Small Molecules Incorporating Privileged Amidine Moiety as Potential Hits Combating Antibiotic-Resistant Bacteria
Abstract
1. Introduction
2. Results and Discussion
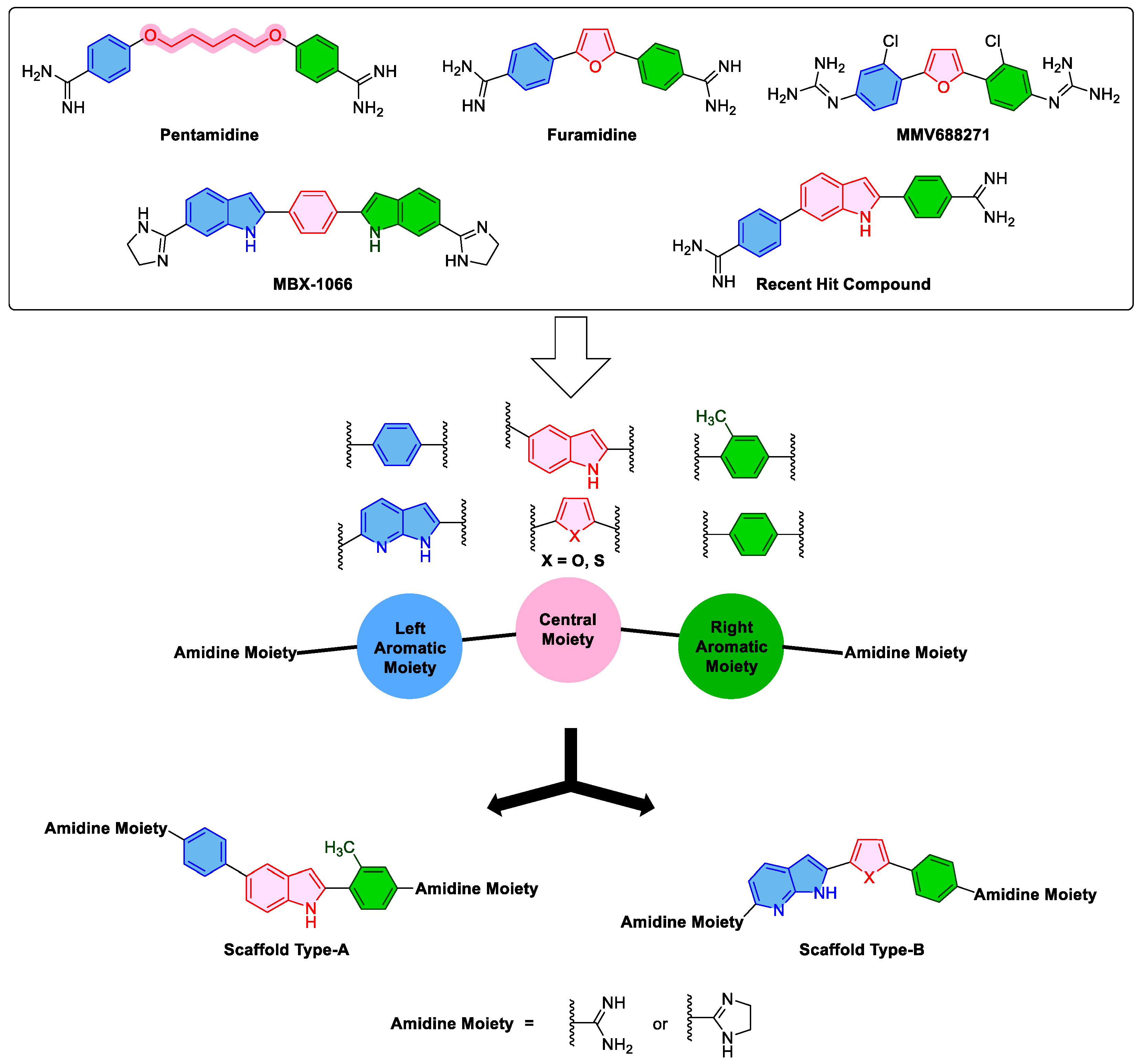
2.1. Design Approach
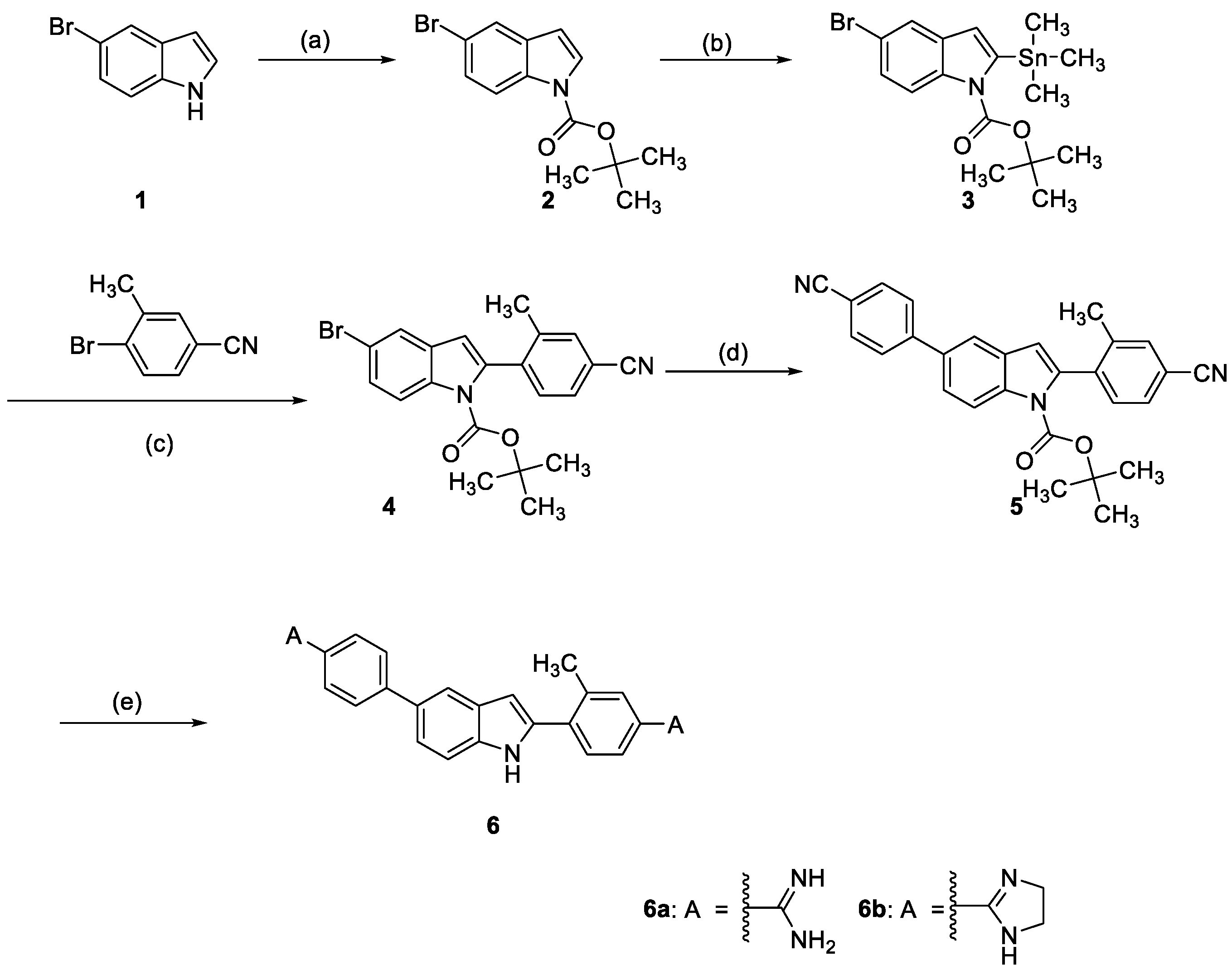
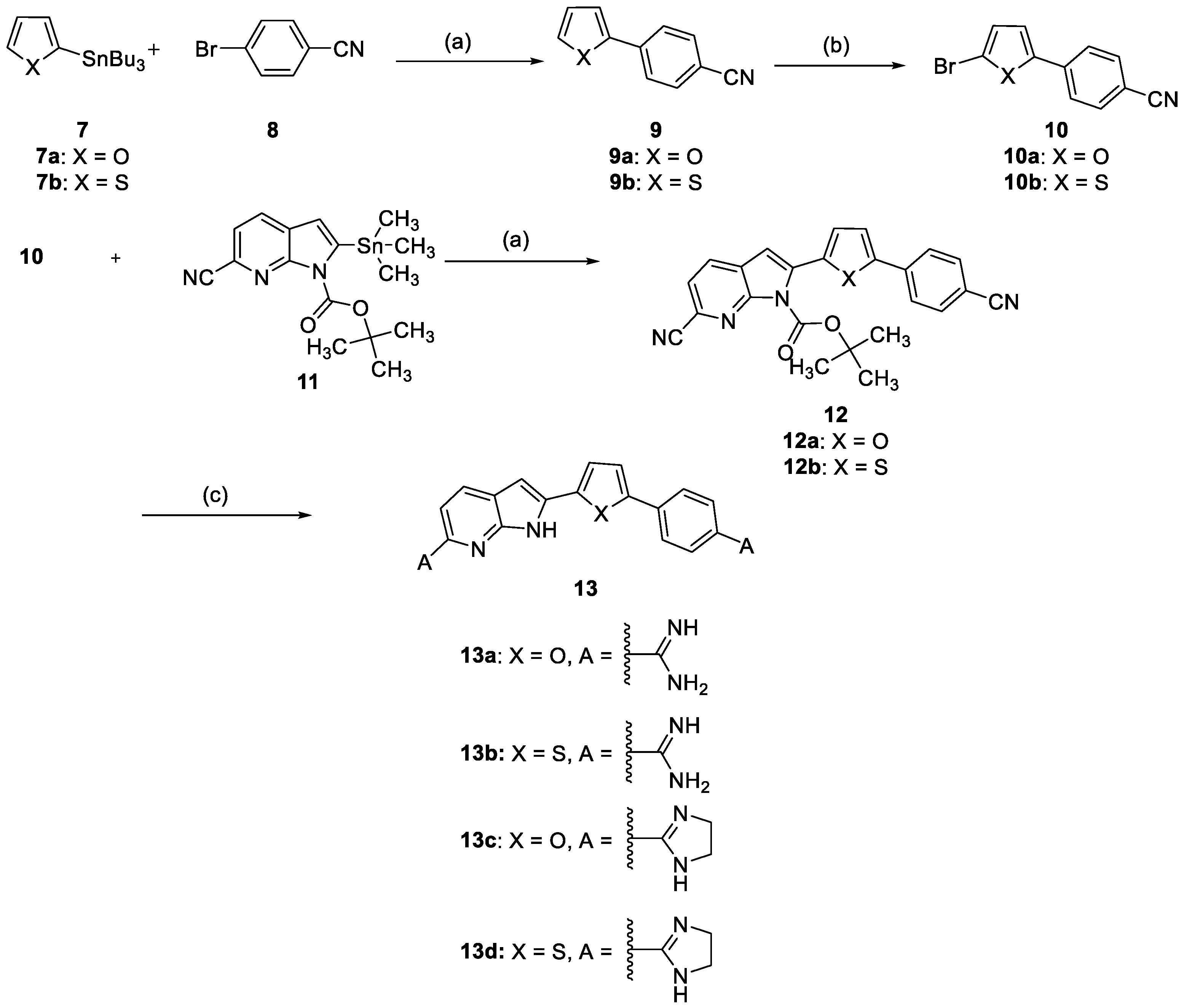
2.2. Chemistry
2.3. Biological Evaluations
2.3.1. Minimum Inhibitory Concentrations and Spectrum of Activity
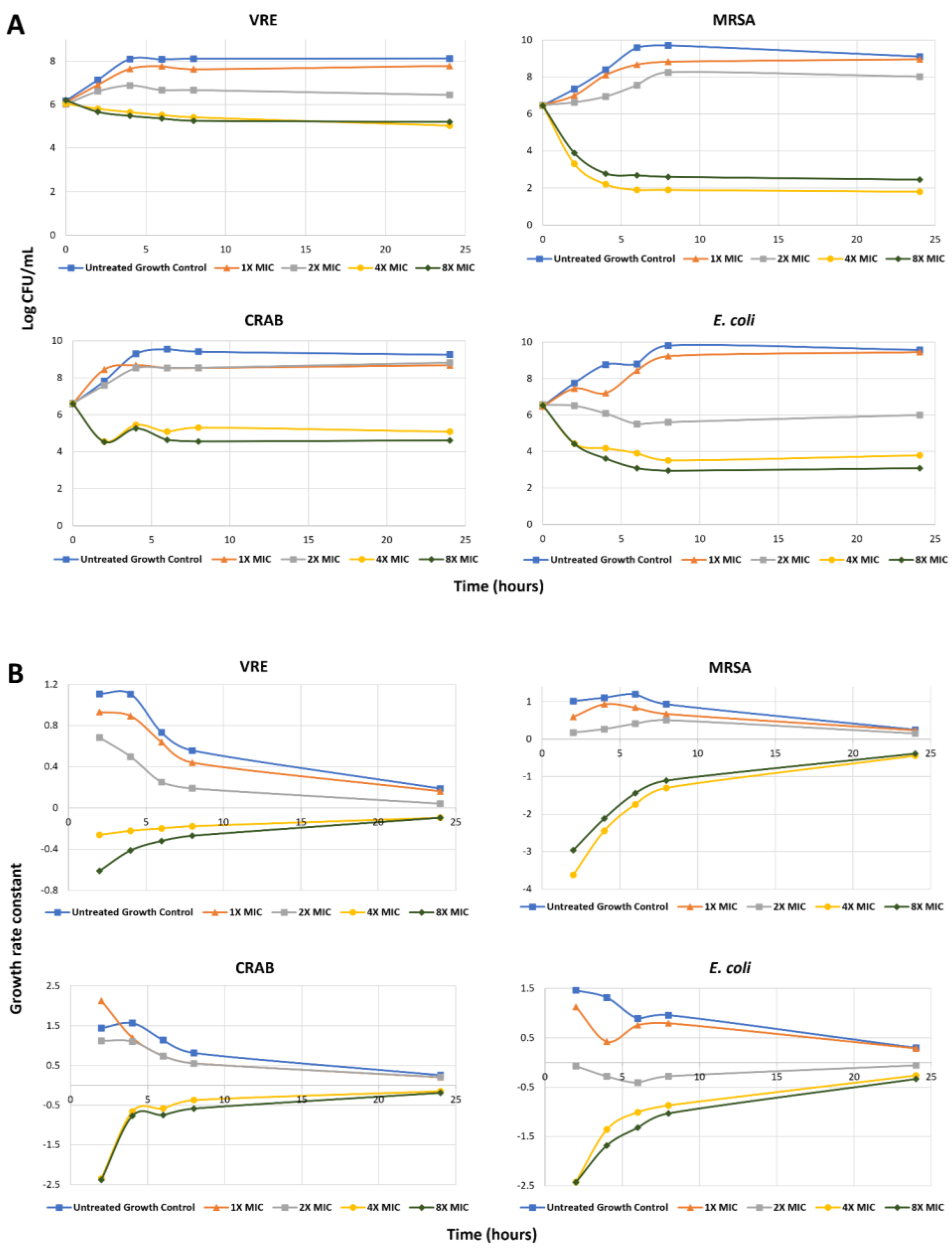
2.3.2. Time–Kill Assay
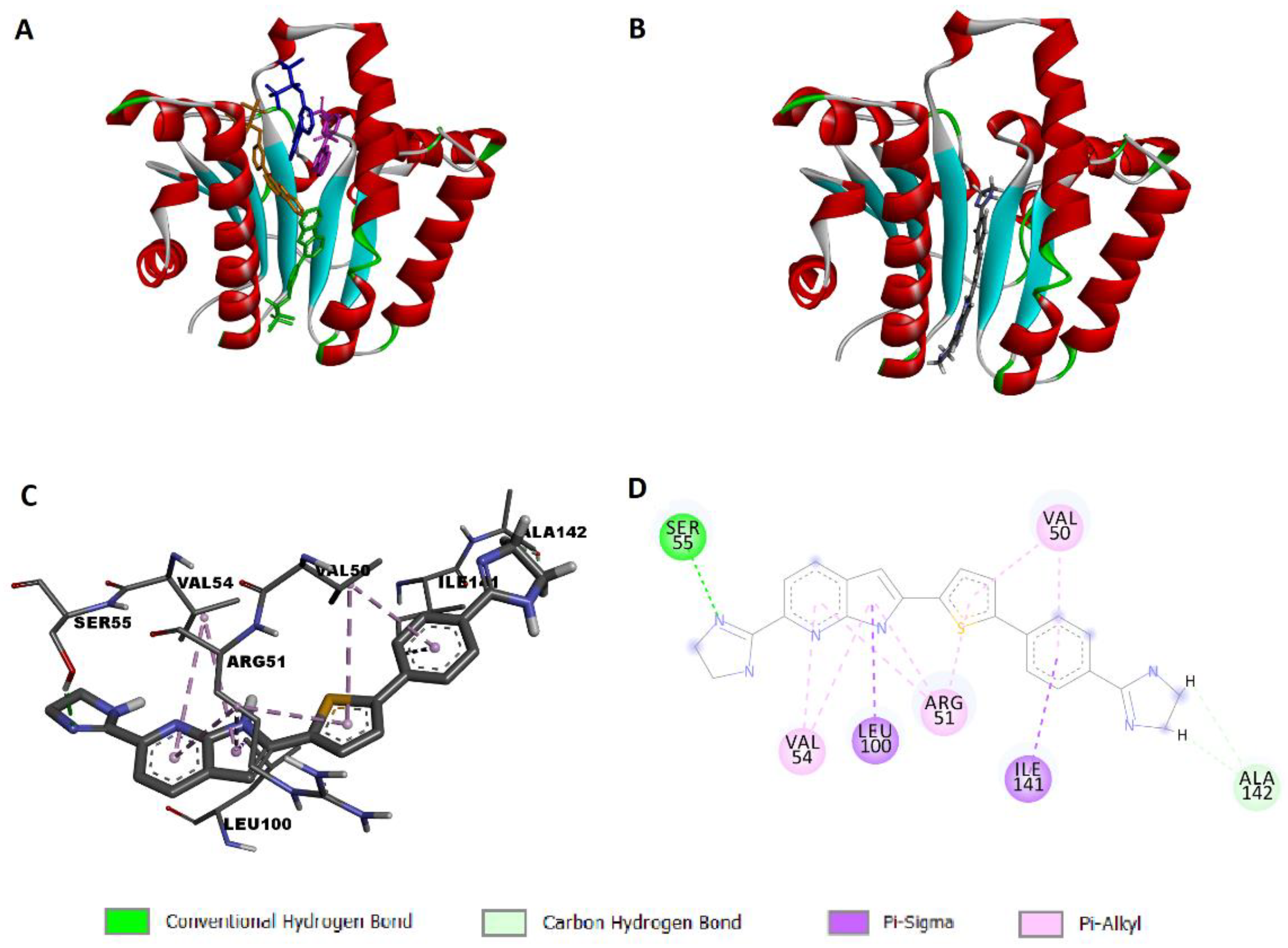
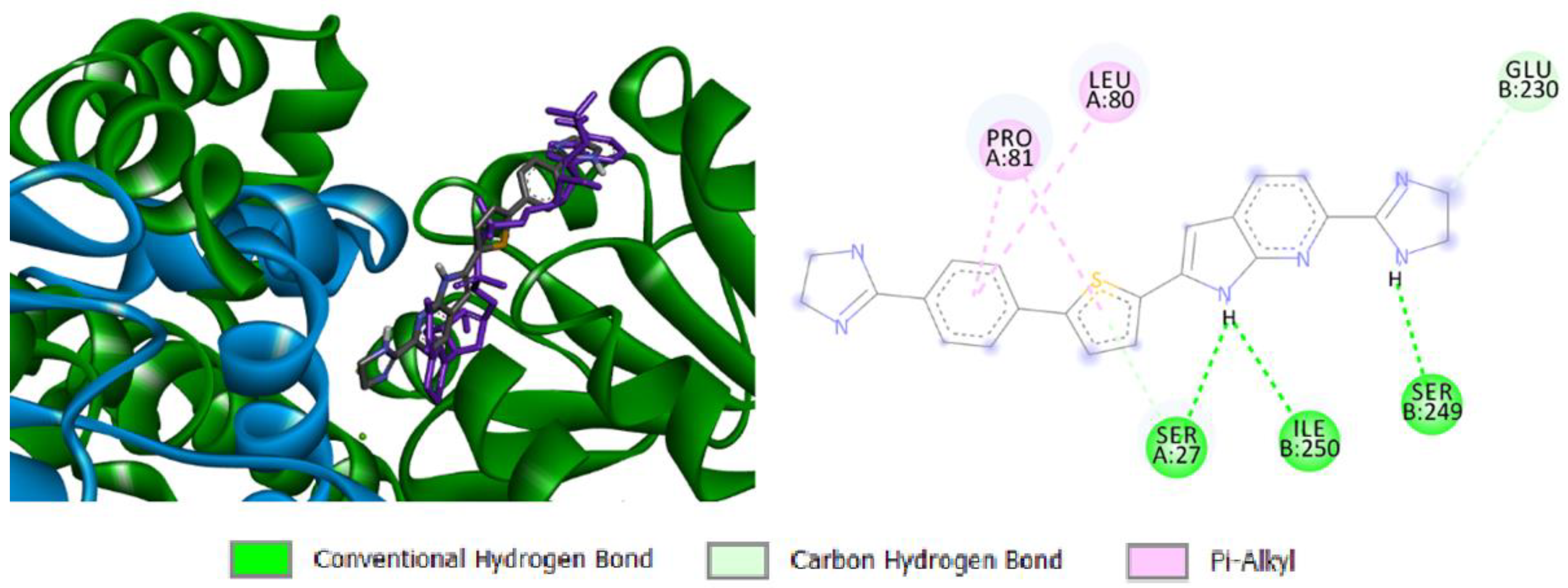
2.4. Docking Study
2.4.1. Docking Simulation of Compound 13d to UPPS
2.4.2. Docking Simulation of Compound 13d to KARI
2.4.3. Docking Simulation of Compound 13d to Bacterial DNA
3. Materials and Methods
3.1. Chemistry
3.2. Biological Evaluations
3.3. Docking Simulations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Davies, J. Where Have all the Antibiotics Gone? Can. J. Infect. Dis. Med. Microbiol. 2006, 17, 707296. [Google Scholar] [CrossRef]
- Theuretzbacher, U.; Outterson, K.; Engel, A.; Karlén, A. The global preclinical antibacterial pipeline. Nat. Rev. Microbiol. 2020, 18, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Kåhrström, C.T. Entering a post-antibiotic era? Nat. Rev. Microbiol. 2013, 11, 146. [Google Scholar] [CrossRef]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Kwon, J.H.; Powderly, W.G. The post-antibiotic era is here. Science 2021, 373, 471. [Google Scholar] [CrossRef]
- Gulia, K.; Hassan, A.H.E.; Lenhard, J.R.; Farahat, A.A. Escaping ESKAPE resistance: In vitro and in silico studies of multifunctional carbamimidoyl-tethered indoles against antibiotic-resistant bacteria. R. Soc. Open Sci. 2023, 10, 230020. [Google Scholar] [CrossRef] [PubMed]
- Denissen, J.; Reyneke, B.; Waso-Reyneke, M.; Havenga, B.; Barnard, T.; Khan, S.; Khan, W. Prevalence of ESKAPE pathogens in the environment: Antibiotic resistance status, community-acquired infection and risk to human health. Int. J. Hyg. Environ. Health. 2022, 244, 114006. [Google Scholar] [CrossRef] [PubMed]
- Zhen, X.; Lundborg, C.S.; Sun, X.; Hu, X.; Dong, H. Economic burden of antibiotic resistance in ESKAPE organisms: A systematic review. Antimicrob. Resist. Infect. Control. 2019, 8, 137. [Google Scholar] [CrossRef]
- Ayobami, O.; Brinkwirth, S.; Eckmanns, T.; Markwart, R. Antibiotic resistance in hospital-acquired ESKAPE-E infections in low- and lower-middle-income countries: A systematic review and meta-analysis. Emerg. Microbes Infect. 2022, 11, 443. [Google Scholar] [CrossRef]
- Idris, F.N.; Nadzir, M.M. Multi-drug resistant ESKAPE pathogens and the uses of plants as their antimicrobial agents. Arch. Microbiol. 2023, 205, 115. [Google Scholar] [CrossRef] [PubMed]
- Mulani, M.S.; Kamble, E.E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging Strategies to Combat ESKAPE Pathogens in the Era of Antimicrobial Resistance: A Review. Front. Microbiol. 2019, 10, 539. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.M.P.D.; Forde, B.M.; Kidd, T.J.; Harris, P.N.A.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walker, M.J. Antimicrobial Resistance in ESKAPE Pathogens. Clin. Microbiol. Rev. 2020, 33, e00181. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Bharath Prasad, A.S.; Mehta, C.H.; Nayak, U.Y. Antimicrobial peptide polymers: No escape to ESKAPE pathogens—A review. World J. Microbiol. Biotechnol. 2020, 36, 131. [Google Scholar] [CrossRef]
- Suckling, C.J.; Hunter, I.S.; Scott, F.J. Multitargeted anti-infective drugs: Resilience to resistance in the antimicrobial resistance era. Future Drug Discov. 2022, 4, FDD73. [Google Scholar] [CrossRef]
- Jukič, M.; Rožman, K.; Sova, M.; Barreteau, H.; Gobec, S. Anthranilic Acid Inhibitors of Undecaprenyl Pyrophosphate Synthase (UppS), an Essential Enzyme for Bacterial Cell Wall Biosynthesis. Front. Microbiol. 2019, 9, 3322. [Google Scholar] [CrossRef] [PubMed]
- Wun, S.J.; Johnson, L.A.; You, L.; McGeary, R.P.; Brueck, T.; Schenk, G.; Guddat, L.W. Inhibition studies of ketol-acid reductoisomerases from pathogenic microorganisms. Arch. Biochem. Biophys. 2020, 692, 108516. [Google Scholar] [CrossRef]
- Tyagi, R.; Duquerroy, S.; Navaza, J.; Guddat, L.W.; Duggleby, R.G. The crystal structure of a bacterial Class II ketol-acid reductoisomerase: Domain conservation and evolution. Protein Sci. 2005, 14, 3089. [Google Scholar] [CrossRef]
- Bayaraa, T.; Kurz, J.L.; Patel, K.M.; Hussein, W.M.; Bilyj, J.K.; West, N.P.; Schenk, G.; McGeary, R.P.; Guddat, L.W. Discovery, Synthesis and Evaluation of a Ketol-Acid Reductoisomerase Inhibitor. Chem. Eur. J. 2020, 26, 8958–8968. [Google Scholar] [CrossRef]
- Boeva, V. Analysis of Genomic Sequence Motifs for Deciphering Transcription Factor Binding and Transcriptional Regulation in Eukaryotic Cells. Front. Genet. 2016, 7, 24. [Google Scholar] [CrossRef]
- Rajewska, M.; Wegrzyn, K.; Konieczny, I. AT-rich region and repeated sequences—The essential elements of replication origins of bacterial replicons. FEMS Microbiol. Rev. 2012, 36, 408. [Google Scholar] [CrossRef]
- Rahman, A.; O’Sullivan, P.; Rozas, I. Recent developments in compounds acting in the DNA minor groove. Medchemcomm 2019, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Barrett, M.P.; Gemmell, C.G.; Suckling, C.J. Minor groove binders as anti-infective agents. Pharmacol. Ther. 2013, 139, 12. [Google Scholar] [CrossRef] [PubMed]
- Larsen, T.A.; Goodsell, D.S.; Cascio, D.; Grzeskowiak, K.; Dickerson, R.E. The Structure of DAPI Bound to DNA. J. Biomol. Struct. Dyn. 1989, 7, 477. [Google Scholar] [CrossRef] [PubMed]
- Tanious, F.A.; Spychala, J.; Kumar, A.; Greene, K.; Boykin, D.W.; Wilson, W.D. Different Binding Mode in AT and GC Sequences for Unfused-Aromatic Dications. J. Biomol. Struct. 1994, 11, 1063. [Google Scholar] [CrossRef]
- Hassan, A.H.E.; El-Sayed, S.M.; Yamamoto, M.; Gohda, J.; Matsumoto, T.; Shirouzu, M.; Inoue, J.-i.; Kawaguchi, Y.; Mansour, R.M.A.; Anvari, A.; et al. In Silico and In Vitro Evaluation of Some Amidine Derivatives as Hit Compounds towards Development of Inhibitors against Coronavirus Diseases. Viruses 2023, 15, 1171. [Google Scholar] [CrossRef]
- Farahat, A.A.; Kumar, A.; Wenzler, T.; Brun, R.; Paul, A.; Guo, P.; Wilson, W.D.; Boykin, D.W. Investigation of the effect of structure modification of furamidine on the DNA minor groove binding and antiprotozoal activity. Eur. J. Med. Chem. 2023, 252, 115287. [Google Scholar] [CrossRef]
- Depauw, S.; Lambert, M.; Jambon, S.; Paul, A.; Peixoto, P.; Nhili, R.; Marongiu, L.; Figeac, M.; Dassi, C.; Paul-Constant, C.; et al. Heterocyclic Diamidine DNA Ligands as HOXA9 Transcription Factor Inhibitors: Design, Molecular Evaluation, and Cellular Consequences in a HOXA9-Dependant Leukemia Cell Model. J. Med. Chem. 2019, 62, 1306–1329. [Google Scholar] [CrossRef]
- Simone, R.; Balendra, R.; Moens, T.G.; Preza, E.; Wilson, K.M.; Heslegrave, A.; Woodling, N.S.; Niccoli, T.; Gilbert-Jaramillo, J.; Abdelkarim, S.; et al. G-quadruplex-binding small molecules ameliorate C9orf72 FTD/ALS pathology in vitro and in vivo. EMBO Mol. Med. 2018, 10, 22–31. [Google Scholar] [CrossRef]
- Hu, L.; Kully, M.L.; Boykin, D.W.; Abood, N. Synthesis and in vitro activity of dicationic bis-benzimidazoles as a new class of anti-MRSA and anti-VRE agents. Bioorg. Med. Chem. Lett. 2009, 19, 1292–1295. [Google Scholar] [CrossRef]
- Hu, L.; Kully, M.L.; Boykin, D.W.; Abood, N. Optimization of the central linker of dicationic bis-benzimidazole anti-MRSA and anti-VRE agents. Bioorg. Med. Chem. Lett. 2009, 19, 3374–3377. [Google Scholar] [CrossRef]
- Hu, L.; Kully, M.L.; Boykin, D.W.; Abood, N. Synthesis and structure–activity relationship of dicationic diaryl ethers as novel potent anti-MRSA and anti-VRE agents. Bioorg. Med. Chem. Lett. 2009, 19, 4626–4629. [Google Scholar] [CrossRef] [PubMed]
- Stokes, J.M.; MacNair, C.R.; Ilyas, B.; French, S.; Côté, J.-P.; Bouwman, C.; Farha, M.A.; Sieron, A.O.; Whitfield, C.; Coombes, B.K.; et al. Pentamidine sensitizes Gram-negative pathogens to antibiotics and overcomes acquired colistin resistance. Nat. Microbiol. 2017, 2, 17028. [Google Scholar] [CrossRef] [PubMed]
- Wesseling, C.M.J.; Slingerland, C.J.; Veraar, S.; Lok, S.; Martin, N.I. Structure–Activity Studies with Bis-Amidines That Potentiate Gram-Positive Specific Antibiotics against Gram-Negative Pathogens. ACS Infect. Dis. 2021, 7, 3314–3335. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Guo, P.; Boykin, D.W.; Wilson, W.D. A New Generation of Minor-Groove-Binding—Heterocyclic Diamidines That Recognize G·C Base Pairs in an AT Sequence Context. Molecules 2019, 24, 946. [Google Scholar] [CrossRef]
- Donkor, I.O.; Clark, A.M. In vitro antimicrobial activity of aromatic diamidines and diimidazolines related to pentamidine. Eur. J. Med. Chem. 1999, 34, 639. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, X.; Wu, Y.; Zhang, W.; Chen, X.; You, X.; Hu, L. Synthesis and structure-activity relationship of novel bisindole amidines active against MDR Gram-positive and Gram-negative bacteria. Eur. J. Med. Chem. 2018, 150, 771. [Google Scholar] [CrossRef]
- Scott, F.J.; Khalaf, A.I.; Giordani, F.; Wong, P.E.; Duffy, S.; Barrett, M.; Avery, V.M.; Suckling, C.J. An evaluation of Minor Groove Binders as anti-Trypanosoma brucei brucei therapeutics. Eur. J. Med. Chem. 2016, 116, 116. [Google Scholar] [CrossRef]
- Zhu, W.; Wang, Y.; Li, K.; Gao, J.; Huang, C.-H.; Chen, C.-C.; Ko, T.-P.; Zhang, Y.; Guo, R.-T.; Oldfield, E. Antibacterial Drug Leads: DNA and Enzyme Multitargeting. J. Med. Chem. 2015, 58, 1215–1227. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, Y.; Sinko, W.; Hensler, M.E.; Olson, J.; Molohon, K.J.; Lindert, S.; Cao, R.; Li, K.; Wang, K.; et al. Antibacterial drug leads targeting isoprenoid biosynthesis. Proc. Natl. Acad. Sci. USA 2013, 110, 123. [Google Scholar] [CrossRef]
- Decker, M. Introduction. In Design of Hybrid Molecules for Drug Development; Decker, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–3. [Google Scholar] [CrossRef]
- Farahat, A.A.; Kumar, A.; Say, M.; Barghash, A.E.-D.M.; Goda, F.E.; Eisa, H.M.; Wenzler, T.; Brun, R.; Liu, Y.; Mickelson, L.; et al. Synthesis, DNA binding, fluorescence measurements and antiparasitic activity of DAPI related diamidines. Bioorg. Med. Chem. 2010, 18, 557–566. [Google Scholar] [CrossRef]
- Kumar, A.; Say, M.; Boykin, D.W. Synthesis of New Substituted 2-(Trimethylstannyl)indoles. Synthesis 2008, 2008, 707–710. [Google Scholar] [CrossRef]
- Guo, P.; Farahat, A.A.; Paul, A.; Harika, N.K.; Boykin, D.W.; Wilson, W.D. Compound Shape Effects in Minor Groove Binding Affinity and Specificity for Mixed Sequence DNA. J. Am. Chem. Soc. 2018, 140, 14761–14769. [Google Scholar] [CrossRef]
- Guo, P.; Paul, A.; Kumar, A.; Farahat, A.A.; Kumar, D.; Wang, S.; Boykin, D.W.; Wilson, W.D. The Thiophene “Sigma-Hole” as a Concept for Preorganized, Specific Recognition of G⋅C Base Pairs in the DNA Minor Groove. Chem. Eur. J. 2016, 22, 15404–15412. [Google Scholar] [CrossRef]
- Laughlin, S.; Wang, S.; Kumar, A.; Farahat, A.A.; Boykin, D.W.; Wilson, W.D. Resolution of Mixed Site DNA Complexes with Dimer-Forming Minor-Groove Binders by Using Electrospray Ionization Mass Spectrometry: Compound Structure and DNA Sequence Effects. Chem. Eur. J. 2015, 21, 5528–5539. [Google Scholar] [CrossRef]
- Paul, A.; Kumar, A.; Nanjunda, R.; Farahat, A.A.; Boykin, D.W.; Wilson, W.D. Systematic synthetic and biophysical development of mixed sequence DNA binding agents. Org. Biomol. Chem. 2017, 15, 827–835. [Google Scholar] [CrossRef]
- Jiang, S.; Zhuang, H.; Zhu, F.; Wei, X.; Zhang, J.; Sun, L.; Ji, S.; Wang, H.; Wu, D.; Zhao, F.; et al. The Role of mprF Mutations in Seesaw Effect of Daptomycin-Resistant Methicillin-Resistant Staphylococcus aureus Isolates. Antimicrob. Agents Chemother. 2022, 66, e01295–e01221. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, G.J.; Domingues, S. Interplay between Colistin Resistance, Virulence and Fitness in Acinetobacter baumannii. Antibiotics 2017, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Lee Yong, H.; Helmann John, D. Reducing the Level of Undecaprenyl Pyrophosphate Synthase Has Complex Effects on Susceptibility to Cell Wall Antibiotics. Antimicrob. Agents Chemother. 2013, 57, 4267–4275. [Google Scholar] [CrossRef]
- Dhanda, G.; Acharya, Y.; Haldar, J. Antibiotic Adjuvants: A Versatile Approach to Combat Antibiotic Resistance. ACS Omega 2023, 8, 10757–10783. [Google Scholar] [CrossRef] [PubMed]
- Balaban, N.Q.; Helaine, S.; Lewis, K.; Ackermann, M.; Aldridge, B.; Andersson, D.I.; Brynildsen, M.P.; Bumann, D.; Camilli, A.; Collins, J.J.; et al. Definitions and guidelines for research on antibiotic persistence. Nat. Rev. Microbiol. 2019, 17, 441. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rojas, A.; Rolff, J. Antimicrobial activity of cationic antimicrobial peptides against stationary phase bacteria. Front. Microbiol. 2022, 13, 1029084. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-Y.; Ko, T.-P.; Liang, P.-H.; Wang, A.H.J. Catalytic Mechanism Revealed by the Crystal Structure of Undecaprenyl Pyrophosphate Synthase in Complex with Sulfate, Magnesium, and Triton. J. Biol. Chem. 2003, 278, 29298. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-Y.; Ko, T.-P.; Chen, A.P.-C.; Wang, A.H.-J.; Liang, P.-H. Substrate binding mode and reaction mechanism of undecaprenyl pyrophosphate synthase deduced from crystallographic studies. J. Mol. Biol. 2004, 13, 971. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.-H.; Lonhienne, T.G.A.; Winzor, D.J.; Schenk, G.; Guddat, L.W. Bacterial and Plant Ketol-Acid Reductoisomerases Have Different Mechanisms of Induced Fit during the Catalytic Cycle. J. Mol. Biol. 2012, 424, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Kurz, J.L.; Patel, K.M.; Wun, S.J.; Hussein, W.M.; Lonhienne, T.; West, N.P.; McGeary, R.P.; Schenk, G.; Guddat, L.W. Discovery of a Pyrimidinedione Derivative with Potent Inhibitory Activity against Mycobacterium tuberculosis Ketol–Acid Reductoisomerase. Chem. Eur. J. 2021, 27, 3130–3141. [Google Scholar] [CrossRef] [PubMed]
- The Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; Fifteenth Informational Supplement. CLSI/NCCLS Document M100-S15; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2005. [Google Scholar]

| Compound | MIC (μg/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Antibiotic-Resistant Gram-Positive | Antibiotic-Susceptible Gram-Positive | Antibiotic-Resistant Gram-Negative | Antibiotic-Susceptible Gram-Negative | ||||||
| E. faecium (VRE) a | S. aureus (MRSA) b | E. faecalis | S. aureus (MSSA) c | K. pneumonia (CRE) d | A. baumannii (CRAB) e | A. baumannii | P. aeruginosa | E. coli | |
| 6a | 16 | 2 | >128 | 2 | >128 | 32 | 32 | 32 | 32 |
| 6b | 32 | 32 | >128 | 32 | >128 | 128 | 128 | >128 | 128 |
| 13a | 16 | 8 | >128 | 32 | >128 | 128 | 32 | 16 | 32 |
| 13b | 64 | 32 | >128 | 32 | >128 | 64 | 64 | 64 | 32 |
| 13c | 32 | 32 | >128 | 32 | >128 | 128 | 128 | >128 | 32 |
| 13d | 0.25 | 0.25 | 1 | 0.5 | 4 | 2 | 32 | >128 | 0.5 |
| Vancomycin | 8 | 1–2 | 2 | 2 | - | - | - | - | - |
| Gentamycin | - | - | - | - | 64 | >128 | 8 | 2 | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Sayed, S.M.; Ahmed, S.A.; Gulia, K.; Lenhard, J.R.; Hassan, A.H.E.; Farahat, A.A. Small Molecules Incorporating Privileged Amidine Moiety as Potential Hits Combating Antibiotic-Resistant Bacteria. Pharmaceuticals 2023, 16, 1040. https://doi.org/10.3390/ph16071040
El-Sayed SM, Ahmed SA, Gulia K, Lenhard JR, Hassan AHE, Farahat AA. Small Molecules Incorporating Privileged Amidine Moiety as Potential Hits Combating Antibiotic-Resistant Bacteria. Pharmaceuticals. 2023; 16(7):1040. https://doi.org/10.3390/ph16071040
Chicago/Turabian StyleEl-Sayed, Selwan M., Samar A. Ahmed, Kanika Gulia, Justin R. Lenhard, Ahmed H.E. Hassan, and Abdelbasset A. Farahat. 2023. "Small Molecules Incorporating Privileged Amidine Moiety as Potential Hits Combating Antibiotic-Resistant Bacteria" Pharmaceuticals 16, no. 7: 1040. https://doi.org/10.3390/ph16071040
APA StyleEl-Sayed, S. M., Ahmed, S. A., Gulia, K., Lenhard, J. R., Hassan, A. H. E., & Farahat, A. A. (2023). Small Molecules Incorporating Privileged Amidine Moiety as Potential Hits Combating Antibiotic-Resistant Bacteria. Pharmaceuticals, 16(7), 1040. https://doi.org/10.3390/ph16071040








