Asthma and COPD: Comparison with International Guidelines and Medication Adherence in Belgium
Abstract
1. Introduction
2. Results
2.1. Population, Most Prescribed Drugs, and Main Prescribers
2.2. Medication Adherence
2.2.1. Influence of Sex, Type of Device and Age
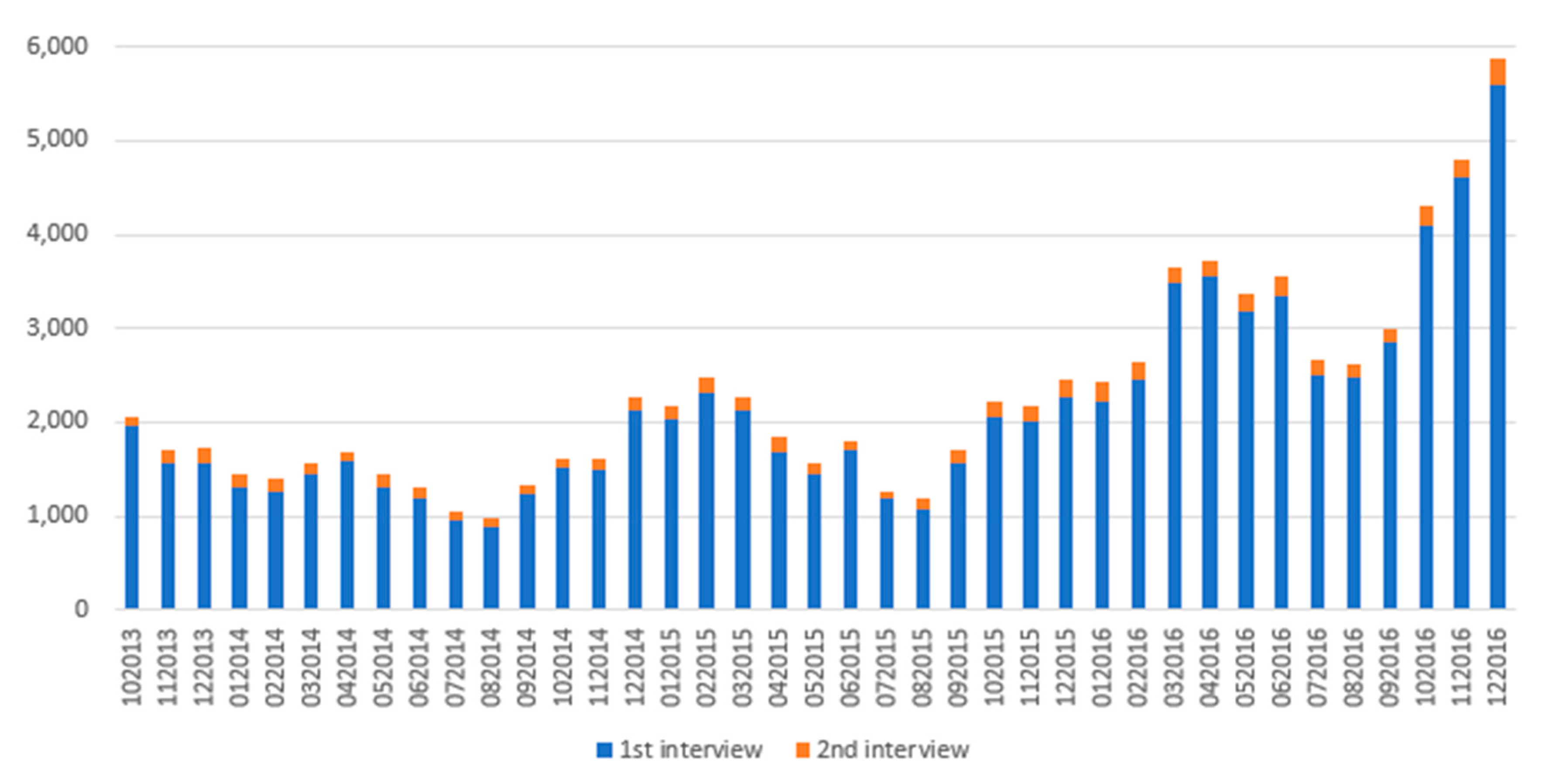
2.2.2. Influence of the New Medicines Service
2.2.3. Persistence
3. Discussion
3.1. Population and Comparaison with GINA and GOLD Guidelines
3.2. Medication Adherence
3.2.1. Influence of Sex
3.2.2. Influence of the Device
3.2.3. The New Medicines Service
3.2.4. Persistence
3.3. Strengths and Weaknesses
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Der Heyden, J.; Charafeddine, R. Maladies et Affections Chroniques—Enquête de Santé. 2018. Available online: https://www.sciensano.be/fr/projets/enquete-de-sante (accessed on 29 September 2021).
- WHO. World Health Organization—Chronic Obstructive Pulmonary Disease (COPD). Available online: https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd) (accessed on 9 November 2022).
- WHO. World Health Organization—Asthma. Available online: https://www.who.int/news-room/fact-sheets/detail/asthma (accessed on 9 November 2022).
- Global Initiative for Asthma: Global Strategy for Asthma Management and Prevention 2018. Available online: https://ginasthma.org/archived-reports/ (accessed on 15 December 2021).
- Global Initiative for Chronic Obstructive Lung Disease: Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease 2016. Available online: https://goldcopd.org/archived-reports/ (accessed on 15 December 2021).
- Sehl, J.; O’doherty, J.; O’connor, R.; O’sullivan, B.; O’regan, A. Adherence to COPD Management Guidelines in General Practice? A Review of the Literature. Ir. J. Med. Sci. 2018, 187, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Palmiotti, G.A.; Lacedonia, D.; Liotino, V.; Schino, P.; Satriano, F.; Di Napoli, P.L.; Sabato, E.; Mastrosimone, V.; Scoditti, A.; Carone, M.; et al. Adherence to GOLD Guidelines in Real-Life COPD Management in the Puglia Region of Italy. Int. J. Chron. Obs. Pulmon Dis. 2018, 13, 2455–2462. [Google Scholar] [CrossRef] [PubMed]
- Grewe, F.A.; Sievi, N.A.; Bradicich, M.; Roeder, M.; Brack, T.; Brutsche, M.H.; Frey, M.; Irani, S.; Leuppi, J.D.; Thurnheer, R.; et al. Compliance of Pharmacotherapy with GOLD Guidelines: A Longitudinal Study in Patients with COPD. Int. J. Chron. Obs. Pulmon Dis. 2020, 15, 627. [Google Scholar] [CrossRef] [PubMed]
- Vrijens, B.; Dima, A.L.; Van Ganse, E.; van Boven, J.F.M.; Eakin, M.N.; Foster, J.M.; de Bruin, M.; Chisholm, A.; Price, D. What We Mean When We Talk About Adherence in Respiratory Medicine. J. Allergy Clin. Immunol. Pract. 2016, 4, 802–812. [Google Scholar] [CrossRef]
- Dima, A.L.; van Ganse, E.; Stadler, G.; de Bruin, M. Does Adherence to Inhaled Corticosteroids Predict Asthma-Related Outcomes over Time? A Cohort Study. Eur. Respir. J. 2019, 54, 1900901. [Google Scholar] [CrossRef]
- World Health Organization: Adherence to Long-Term Therapies—Evidence for Action (2003). Available online: https://apps.who.int/iris/handle/10665/42682 (accessed on 5 June 2021).
- Jüngst, C.; Gräber, S.; Simons, S.; Wedemeyer, H.; Lammert, F. Medication Adherence among Patients with Chronic Diseases: A Survey-Based Study in Pharmacies. QJM 2019, 112, 505–512. [Google Scholar] [CrossRef]
- Krass, I.; Schieback, P.; Dhippayom, T. Adherence to Diabetes Medication: A Systematic Review. Diabet. Med. 2015, 32, 725–737. [Google Scholar] [CrossRef]
- Janjua, S.; Pike, K.C.; Carr, R.; Coles, A.; Fortescue, R.; Batavia, M. Interventions to Improve Adherence to Pharmacological Therapy for Chronic Obstructive Pulmonary Disease (COPD). Cochrane Database Syst. Rev. 2021, 9, CD013381. [Google Scholar] [CrossRef]
- Vrijens, B.; De Geest, S.; Hughes, D.A.; Przemyslaw, K.; Demonceau, J.; Ruppar, T.; Dobbels, F.; Fargher, E.; Morrison, V.; Lewek, P.; et al. A New Taxonomy for Describing and Defining Adherence to Medications. Br. J. Clin. Pharmacol. 2012, 73, 691–705. [Google Scholar] [CrossRef]
- Mannino, D.; Bogart, M.; Wu, B.; Germain, G.; Laliberté, F.; MacKnight, S.D.; Jung, Y.; Stiegler, M.; Duh, M.S. Adherence and Persistence to Once-Daily Single-Inhaler versus Multiple-Inhaler Triple Therapy among Patients with Chronic Obstructive Pulmonary Disease in the USA: A Real-World Study. Respir. Med. 2022, 197, 106807. [Google Scholar] [CrossRef]
- Gregoriano, C.; Dieterle, T.; Breitenstein, A.-L.; Dürr, S.; Baum, A.; Maier, S.; Arnet, I.; Hersberger, K.E.; Leuppi, J.D. Use and Inhalation Technique of Inhaled Medication in Patients with Asthma and COPD: Data from a Randomized Controlled Trial. Respir. Res. 2018, 19, 237. [Google Scholar] [CrossRef] [PubMed]
- Usmani, O.S.; Hickey, A.J.; Guranlioglu, D.; Rawson, K.; Stjepanovic, N.; Siddiqui, S.; Dhand, R. The Impact of Inhaler Device Regimen in Patients with Asthma or COPD. J. Allergy Clin. Immunol. Pract. 2021, 9, 3033–3040. [Google Scholar] [CrossRef]
- Association Pharmaceutique Belge ENM? Késako? Available online: https://www.apb.be/fr/corp/media-room/Relations-publiques/communique-de-presse/Pages/ENM-BNM-KESAKO.aspx (accessed on 12 August 2022).
- Fraeyman, J.; Foulon, V.; Mehuys, E.; Boussery, K.; Saevels, J.; De Vriese, C.; Dalleur, O.; Housiaux, M.; Steurbaut, S.; Naegels, M.; et al. Evaluating the Implementation Fidelity of New Medicines Service for Asthma Patients in Community Pharmacies in Belgium. Res. Soc. Adm. Pharm. 2017, 13, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Global Initiative for Asthma: Global Strategy for Asthma Management and Prevention 2016. Available online: https://ginasthma.org/archived-reports/ (accessed on 6 July 2022).
- Biset, N.; Kestens, W.; Detemmerman, D.; Lona, M.; Karakaya, G.; Ceuppens, A.; Pochet, S.; De Vriese, C. Analysis of the Consumption of Drugs Prescribed for the Treatment of Asthma in Belgian Children. Int. J. Environ. Res. Public. Health 2022, 19, 548. [Google Scholar] [CrossRef] [PubMed]
- NICE Overview|Asthma: Diagnosis, Monitoring and Chronic Asthma Management|Guidance|NICE. Available online: https://www.nice.org.uk/guidance/ng80 (accessed on 29 September 2021).
- Schmiedl, S.; Fischer, R.; Ibáñez, L.; Fortuny, J.; Klungel, O.H.; Reynolds, R.; Gerlach, R.; Tauscher, M.; Thürmann, P.; Hasford, J.; et al. Utilisation and Off-Label Prescriptions of Respiratory Drugs in Children. PLoS ONE 2014, 9, e105110. [Google Scholar] [CrossRef]
- Bollmeier, S.G.; Hartmann, A.P. Management of Chronic Obstructive Pulmonary Disease: A Review Focusing on Exacerbations. Am. J. Health-Syst. Pharm. AJHP 2020, 77, 259. [Google Scholar] [CrossRef]
- Fickweiler, F.; Fickweiler, W.; Urbach, E. Interactions between Physicians and the Pharmaceutical Industry Generally and Sales Representatives Specifically and Their Association with Physicians’ Attitudes and Prescribing Habits: A Systematic Review. BMJ Open 2017, 7, e016408. [Google Scholar] [CrossRef]
- Institut National D’assurance Maladie-Invalidité (INAMI). Nombre de Dispensateurs de Soins Individuels—INAMI. Available online: https://www.inami.fgov.be/fr/statistiques/soinsdesante/2017/Pages/nombre-dispensateurs-soins-individuels.aspx (accessed on 28 July 2022).
- Engelkes, M.; Janssens, H.M.; De Jongste, J.C.; Sturkenboom, M.C.J.M.; Verhamme, K.M.C. Medication Adherence and the Risk of Severe Asthma Exacerbations: A Systematic Review. Eur. Respir. J. 2015, 45, 396–407. [Google Scholar] [CrossRef]
- Bidwal, M.; Lor, K.; Yu, J.; Ip, E. Evaluation of Asthma Medication Adherence Rates and Strategies to Improve Adherence in the Underserved Population at a Federally Qualified Health Center. Res. Social. Adm. Pharm. 2017, 13, 759–766. [Google Scholar] [CrossRef]
- George, M.; Topaz, M.; Rand, C.; Sommers, M.S.; Glanz, K.; Pantalon, M.V.; Mao, J.J.; Shea, J.A. Inhaled Corticosteroid Beliefs, Complementary and Alternative Medicine and Uncontrolled Asthma in Urban Minority Adults. J. Allergy Clin. Immunol. 2014, 134, 1252. [Google Scholar] [CrossRef]
- Ulrik, C.S.; Søes-Petersen, U.; Backer, V.; Lange, P.; Harving, H.; Plaschke, P. Disease Variability in Asthma: How Do the Patients Respond? And Why? J. Asthma 2008, 45, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Rolnick, S.J.; Pawloski, P.A.; Hedblom, B.D.; Asche, S.E.; Bruzek, R.J. Patient Characteristics Associated with Medication Adherence. Clin. Med. Res. 2013, 11, 54. [Google Scholar] [CrossRef] [PubMed]
- Averell, C.M.; Laliberté, F.; Germain, G.; Duh, M.S.; Rousculp, M.D.; MacKnight, S.D.; Slade, D.J. Impact of Adherence to Treatment with Inhaled/Long-Acting β-Agonists on Asthma Outcomes in the United. Ther. Adv. Respir. Dis. 2022, 16, 17534666221116997. [Google Scholar] [CrossRef] [PubMed]
- Parimi, M.; Svedsater, H.; Ann, Q.; Gokhale, M.; Gray, C.M.; Hinds, D.; Nixon, M.; Boxall, N. Persistence and Adherence to ICS/LABA Drugs in UK Patients with Asthma: A Retrospective New-User Cohort Study. Adv. Ther. 2020, 37, 2916–2931. [Google Scholar] [CrossRef] [PubMed]
- Moretz, C.; Cole, A.L.; Mu, G.; Wu, B.; Guisinger, A.; Liu, Y.; Hahn, B.; Baylis, L. Evaluation of Medication Adherence and Rescue Medication Use in Non-Exacerbating Patients with COPD Receiving Umeclidinium/Vilanterol or Budesonide/Formoterol as Initial Maintenance Therapy. Int. J. Chron. Obstruct Pulmon Dis. 2020, 15, 2207. [Google Scholar] [CrossRef] [PubMed]
- Bårnes, C.B.; Ulrik, C.S. Asthma and Adherence to Inhaled Corticosteroids: Current Status and Future Perspectives. Respir. Care 2015, 60, 455–468. [Google Scholar] [CrossRef]
- Izquierdo, J.L.; Paredero, J.M.; Piedra, R. Relevance of Dosage in Adherence to Treatment with Long-Acting Anticholinergics in Patients with COPD. Int. J. Chron. Obstruct Pulmon Dis. 2016, 11, 289. [Google Scholar] [CrossRef]
- Bogart, M.; Stanford, R.H.; Laliberté, F.; Germain, G.; Wu, J.W.; Duh, M.S. Medication Adherence and Persistence in Chronic Obstructive Pulmonary Disease Patients Receiving Triple Therapy in a USA Commercially Insured Population. Int. J. Chron. Obstruct Pulmon Dis. 2019, 14, 343. [Google Scholar] [CrossRef]
- Covvey, J.R.; Mullen, A.B.; Ryan, M.; Steinke, D.T.; Johnston, B.F.; Wood, F.T.; Boyter, A.C. A Comparison of Medication Adherence/Persistence for Asthma and Chronic Obstructive Pulmonary Disease in the United Kingdom. Int. J. Clin. Pract. 2014, 68, 1200–1208. [Google Scholar] [CrossRef]
- Moretz, C.; Bengtson, L.G.; Sharpsten, L.; Koep, E.; Le, L.; Tong, J.; Stanford, R.H.; Hahn, B.; Ray, R. Evaluation of Rescue Medication Use and Medication Adherence Receiving Umeclidinium/Vilanterol versus Tiotropium Bromide/Olodaterol. Int. J. Chron. Obstruct Pulmon Dis. 2019, 14, 2047. [Google Scholar] [CrossRef]
- Toy, E.L.; Beaulieu, N.U.; McHale, J.M.; Welland, T.R.; Plauschinat, C.A.; Swensen, A.; Duh, M.S. Treatment of COPD: Relationships between Daily Dosing Frequency, Adherence, Resource Use, and Costs. Respir. Med. 2011, 105, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Stanford, R.H.; Parker, E.D.; Reinsch, T.K.; Buikema, A.R.; Blauer-Peterson, C. Assessment of COPD-Related Outcomes in Patients Initiating a Once Daily or Twice Daily ICS/LABA. Respir. Med. 2019, 150, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Stanford, R.H.; Averell, C.; Parker, E.D.; Blauer-Peterson, C.; Reinsch, T.K.; Buikema, A.R. Assessment of Adherence and Asthma Medication Ratio for a Once-Daily and Twice-Daily Inhaled Corticosteroid/Long-Acting β-Agonist for Asthma. J. Allergy Clin. Immunol. Pract. 2019, 7, 1488–1496.e7. [Google Scholar] [CrossRef]
- Averell, C.M.; Laliberté, F.; Germain, G.; Slade, D.J.; Duh, M.S.; Spahn, J. Disease Burden and Treatment Adherence among Children and Adolescent Patients with Asthma. J. Asthma 2022, 59, 1687–1696. [Google Scholar] [CrossRef]
- Landis, S.H.; Muellerova, H.; Mannino, D.M.; Menezes, A.M.; Han, M.L.K.; van der Molen, T.; Masakazu, I.; Aisanov, Z.; Oh, Y.M.; Davis, K.J. Continuing to Confront COPD International Patient Survey: Methods, COPD Prevalence, and Disease Burden in 2012–2013. Int. J. Chron Obstruct. Pulmon Dis. 2014, 9, 597. [Google Scholar] [CrossRef]
- Soriano, J.B.; Kendrick, P.J.; Paulson, K.R.; Gupta, V.; Abrams, E.M.; Adedoyin, R.A.; Adhikari, T.B.; Advani, S.M.; Agrawal, A.; Ahmadian, E.; et al. Prevalence and Attributable Health Burden of Chronic Respiratory Diseases, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet Respir. Med. 2020, 8, 585. [Google Scholar] [CrossRef]
- Jenkins, C.R.; Chapman, K.R.; Donohue, J.F.; Roche, N.; Tsiligianni, I.; Han, M.K. Improving the Management of COPD in Women. Chest J. 2017, 151, 686–696. [Google Scholar] [CrossRef]
- Silveyra, P.; Fuentes, N.; Rodriguez Bauza, D.E. Sex and Gender Differences in Lung Disease. Adv. Exp. Med. Biol. 2021, 1304, 227. [Google Scholar] [CrossRef]
- Schnoor, K.; Versluis, A.; Bakema, R.; van Luenen, S.; Kooij, M.J.; van den Heuvel, J.M.; Teichert, M.; Honkoop, P.J.; van Boven, J.F.M.; Chavannes, N.H.; et al. A Pharmacy-Based EHealth Intervention Promoting Correct Use of Medication in Patients with Asthma and COPD: Nonrandomized Pre-Post Study. J. Med. Internet Res. 2022, 24, e32396. [Google Scholar] [CrossRef]
- Raparelli, V.; Proietti, M.; Romiti, G.F.; Lenzi, A.; Basili, S.; Tiberti, C.; Panimolle, F.; Isidori, A.; Giannetta, E.; Venneri, M.A.; et al. The Sex-Specific Detrimental Effect of Diabetes and Gender-Related Factors on Pre-Admission Medication Adherence Among Patients Hospitalized for Ischemic Heart Disease: Insights from EVA Study. Front. Endocrinol. 2019, 10, 107. [Google Scholar] [CrossRef]
- Lopes, J.; Santos, P. Determinants of Non-Adherence to the Medications for Dyslipidemia: A Systematic Review. Patient Prefer. Adherence 2021, 15, 1853. [Google Scholar] [CrossRef] [PubMed]
- Manteuffel, M.; Williams, S.; Chen, W.; Verbrugge, R.R.; Pittman, D.G.; Steinkellner, A. Influence of Patient Sex and Gender on Medication Use, Adherence, and Prescribing Alignment with Guidelines. J. Womens Health 2014, 23, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Battle, K.; Lurslurchachai, L.; Halm, E.A.; Wisnivesky, J.P. Inhaler Device, Administration Technique, and Adherence to Inhaled Corticosteroids in Patients with Asthma. Prim. Care Respir. J. 2011, 20, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Darbà, J.; Ramírez, G.; Sicras, A.; Francoli, P.; Torvinen, S.; Sánchez-de la Rosa, R. The Importance of Inhaler Devices: The Choice of Inhaler Device May Lead to Suboptimal Adherence in COPD Patients. Int. J. Chron. Obstruct Pulmon Dis. 2015, 10, 2335–2345. [Google Scholar] [CrossRef]
- Price, D.; Roche, N.; Christian Virchow, J.; Burden, A.; Ali, M.; Chisholm, A.; Lee, A.J.; Hillyer, E.V.; Von Ziegenweidt, J. Device Type and Real-World Effectiveness of Asthma Combination Therapy: An Observational Study. Respir. Med. 2011, 105, 1457–1466. [Google Scholar] [CrossRef]
- Koehorst-Ter Huurne, K.; Movig, K.; Van Der Valk, P.; Van Der Palen, J.; Brusse-Keizer, M. The Influence of Type of Inhalation Device on Adherence of COPD Patients to Inhaled Medication. Expert Opin. Drug Deliv. 2016, 13, 469–475. [Google Scholar] [CrossRef]
- Imamura, Y.; Kawayama, T.; Kinoshita, T.; Sakazaki, Y.; Yoshida, M.; Takahashi, K.; Fujii, K.; Ando, M.; Hoshino, T.; Iwanaga, T.; et al. Poor Pharmacological Adherence to Inhaled Medicines Compared with Oral Medicines in Japanese Patients with Asthma and Chronic Obstructive Pulmonary Disease. Allergol. Int. 2017, 66, 482–484. [Google Scholar] [CrossRef]
- Thomas, M.; Price, D.; Chrystyn, H.; Lloyd, A.; Williams, A.E.; von Ziegenweidt, J. Inhaled Corticosteroids for Asthma: Impact of Practice Level Device Switching on Asthma Control. BMC Pulm. Med. 2009, 9, 1. [Google Scholar] [CrossRef]
- Rogliani, P.; Ora, J.; Puxeddu, E.; Matera, M.G.; Cazzola, M. Adherence to COPD Treatment: Myth and Reality. Respir. Med. 2017, 129, 117–123. [Google Scholar] [CrossRef]
- Dekhuijzen, P.N.R.; Levy, M.L.; Corrigan, C.J.; Hadfield, R.M.; Roche, N.; Usmani, O.S.; Barnes, P.J.; Scullion, J.E.; Lavorini, F.; Corbetta, L.; et al. Is Inhaler Technique Adequately Assessed and Reported in Clinical Trials of Asthma and Chronic Obstructive Pulmonary Disease Therapy? A Systematic Review and Suggested Best Practice Checklist. J. Allergy Clin. Immunol. Pract. 2022, 10, 1813–1824.e1. [Google Scholar] [CrossRef]
- Institut National D’assurance Maladie-Invalidité (INAMI) Un Nouveau Service Du Pharmacien Pour Le Patient Asthmatique Chronique: L’entretien D’accompagnement de Bon Usage Des Médicaments (BUM)—INAMI. Available online: https://www.riziv.fgov.be/fr/professionnels/sante/pharmaciens/Pages/entretien-pharmacien-patient-asthmatique-info-pharmacien.aspx (accessed on 12 August 2022).
- Turi, K.N.; Gebretsadik, T.; Lee, R.L.; Hartert, T.V.; Evans, A.M.; Stone, C.; Sicignano, N.M.; Wu, A.C.; Iribarren, C.; Butler, M.G.; et al. Seasonal Patterns of Asthma Medication Fills among Diverse Populations of the United States. J. Asthma 2018, 55, 764. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.; Wilke, T.; Bechtel, B.; Punekar, Y.S.; Mitzner, K.; Virchow, J.C. Non-Persistence and Non-Adherence to Long-Acting COPD Medication Therapy: A Retrospective Cohort Study Based on a Large German Claims Dataset. Respir. Med. 2017, 122, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jansen, E.M.; van de Hei, S.J.; Dierick, B.J.H.; Kerstjens, H.A.M.; Kocks, J.W.H.; van Boven, J.F.M. Global Burden of Medication Non-Adherence in Chronic Obstructive Pulmonary Disease (COPD) and Asthma: A Narrative Review of the Clinical and Economic Case for Smart Inhalers. J. Thorac. Dis. 2021, 13, 3846. [Google Scholar] [CrossRef]
- Tommelein, E.; Mehuys, E.; Van Hees, T.; Adriaens, E.; Van Bortel, L.; Christiaens, T.; Van Tongelen, I.; Remon, J.P.; Boussery, K.; Brusselle, G. Effectiveness of Pharmaceutical Care for Patients with Chronic Obstructive Pulmonary Disease (PHARMACOP): A Randomized Controlled Trial. Br. J. Clin. Pharmacol. 2014, 77, 756. [Google Scholar] [CrossRef]
- Pazzagli, L.; Linder, M.; Zhang, M.; Vago, E.; Stang, P.; Myers, D.; Andersen, M.; Bahmanyar, S. Methods for Time-Varying Exposure Related Problems in Pharmacoepidemiology: An Overview. Pharmacoepidemiol. Drug Saf. 2018, 27, 148–160. [Google Scholar] [CrossRef]
- Meaidi, M.; Støvring, H.; Rostgaard, K.; Torp-Pedersen, C.; Kragholm, K.H.; Andersen, M.; Sessa, M. Pharmacoepidemiological Methods for Computing the Duration of Pharmacological Prescriptions Using Secondary Data Sources. Eur. J. Clin. Pharmacol. 2021, 77, 1805–1814. [Google Scholar] [CrossRef]
- Baumgartner, P.C.; Haynes, R.B.; Hersberger, K.E.; Arnet, I. A Systematic Review of Medication Adherence Thresholds Dependent of Clinical Outcomes. Front. Pharmacol. 2018, 9, 1290. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.A.; Stedman, M.R.; Lii, J.; Vogeli, C.; Shrank, W.H.; Brookhart, M.A.; Weissman, J.S. Primary Medication Non-Adherence: Analysis of 195,930 Electronic Prescriptions. J. Gen. Intern. Med. 2010, 25, 284–290. [Google Scholar] [CrossRef]
- Vollmer, W.M.; Xu, M.; Feldstein, A.; Smith, D.; Waterbury, A.; Rand, C. Comparison of Pharmacy-Based Measures of Medication Adherence. BMC Health Serv. Res. 2012, 12, 155. [Google Scholar] [CrossRef]
- Dima, A.L.; Dediu, D. Computation of Adherence to Medication and Visualization of Medication Histories in R with AdhereR: Towards Transparent and Reproducible Use of Electronic Healthcare Data. PLoS ONE 2017, 12, e0174426. [Google Scholar] [CrossRef]
- WHOCC WHO. Collaborating Center for Drug Statistics Methodology—Definition and General Considerations. Available online: https://www.whocc.no/ddd/definition_and_general_considera/ (accessed on 8 November 2022).
| ICS | LABA | LAMA | LABA-ICS | LABA-LAMA | |
|---|---|---|---|---|---|
| Number of patients | 521,634 | 77,747 | 96,462 | 741,284 | 24,694 |
| Sex | |||||
| Males (%) | 48.8 | 56.7 | 63.9 | 47.5 | 64.6 |
| Females (%) | 51.2 | 43.3 | 36.1 | 52.5 | 35.4 |
| Age | |||||
| Mean (years) | 31 | 64 | 68 | 53 | 68 |
| Median (years) | 14 | 67 | 69 | 56 | 68 |
| ICS | LABA | LAMA | LABA-ICS | LABA-LAMA | |
|---|---|---|---|---|---|
| 1. | Budesonide (54.73%) | Indacaterol (54.22%) | Tiotropium bromide (91.38%) | Salmeterol and fluticasone (38.98%) | Indacaterol and glycopyrronium bromide (76.97%) |
| 2. | Fluticasone (35.65%) | Formoterol (37.21%) | Glycopyrronium bromide (6.37%) | Formoterol and budesonide (36.09%) | Vilanterol and umeclidinium bromide (19.90%) |
| 3. | Beclometasone (9.62%) | Salmeterol (8.57%) | Aclidinium bromide (1.83%) | Formoterol and beclometasone (17.51%) | Olodaterol and tiotropium bromide (2.27%) |
| ICS | LABA | LAMA | LABA-ICS | LABA-LAMA | |
|---|---|---|---|---|---|
| 1. | GPs (72.0%) | GPs (85.5%) | GPs (83.1%) | GPs (83.5%) | GPs (68.2%) |
| 2. | Pediatricians (20.0%) | Specialists in respiratory medicine (9.1%) | Specialists in respiratory medicine (12.6%) | Specialists in respiratory medicine (10.4%) | Specialists in respiratory medicine (27.2%) |
| 3. | Specialists in respiratory medicine (4.4%) | Pediatricians (1.2%) | Specialists in internal medicine (1.0%) | Pediatricians (1.7%) | Specialists in internal medicine (1.6%) |
| CMA | ICS | LABA | LAMA | LABA-ICS | LABA-LAMA |
|---|---|---|---|---|---|
| Mean ± σ | 0.211 ± 0.274 | 0.692 ± 0.318 | 0.797 ± 0.236 | 0.420 ± 0.310 | 0.872 ± 0.192 |
| 25th percentile | 0.039 | 0.421 | 0.664 | 0.151 | 0.806 |
| Median | 0.089 | 0.800 | 0.895 | 0.333 | 0.978 |
| 75th percentile | 0.247 | 1.000 | 0.999 | 0.637 | 1.000 |
| % of adherent patients (CMA ≥ 0.8) | 7.98 | 50.01 | 62.83 | 17.56 | 75.66 |
| CMA | ICS | LABA | LAMA | LABA-ICS | LABA-LAMA | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sex | F | M | F | M | F | M | F | M | F | M |
| Mean | 0.219 * | 0.202 * | 0.664 * | 0.714 * | 0.787 * | 0.802 * | 0.402 * | 0.440 * | 0.867 | 0.874 |
| Std. deviation | 0.281 | 0.266 | 0.326 | 0.311 | 0.244 | 0.232 | 0.306 | 0.313 | 0.197 | 0.189 |
| Median | 0.092 | 0.085 | 0.740 | 0.841 | 0.887 | 0.899 | 0.307 | 0.363 | 0.978 | 0.978 |
| % of adherent patients (CMA ≥ 0.8) | 8.69 | 7.24 | 46.15 | 52.93 | 61.09 | 63.82 | 16.07 | 19.18 | 74.71 | 76.19 |
| Device | DPI | pMDI | DPI | pMDI | DPI | pMDI | DPI | pMDI | DPI | pMDI |
| Number of patients | 85,985 | 200,738 | 74,468 | 3451 | 90,912 | 19,140 | 555,151 | 206,691 | 23,704 | 1071 |
| Mean | 0.377 * | 0.116 * | 0.701 * | 0.502 * | 0.796 * | 0.856 * | 0.417 * | 0.480 * | 0.883 * | 0.654 * |
| Std. deviation | 0.300 | 0.170 | 0.316 | 0.300 | 0.236 | 0.200 | 0.308 | 0.323 | 0.183 | 0.221 |
| Median | 0.282 | 0.056 | 0.820 | 0.441 | 0.892 | 0.960 | 0.334 | 0.403 | 0.984 | 0.612 |
| % of adherent patients (CMA ≥ 0.8) | 14.27 | 1.93 | 51.34 | 21.44 | 62.69 | 72.06 | 17.13 | 23.21 | 77.94 | 27.16 |
| No Interview | One or Two Interviews | |
|---|---|---|
| ICS | ||
| Mean CMA ± σ | 0.211 * ± 0.274 | 0.218 * ± 0.264 |
| % of adherent patients (CMA ≥ 0.8) | 8.01 | 7.25 |
| LABA-ICS | ||
| Mean CMA ± σ | 0.420 * ± 0.310 | 0.431 * ± 0.314 |
| % of adherent patients (CMA ≥ 0.8) | 17.55 | 18.44 |
| Only 1st Interview | 1st and 2nd Interviews | |
|---|---|---|
| Number of interviews delivered | 76,282 | 5731 |
| Number of patients | 70,949 | 5284 |
| Female | 38,413 | 2852 |
| Male | 32,536 | 2432 |
| Mean age (years) | 45 | 47 |
| Female | 46 | 49 |
| Male | 43 | 45 |
| Specialties of the main prescribers | GP (80.1%) | GP (79.6%) |
| Specialist in respiratory | Specialist in respiratory | |
| medicine (10.5%) | medicine (13.5%) | |
| Pediatrician (5.8%) | Pediatrician (7.5%) | |
| ICS | ||
| Mean CMA ± σ | 0.213 * ± 0.263 | 0.244 * ± 0.270 |
| % of adherent patients (CMA ≥ 0.8) | 7.40 | 7.83 |
| LABA-ICS | ||
| Mean CMA ± σ | 0.420 * ± 0.313 | 0.484 * ± 0.311 |
| % of adherent patients (CMA ≥ 0.8) | 18.71 | 22.99 |
| CMA | ICS | LABA | LAMA | LABA-ICS | LABA-LAMA |
|---|---|---|---|---|---|
| First episode | |||||
| Mean ± σ | 0.432 * ± 0.356 | 0.913 * ± 0.178 | 0.837 * ± 0.200 | 0.632 * ± 0.283 | 0.914 ± 0.154 |
| % of adherent patients (CMA ≥ 0.8) | 24.42 | 83.05 | 68.30 | 35.34 | 83.25 |
| Mean episode duration (days) | 85 | 250 | 461 | 214 | 293 |
| Second episode | |||||
| N | 358,651 | 44,784 | 25,885 | 527,985 | 10,631 |
| N with at least two deliveries during episode | 85,803 | 24,459 | 17,593 | 217,316 | 6617 |
| Mean ± σ | 0.412 * ± 0.366 | 0.801 * ± 0.257 | 0.859 * ± 0.208 | 0.694 * ± 0.298 | 0.911 ± 0.153 |
| % CMA ≥ 0.8 | 24.51 | 63.86 | 72.53 | 37.12 | 80.66 |
| Mean episode duration (days) | 55 | 175 | 186 | 113 | 218 |
| Third or more episode | |||||
| N | 234,471 | 39,211 | 14,367 | 342,039 | 7014 |
| N with at least two deliveries during episode | 75,091 | 24,807 | 8265 | 191,263 | 4062 |
| Mean ± σ | 0.509 * ± 0.389 | 0.861 * ± 0.230 | 0.912 * ± 0.160 | 0.816 * ± 0.249 | 0.921 * ± 0.151 |
| % CMA ≥ 0.8 | 35.11 | 74.34 | 82.61 | 65.08 | 84.59 |
| Mean episode duration (days) | 62 | 153 | 140 | 125 | 168 |
| Mean number of episodes | 1.999 | 2.059 | 1.466 | 1.810 | 1.693 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biset, N.; Lelubre, M.; Pochet, S.; De Vriese, C. Asthma and COPD: Comparison with International Guidelines and Medication Adherence in Belgium. Pharmaceuticals 2023, 16, 1030. https://doi.org/10.3390/ph16071030
Biset N, Lelubre M, Pochet S, De Vriese C. Asthma and COPD: Comparison with International Guidelines and Medication Adherence in Belgium. Pharmaceuticals. 2023; 16(7):1030. https://doi.org/10.3390/ph16071030
Chicago/Turabian StyleBiset, Natacha, Mélanie Lelubre, Stéphanie Pochet, and Carine De Vriese. 2023. "Asthma and COPD: Comparison with International Guidelines and Medication Adherence in Belgium" Pharmaceuticals 16, no. 7: 1030. https://doi.org/10.3390/ph16071030
APA StyleBiset, N., Lelubre, M., Pochet, S., & De Vriese, C. (2023). Asthma and COPD: Comparison with International Guidelines and Medication Adherence in Belgium. Pharmaceuticals, 16(7), 1030. https://doi.org/10.3390/ph16071030






