonas aeruginosa, Shigella flexneri, Klebsiella pneumoniae, Salmonella typhimurium, Staphylococcus aureus | [241] |
| alkanin hoslundal, | |||
| artemidiol, | |||
| ganoderiol, | |||
| 2-hexaprenyl-6-hydroxyphenol | |||
| Artemisia dubia Wall. ex Besser | Chrysanthenone, coumarin, camphor | Aspergillus niger | [242] |
| Artemisia indica Willd. | Isoascaridole, trans-p-mentha-2,8-dien-1-ol, trans-verbenol, artemisia ketone, germacrene B, borneol, cis-chrysanthenyl acetate, davanone, β-pinene. | Bacillus subtilis, Staphylococcus epidermidis, Pseudomonas aeruginosa, Salmonella typhi, Klebsiella pneumoniae, Penicillium chrysogenum, Aspergillus niger | [242,243] |
| Asparagus racemosus Willd. | Catecholic tannin, saponin, gallic tannin | Escherichia coli, Salmonella typhimurium, Bacillus subtilis, Pseudomonas aeruginosa, Staphylococcus aureus, Klebsiella pneumoniae, Enterococcus faecalis, Saccharomyces cerevisiae | [244] |
| Atropa acuminata Royle ex Lindl. | Aqueous extract | Bacillus Subtilis, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Salmonella typhimurium, Staphylococcus aureus | [245] |
| Atropa bella-donna L. | Ethanolic extracts | Staphylococcus aureus, Escherichia coli | [246] |
| Bacopa monnieri (L.) Wettst. | Luteolin | Staphylococcus aureus, Alternaria alternate, Fusarium acuminatum | [247] |
| Baliospermum montanum (Willd.) Müll.Arg. | Leaf (methanolic and aqueous extract), callus (acetone and ethanolic extract) | Bacillus subtilis, Klebsiella pneumoniae, Staphylococcus aureus, Escherichia coli | [248] |
| Berberis lyceum Royle | Berberine | Streptococcus agalactiae, Staphylococcus aureus, Streptococcus mutans, Streptococcus pyogenes, Corynebacterium diphtheriae | [110] |
| Bergenia ciliate (Haw.) Sternb. | Pyrogallol, | Staphylococcus aureus, Bacillus subtilis, Bacillus megaterium, Escherichia coli, Serratia marcescens, Nocardia tenerifensis, Streptomyces sp., Aspergillus niger, Fusarium oxysporum | [249] |
| rutin, | |||
| morin, | |||
| bergenin, | |||
| catechin, | |||
| gallic acid | |||
| Betula utilis D.Don | Geranic acid, | Staphylococcus aureus, Bacillus subtilis, Pseudomonas aeruginosa, Escherichia coli | [250] |
| β-seleneol, | |||
| β-linalool, | |||
| β-sesquiphellendrene, | |||
| champacol, | |||
| 1,8-cineol. | |||
| Bidens biternate (Lour.) Merr. & Sherff | Methanolic extract | Escherichia coli, Klebsiella pneumoniae, Pseudomonas sp., Staphylococcus aureus, Staphylococcus epidermidis | [251] |
| Blumea lacera (Burm.f.) DC. | Lachnophyllum ester, lachnophyllic acid, germacrene d, β-farnesene. | Staphylococcus aureus, Candida albicans, Aspergillus niger | [252] |
| Bridelia retusa (L.) A.Juss. | Ethanolic extract | Pseudomonas aeruginosa, Escherichia coli | [253] |
| Calendula officinalis L. | Selenium nanoparticles of methanolic extract of flowers | Serratia marcescens, Enterobacter cloacae, Alcaligenes faecalis | [254] |
| Calotropis procera (Aiton) W.T.Aiton | α-amyrin, lupeol acetate, phytol, hexadecanoic acid, stigmasterol, linolenic acid, gombasterol A | Staphylococcus aureus, Klebsiella pneumoniae, Escherichia coli | [255] |
| Caltha palustris L. | Methanolic extract | Staphylococcus epidermidis, Proteus vulgaris | [256] |
| Cannabis sativa L. | Cannabidiol (cannabinoids) | Staphylococcus aureus (MDR, MRSA), Staphylococcus epidermidis, Streptococcus pneumoniae, Streptococcus pyogenes, Enterococcus faecium, Cutibacterium acnes, Clostridium difficile, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Acinetobacter baumannii, Serratia marcescens, Proteus mirabilis, Salmonella typhimurium | [257] |
| Cassia fistula L. | Eugenol, phytol, camphor, linonene, salicyl alcohol, 4-hydroxybenzyl alcohol | Aspergillus niger, Candida albicans | [229] |
| Cassia tora L. | Elemol, linalool, palmitic acid | Bacillus cereus, Staphylococcus aureus | [229] |
| Cedrus deodara (Roxb. ex D.Don) G.Don | Wikstromal, matairesinol, dibenzylbutyrolactol, berating, isopimpillin, lignans 1, 4 diaryl butane, benzofuranoid neo lingam, isohemacholone, sesquiterpenes, deodarone, atlantone, deodarin, deodardione, limonenecarboxylic acid, α-himacholone, β-himacholone, α-pinene, β-pinene, myrcene, cedrin (6-methyldihydromyricetin), taxifolin, cedeodarin (6-methyltaxifolin), dihydromyricetin and cedrinoside | Escherichia coli | [258] |
| Chaerophyllum villosum Wall. ex DC. | γ-terpinene, p-cymene, carvacrol methyl ether, myristicin, thymol | Staphylococcus aureus, Streptococcus mutans, Candida albicans, Candida glabrata | [259] |
| Chenopodium ambrosioides L. | Rutin (3,3′,4′,5,7-pentahydroxyflavone-3-rhamnoglucoside) | Staphylococcus aureus, Pseudomonas aeruginosa, Enterococcus faecalis, Paenibacillus apiarius, Paenibacillus thiaminolyticus | [260] |
| Cichorium intybus L. | Triterpenois, cichoridiol, intybusoloid, lupeol, fridelin, β- sitosterol, sigmasterol, betulinic acid, betunaldehyde, syringic acid, vanilic acid | Pseudomonas aeruginosa, Staphylococcus aureus | [260] |
| Cinnamomum glanduliferum (Wall.) Meisn. | 1,8-cineole, α-pinene, α-terpineol, germacrene d-4-ol, α-thujene | Micrococcus luteus, Escherichia coli, Pseudomonas aeruginosa, Aeromonas salmonicida | [261] |
| Cissampelos pareira L. | Bis-benzylisoquinoline, benzylisoquinoline, tropoloisoquinoline, aporphine, azafluoranthene, protoberberine | Staphylococcus aureus, Streptococcus pneumoniae, Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumoniae, Proteus vulgaris | [262] |
| Convolvulus arvensis L. | Butanolic extract | Staphylococcus aureus, Acinetobacter junii, Klebsiella pneumoniae, Acinetobacter baumannii, Escherichia coli, Enterococcus faecalis, Pseudomonas aeruginosa, Salmonella dysenteriae, Vibrio cholera, Proteus mirabilis, Salmonella paratyphi, Serratia marcescens, Enterobacter cloacae | [263] |
| Coriandrum sativum L. | β-linalool (essential oil) | Bacillus subtilis, Stenotrophomonas maltophilia | [264] |
| Crocus sativus L. | Crocin, safranal semi-synthetic safranal derivatives | Helicobacter pylori, Staphylococcus aureus, Listeria spp. Bacillus subtilis, Bacillus cereus, Salmonella enterica, Shigella dysenteriae, Escherichia coli | [265] |
| Curcuma Longa L. | α-turmerone, β-turmerone, α-phellandrene, 1,8-cineole, p-cymene, terpinolene | Bacillus cereus, Staphylococcus aureus, Aspergillus niger | [266] |
| Cuscuta reflexa Roxb. | cis-3-butyl-4-vinylcyclopentane, limonene, (e)-nerolidol | Aspergillus niger | [267] |
| Cymbopogon citratus (DC.) Stapf | α-citral, | Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, Staphylococcus aureus, Bacillus subtilis | [268] |
| β-citral, | |||
| myrcene | |||
| Cyperus rotundus L. | α.-pinene, camphene, D-limonene, | Bacillus subtilis, Pseudomonas aeruginosa, Escherichia coli, Staphylococcus aureus (ARSA) | [269,270] |
| camphenol, p-mentha-1,5-dien-8-ol, | |||
| thymol, myrtenal, carveol, copaene, | |||
| caryophyllene, naphthalene, | |||
| 1,6-dimethyl-4-(1-methylethyl). | |||
| Datura metel L. | Daturaolone | Escherichia coli, Staphylococcus aureus, Bacillus subtilis, Klebsiella pneumoniae, Staphylococcus epidermidis | [271] |
| Datura stramonium L. | Chloroform extracts | Staphylococcus aureus | [272] |
| Daucus carota L. | Methylisoeugenol | Campylobacter jejuni | [273] |
| Dioscorea bulbifera L. | Bafoudiosbulbins, 2,7-dihydroxy-4-methoxyphenanthrene | Escherichia coli, Enterobacter aerogenes, Klebsiella pneumoniae, Pseudomonas aeruginosa | [274] |
| Dodecadenia grandiflora Nees | Germacrene D, furanodiene | Staphylococcus aureus, Pasteurella multocida | [275] |
| Dodonaea viscosa Jacq. | Hautriwaic acid, dodonoside B, dodonic acid, kaempferol, sakuranetin, dehydrohautriwaic acid, hautriwaic lactone, alizarin, penduletin, 3,5,7-trihydroxy-4′-methoxyflavone, isorhamnetin-3-rhamnosylgalactoside, donoside a, 5- hydroxy-3,6,7,4′-tetra methoxy flavone | Streptococcus pyogenes, Escherichia coli, Klebsiella pneumonia, Pseudomonas fluorescens, Staphylococcus aureus, Bacillus subtilis | [276] |
| Epimedium grandiflorum C. Morren | Hydroethanolic extract | Bacillus subtilis, Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae, Acinetobacter sp., Pseudomonas sp., Salmonella sp. | [277] |
| Equisetum diffusum D. Don | Aqueous extract | Escherichia coli, Micrococcus luteus, Pseudomonas aeruginosa, Bacillus pumilus, Bacillus cereus, Bacillus licheniformis, Salmonella typhi, Streptococcus mutans | [278] |
| Eupatorium adenophorum Spreng. | p-cymene, bornyl acetate, amorph-4-en-7-ol, camphene | Arthrobacter protophormiae, Escherichia coli, Micrococcus luteus, Rhodococcus rhodochrous, Staphylococcus aureus | [233] |
| Euphorbia helioscopia L. | Euphoheliosnoid E | Streptococcus mutans, Actinomyces viscosus | [279] |
| Euphorbia wallichii Hook. f. | Acorenone B, cycloisosativene, β-cedrene, copaene, 3β-hydroxy-5α-androstane, palmitic acid | Staphylococcus aureus | [279] |
| Foeniculum vulgare Mill. | Dillapional | Bacillus subtilis | [280] |
| Fritillaria roylei Hook. | Peonidin | Escherichia coli, Klebsiella pneumoniae, Micrococcus luteus, Staphylococcus pneumonia, Haemophilus influenza, Neisseria mucosa | [281] |
| Fumaria indica (Hausskn.) Pugsley | n-octacosan-7β-ol | Leishmania donovani promastigotes, Staphylococcus epidermidis, Escherichia coli, Candida albicans, Aspergillus niger | [282] |
| Galium aparine L. | Chlorogenic acid, p-coumaric acid, ferulic acid, luteolin, rutin | Staphylococcus aureus, Listeria monocytogenes | [283] |
| Gentiana kurroo Royle | Flavonoids and phenols | Proteus mirabilis, Streptococcus faecalis, Escherichia coli, Salmonella enteritidis, Micrococcus luteus, Enterobacter cloacae | [284] |
| Geranium wallichianum D.Don ex Sweet | Leaf extracts conjugated with zinc oxide nanoparticles | Bacillus subtilis, Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae | [285] |
| Girardinia diversifolia (Link) Friis | β-sitosterol, 3-hydroxystigmast-5-en-7-one, 7-hydroxysitosterol | Bacillus pumilus, Escherichia coli, Staphylococcus aureus | [286] |
| Gaultheria fragrantissima Wall. | Methyl salicylate | Staphylococcus aureus | [259] |
| Hedychium spicatum G.Lodd. | Hedychenone, spicatanol, 6-endo-hydroxycineole | Shigella boydii, Shigella sonnei, Shigella flexneri, Bacillus cereus, Vibrio cholera, Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, Klebsiella pneumoniae | [287,288] |
| Holarrhena antidysenterica Wall. | Conessine | Acinetobacter baumannii, Pseudomonas aeruginosa | [120] |
| Hyoscyamus niger L. | Non-alkaloidal seed extract | Bacillus subtilis, Escherichia coli, Staphylococcus aureus | [289] |
| Hypericum perforatum L. | Hypericin | Methicillin-resistant Staphylococcus aureus, methicillin-sensitive Staphylococcus aureus, Escherichia coli | [290] |
| Inula cappa (Buch.-Ham. ex D.Don) DC. | β-caryophyllene, cis-dihydro-mayurone, β-bisabolene, (E)-β-farnesene | Enterococcus faecalis, Klebsiella pneumoniae, Xanthomonas phaseoli and Bacillus subtilis | [291] |
| Inula racemose Hook.f. | Isoalantolactone | Bacillus subtilis, Escherichia coli, Pseudomonas fluorescens, Staphylococcus lentus, Staphylococcus aureus | [292] |
| Iris ensata Thunb. | Methanolic extracts | Bacillus cereus, Pseudomonas aeruginosa, Proteus vulgaris, Escherichia coli | [293] |
| Iris kashmiriana Baker | Irigenin, iridin, junipeginin-c, | Bacillus subtilis, Staphylococcus epidermidis, Proteus vulgaris, Pseudomonas aeruginosa, Staphylococcus aureus, Staphylococcus typhimurium, Escherichia coli, Shigella dysenteriae, Klebsiella pneumoniae | [293] |
| resveratrol, piecid, resveratroloside, | |||
| isorhamnetin-3-oneohesperidoside | |||
| Iris nepalensis D.Don | Methanolic extract | Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa | [294] |
| Jasminum officinale L. | Ethanolic extract | Methicillin-resistant Staphylococcus aureus | [295] |
| Juglans regia L. | α-pinene, β-pinene, β-caryophyllene, germacrene d, limonene, eugenol, methyl salicylate, germacrene d, (e)-β-farnesene | Bacillus subtilis, Staphylococcus epidermidis, Proteus vulgaris, Pseudomonas aeruginosa, Staphylococcus aureus, Salmonella typhi, Escherichia coli, Shigella dysenteriae, Klebsiella pneumoniae | [296] |
| Juniperus macropoda Boiss. | Sabinene, terpinen-4-ol, cedrol, β-elemene, trans-sabinene hydrate, α-cubebene, α-thujone, biformene | Candida albicans, Colletotrichum fragariae, Colletotrichum gloeosporioides | [297] |
| Lagenaria siceraria (Molina) Standl. | β-carotene, 22-deoxocurcubitacin-d, 22-deoxoisocurcubitacin D, avenasterol, codisterol, elesterol, isofucasterol, stigmasterol, sitosterol, compesterol, spinasterol, 7-0-glucosyl-6-c-glucoside apigenin, 6-c-glucoside apigenin, 6-cglucoside luteolin, 7,4′-o-diglucosyl- 6-c-glucoside, apigenin | Staphylococcus aureus, Pseudomonas sp., Escherichia coli, Bacillus subtilis, Candida sp., Aspergillus niger | [298] |
| Lantana camara (Hayek) R.W.Sanders | Germacrene B, β-caryophyllen, 3,7,11-trimethyl-1,6,10-dodecatriene, β-caryophyllene, zingiberene, γ-curcumene, davanone, (E)-nerolidol | Arthrobacter protophormiae, Micrococcus luteus, Rhodococcus rhodochrous, Staphylococcus aureus | [233] |
| Lavandula stoechas L. | 1,8-cineole, | Methicillin-resistant Staphylococcus aureus, Klebsiella pneumoniae, Salmonella typhimurium | [299] |
| fenchone, | |||
| camphor | |||
| Lindera neesiana (Wall. ex Nees) Kurz | Geranial, neral, citronellal, 1,8-cineole, α-pinene, β-pinene, methyl chavicol, safrole | Staphylococcus aureus, Candida albicans | [300] |
| Lindera pulcherrima (Nees) Benth. ex Hook.f. | Curzerenone, furanodienone | Staphylococcus aureus, Salmonella enterica | [275] |
| Mallotus philippensis (Lam.) Müll.Arg. | Bergenin, mallotophilippinens, rottlerin, and isorottlerin | Bacillus cereus var mycoides, Bacillus pumilus, Bacillus subtilis, Bordetella bronchiseptica, Micrococcus luteus, Staphylococcus aureus, Staphylococcus epidermidis, Escherichia coli, Klebsiella pneumoniae, Candida albicans, Saccharomyces cerevisiae | [301,302] |
| Malva neglecta Wallr. | Hydrotyrosol, coumaroylhexoside, kaempferol-3-(p-coumaroyldiglucoside)-7-glucoside, quercetin-3-o-rutinoside, epicatechin-3-o-(4-o-methyl)-gallate, oleic acid, taurine, ethylene dimercaptan, isoeugenol, patchoulane, methyl 12-methyltetradecanoate, isopropyl myristate | Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae, Salmonella typhi, Bacillus subtilis, Aspergillus fumigatus, Aspergillus flavus, Aspergillus niger, Fusarium solani | [303] |
| Marrubium vulgare L. | Methanolic extract | Escherichia coli, Bacillus subtilis, Staphylococcus aureus, Staphylococcus epidermidis, Pseudomonas aeruginosa, Proteus vulgaris, Candida albicans | [304] |
| Melia azedarach L. | Crude extract | Bacillus subtilis, Proteus mirabilis, Shigella flexneri, Proteus mirabilis, Shigella flexneri, Staphylococcus aureus, Bacillus subtilis, Pseudomonas aeruginosa, Shigella flexneri | [305] |
| Morina longifolia Wall. ex DC. | Germacrene d, α-pinene, bicyclogermacrene, α-cadinol, (e)-citronellyl tiglate, β-phellandrene | Escherichia coli, Staphylococcus aureus, Proteus vulgaris, Klebsiella pneumoniae, Bacillus subtilis, Pseudomonas aeruginosa, Alternaria alternata, Aspergillus flavus, Aspergillus fumigatus, Fusarium solani | [306,307] |
| Nepeta cataria L. | Nepetalactone, | Neisseria subflava, Citrobacter freundii, Branhamella ovis, Aeromonas caviae, Escherichia coli, Serratia marcescens, Enterococcus species, Staphylococcus aureus | [308,309] |
| β-caryophyllene, | |||
| thymol | |||
| Nardostachys jatamansi (D.Don) DC. | β-gurjunene, valerena-4,7(11)-diene (7.1%), nardol a, 1(10)-aristolen-9β-ol, jatamansone | Bacillus cereus, Escherichia coli, Candida albicans | [310] |
| Oxalis corniculate L. | Methanolic extract | Staphylococcus aureus, Escherichia coli, Shigella dysenteriae, Shigella flexneri, Shigella boydii, Shigella sonnei | [311] |
| Paeonia emodi Royle | Leaf extract nanoparticles | Staphylococcus aureus, Bacillus subtilis, Escherichia coli, Salmonella typhi, Pseudomonas aeruginosa, Klebsiella pneumoniae | [312] |
| Phoebe lanceolata (Nees) Nees | 1,8-cineole, β-caryophyllene | Escherichia coli | [275] |
| Persicaria hydropiper (L.) Delarbre | Confertifolin, polygodial | Enterococcus faecalis, Bacillus subtilis, Staphylococcus aureus, Escherichia coli, Salmonella enterica, Epidermophyton floccosum, Curvularia lunata, Scopulariopsis sp., Candida albicans, Candida utilis, Candida krusei, Cryptococcus neoformans, Saccharomyces cerevisiae, Epidermophyton floccosum, Trichophyton mentagrophytes, Penicillium marneffei | [313] |
| Plantago lanceolata L. | Luteolin 7-glucoside, hispidulin 7-glucuronide, luteolin 7-diglucoside, apigenin 7-glucoside, nepetin 7-glucoside and luteolin 6-hydroxy 4′-methoxy 7-galactoside, oleanolic acid, sitosterol acid, 18β-glycyrrhetinic, plantamajoside, verbacoside, 10-hydroxymajoroside, 10-acetoxymajoroside | Bacillus subtilis, Staphylococcus aureus, Candida albicans, Candida tropicalis, Escherichia coli, Streptococcus pneumoniae | [314] |
| Podophyllum hexandrum Royle | Phthalic acid, | Bacillus megaterium, Pseudomonas aeruginosa, Aspergillus flavus, Fusarium solani, Staphylococcus aureus, Salmonella typhi, Klebsiella pneumoniae, Enterococcus faecalis | [315,316] |
| di-isobutyl ester, | |||
| 1,2-benzenedicarboxylic acid, | |||
| diisooctyl ester, | |||
| polyneuridine, | |||
| podophyllotoxin, | |||
| β-sitosterol | |||
| Punica granatum L. | Punicalagin | Pseudomonas aeruginosa, Salmonella enteritidis, Escherichia coli, Staphylococcus epidermidis, Staphylococcus xylosus, Staphylococcus aureus, Bacillus cereus, Enterococcus faecium, Enterococcus faecalis | [317] |
| Prunus domestica L. | Quercetin-3-o-galactoside | Campylobacter jejuni, Salmonella typhimurium, Escherichia coli, Staphylococcus aureus, Listeria monocytogenes | [318] |
| Rheum emodi Wall. | Emodin, rhein, chrysophanol dimethyl ether, resveratrol, revandchinone-4 | Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae, Bacillus subtilis, Pseudomonas aeruginosa, Klebsiella aerogenes, Bacillus sphaericus, Chromobacterium violaceum | [319] |
| Rhododendron anthopogon D.Don | α-pinene, β-pinene, limonene, δ-cadinene | Bacillus subtilis, Mycobacterium tuberculosis, Candida pseudotropicalis | [320] |
| Rumex dentatus L. | Musizin, torachrysone-glucoside, 2-methoxystypandrone | Escherichia coli, Klebsiella pneumoniae, Salmonella typhi, Pseudomonas aeruginosa, Bacillus subtilis, Streptococcus pneumoniae, Listeria monocytogenes, Staphylococcus epidermidis, Staphylococcus aureus, Bacillus cereus | [321] |
| Salix alba L. | Anthocyanins, p-hydroxybenzoic, gallic acid, gentisic acid, sisymbrifolin, catechol | Pseudomonas aeruginosa, Escherichia coli, Salmonella enterica, Staphylococcus aureus | [322] |
| Salvia sclarea L. | Essential oil | Escherichia coli, Staphylococcus aureus, Methicillin-resistant Staphylococcus epidermidis | [323] |
| Sambucus wightiana Wall. ex Wight & Arn. | Gold nanoparticles of whole-plant extract | Escherichia coli, Staphylococcus epidermidis, Salmonella enteritidis | [324] |
| Saussurea lappa (Decne.) Sch.Bip. | Sesquiterpene lactones, zinc oxide nanoparticles of rhizome methanolic extract | Staphylococcus aureus, Sphingobacterium thalpophilum, Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Sphingobacterium sp., Acinetobacter sp., Ochrobactrum sp. | [325] |
| Skimmia laureola (DC.) Decne. | Linalyl acetate, linalool, limonene, α-terpineol, geranyl acetate | Staphylococcus aureus, Staphylococcus epidermidis, Aspergillus niger, Penicillium chrysogenum | [326] |
| Solanum tuberosum L. | Potide-g, afp-j, potamin-1 or pg-2 | Staphylococcus aureus, Listeria monocytogenes, Escherichia coli, Candida albicans | [327] |
| Sonchus arvensis L. | Phenols and flavonoids | Escherichia coli, Salmonella enterica, Vibrio parahaemolyticus, Staphylococcus aureus | [328] |
| Stephania glabra (Roxb.) Miers | Glabradine | Staphylococcus aureus, Streptococcus. mutans, Microsporum gypseum, Microsporum canis, Trichophyton rubrum | [329] |
| Taraxacum officinale F.H.Wigg. | 9-hydroxyoctadecatrienoic acid, 9-hydroxyoctadecadienoic acid, vanillin, coniferaldehyde, p-methoxyphenylglyoxylic acid | Staphylococcus aureus, Methicillin-resistant Staphylococcus aureus, Bacillus cereus | [330] |
| Terminalia arjuna (Roxb. ex DC.) Wight & Arn. | Silver nanoparticles of bark extract | Escherichia coli | [331] |
| Terminalia chebula Retz. | 1,2,6-tri-o-galloyl-β-d-glucopyranose | Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumoniae, Staphylococcus aureus, methicillin-resistant Staphylococcus aureus, Proteus mirabilis, Acinetobacter baylyi, Bacillus megaterium | [332,333,334] |
| Valeriana jatamansi (D.Don) Wall. | Maaliol, 3-methylvaleric acid, β-gurjunene | Microsporum canis, Fusarium solani | [335] |
| Verbascum Thapsus L. | 1-hexzanol 2-hexene | Klebsiella pneumoniae, Staphylococcus aureus, Escherichia coli, Mycobacterium phlei, methicillin-resistant Staphylococcus aureus | [336] |
| Viola odorata L. | 3-(2′,4′,6′,6′-tetramethylcyclohexa-1′,4′-dienyl) acrylic acid | Haemophilus influenzae, Pseudomonas aeruginosa, Staphylococcus aureus, Streptococcus pneumoniae | [337] |
| Viscum album L. | Hydroxycinnamic acids | Xanthomonas campestris, Clavibacter michiganensis, Alternaria alternate, Fusarium oxysporum | [338] |
| Vitex negundo L. | Methanolic extract | Vibrio cholerae, Vibrio parahaemolyticus, Vibrio mimicus, Escherichia coli, Shigella sp., Aeromonas sp. | [339] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashraf, M.V.; Pant, S.; Khan, M.A.H.; Shah, A.A.; Siddiqui, S.; Jeridi, M.; Alhamdi, H.W.S.; Ahmad, S. Phytochemicals as Antimicrobials: Prospecting Himalayan Medicinal Plants as Source of Alternate Medicine to Combat Antimicrobial Resistance. Pharmaceuticals 2023, 16, 881. https://doi.org/10.3390/ph16060881
Ashraf MV, Pant S, Khan MAH, Shah AA, Siddiqui S, Jeridi M, Alhamdi HWS, Ahmad S. Phytochemicals as Antimicrobials: Prospecting Himalayan Medicinal Plants as Source of Alternate Medicine to Combat Antimicrobial Resistance. Pharmaceuticals. 2023; 16(6):881. https://doi.org/10.3390/ph16060881
Chicago/Turabian StyleAshraf, Mohammad Vikas, Shreekar Pant, M. A. Hannan Khan, Ali Asghar Shah, Sazada Siddiqui, Mouna Jeridi, Heba Waheeb Saeed Alhamdi, and Shoeb Ahmad. 2023. "Phytochemicals as Antimicrobials: Prospecting Himalayan Medicinal Plants as Source of Alternate Medicine to Combat Antimicrobial Resistance" Pharmaceuticals 16, no. 6: 881. https://doi.org/10.3390/ph16060881
APA StyleAshraf, M. V., Pant, S., Khan, M. A. H., Shah, A. A., Siddiqui, S., Jeridi, M., Alhamdi, H. W. S., & Ahmad, S. (2023). Phytochemicals as Antimicrobials: Prospecting Himalayan Medicinal Plants as Source of Alternate Medicine to Combat Antimicrobial Resistance. Pharmaceuticals, 16(6), 881. https://doi.org/10.3390/ph16060881





