Riluzole, a Derivative of Benzothiazole as a Potential Anti-Amoebic Agent against Entamoeba histolytica
Abstract
1. Introduction
2. Results
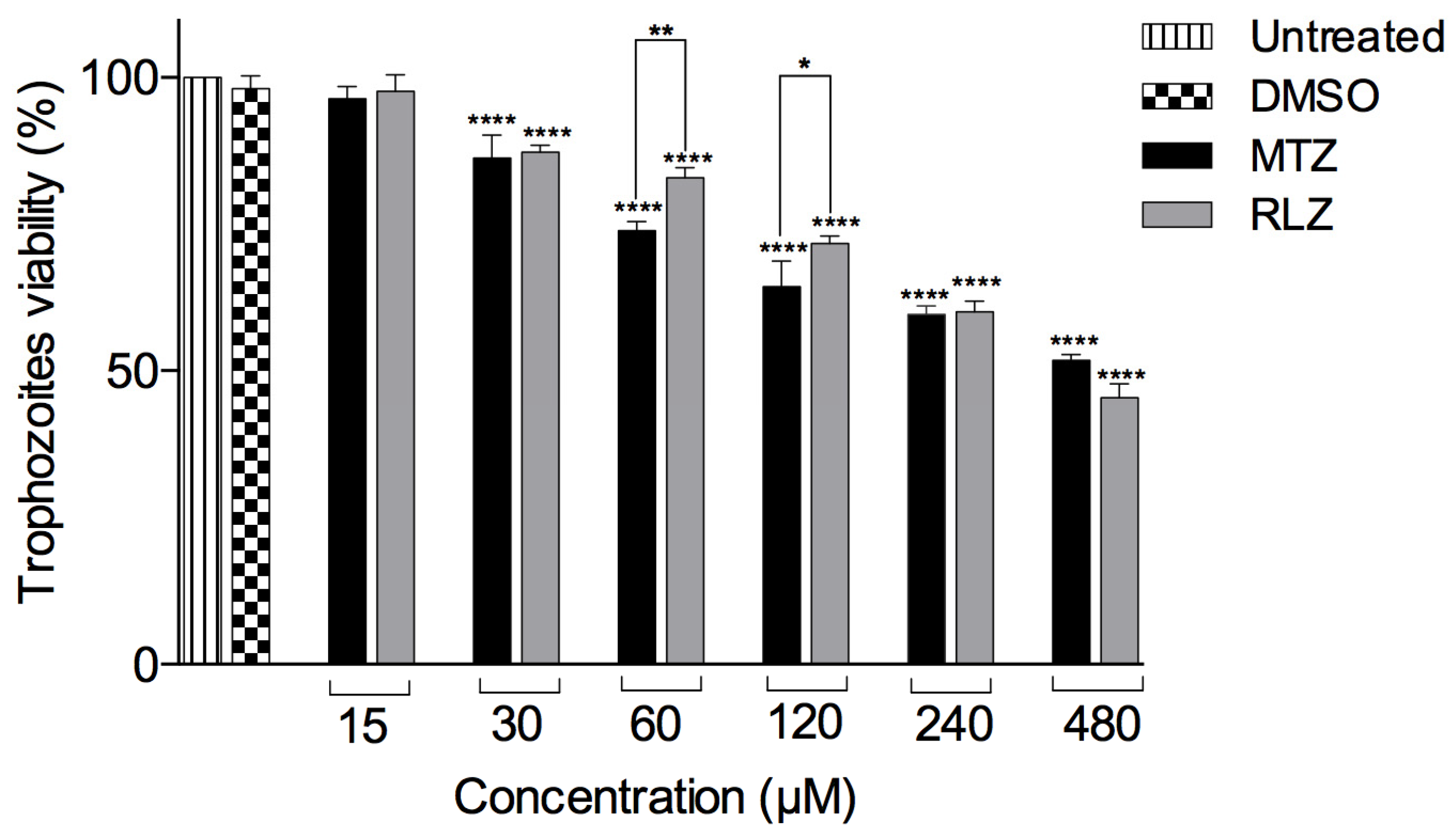
2.1. Riluzole Decreases the Viability of E. histolytica Trophozoites
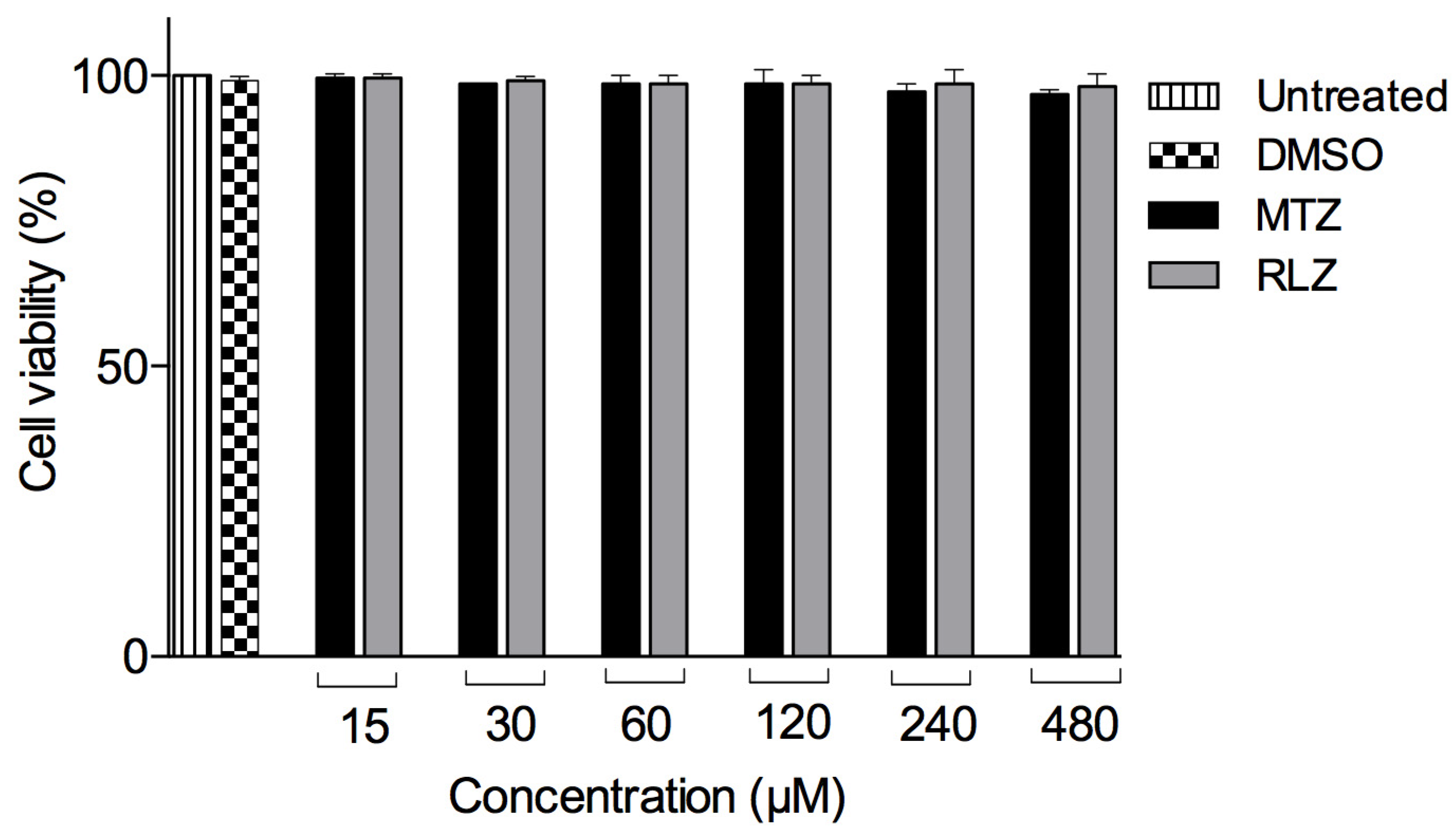
2.2. Riluzole Treatment Does Not Affect the VERO Cell Line
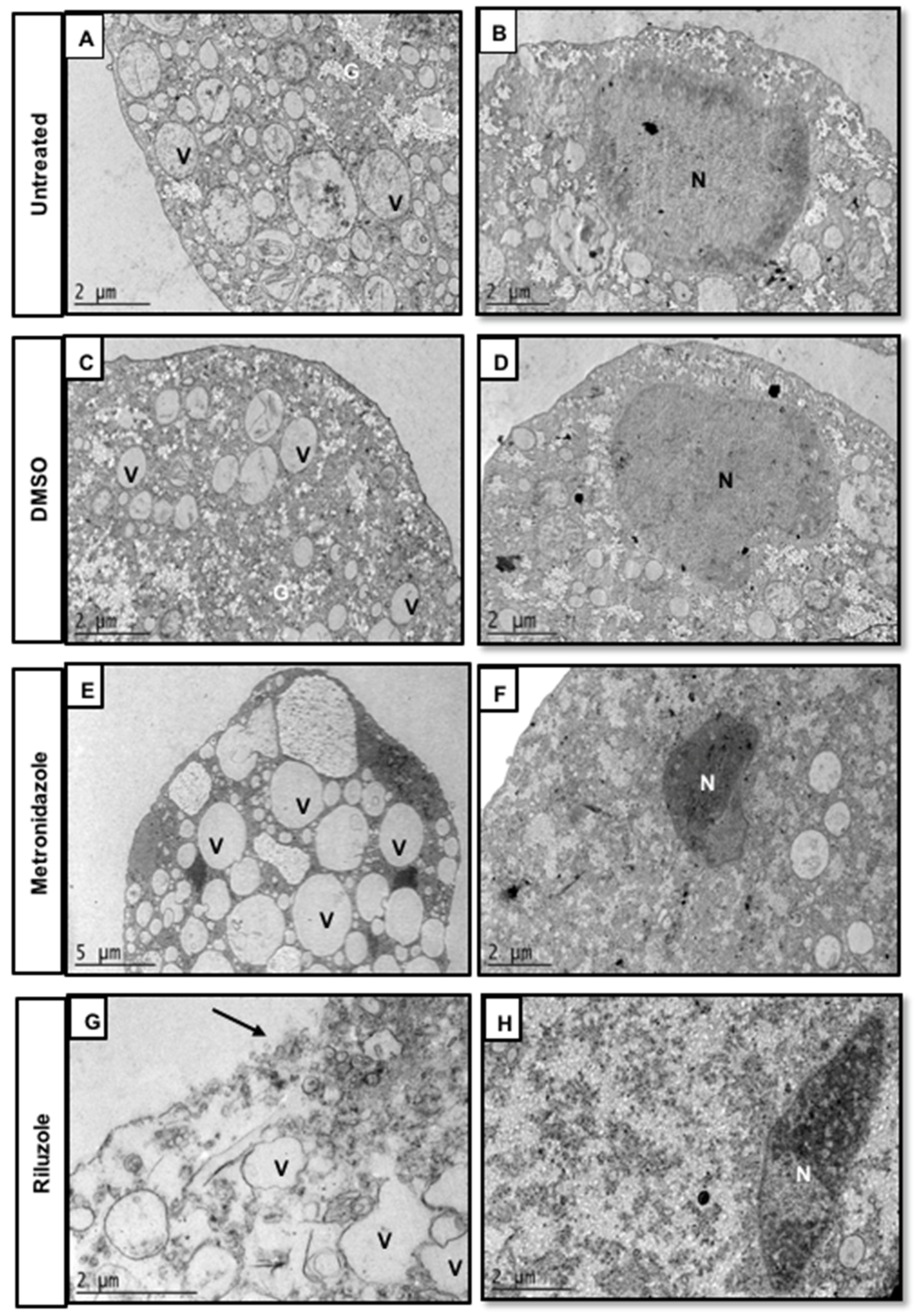
2.3. Riluzole Treatment Induces Ultrastructural Changes in E. histolytica Trophozoites
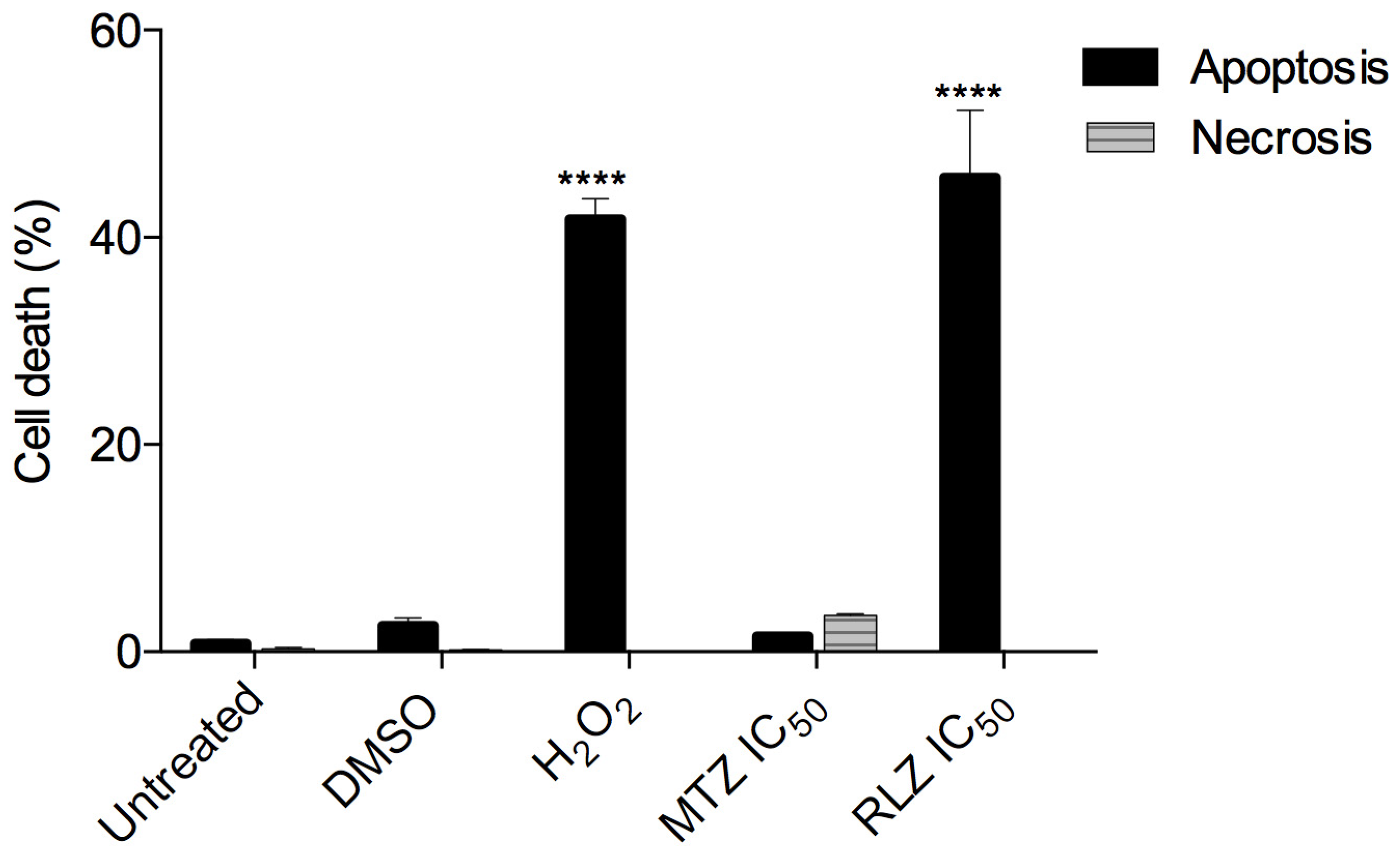
2.4. Riluzole Treatment Induces Apoptosis in E. histolytica Trophozoites
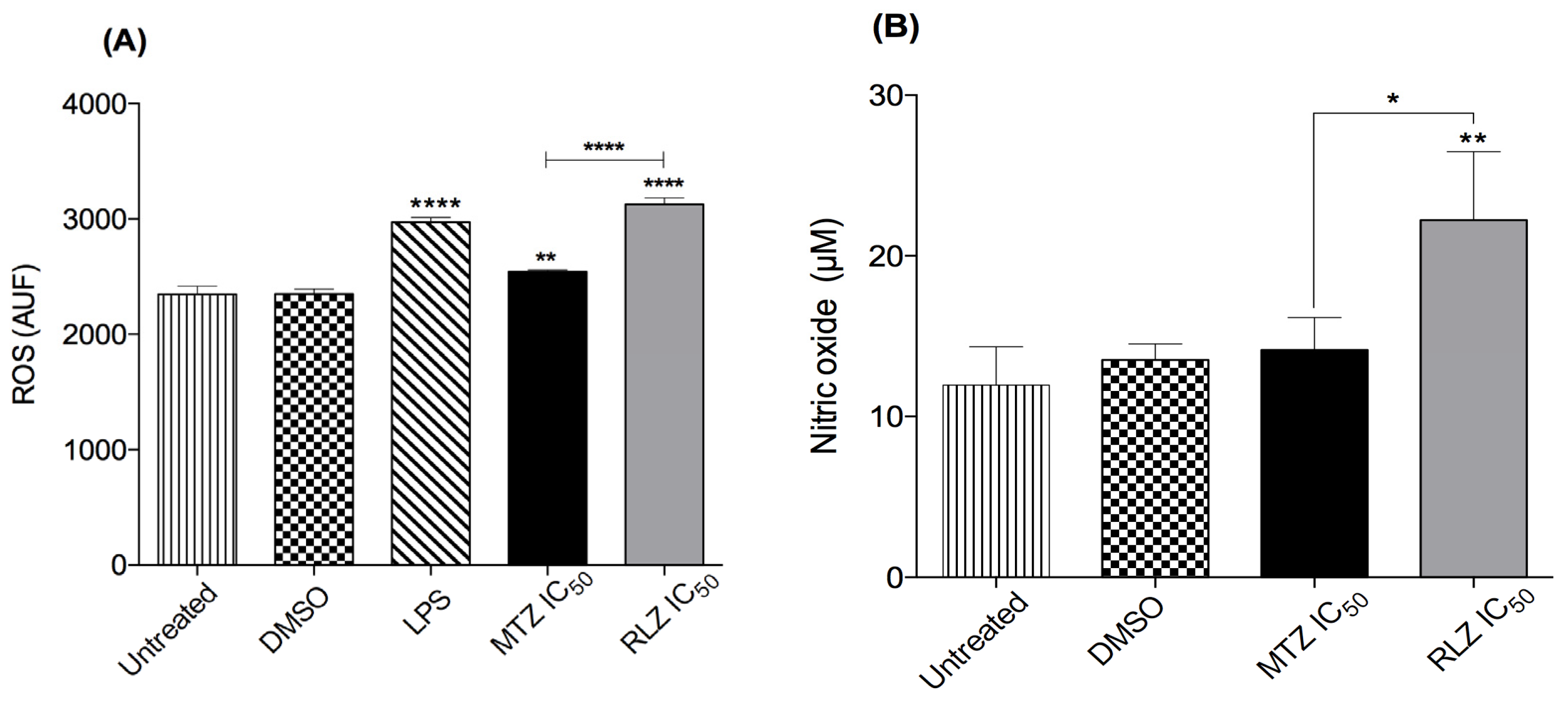
2.5. Riluzole Produces ROS and ON in E. histolytica Trophozoites
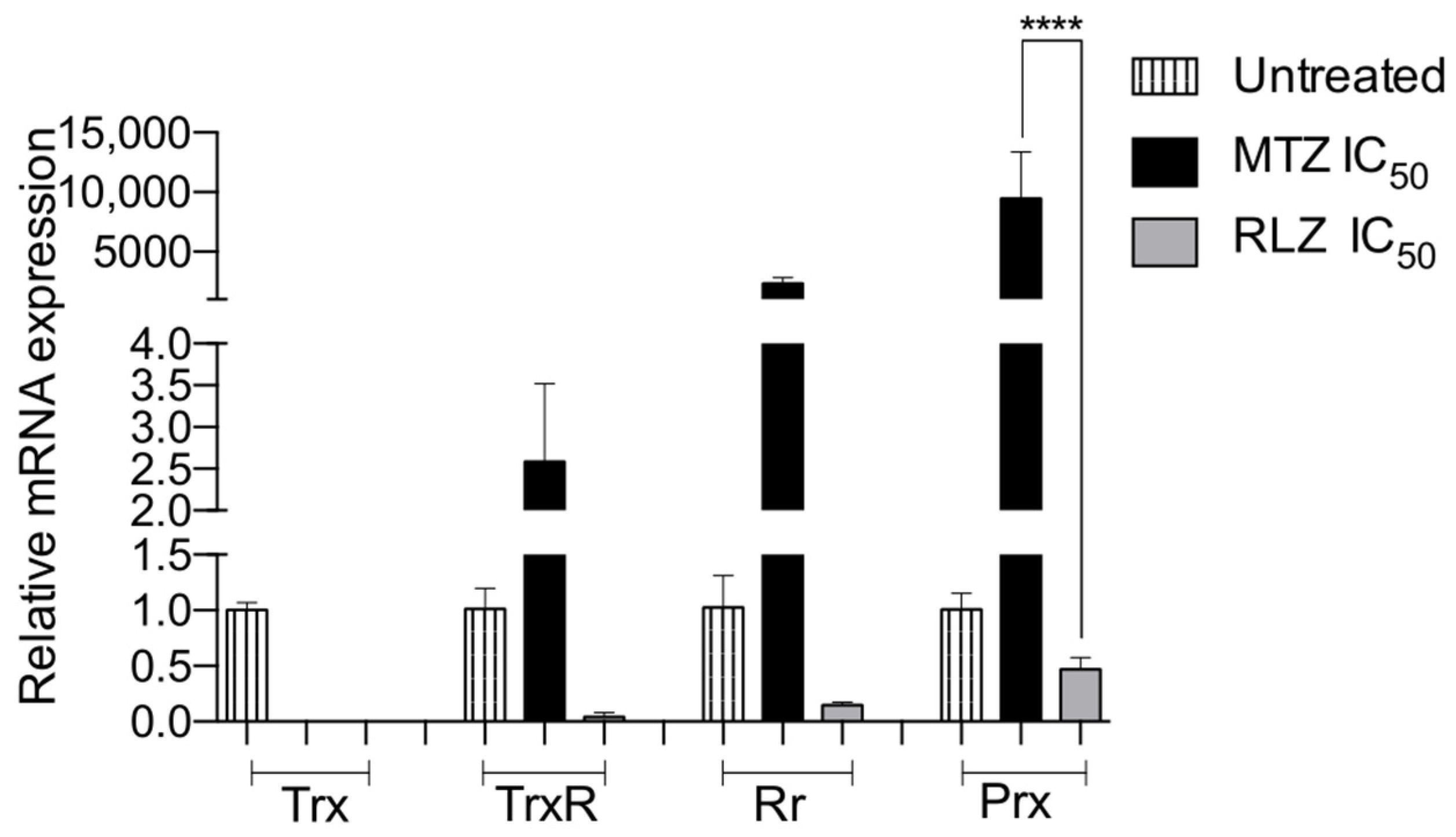
2.6. Riluzole Decreased the Relative Gene Expression of Antioxidant Enzymes
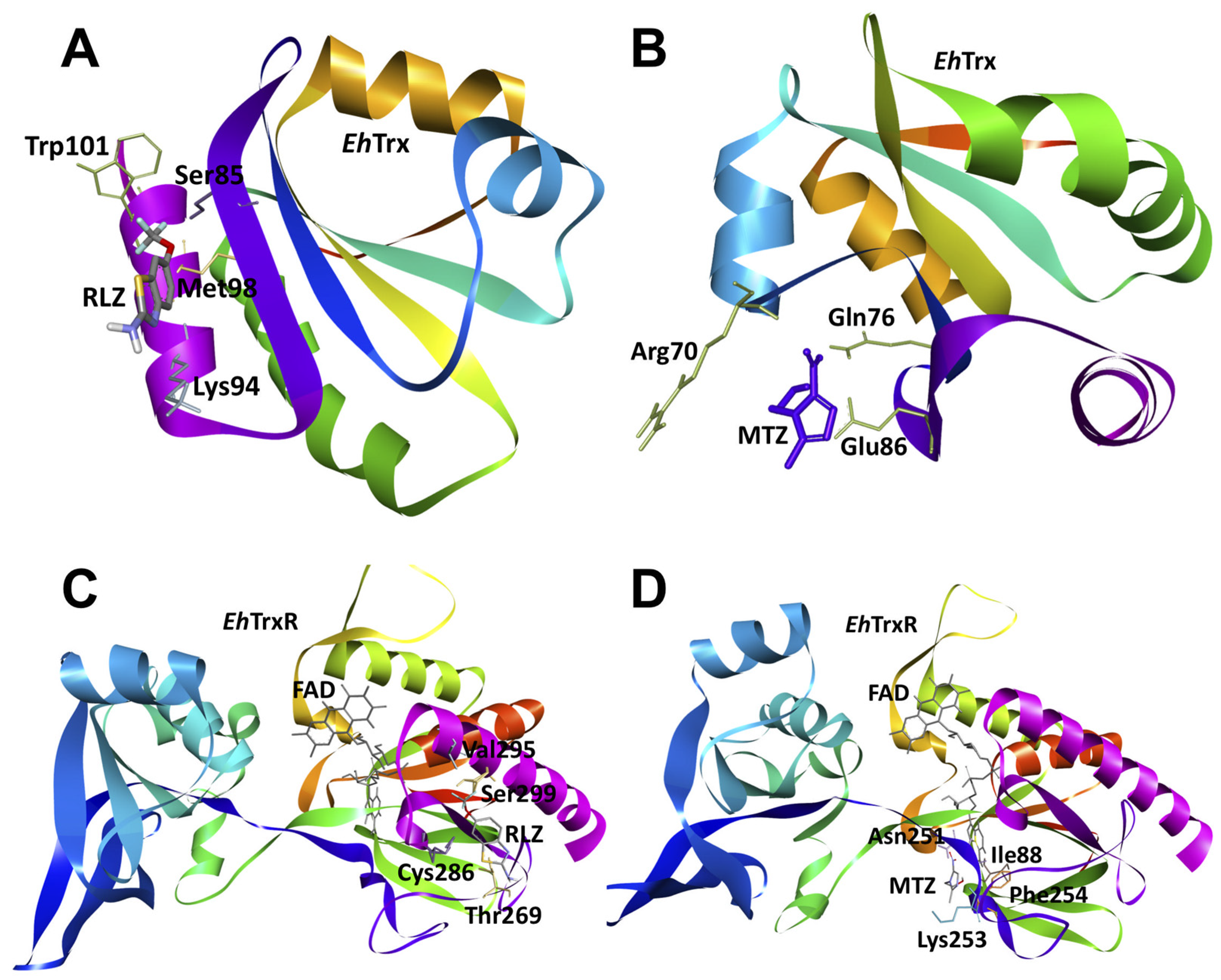
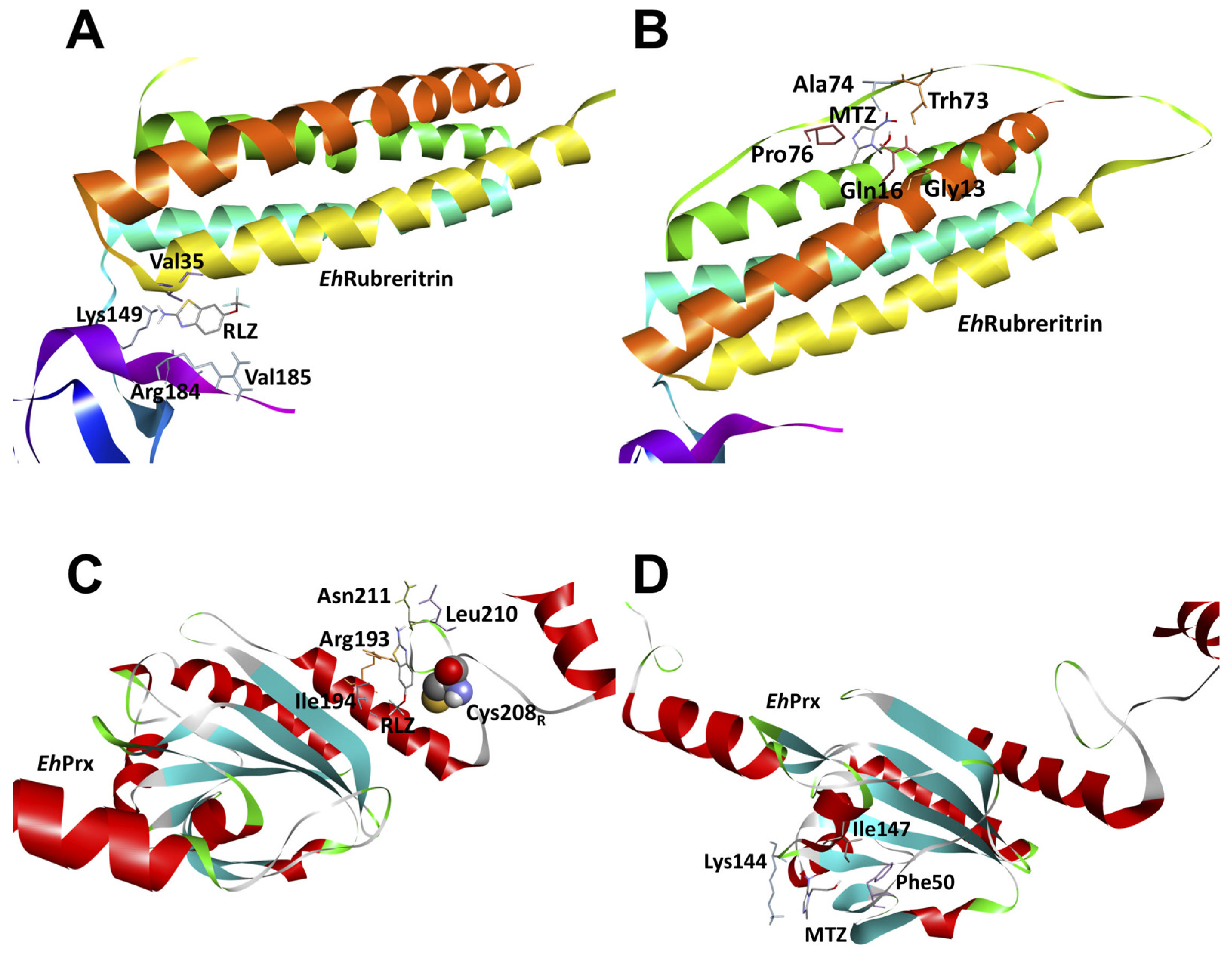
2.7. In Silico Molecular Docking Show That Riluzole Has a High Affinity for the Antioxidant Enzymes of E. histolytica
3. Discussion
4. Materials and Methods
4.1. E. histolytica Trophozoite Cultures
VERO Cell Culture
4.2. Evaluation of the Anti-Amoebic Activity of Riluzole and Its IC50 Value
4.3. Evaluation of the Cytotoxic Effect of Riluzole on VERO Cells
4.4. Evaluation of E. histolytica Trophozoites Ultrastructure
4.5. Determination of the Cell Death Type of E. histolytica Trophozoites
4.6. Determination of Reactive Oxygen Species (ROS)
4.7. Determination of Nitric Oxide (NO)
4.8. Relative Gene Expression of E. histolytica Antioxidant Enzymes
4.9. Molecular Docking Simulation
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pacheco-Yépez, J.; Martínez-Castillo, M.; Cruz-Baquero, A.; Serrano-Luna, J.; Shibayama, M. Role of Cytokines and Reactive Oxygen Species in the Amebic Liver Abscess Produced by Entamoeba histolytica. In Liver Pathophysiology: Therapies and Antioxidants; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Espinosa-Cantellano, M.; Martínez-Palomo, A. Pathogenesis of Intestinal Amebiasis: From Molecules to Disease. Clin. Microbiol. Rev. 2000, 13, 318–331. [Google Scholar] [CrossRef]
- Ansari, M.F.; Siddiqui, S.M.; Agarwal, S.M.; Vikramdeo, K.S.; Mondal, N.; Azam, A. Metronidazole Hydrazone Conjugates: Design, Synthesis, Antiamoebic and Molecular Docking Studies. Bioorg. Med. Chem. Lett. 2015, 25, 3545–3549. [Google Scholar] [CrossRef]
- Haque, R.; Huston, C.D.; Hughes, M.; Houpt, E.; Petri, W.A., Jr. Amebiasis. N. Engl. J. Med. 2003, 348, 1565–1573. [Google Scholar] [CrossRef] [PubMed]
- Bendesky, A.; Menéndez, D.; Ostrosky-Wegman, P. Is Metronidazole Carcinogenic? Mutat. Res. Rev. Mutat. Res. 2002, 511, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Leitsch, D.; Kolarich, D.; Wilson, I.B.H.; Altmann, F.; Duchêne, M. Nitroimidazole Action in Entamoeba histolytica: A Central Role for Thioredoxin Reductase. PLoS Biol. 2007, 5, e211. [Google Scholar] [CrossRef]
- Boechat, N.; Carvalho, A.S.; Salomão, K.; de Castro, S.L.; Araujo-Lima, C.F.; Mello, F.V.; Felzenszwalb, I.; Aiub, C.A.; Conde, T.R.; Zamith, H.P.; et al. Studies of Genotoxicity and Mutagenicity of Nitroimidazoles: Demystifying This Critical Relationship with the Nitro Group. Mem. Inst. Oswaldo Cruz 2015, 110, 492–499. [Google Scholar] [CrossRef]
- Agarwal, A.; Kanekar, S.; Sabat, S.; Thamburaj, K. Metronidazole-Induced Cerebellar Toxicity. Neurol. Int. 2016, 8, 6365. [Google Scholar] [CrossRef] [PubMed]
- Marie, C.; Petri, W.A. Amoebic Dysentery. BMJ Clin. Evid. 2013, 2013, 918. [Google Scholar]
- Wassmann, C.; Hellberg, A.; Tannich, E.; Bruchhaus, I. Metronidazole Resistance in the Protozoan Parasite Entamoeba histolytica Is Associated with Increased Expression of Iron-Containing Superoxide Dismutase and Peroxiredoxin and Decreased Expression of Ferredoxin 1 and Flavin Reductase. J. Biol. Chem. 1999, 274, 26051–26056. [Google Scholar] [CrossRef] [PubMed]
- Hanna, R.M.; Dahniya, M.H.; Badr, S.S.; El-Betagy, A. Percutaneous Catheter Drainage in Drug-Resistant Amoebic Liver Abscess. Trop. Med. Int. Health 2000, 5, 578–581. [Google Scholar] [CrossRef]
- Keri, R.S.; Patil, M.R.; Patil, S.A.; Budagupi, S. A Comprehensive Review in Current Developments of Benzothiazole-Based Molecules in Medicinal Chemistry. Eur. J. Med. Chem. 2015, 89, 207–251. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.; Syed, M.A.H.; Mohammed, S.M. Therapeutic Potential of Benzothiazoles: A Patent Review (2010–2014). Expert Opin. Ther. Pat. 2015, 25, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Blyufer, A.; Lhamo, S.; Tam, C.; Tariq, I.; Thavornwatanayong, T.; Mahajan, S.S. Riluzole: A Neuroprotective Drug with Potential as a Novel Anti-Cancer Agent. Int. J. Oncol. 2021, 59, 1–11. [Google Scholar] [CrossRef]
- Haroun, M.; Tratrat, C.; Kositzi, K.; Tsolaki, E.; Petrou, A.; Aldhubiab, B.; Attimarad, M.; Harsha, S.; Geronikaki, A.; Venugopala, K.N.; et al. New Benzothiazole-Based Thiazolidinones as Potent Antimicrobial Agents. Design, Synthesis and Biological Evaluation. Curr. Top. Med. Chem. 2018, 18, 75–87. [Google Scholar] [CrossRef]
- Bhutani, R.; Pathak, D.P.; Kapoor, G.; Husain, A.; Iqbal, M.A. Novel Hybrids of Benzothiazole-1,3,4-Oxadiazole-4-Thiazolidinone: Synthesis, in silico ADME Study, Molecular Docking and in vivo Anti-Diabetic Assessment. Bioorg. Chem. 2019, 83, 6–19. [Google Scholar] [CrossRef]
- Khokra, S.L.; Arora, K.; Khan, S.A.; Kaushik, P.; Saini, R.; Husain, A. Synthesis, Computational Studies and Anticonvulsant Activity of Novel Benzothiazole Coupled Sulfonamide Derivatives. Iran. J. Pharm. Res. 2019, 18, 1. [Google Scholar]
- Asif, M.; Imran, M. A Mini-Review on Pharmacological Importance of Benzothiazole Scaffold. Mini Rev. Org. Chem. 2020, 18, 1086–1097. [Google Scholar] [CrossRef]
- Asiri, Y.I.; Alsayari, A.; Muhsinah, A.B.; Mabkhot, Y.N.; Hassan, M.Z. Benzothiazoles as Potential Antiviral Agents. J. Pharm. Pharmacol. 2020, 72, 1459–1480. [Google Scholar] [CrossRef]
- Patel, R.V.; Patel, P.K.; Kumari, P.; Rajani, D.P.; Chikhalia, K.H. Synthesis of Benzimidazolyl-1,3,4-Oxadiazol-2ylthio-N-Phenyl (Benzothiazolyl) Acetamides as Antibacterial, Antifungal and Antituberculosis Agents. Eur. J. Med. Chem. 2012, 53, 41–51. [Google Scholar] [CrossRef]
- Mahran, M.A.; El-Nassry, S.M.F.; Allam, S.R.; El-Zawawy, L.A. Synthesis of Some New Benzothiazole Derivatives as Potential Antimicrobial and Antiparasitic Agents. Pharmazie 2003, 58, 527–530. [Google Scholar] [CrossRef]
- Singh, G.; Chowdhary, K.; Satija, P.; Singh, A.; Singh, B.; Singh, K.; Espinosa, C.; Esteban, M.A.; Sehgal, R.; Verma, V. Synthesis and Immobilization of Benzothiazole-Appended Triazole-Silane: Biological Evaluation and Molecular Docking Approach. ChemistrySelect 2018, 3, 1609–1614. [Google Scholar] [CrossRef]
- Ozpinar, N.; Culha, G.; Kaya, T.; Yucel, H. The Amoebicidal Activity of Three Substances Derived from Benzothiazole on Acanthamoeba castellanii Cysts and Trophozoites and Its Cytotoxic Potentials. Acta Trop. 2021, 220, 105981. [Google Scholar] [CrossRef] [PubMed]
- Guerrieri, D.; Ferrari, S.; Costi, M.P.; Michels, P.A.M. Biochemical Effects of Riluzole on Leishmania Parasites. Exp. Parasitol. 2013, 133, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.; Morandi, F.; Motiejunas, D.; Nerini, E.; Henrich, S.; Luciani, R.; Venturelli, A.; Lazzari, S.; Calò, S.; Gupta, S.; et al. Virtual Screening Identification of Nonfolate Compounds, including a CNS Drug, as Antiparasitic Agents Inhibiting Pteridine Reductase. J. Med. Chem. 2011, 54, 211–221. [Google Scholar] [CrossRef]
- Suresh, C.H.; Rao, J.V.; Jayaveera, K.; Subudhi, S. Synthesis and Anthelminitc Activity of 3 (2-Hydrazino Benzothiazoles)-Substituted Indole-2-One. IJPR 2011, 2, 257–261. [Google Scholar]
- Thakkar, S.S.; Thakor, P.; Ray, A.; Doshi, H.; Thakkar, V.R. Benzothiazole Analogues: Synthesis, Characterization, MO Calculations with PM6 and DFT, in silico Studies and in vitro Antimalarial as DHFR Inhibitors and Antimicrobial Activities. Bioorg. Med. Chem. 2017, 25, 5396–5406. [Google Scholar] [CrossRef]
- Cuevas-Hernández, R.I.; Girard, R.M.B.M.; Martínez-Cerón, S.; da Silva, M.S.; Elias, M.C.; Crispim, M.; Trujillo-Ferrara, J.G.; Silber, A.M. A Fluorinated Phenylbenzothiazole Arrests the Trypanosoma cruzi Cell Cycle and Diminishes the Infection of Mammalian Host Cells. Antimicrob. Agents Chemother. 2020, 64, e01742-19. [Google Scholar] [CrossRef]
- Cuevas-Hernández, R.I.; Correa-Basurto, J.; Flores-Sandoval, C.A.; Padilla-Martínez, I.I.; Nogueda-Torres, B.; Villa-Tanaca, M.D.L.; Tamay-Cach, F.; Nolasco-Fidencio, J.J.; Trujillo-Ferrara, J.G. Fluorine-Containing Benzothiazole as a Novel Trypanocidal Agent: Design, in silico Study, Synthesis and Activity Evaluation. Med. Chem. Res. 2016, 25, 211–224. [Google Scholar] [CrossRef]
- Martínez-Cerón, S.; Gutiérrez-Nágera, N.A.; Mirzaeicheshmeh, E.; Cuevas-Hernández, R.I.; Trujillo-Ferrara, J.G. Phenylbenzothiazole Derivatives: Effects against a Trypanosoma cruzi Infection and Toxicological Profiles. Parasitol. Res. 2021, 120, 2905–2918. [Google Scholar] [CrossRef]
- Doble, A. The Pharmacology and Mechanism of Action of Riluzole. Neurology 1996, 47, 233S–241S. [Google Scholar] [CrossRef]
- Vázquez-Jiménez, L.K.; Moreno-Herrera, A.; Juárez-Saldivar, A.; González-González, A.; Ortiz-Pérez, E.; Paz-González, A.D.; Palos, I.; Ramírez-Moreno, E.; Rivera, G. Recent Advances in the Development of Triose Phosphate Isomerase Inhibitors as Antiprotozoal Agents. Curr. Med. Chem. 2021, 29, 2504–2529. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Ghoshal, N. Exploration of a Binding Mode of Benzothiazol-2-Yl Acetonitrile Pyrimidine Core Based Derivatives as Potent c-Jun N-Terminal Kinase-3 Inhibitors and 3D-QSAR Analyses. J. Chem. Inf. Model. 2006, 46, 1763–1774. [Google Scholar] [CrossRef] [PubMed]
- Guédat, P.; Colland, F. Patented Small Molecule Inhibitors in the Ubiquitin Proteasome System. BMC Biochem. 2007, 8, 1–12. [Google Scholar] [CrossRef]
- Choi, S.J.; Park, H.J.; Lee, S.K.; Kim, S.W.; Han, G.; Choo, H.Y.P. Solid Phase Combinatorial Synthesis of Benzothiazoles and Evaluation of Topoisomerase II Inhibitory Activity. Bioorg. Med. Chem. 2006, 14, 1229–1235. [Google Scholar] [CrossRef]
- Pinar, A.; Yurdakul, P.; Yildiz, I.; Temiz-Arpaci, O.; Acan, N.L.; Aki-Sener, E.; Yalcin, I. Some Fused Heterocyclic Compounds as Eukaryotic Topoisomerase II Inhibitors. Biochem. Biophys. Res. Commun. 2004, 317, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Gillis, E.P.; Eastman, K.J.; Hill, M.D.; Donnelly, D.J.; Meanwell, N.A. Applications of Fluorine in Medicinal Chemistry. J. Med. Chem. 2015, 58, 5822–5880. [Google Scholar] [CrossRef]
- Tamaian, R.; Moţ, A.; Silaghi-Dumitrescu, R.; Ionuţ, I.; Stana, A.; Oniga, O.; Nastasə, C.; Benedec, D.; Tiperciuc, B.; McPhee, D.J. Study of the Relationships between the Structure, Lipophilicity and Biological Activity of Some Thiazolyl-Carbonyl-Thiosemicarbazides and Thiazolyl-Azoles. Molecules 2015, 20, 19841. [Google Scholar] [CrossRef]
- Purser, S.; Moore, P.R.; Swallow, S.; Gouverneur, V. Fluorine in Medicinal Chemistry. Chem. Soc. Rev. 2008, 37, 320–330. [Google Scholar] [CrossRef]
- Smart, B.E. Fluorine Substituent Effects (on Bioactivity). J. Fluor. Chem. 2001, 109, 3–11. [Google Scholar] [CrossRef]
- Ongarora, D.S.B.; Gut, J.; Rosenthal, P.J.; Masimirembwa, C.M.; Chibale, K. Benzoheterocyclic Amodiaquine Analogues with Potent Antiplasmodial Activity: Synthesis and Pharmacological Evaluation. Bioorg. Med. Chem. Lett. 2012, 22, R713–R715. [Google Scholar] [CrossRef]
- Racané, L.; Rep, V.; Kraljević Pavelić, S.; Grbčić, P.; Zonjić, I.; Radić Stojković, M.; Taylor, M.C.; Kelly, J.M.; Raić-Malić, S. Synthesis, Antiproliferative and Antitrypanosomal Activities, and DNA Binding of Novel 6-Amidino-2-Arylbenzothiazoles. J. Enzym. Inhib. Med. Chem. 2021, 36, 1952–1967. [Google Scholar] [CrossRef]
- Cuevas-Hernández, R.I.; Padilla-Martínez, I.I.; Martínez-Cerón, S.; Vásquez-Moctezuma, I.; Trujillo-Ferrara, J.G. Helical Arrangement of 2-(4-Hydroxy-3-Methoxyphenyl)-Benzothiazole in Crystal Formation and Biological Evaluation on HeLa Cells. Crystals 2017, 7, 171. [Google Scholar] [CrossRef]
- Pais-Morales, J.; Betanzos, A.; Garcia-Rivera, G.; Chavez-Munguia, B.; Shibayama, M.; Orozco, E. Resveratrol Induces Apoptosis-like Death and Prevents in vitro and in vivo Virulence of Entamoeba histolytica. PLoS ONE 2016, 11, 146287. [Google Scholar] [CrossRef]
- Jarillo Luna, R.A.; Gutiérrez Meza, J.M.; Barbosa Cabrera, E.; Luna Palencia, G.R.; Rosales Hernandez, M.C.; Nequiz, M.; Pacheco Yepez, J. Kaempferol Induces Prooxidant Effect and Ultrastructural Changes as Amoebicidal Mechanism. Rev. Méd. Univ. Autón. Sinaloa REVMEDUAS 2022, 12, 195–205. [Google Scholar]
- Villalba, J.D.A.; Gómez, C.; Medel, O.; Sánchez, V.; Carrero, J.C.; Shibayama, M.; Pérez Ishiwara, D.G. Programmed Cell Death in Entamoeba histolytica Induced by the Aminoglycoside G418. Microbiology 2007, 153, 3852–3863. [Google Scholar] [CrossRef] [PubMed]
- Nandi, N.; Sen, A.; Banerjee, R.; Kumar, S.; Kumar, V.; Ghosh, A.N.; Das, P. Hydrogen Peroxide Induces Apoptosis-like Death in Entamoeba histolytica Trophozoites. Microbiology 2010, 156, 1926–1941. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Qiao, X.; Liu, X.M.; Shi, S.H.; Xu, J.Y. Blocking XCT and PI3K/Akt Pathway Synergized with DNA Damage of Riluzole-Pt (IV) Prodrugs for Cancer Treatment. Eur. J. Med. Chem. 2023, 250, 115233. [Google Scholar] [CrossRef] [PubMed]
- Rufino-González, Y.; Ponce-Macotela, M.; García-Ramos, J.C.; Martínez-Gordillo, M.N.; Galindo-Murillo, R.; González-Maciel, A.; Reynoso-Robles, R.; Tovar-Tovar, A.; Flores-Alamo, M.; Toledano-Magaña, Y.; et al. Antigiardiasic Activity of Cu (II) Coordination Compounds: Redox Imbalance and Membrane Damage after a Short Exposure Time. J. Inorg. Biochem. 2019, 195, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Godínez, C.; Ontiveros-Rodríguez, J.C.; Ríos-Valencia, D.G.; Herbert-Pucheta, J.E.; Zepeda-Vallejo, L.G.; Carrero, J.C. Anti-Amoebic Activity of Leaf Extracts and Aporphine Alkaloids Obtained from Annona Purpurea. Planta Med. 2020, 86, 425–433. [Google Scholar] [CrossRef]
- Velázquez-Domínguez, J.A.; Hernández-Ramírez, V.I.; Calzada, F.; Varela-Rodríguez, L.; Pichardo-Hernández, D.L.; Bautista, E.; Herrera-Martínez, M.; Castellanos-Mijangos, R.D.; Matus-Meza, A.S.; Chávez-Munguía, B.; et al. Linearolactone and Kaempferol Disrupt the Actin Cytoskeleton in Entamoeba histolytica: Inhibition of Amoebic Liver Abscess Development. J. Nat. Prod. 2020, 83, 3671–3680. [Google Scholar] [CrossRef]
- Seol, H.S.; Lee, S.E.; Song, J.S.; Lee, H.Y.; Park, S.; Kim, I.; Singh, S.R.; Chang, S.; Jang, S.J. Glutamate Release Inhibitor, Riluzole, Inhibited Proliferation of Human Hepatocellular Carcinoma Cells by Elevated ROS Production. Cancer Lett. 2016, 382, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Shahi, P.; Trebicz-Geffen, M.; Nagaraja, S.; Alterzon-Baumel, S.; Hertz, R.; Methling, K.; Lalk, M.; Ankri, S. Proteomic Identification of Oxidized Proteins in Entamoeba histolytica by Resin-Assisted Capture: Insights into the Role of Arginase in Resistance to Oxidative Stress. PLoS Negl. Trop. Dis. 2016, 10, e0004340. [Google Scholar] [CrossRef] [PubMed]
- Konstantinova, L.S.; Knyazeva, E.A.; Gatilov, Y.V.; Zlotin, S.G.; Rakitin, O.A. Nitro Derivatives of 2,1,3-Benzothiadiazole 1-Oxides: Synthesis, Structural Study, and NO Release. Russ. Chem. Bull. 2018, 67, 95–101. [Google Scholar] [CrossRef]
- Fahey, R.C.; Newton, G.L.; Arrick, B.; Overdank-Bogart, T.; Aley, S.B. Entamoeba histolytica: A Eukaryote without Glutathione Metabolism. Science 1984, 224, 6322306. [Google Scholar] [CrossRef]
- Mehlotra, R.K. Antioxidant Defense Mechanisms in Parasitic Protozoa. Crit. Rev. Microbiol. 1996, 22, 295–314. [Google Scholar] [CrossRef]
- Loftus, B.; Anderson, I.; Davies, R.; Alsmark, U.C.M.; Samuelson, J.; Amedeo, P.; Roncaglia, P.; Berriman, M.; Hirt, R.P.; Mann, B.J.; et al. The Genome of the Protist Parasite Entamoeba histolytica. Nature 2005, 433, 865–868. [Google Scholar] [CrossRef]
- Jeelani, G.; Nozaki, T. Entamoeba Thiol-Based Redox Metabolism: A Potential Target for Drug Development. Mol. Biochem. Parasitol. 2016, 206, 39–45. [Google Scholar] [CrossRef]
- Arias, D.G.; Gutierrez, C.E.; Iglesias, A.A.; Guerrero, S.A. Thioredoxin-Linked Metabolism in Entamoeba histolytica. Free Radic. Biol. Med. 2007, 42, 1496–1505. [Google Scholar] [CrossRef]
- Arias, D.G.; Regner, E.L.; Iglesias, A.A.; Guerrero, S.A. Entamoeba histolytica Thioredoxin Reductase: Molecular and Functional Characterization of Its Atypical Properties. Biochim. Biophys. Acta Gen. Subj. 2012, 1820, 2696–2705. [Google Scholar] [CrossRef]
- Arias, D.G.; Carranza, P.G.; Lujan, H.D.; Iglesias, A.A.; Guerrero, S.A. Immunolocalization and Enzymatic Functional Characterization of the Thioredoxin System in Entamoeba histolytica. Free Radic. Biol. Med. 2008, 45, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Tazreiter, M.; Leitsch, D.; Hatzenbichler, E.; Mair-Scorpio, G.E.; Steinborn, R.; Schreiber, M.; Duchêne, M. Entamoeba histolytica: Response of the Parasite to Metronidazole Challenge on the Levels of MRNA and Protein Expression. Exp. Parasitol. 2008, 120, 403–410. [Google Scholar] [CrossRef]
- Rivera-Santiago, L.; Martínez, I.; Arroyo-Olarte, R.; Díaz-Garrido, P.; Cuevas-Hernandez, R.I.; Espinoza, B. Structural New Data for Mitochondrial Peroxiredoxin from Trypanosoma cruzi Show High Similarity with Human Peroxiredoxin 3: Repositioning Thiostrepton as Antichagasic Drug. Front Cell Infect. Microbiol. 2022, 12, 847. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.H.; Sajed, D.; Poole, L.; Hirata, K.; Herdman, S.; Torian, B.E.; Reed, S.L. An Unusual Surface Peroxiredoxin Protects Invasive Entamoeba histolytica from Oxidant Attack. Mol. Biochem. Parasitol. 2005, 143, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Bruchhaus, I.; Richter, S.; Tannich, E. Removal of Hydrogen Peroxide by the 29 KDa Protein of Entamoeba histolytica. Biochem. J. 1997, 326, 785–789. [Google Scholar] [CrossRef]
- Andrade, R.M.; Reed, S.L. New Drug Target in Protozoan Parasites: The Role of Thioredoxin Reductase. Front. Microbiol. 2015, 6, 975. [Google Scholar] [CrossRef]
- Becker, K.; Gromer, S.; Heiner Schirmer, R.; Müller, S. Thioredoxin Reductase as a Pathophysiological Factor and Drug Target. Eur. J. Biochem. 2000, 267, 6118–6125. [Google Scholar] [CrossRef]
- Debnath, A.; Parsonage, D.; Andrade, R.M.; He, C.; Cobo, E.R.; Hirata, K.; Chen, S.; García-Rivera, G.; Orozco, E.; Martínez, M.B.; et al. A High-Throughput Drug Screen for Entamoeba histolytica Identifies a New Lead and Target. Nat. Med. 2012, 18, 956–960. [Google Scholar] [CrossRef] [PubMed]
- Jeelani, G.; Nozaki, T. Oxidative Stress and Antioxidant Defense Mechanism in the Human Enteric Protozoan Parasite Entamoeba histolytica In Oxidative Stress in Microbial Diseases; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Ondarza, R. Drug Targets from Human Pathogenic Amoebas: Acanthamoeba Polyphaga and Naegleria fowleri. Infect. Disord. Drug Targets 2008, 7, 266–280. [Google Scholar] [CrossRef] [PubMed]
- Maralikova, B.; Ali, V.; Nakada-Tsukui, K.; Nozaki, T.; van der Giezen, M.; Henze, K.; Tovar, J. Bacterial-Type Oxygen Detoxification and Iron-Sulfur Cluster Assembly in Amoebal Relict Mitochondria. Cell Microbiol. 2010, 12, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Müller, M. Energy Metabolism: Part I: Anaerobic Protozoa. Mol. Med. Parasitol. 2003, 2003, 125–139. [Google Scholar] [CrossRef]
- Diamond, L.S.; Harlow, D.R.; Cunnick, C.C. A New Medium for the Axenic Cultivation of Entamoeba histolytica and Other Entamoeba. Trans. R. Soc. Trop. Med. Hyg. 1978, 72, 431–432. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.S.; Dutta, S.; Raha, S. Hydrogen Peroxide-Induced Apoptosis-like Cell Death in Entamoeba histolytica. Parasitol. Int. 2010, 59, 166–172. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2-ΔΔCT Method. Methods 2001, 25, 2200. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef] [PubMed]
- Sanner, M.F. Python: A Programming Language for Software Integration and Development. J. Mol. Graph. Model. 1999, 17, 57–61. [Google Scholar] [PubMed]
| Gene | Accession | Forward (5′ → 3′) | Reverse (5′ → 3′) |
|---|---|---|---|
| Thioredoxin | XM_649815.1 | TAT GC AGA GTG GTG TGG TCC AT | AAA TGT CGG CAT ACA ACG AAT ACC |
| Rubrerythrin | XM_647039.2 | ATG CTC AAA TTG CTG CTA GAC TT | ATA TCC ACA TTC TCT ACA AAC CCA AA |
| Thioredoxin reductase | XM_650656.2 | ATG AGA ACA CAA TCA GAG AAG TAT GGA | AGC TGT AGC ACC TGT TGC AAT AAT |
| Peroxiredoxin | XM_646911.2 XM_644418.2 XM_643430.2 | CGA AGC AGG AAT TGC AAG AAG | GCT CCA TGT TCA TCA CTG AAT TG |
| GAPDH | AB002800.1 | TTC ATG GAT CCA AAA TAC ATG GTT | GCC AAT TTG AGC TGG ATC TCT T |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velásquez-Torres, M.; Trujillo-Ferrara, J.G.; Godínez-Victoria, M.; Jarillo-Luna, R.A.; Tsutsumi, V.; Sánchez-Monroy, V.; Posadas-Mondragón, A.; Cuevas-Hernández, R.I.; Santiago-Cruz, J.A.; Pacheco-Yépez, J. Riluzole, a Derivative of Benzothiazole as a Potential Anti-Amoebic Agent against Entamoeba histolytica. Pharmaceuticals 2023, 16, 896. https://doi.org/10.3390/ph16060896
Velásquez-Torres M, Trujillo-Ferrara JG, Godínez-Victoria M, Jarillo-Luna RA, Tsutsumi V, Sánchez-Monroy V, Posadas-Mondragón A, Cuevas-Hernández RI, Santiago-Cruz JA, Pacheco-Yépez J. Riluzole, a Derivative of Benzothiazole as a Potential Anti-Amoebic Agent against Entamoeba histolytica. Pharmaceuticals. 2023; 16(6):896. https://doi.org/10.3390/ph16060896
Chicago/Turabian StyleVelásquez-Torres, Maritza, José Guadalupe Trujillo-Ferrara, Marycarmen Godínez-Victoria, Rosa Adriana Jarillo-Luna, Víctor Tsutsumi, Virginia Sánchez-Monroy, Araceli Posadas-Mondragón, Roberto Issac Cuevas-Hernández, José Angel Santiago-Cruz, and Judith Pacheco-Yépez. 2023. "Riluzole, a Derivative of Benzothiazole as a Potential Anti-Amoebic Agent against Entamoeba histolytica" Pharmaceuticals 16, no. 6: 896. https://doi.org/10.3390/ph16060896
APA StyleVelásquez-Torres, M., Trujillo-Ferrara, J. G., Godínez-Victoria, M., Jarillo-Luna, R. A., Tsutsumi, V., Sánchez-Monroy, V., Posadas-Mondragón, A., Cuevas-Hernández, R. I., Santiago-Cruz, J. A., & Pacheco-Yépez, J. (2023). Riluzole, a Derivative of Benzothiazole as a Potential Anti-Amoebic Agent against Entamoeba histolytica. Pharmaceuticals, 16(6), 896. https://doi.org/10.3390/ph16060896







